Abstract
Arsenic trioxide (ATO) induces disease remission in acute promyelocytic leukemia (APL) patients, but not in non-APL acute myeloid leukemia (AML) patients. ATO at therapeutic concentrations (1-2 μM) induce APL NB4, but not non-APL HL-60, cells to undergo apoptosis through the mitochondrial pathway. The role of antiapoptotic protein Mcl-1 in ATO-induced apoptosis was determined. The levels of Mcl-1 were decreased in NB4, but not in HL-60, cells after ATO treatment through proteasomal degradation. Both GSK3β inhibitor SB216763 and siRNA blocked ATO-induced Mcl-1 reduction as well as attenuated ATO-induced apoptosis in NB4 cells. Silencing Mcl-1 sensitized HL-60 cells to ATO-induced apoptosis. Both ERK and AKT inhibitors decreased Mcl-1 levels and enhanced ATO-induced apoptosis in HL-60 cells. Sorafenib, a Raf inhibitor, activated GSK3β by inhibiting its phosphorylation, decreased Mcl-1 levels, and decreased intracellular glutathione levels in HL-60 cells. Sorafenib plus ATO augmented ROS production and apoptosis induction in HL-60 cells and in primary AML cells. These results indicate that ATO induces Mcl-1 degradation through activation of GSK3β in APL cells and provide a rationale for utilizing ATO in combination with sorafenib for the treatment of non-APL AML patients.
Keywords: Arsenic trioxide, Mcl-1, glycogen synthase kinase-3β, myeloid leukemia, apoptosis
Introduction
Arsenic trioxide (ATO) alone successfully induces remission in acute promyelocytic leukemia (APL) patients with the PML-RARα fusion protein and is approved for relapsed APL treatment (1). The induction of apoptosis and partial differentiation has been found to be the mechanism of action of ATO in APL (2-4). Although ATO-induced PML-RARα degradation occurs during therapy for APL, ATO induces APL-cell apoptosis by a process that is independent of PML-RARα degradation (5, 6). ATO, as a single agent, has not been successful in treatment of other types of acute myeloid leukemia (AML). Considering the minimal toxicity of ATO in APL patients, it has been suggested that ATO could be combined with other agents for AML treatment (7). Previously we, and other groups, have found that ATO-induced apoptosis in APL cells is, at least in part, mediated through H2O2 accumulation (8, 9), which is followed by changes in mitochondrial transmembrane permeability, cytochrome c release, and caspase activation (8). Moreover, our studies showed that the remarkable sensitivity of APL cells to ATO-induced apoptosis, compared to cells isolated from other types of myeloid leukemia such as HL-60 and U937, was correlated with greater H2O2 accumulation (8). Although it has been found that agents such as ascorbic acid, which increase the levels of H2O2, enhanced ATO apoptosis induction of non-APL malignant cells (7), a report noted that reactive oxygen species (ROS) seem not to be required for ATO-induced apoptosis (10). Multiple signaling pathways appear to be regulated by ATO in APL cells (11). We thought that signaling pathways, in addition to ROS production, might be involved in ATO-induced apoptosis in APL cells.
The mitochondrial apoptotic pathway is controlled by three main antiapoptotic proteins, Bcl-2, Bcl-xL, and Mcl-1, which block the functions of the proapoptotic proteins Bax and Bak (12). Recently we found that APL NB4 cells expressed Bcl-2 and Mcl-1, but not Bcl-xL (13). Mcl-1 has been found to play a critical role in the regulation of neutrophil apoptosis and to be essential for the survival of hematopoietic stem cells (14, 15). Therefore, Mcl-1 could play an important role in protecting cells from apoptotic death in APL cells. Activated PI3K/AKT/mTOR signaling occurs in AML cells (16). Activated mTOR signaling was found to promote cell survival by increasing translation of proteins, including Mcl-1 (17). Mcl-1 is a short-lived protein due to rapid degradation after post-transcriptional phosphorylation by ERK and AKT kinases (18). It has been found that ATO treatment decreased AKT levels in APL cells and that inhibitors of ERK and AKT enhanced ATO-induced apoptosis in non-APL leukemia cells (19, 20). Recently, it has been found that activated glycogen synthase kinase-3 (GSK3) phosphorylated Mcl-1 and led to proteasomal degradation of Mcl-1 (21). Since GSK3 is inhibited by AKT (22), we suspected that Mcl-1 levels are regulated by ATO and that Mcl-1 might have a role in ATO-induced apoptosis of APL cells.
APL NB4 cells, but not non-APL HL-60 cells, respond to apoptosis induction following ATO treatment at therapeutic concentrations (1-2 μM) (8). We compared the regulation of Mcl-1 protein levels due to ATO treatment in NB4 and HL-60 cells and found that the Mcl-1 protein was decreased in NB4 cells, but not in HL-60 cells. The mechanism of Mcl-1 down-regulation by ATO treatment in NB4 cells was explored by examining the signaling pathways of ERK, mTOR, AKT and GSK3β. We found that ATO decreased Mcl-1 levels by activating GSK3β by inhibition of ERK and AKT in APL cells. The role of decreased Mcl-1 levels in ATO-induced apoptosis was studied in HL-60 cells by silencing Mcl-1 using siRNA. To improve the apoptotic effects of ATO in non-APL cells, we tested the combined apoptotic effects of ATO with an AKT or an ERK inhibitor in HL-60 cells and investigated the potential mechanisms of apoptosis induction of these combinations. We found that sorafenib, a Raf inhibitor, decreased Mcl-1 levels, decreased intracellular reduced glutathione (GSH) levels, and augmented ATO-induced ROS production and apoptosis in HL-60 cells as well as in primary AML cells. Our data indicate that treatment with ATO plus sorafenib should benefit non-APL AML patients.
Materials and Methods
Reagents
ATO solution was obtained from the pharmacy of our hospital. MEK inhibitors, U0126 and PD184352, and the Raf inhibitor sorafenib were purchased from LC Laboratories (Woburn, MA). The mTOR inhibitor rapamycin, the PI3K inhibitor LY290024, and the GSK-3β inhibitor SB216763 were purchased from Sigma Chemical Co. (St. Louis, MO). Antibody to poly-(ADP-ribose)-polymerase (PARP) was obtained from Boehringer Mannheim (Indianapolis, IN); to pro-caspase-3 was from BD Biosciences (San Diego, CA); to Mcl-1, ERK1, Bcl-2, p-ERK, and β-actin were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); to Bak, p-MEK (Ser217/221), p-Mcl-1 (Thr163), p70S6K, p-p70S6K (Thr389), p-p70S6K (Thr421/Ser424), p-S6 ribosomal protein (Ser235/236), p-GSK-3β, and GSK-3β (Ser9) were from Cell Signaling Technology, Inc. (Beverly, MA).
Cell lines
NB4 and HL-60 cells were cultured in RPMI 1640 medium supplemented with 100 units/mL penicillin, 100 μg/mL streptomycin, 1 mmol/L L-glutamine, and 10% (v/v) heat-inactivated fetal bovine serum (FBS) as we reported before (8). HL-60 cells were developed from an APL patient without the t(15;17) translocation and are considered a non-APL AML cell line (23). HP100-1 cells, a H2O2-resitant derivative of HL-60 cells (24), were obtained from the Japanese Cell Bank.
Isolation of patient-derived leukemic blasts
Leukemic blasts were obtained from one bone marrow and three peripheral blood samples of AML patients with AML of FAB subtypes M1 and M2. These studies have been sanctioned by the Investigational Review Board of Mount Sinai School of Medicine and all patients provided informed consent. Whole blood or bone marrow was collected in heparinized tubes. The leukemia cells were isolated using Ficoll-Hypaque density gradient separation. The blasts were washed twice with PBS, resuspended in RPMI 1640, supplemented with 20% FBS, and maintained at 1 × 107 cells/mL.
Quantitation of apoptotic cells, determination of H2O2 production, Western blot analysis, analysis of Bak conformational change, and RNA interference were performed as we reported before (13).
Measurement of intracellular GSH content
The levels of intracellular GSH were measured by a monochlorobimane (mBCl) fluorometric method in which mBCl was used as a sensitive and specific probe to analyze GSH in intact cells (25). Briefly, 3×106 cells were washed once in PBS, resuspended in 1 mL PBS containing 100 μmol/L mBCl, and maintained at 37°C in the dark for 30 min before analysis. The formation of the fluorescent adduct (GS-mBCl) was monitored with a Multi-mode microplate reader (Spectra Max M5e, Molecular devices, LLC) using excitation and emission wavelengths of 395 and 482 nm, respectively. The GSH content was calculated as nanomoles per 106 cells based on a GSH standard curve.
Statistical analysis
Data were analyzed for statistical significance using the Student's t test (Microsoft Excel, Microsoft Corp.). A p-value of less than 0.05 was considered statistically significant.
Results
ATO decreases Mcl-1 levels in NB4 cells, but not in HL-60 cells
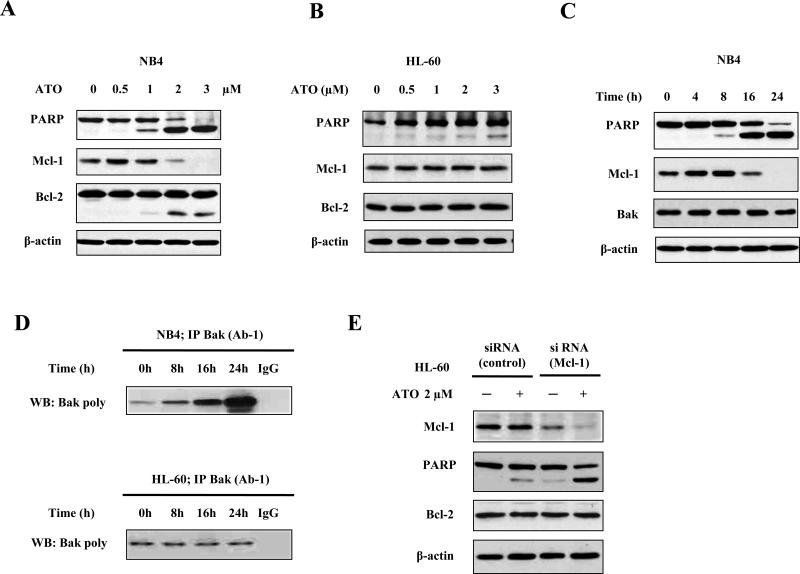
NB4 and HL-60 cells were treated with various concentrations of ATO for 24 h. The levels of Mcl-1, Bcl-2, and PARP were determined and compared. In NB4 cells, ATO at the lowest concentration tested (0.5 μM) slightly increased the level of Mcl-1 protein, but at increased concentrations (2 μM and 3 μM) significantly decreased Mcl-1 levels (Fig. 1A). The levels of Bcl-2 were not significantly changed, except that a small portion of cleaved fragment was observed by treatment with higher concentrations of ATO (Fig. 1A). Unlike in NB4 cells, in HL-60 cells ATO treatment did not change the levels of Mcl-1 protein (Fig. 1B). In NB4 cells after ATO treatment, PARP was cleaved which correlated with decreases in the Mcl-1 levels (Fig. 1A). In the time-course study of Mcl-1 levels in NB4 cells treated with 2 μM ATO, decreases in Mcl-1 levels were detected after treatment for 16 h (Fig. 1C). Mcl-1 is known to preferably bind to Bak to block mitochondrial apoptosis (12). We used the antibody Bak (Ab-1), which specifically recognizes the active form of Bak, to compare the levels of active Bak to the amount of total Bak present after treatment with 2 μM ATO in both NB4 and HL-60 cells. After treatment with 2 μM ATO for 16 h, the levels of active Bak were significantly increased in NB4 cells, but not in HL-60 cells (Fig. 1D). To further test if Mcl-1 down-regulation contributes to ATO-induced apoptosis, Mcl-1 was knocked-down using siRNA in HL-60 cells. HL-60 cells transfected with Mcl-1 siRNA have decreased Mcl-1 levels and enhanced response to ATO-induced apoptosis based on the detection of PARP cleavage (Fig. 1E). These data suggest that reduction of Mcl-1 protein contributes to ATO-induced apoptosis.
Fig. 1. Down-regulation of Mcl-1 contributes to ATO-induced apoptosis in leukemia cells.
(A) Down-regulation of Mcl-1 levels by ATO treatment in NB4 cells. (B) Down-regulation of Mcl-1 levels by ATO treatment in HL-60 cells. (C) Time-dependent down-regulation of Mcl-1 by ATO treatment in NB4 cells. NB4 and HL-60 cells were treated with ATO at the indicated concentrations for 24 h or with 2 μM ATO for the indicated times. The relative levels of PARP, Mcl-1, Bcl-2, and β-actin were determined using specific antibodies with Western blot analysis. β-actin was used as a loading control. (D) Bak activation by ATO treatment in both NB4 and HL-60 cells. NB4 and HL-60 cells were treated with ATO at 2 μM for the indicated times and lysed in CHAPS lysis buffer. Total Bak protein was immunoprecipitated with anti-Bak (AB-1) antibody and conformationally changed Bak was probed using poly anti-Bak antibody. (E) Silencing Mcl-1 enhances ATO-induced apoptosis in HL-60 cells. HL-60 cells transfected with Mcl-1 siRNA or control siRNA were treated with 2 μM ATO for 24 h. The relative levels of PARP, Mcl-1, Bcl-2, and β-actin were determined using specific antibodies with Western blot analysis.
The ATO-induced reduction of Mcl-1 protein levels in NB4 cells is correlated with inhibition of ERK signaling
It has been found that Mcl-1 phosphorylation at the Thr163 site by ERK leads to a prolonged Mcl-1 half-life by preventing its degradation (26). We studied the levels of p-Mcl-1(Thr163) in NB4 cells treated with ATO. ATO treatment at high concentrations reduced p-Mcl-1(Thr163) levels. This is associated with decreases in p-ERK levels (Fig. 2A). ERK is activated due to phosphorylation by MEK which itself is phosphorylated by Raf (27). ATO treatment also reduced p-MEK levels in NB4 cells. In a time course study in NB4 cells after treatment with 2 μM ATO, reduced p-MEK, p-ERK, and p-Mcl-1(Thr163) levels occurred at 8 h and reductions in Mcl-1 levels occurred after 16 h (Fig. 2B). So the inhibition of MEK/ERK phosphorylation occurs earlier than the decreases in Mcl-1 levels. To confirm the role of ERK inhibition in Mcl-1 regulation due to ATO, two ERK inhibitors, U0126 and PD184352, and one Raf inhibitor, sorafenib, were used to test if they decrease Mcl-1 levels and enhance ATO-induced apoptosis in NB4 cells. Pretreatment of NB4 cells with U0126, PD184352, or sorafenib decreased Mcl-1 levels, but did not induce apoptosis. When ATO was combined with any one of these three agents, augmented PARP cleavage and Mcl-1 decreases were obtained (Fig. 2C, 2D). Using sorafenib with ATO as a representative combination, the enhanced apoptotic effect was confirmed by Annexin V assay. More than 58% of apoptotic cells were obtained following combination treatment while using 1 μM ATO alone induced only 13% and using 5 μM sorafenib alone induced only 7% of the cells to undergo apoptosis (Fig. 2E). Since further reduction in Mcl-1 levels did not correlate with decreases in p-ERK levels, other mechanisms could also contribute to reduction in Mcl-1 levels.
Fig. 2. ATO reduces Mcl-1 and phosphorylated ERK levels in NB4 cells.
(A, B) Reduction of phosphorylated ERK and MEK following ATO treatment in NB4 cells. NB4 cells were treated with ATO at the indicated concentrations for 24 h or treated with 2 μM ATO for the indicated times. The levels of Mcl-1, p-MER, ERK, p-ERK, p-Mcl-1(Thr163), and β-actin were determined using specific antibodies with Western blot analysis. (C, D, E) The combined effects of ATO with MEK/ERK inhibitors on Mcl-1 levels. NB4 cells were pretreated with 5 μM U0126 (C), 1 μM PD184352 (C), or 5 μM sorafenib (D) for 2 h and then treated with 1 μM ATO for another 24 h. The levels of PARP, Mcl-1, p-Mcl-1(Thr163), p-MEK, p-ERK, and β-actin were determined using specific antibodies with Western blot analysis. (E) The effect of ATO plus sorafenib on inducing apoptosis. NB4 were treated with 5 μM sorafenib, 1 μM ATO, or their combination and apoptotic cells were analyzed with annexin V-FITC using flow cytometry.
Inhibition of mTOR does not contribute to ATO-induced reduction in Mcl-1 levels and apoptosis in NB4 cells
There is accumulating evidence that Mcl-1 is translationally up-regulated by mTORC1, a downstream target of PI3K/AKT (17). mTOR is activated by AKT and it stimulates protein translation by phosphorylating eIF4E binding protein (4E-BP1) as well as p70S6K which phosphorylates S6. In addition, p70S6K is also activated by ERK. The phosphorylation sites of p70S6K by mTOR and ERK differ. ERK phostorylates p70S6K at Thr421/Ser424, while mTOR phosphorylates p70S6K at Thr389. To determine if reduction of Mcl-1 levels by ATO treatment is due to the inhibition of mTOR signaling, the relative levels of phosphorylated mTOR, p70S6K, 4EBP1, and S6 were determined. Consistent with a previously report (19) we found that AKT levels were decreased following ATO treatment at concentration higher than 2 μM (Fig. 3A). Correlated with decreases in AKT levels, the levels of p-mTOR, p-p70S6K, and p-4E-BP1 were also decreased after ATO treatment (Fig. 3A). It should be pointed out that p70S6K levels were also decreased by ATO treatment at concentrations above 2 μM for 24 h. However, the p-S6 level was decreased by ATO treatment at a concentration of only 1 μM. A time-dependent study indicated that the level of p-p70S6K(Thr389) was decreased at 8 h treatment without reduction in Mcl-1 levels which suggests that inhibition of mTOR does not mediate the reduction of Mcl-1 levels (Fig. 3B). To study if inhibition of mTOR affected ATO-induced Mcl-1 protein reduction and apoptosis, rapamycin, an mTOR inhibitor, was used. Rapamycin at a concentration of 40 nM decreased p-p70S6K(Thr389) and p-S6, but not p-p70S6K(Thr421/Ser424) and Mcl-1 levels (Fig. 3C). Rapamycin failed to be synergistic with ATO in reducing Mcl-1 levels in NB4 cells, although it effectively led to reduction in p-p70S6K (Thr389) levels (Fig. 3C). Moreover, rapamycin pretreatment did not enhance 1 μM ATO-induced apoptosis as determined by both PARP cleavage (Fig. 3C) and annexin V assay (Fig. 3D). These data suggest that translational regulation by mTOR signaling is not the key signaling pathway by which ATO treatment leads to decreased Mcl-1 protein levels.
Fig. 3. Inhibition of the AKT/mTOR pathway does not enhance ATO-induced apoptosis.
(A, B) Inhibition of the AKT/mTOR signaling pathway by ATO in a dose- and time-dependent manner. NB4 cells were treated with ATO at the indicated concentrations for 24 h or treated with 2 μM ATO for the indicated times. The levels of Mcl-1, AKT, p-mTOR, p-p70S6K(Thr389), p-4E-BP1, p-S6(Ser235/236), and β-actin were determined using specific antibodies with Western blot analysis. (C, D) The combined effects of rapamycin plus ATO on both Mcl-1 levels and apoptosis. NB4 cells were pretreated with 40 nM rapamycin for 2 h and then treated with 1 μM ATO for another 24 h. The levels of PARP, Mcl-1, p-ERK, p-p70S6K (Thr421/Ser424), p-p70S6K (Thr389), p-S6 (Ser235/236), and β-actin were determined using specific antibodies with Western blot analysis (C). Apoptotic cells were detected with annexin V-FITC using flow cytometry (D).
GSK-3β activation is required for Mcl-1 degradation and apoptosis induction by ATO treatment in NB4 cells
Recently it has been found that Mcl-1 can be phosphorylated by GSK-3β at Ser159, resulting in Mcl-1 ubiquitination and its rapid proteasomal degradation (21, 28). Both AKT and ERK can phosphorylate GSK-3β on the Ser9 residue which leads to GSK-3β inactivation (29). The levels of p-GSK-3β on ser9, GSK-3β and Mcl-1 protein were determined in NB4 cells after ATO treatment. ATO treatment led to reduction in levels of p-GSK-3β on ser9 and Mcl-1 without changing GSK-3β protein levels (Fig. 4A). Since ATO inhibited AKT and ERK in NB4 cells (Fig, 2A, 3A), it suggests that phosphorylation of GSK-3β on the Ser9 residue by AKT/ERK leads to its inactivation and that ATO decreases Mcl-1 level through activation of GSK3β due to inhibition of its phosphorylation. To determine if GSK-3β activation is required for the reduction in Mcl-1 levels upon ATO treatment in NB4 cells, a cell-permeable inhibitor of GSK3β, SB216763, was used (30). Pretreatment of NB4 cells with SB216763 totally blocked reduction of Mcl-1 levels in cells treated with 2 μM ATO. The reductions in p-ERK and AKT levels by ATO were not blocked by SB216763 (Fig. 4B). SB 216763 alone decreased the levels of p-ERK, but not AKT (Fig. 4B). The apoptosis induced by ATO at 2 μM was significantly attenuated, although not completely, by SB 216763 treatment as determined by PARP cleavage (Fig. 4B). To further test the requirement of GSK3β activation for Mcl-1 degradation, GSK3β was silenced using a siRNA. Silencing GSK3β blocked the Mcl-1 reduction in ATO treated NB4 cells (Fig.4C). To test if the Mcl-1 decrease is through a proteasomal pathway, NB4 cells were pretreated with a proteasome inhibitor MG132. MG132 partially blocked the decrease in the levels of Mcl-1 due to ATO treatment (Fig. 4D). To confirm the role of AKT in decreasing p-GSK-3β(Ser9) and Mcl-1 levels, the AKT inhibitor, LY294002, was used. LY294002 treatment led to reduction in p-GSK-3β(Ser9) and in Mcl-1 levels and enhanced ATO-induced apoptosis as determined by PARP cleavage (Suppl. Fig. 1A). ERK inhibitors, U0126 and PD184352, decreased p-GSK-3β(Ser9) and Mcl-1 levels. The reduction of Mcl-1 levels was further augmented by adding ATO together with both agents (Suppl. Fig. 1B). These data suggest that inhibition of ERK leads to reduced Mcl-1 levels not only by decreasing Mcl-1 phosphorylation at Thr163, but also by promoting phosphorylation at Ser159. Based on these results, we propose that ATO treatment leads to reduction in Mcl-1 levels primarily by promoting its proteasomal degradation after phosphorylation by activated GSK-3β due to inhibiting ERK activation and reduction of AKT levels in NB4 cells (Fig. 4E).
Fig. 4. Inhibition of GSK-3β phosphorylation contributes to reduction in Mcl-1 levels in NB4 cells.
(A) ATO treatment decreases p-GSK-3β(Ser9) levels. NB4 cells were treated with ATO at the indicated concentrations for 24 h. (B) The GSK-3β inhibitor, SB216763, blocks ATO-induced decreases in Mcl-1 levels. NB4 cells were pretreated with 5 μM SB216763 for 2 h and then treated with 2 μM ATO for another 24 h. (C) Silencing GSK-3β blocks ATO-induced reduction of Mcl-1 levels. NB4 cells transfected with GSK-3β siRNA or control siRNA were treated with 2 μM ATO for 24 h. (D) Proteasome inhibitor MG132 blocks ATO-induced reduction of Mcl-1 levels. NB4 cells were pretreated with 0.5 μM MG132 for 2 h and then treated with 2 μM ATO for another 24 h. The relative levels of Mcl-1, PARP, GSK-3β, p-ERK, AKT and β-actin were determined with Western blot analyses using specific antibodies. (E) The potential mechanisms of ATO-induced reduction of Mcl-1 levels in NB4 cells.
ERK and AKT inhibitors plus ATO augment Mcl-1 reduction and apoptosis induction in HL-60 cells
The levels of p-ERK, AKT and p-GSK-3β were analyzed in HL-60 cells after ATO treatment at 0.5-3 μM. The levels of these proteins were not decreased after ATO treatment (Fig. 5A). Moreover, AKT levels were increased following ATO treatment (Fig. 5A). To test if inhibition of ERK or AKT activity enhances ATO-induced apoptosis in HL-60 cells, HL-60 cells were treated with 5 μM sorafenib, 1 μM PD184352, or 20 μM LY294002 alone or in combination with 2 μM ATO. Only less than 15% of the cells became apoptotic following treatment with each agent alone, but more than 58% of the cells underwent apoptosis after treatment with ATO in combination with any of the three inhibitors (Fig. 5B). The levels of Mcl-1, GSK-3β, and p-GSK-3β were analyzed in HL-60 cells treated with each inhibitor alone or in combination with ATO. Five μM sorafenib, 1 μM PD184352, or 20 μM LY294002 alone led to significant reduction of p-GSK-3β and Mcl-1 levels without influencing GSK-3β levels (Fig. 5C-E). The addition of 2 μM ATO with any of the three inhibitors led to further reduction in p-GSK-3β and Mcl-1 levels which was associated with increased levels of PARP cleavage (Fig. 5C, 5D, 5E).
Fig. 5. ERK and AKT inhibitors enhance ATO-induced apoptosis in HL-60 cells.
(A) The regulation by ATO treatment on the levels of AKT, p-ERK, and p-GSK-3β in HL-60 cells. HL-60 cells were treated with ATO at the indicated concentrations for 24 h and the levels of Mcl-1, p-ERK, AKT, GSK-3β, p-GSK-3β(Ser9), and β-actin were determined with Western blot analysis using specific antibodies. (B) The combined effects of an ERK inhibitor or an AKT inhibitor with ATO on apoptosis induction. HL-60 cells were pretreated with 20 μM LY294002, 1 μM PD184352, or 5 μM sorafenib for 2 h and then treated with or without 2 μM ATO for another 24 h. Apoptotic cells were detected with annexin V-FITC using flow cytometry. (C, D, E) The combined effects of either ERK inhibitors or an AKT inhibitor with ATO on the regulation of Mcl-1 levels and PARP cleavage. HL-60 cells were pretreated with 20 μM LY294002 (C), 1 μM PD184352 (D), or 5 μM sorafenib (E) for 2 h and then treated with or without 2 μM ATO for another 24 h. The levels of PARP, Mcl-1, GSK-3β, GSK-3β(Ser9), and β-actin were determined with Western blot analysis using specific antibodies.
Sorafenib decreased the levels of GSH and enhanced H2O2 production in ATO-treated HL-60 cells
Previously we found that ROS is required for ATO-induced apoptosis in APL cells and that APL cells have low levels of GSH (5, 8). It has been found that LY294002 enhanced ATO-induced apoptosis by both increasing production of ROS and decreasing GSH levels (20). We measured the effects of sorafenib with ATO on ROS production and GSH depletion. Sorafenib, but not ATO, decreased the level of GSH in HL-60 cells (Fig. 6A). The level of ROS was increased by treatment with either sorafenib or ATO alone and further augmented by the combination (Fig. 6B). To test the effect of ROS in apoptosis induction by ATO plus sorafenib, an H2O2-resistant HL-60 subclone, HP100-1, was used. There was less apoptosis following treatment with sorafenib plus ATO (Fig. 6C), although Mcl-1 level was reduced (Fig. 6D). These data suggest that sorafenib enhances the apoptotic effects of ATO not only by decreasing Mcl-1 levels, but also by decreasing GSH levels which augment the ROS production by ATO.
Fig. 6. Sorafenib decreases intracellular GSH levels and enhances ROS production due to ATO treatment.
(A) Sorafenib decreases GSH levels in HL-60 cells. HL-60 cells were pretreated with 5 μM sorafenib for 2 h and then treated with or without 2 μM ATO for 12 h. Then the intracellular GSH levels were determined as described in Material and Methods. (B) ROS levels of HL-60 cells treated with ATO plus sorafenib. HL-60 cells were pretreated with 5 μM sorafenib for 2 h and then treated with or without 2 μM ATO for another 16 h. The intracellular H2O2 content was determined by DCFH-DA staining with FACS. (C) The combined effects of sorafenib plus ATO on apoptosis induction. HL-60 and HP100-1 cells were treated with 5 μM sorafenib with or without 2 μM ATO for 24 h. Apoptotic cells were detected with annexin V-FITC using flow cytometry. (D) The combined effects of sorafenib plus ATO on Mcl-1 levels. HL-60 and HP100-1 cells were treated by 5 μM sorafenib with or without 2 μM ATO for 24 h. Then the levels of PARP, Mcl-1, p-GSK-3β(Ser9), and β-actin were determined with Western blot analysis using specific antibodies.
ATO plus sorafenib augment apoptosis induction in primary non-APL AML cells
The combined apoptotic effects of ATO plus sorafenib were tested in primary leukemia cells isolated from one FAB M1 AML patient and three FAB M2 AML patients. After 24 h of culture, 16.7% apoptotic cells was detected without any treatment. Treatment with 2 μM ATO and 5 μM sorafenib induced 25.3% and 28.3% apoptotic cells, respectively. Apoptosis significantly increased to 65.9% when ATO was added together with sorafenib (Fig. 7A). Sorafenib by itself decreased the levels of p-GSK3β and Mcl-1, and when added together with ATO and enhanced the leavage of PARP (Fig. 7B).
Fig. 7. Sorafenib plus ATO augment apoptosis induction in primary AML cells.
Primary AML cells were isolated as described in Material and Methods from four AML patients and treated with 5 μM sorafenib, 2 μM ATO and their combination for 24 h. Apoptotic cells were detected with annexin V-FITC using flow cytometry (A). The number listed are average and ** p < 0.01 compared to cells treated with either ATO or sorafenib alone (A). The levels of PARP, Mcl-1, p-GSK-3β(Ser9), and β-actin were determined with Western blot analysis using specific antibodies in cells isolated from AML#2 patient (B).
Discussion
Although several parameters, including lower levels of GSH, glutathione S-transferase π and catalase (5, 8, 31, 32), have been found to mediate different responses to ATO in APL cells compared to other types of AML cells, the roles of antiapoptotic proteins in the action of ATO in APL cells have rarely been studied. Bcl-2, Bcl-xL, and Mcl-1 are three principle antiapoptotic proteins which block the functions of the proapoptotic proteins Bax and Bak and control the mitochondrial membrane potential (12). We found that APL NB4 cells do not express Bcl-xL (13), suggesting that either Bcl-2 and/or Mcl-1 might play an important role in protecting against ATO-induced apoptosis. Previously it was found that ATO treatment decreased the levels of Bcl-2 in NB4 cells (33), but that was not consistent with later studies (5, 34-37). The difference may be due to concentration and time of treatment. It was found that ATO at 1 μM did not decrease the level of Bcl-2 in NB4 cells after 24 h treatment, but the Bcl-2 level could be decreased at increased concentrations of ATO or longer exposure to ATO (34-37). We found here that Bcl-2 level was not decreased after 1 μM ATO treatment, but a cleaved fragment of Bcl-2 was detected in NB4 cells treated with higher concentrations of ATO (Fig. 1A). Bcl-2 cleavage was also found in HL-60 cells treated with ATO plus PD184352 or sorafenib (Fig. 5D, 5E). The cleavage of Bcl-2 is correlated with PARP cleavage. These data suggest that Bcl-2 decrease by ATO at higher concentration might follow apoptosis since Bcl-2 is cleaved by caspase-3 (38). After ATO treatment Mcl-1 levels were decreased starting at 2 μM in NB4 cells (Fig. 1A). Neither Bcl-2 nor Mcl-1 protein levels were decreased after ATO treatment in HL-60 cells (Fig. 1B). Since Mcl-1 blocks mitochondrial apoptosis by binding to Bak, the reduction in Mcl-1 levels should lead to Bak activation in NB4 cells. The active form of Bak was significantly increased in NB4 cells, but not in HL-60 cells, which correlated with the cleavage of PARP (Fig. 1C, 1D). Silencing Mcl-1 with siRNA significantly enhanced ATO-induced apoptosis in HL-60 cells (Fig. 1E) which suggests that reduction of Mcl-1 levels plays an important role in ATO-induced apoptosis.
It was found that the Mcl-1 synthesis is regulated by mTOR signaling which promotes cell survival (17). mTOR signaling is regulated by AKT and it has been found that AKT is down-regulated by ATO treatment in NB4 cells (19, 39). We determined the levels of up- and down-stream factors of mTOR signaling, AKT, p-mTOR, p-4E-BP1, p-p70S6K, and p-S6 in NB4 cells. The levels of AKT, p-mTOR, p-4E-BP1, and p-p70S6K were decreased by ATO treatment at a concentration of 2 μM, but not by ATO at a concentration of 1 μM (Fig. 3A). Rapamycin neither enhanced ATO-induced reduction of Mcl-1 levels (Fig. 3C) nor ATO-induced apoptosis (Fig. 3D). These data suggest that the reduction of Mcl-1 levels by ATO treatment is not due to inhibition of Mcl-1 protein synthesis through mTOR signaling.
MEK/ERK/S6K signaling also plays a critical role in protein translational regulation (40). ERK phosphorylates S6K at Thr421. The levels of p-p70S6K(Thr421/Ser424) were decreased by ATO treatment, but not by rapamycin treatment (Fig. 3C) which suggests that ERK activity is inhibited by ATO treatment. Recently it was found that ERK phosphorylates Mcl-1 at Thr163 which stabilizes it (26). The levels of p-ERK were decreased by ATO treatment at a concentration of 1 μM (Fig. 2A). Since the levels of Mcl-1 were not decreased by ATO at 1 μM (Fig. 2A), the inhibition of ERK activity seems to be an early event leading to Mcl-1 reduction by decreasing its phosphorylation. Treatment with ERK inhibitors, U0126 and PD184352, decreased p-Mcl-1 and Mcl-1 levels (Fig. 2C). Sorafenib, a Raf inhibitor, decreased the levels of p-MEK and Mcl-1 and acted synergistically with ATO to induce apoptosis in NB4 cells (Fig. 2D). Treatment with sorafenib alone did not significantly decrease p-ERK levels which could be due to feedback activation by inhibiting p-MEK. It has been found that sorafenib decreases the levels of Mcl-1 through inhibition of translation (41, 42). It also has been found that sorafenib can decrease Mcl-1 phosphorylation levels by inhibiting ERK activity (43). Therefore, it seems that inhibition of both new protein synthesis and Mcl-1 phosphorylation may contribute to the combined effects of sorafenib plus ATO in decreasing Mcl-1 levels in NB4 cells (Fig. 2D).
Recently it was found that GSK-3β modulated Mcl-1 degradation by phosphorylating Mcl-1 at sites differing from those phosphorylated by ERK (21, 22). The activity of GSK-3β is controlled by phosphorylation which maintains it in an inactive form. Both ERK and AKT phosphorylate GSK-3β (44). The level of p-GSK-3β was reduced in NB4 cells after ATO treatment (Fig. 4A). Since an antibody to test the levels of phosphorylated Mcl-1 at Ser159 due to GSK-3β activation is not available, we used a GSK-3β inhibitor and GSK-3β siRNA to determine the effect on ATO-induced Mcl-1 reduction. Both the GSK-3β inhibitor SB216763 and GSK-3β siRNA blocked Mcl-1 reduction by ATO (2 μM) (Fig. 4B, 4C). It is known that GSK-3β phosphorylates Mcl-1 which leads to its proteasomal degradation (45). We found that the proteasome inhibitor, MG132, blocked ATO-induced Mcl-1 reduction in NB4 cells (Fig. 4D). These data suggest that the decrease in Mcl-1 levels following ATO treatment is due to two pathways: 1) activation of GSK3β by reducing p-ERK and AKT levels which promotes Mcl-1 phosphorylation at Ser159 and degradation and 2) direct inhibition of ERK-induced phosphorylation of Mcl-1 at Thr163 which destabilizes Mcl-1 (Fig. 4E).
Since silencing Mcl-1 sensitizes ATO-induced apoptosis in HL-60 cells (Fig. 1E), it seems that Mcl-1 plays an important role in protecting cells from ATO-induced apoptosis. ERK and AKT inhibitors, sorafenib, PD184352, and LY294002, all decreased the levels of p-GSK-3β and Mcl-1 protein and augmented ATO-induced apoptosis (Fig. 5). Since treatments with sorafenib, PD184352, or LY294002 significantly decreased Mcl-1 levels and by themselves did not induce apoptosis, the apoptotic effects of combinations of these inhibitors with ATO seem not to be induced due only to decreases in Mcl-1 levels. The GSK-3β inhibitor SB216763 completely blocked ATO-induced Mcl-1 reduction, but only partly inhibited ATO-induced apoptosis (Fig. 4B). Previously we have found that ROS are required for ATO apoptosis induction in NB4 cells (8). GSH levels determine the ability of ATO to produce ROS and it has been found that LY294002 and another ERK inhibitor, PD98059, decrease GSH levels (20, 46). In addition, sorafenib has been found to decrease GSH levels in hepatocellular carcinoma cells (47). We found that sorafenib alone decreased GSH level (Fig. 6A) and enhanced ROS production by ATO treatment in HL-60 cells (Fig. 6B). These results support our previous report that decreased intracellular GSH levels enhance the ability of ATO to produce ROS (5). HP100-1 cells, a H2O2-resistant HL-60 subclone, have a decreased response to ATO plus sorafenib-induced apoptosis compared to parental HL-60 cells (Fig. 6C). Since treatment with ATO plus sorafenib decreased Mcl-1 and p-GSK-3β levels in HP100-1 cells (Fig. 6D), it indicates that both ROS production and reduction of Mcl-1 levels are required for ATO apoptosis induction. Previously, we, and other groups, have found that buthionine sulfoximine (BSO), which completely depletes GSH levels by inhibiting the activity of glutathione synthase, enhanced ATO-induced apoptosis in cancer cells without selectivity (48, 49). It has been shown that ERK and AKT activation increases GSH levels by increasing the transcription of glutamate cysteine ligase (GCL), the initial enzyme in glutathione synthesis (50-52). ERK and AKT inhibitors decrease GSH levels by inhibiting GCL transcription. This decrease in GSH levels depends on the activities of ERK and AKT. Therefore, inhibitors of ERK and AKT have an advantage over BSO in ATO combination therapy. The question, unanswered thus far, is the mechanism by which silenced Mcl-1, using siRNA, enhances ATO-induced apoptosis (Fig. 1E). It has been found that Bcl-2 increases GSH levels (53) and functions as an antioxidant (54). It is possible that Mcl-1 works in a pathway similar to that of Bcl-2 to maintain GSH levels. By testing GSH and ROS levels, we found that silencing Mcl-1by using siRNA decreased GSH levels and enhanced ATO production of ROS in HL-60 cells (Suppl. Fig 2.).
In summary, we found that ATO treatment leads to reduction in Mcl-1 levels in APL cells primarily through activation of GSK3β by inhibiting p-ERK and AKT (Fig. 4E). ERK and AKT inhibitors enhance ATO-induced apoptosis in non-APL AML cells by 1) decreasing Mcl-1 levels and 2) by depleting GSH levels which then enhances ATO-induced ROS production (Fig. 8). Sorafenib is being tested in AML patients with limited efficacy (55). ATO plus sorafenib enhance apoptosis induction in non-APL HL-60 (Fig. 6) and primary AML cells (Fig. 7). Sorafenib plus ATO should be more effective than either agent alone. This combination treatment could possibly be developed as a novel combination therapy in non-APL AML patients, therefore, is worthy of clinical trials.
Fig. 8. The mechanisms of augmented apoptosis induction by ATO in combination with inhibitors of ERK or AKT in AML cells.
Inhibitors of ERK and AKT activate GSK3β by inhibiting phosphorylation due to ERK/AKT. Inhibitors of ERK and AKT also decrease intracellular GSH levels which enhances ATO production of ROS as well as ATO inhibition of ERK/AKT. Activated GSK3β phosphorylates Mcl-1 and leads to its proteasomal degradation. Enhanced ROS production plus decreased Mcl-1 levels by these combination treatment result in synergistic induction of apoptosis via activation of Bak.
Supplementary Material
Acknowledgments
This work was supported by NIH grant R01-CA93533. Critical reading of the manuscript by William Scher is appreciated. We would like to thank Jossela Aguilar and Lisa Arabova for consenting patients and collecting blood samples.
Footnotes
Authorship:
Contribution: R.W. designed and performed the research, analyzed the data, and wrote the paper; Y.J. designed the research, analyzed the data, and wrote the paper; J.G. and S.W. analyzed the data; and L.X. performed research.
Conflict of interest disclosure: JG is one of named inventors for the formulation of arsenic trioxide and receives royalty payment for the use of arsenic trioxide in patients with acute promyelocytic leukemia.
Supplementary information is available at Leukemia's website.
References
- 1.Park JH, Tallman MS. Treatment of acute promyelocytic leukemia without cytotoxic chemotherapy. Oncology (Williston Park) 2011;25:733–741. [PubMed] [Google Scholar]
- 2.Soignet SL, Maslak P, Wang ZG, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–1348. doi: 10.1056/NEJM199811053391901. [DOI] [PubMed] [Google Scholar]
- 3.Jing Y, Wang L, Xia L, Chen GQ, Chen Z, Miller WH, et al. Combined effect of all-trans retinoic acid and arsenic trioxide in acute promyelocytic leukemia cells in vitro and in vivo. Blood. 2001;97:264–269. doi: 10.1182/blood.v97.1.264. [DOI] [PubMed] [Google Scholar]
- 4.Chen GQ, Shi XG, Tang W, Xiong SM, Zhu J, Cai X, et al. Use of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia (APL): I. As2O3 exerts dose-dependent dual effects on APL cells. Blood. 1997;89:3345–3353. [PubMed] [Google Scholar]
- 5.Dai J, Weinberg RS, Waxman S, Jing Y. Malignant cells can be sensitized to undergo growth inhibition and apoptosis by arsenic trioxide through modulation of the glutathione redox system. Blood. 1999;93:268–277. [PubMed] [Google Scholar]
- 6.Shao W, Fanelli M, Ferrara FF, Riccioni R, Rosenauer A, Davison K, et al. Arsenic trioxide as an inducer of apoptosis and loss of PML/RAR alpha protein in acute promyelocytic leukemia cells. J Natl Cancer Inst. 1998;90:124–133. doi: 10.1093/jnci/90.2.124. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi S. Combination therapy with arsenic trioxide for hematological malignancies. Anticancer Agents Med Chem. 2010;10:504–510. doi: 10.2174/1871520611009060504. [DOI] [PubMed] [Google Scholar]
- 8.Jing Y, Dai J, Chalmers-Redman RM, Tatton WG, Waxman S. Arsenic trioxide selectively induces acute promyelocytic leukemia cell apoptosis via a hydrogen peroxide-dependent pathway. Blood. 1999;94:2102–2111. [PubMed] [Google Scholar]
- 9.Chou WC, Jie C, Kenedy AA, Jones RJ, Trush MA, Dang CV. Role of NADPH oxidase in arsenic-induced reactive oxygen species formation and cytotoxicity in myeloid leukemia cells. Proc Natl Acad Sci U S A. 2004;101:4578–4583. doi: 10.1073/pnas.0306687101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morales AA, Gutman D, Cejas PJ, Lee KP, Boise LH. Reactive oxygen species are not required for an arsenic trioxide-induced antioxidant response or apoptosis. J Biol Chem. 2009;284:12886–12895. doi: 10.1074/jbc.M806546200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Platanias LC. Biological responses to arsenic compounds. J Biol Chem. 2009;284:18583–18587. doi: 10.1074/jbc.R900003200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dai Y, Grant S. Targeting multiple arms of the apoptotic regulatory machinery. Cancer Res. 2007;67:2908–2911. doi: 10.1158/0008-5472.CAN-07-0082. [DOI] [PubMed] [Google Scholar]
- 13.Yin S, Wang R, Zhou F, Zhang H, Jing Y. Bcl-xL is a dominant antiapoptotic protein that inhibits homoharringtonine-induced apoptosis in leukemia cells. Mol Pharmacol. 2011;79:1072–1083. doi: 10.1124/mol.110.068528. [DOI] [PubMed] [Google Scholar]
- 14.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell CJ, Lee JB, Levadoux-Martin M, Wynder T, Xenocostas A, Leber B, et al. The human stem cell hierarchy is defined by a functional dependence on Mcl-1 for self-renewal capacity. Blood. 2010;116:1433–1442. doi: 10.1182/blood-2009-12-258095. [DOI] [PubMed] [Google Scholar]
- 16.Martelli AM, Evangelisti C, Chiarini F, Grimaldi C, Manzoli L, McCubrey JA. Targeting the PI3K/AKT/mTOR signaling network in acute myelogenous leukemia. Expert Opin Investig Drugs. 2009;18:1333–1349. doi: 10.1517/14728220903136775. [DOI] [PubMed] [Google Scholar]
- 17.Mills JR, Hippo Y, Robert F, Chen SM, Malina A, Lin CJ, et al. mTORC1 promotes survival through translational control of Mcl-1. Proc Natl Acad Sci U S A. 2008;105:10853–10858. doi: 10.1073/pnas.0804821105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas LW, Lam C, Edwards SW. Mcl-1; the molecular regulation of protein function. FEBS Lett. 2010;584:2981–2989. doi: 10.1016/j.febslet.2010.05.061. [DOI] [PubMed] [Google Scholar]
- 19.Mann KK, Colombo M, Miller WH., Jr Arsenic trioxide decreases AKT protein in a caspase-dependent manner. Mol Cancer Ther. 2008;7:1680–1687. doi: 10.1158/1535-7163.MCT-07-2164. [DOI] [PubMed] [Google Scholar]
- 20.Ramos AM, Fernandez C, Amran D, Sancho P, de Blas E, Aller P. Pharmacologic inhibitors of PI3K/Akt potentiate the apoptotic action of the antileukemic drug arsenic trioxide via glutathione depletion and increased peroxide accumulation in myeloid leukemia cells. Blood. 2005;105:4013–4020. doi: 10.1182/blood-2004-07-2802. [DOI] [PubMed] [Google Scholar]
- 21.Maurer U, Charvet C, Wagman AS, Dejardin E, Green DR. Glycogen synthase kinase-3 regulates mitochondrial outer membrane permeabilization and apoptosis by destabilization of MCL-1. Mol Cell. 2006;21:749–760. doi: 10.1016/j.molcel.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Opferman JT. Unraveling MCL-1 degradation. Cell Death Differ. 2006;13:1260–1262. doi: 10.1038/sj.cdd.4401978. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–733. [PubMed] [Google Scholar]
- 24.Yamada M, Hashinaka K, Inazawa J, Abe T. Expression of catalase and myeloperoxidase genes in hydrogen peroxide-resistant HL-60 cells. DNA Cell Biol. 1991;10:735–742. doi: 10.1089/dna.1991.10.735. [DOI] [PubMed] [Google Scholar]
- 25.Fernandez-Checa JC, Kaplowitz N. The use of monochlorobimane to determine hepatic GSH levels and synthesis. Anal Biochem. 1990;190:212–219. doi: 10.1016/0003-2697(90)90183-a. [DOI] [PubMed] [Google Scholar]
- 26.Domina AM, Vrana JA, Gregory MA, Hann SR, Craig RW. MCL1 is phosphorylated in the PEST region and stabilized upon ERK activation in viable cells, and at additional sites with cytotoxic okadaic acid or taxol. Oncogene. 2004;23:5301–5315. doi: 10.1038/sj.onc.1207692. [DOI] [PubMed] [Google Scholar]
- 27.Grant S. Cotargeting survival signaling pathways in cancer. J Clin Invest. 2008;118:3003–3006. doi: 10.1172/JCI36898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ding Q, He X, Hsu JM, Xia W, Chen CT, Li LY, et al. Degradation of Mcl-1 by beta-TrCP mediates glycogen synthase kinase 3-induced tumor suppression and chemosensitization. Mol Cell Biol. 2007;27:4006–4017. doi: 10.1128/MCB.00620-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culbert AA, Brown MJ, Frame S, Hagen T, Cross DA, Bax B, et al. GSK-3 inhibition by adenoviral FRAT1 overexpression is neuroprotective and induces Tau dephosphorylation and beta-catenin stabilisation without elevation of glycogen synthase activity. FEBS Lett. 2001;507:288–294. doi: 10.1016/s0014-5793(01)02990-8. [DOI] [PubMed] [Google Scholar]
- 30.Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- 31.Coe E, Schimmer AD. Catalase activity and arsenic sensitivity in acute leukemia. Leuk Lymphoma. 2008;49:1976–1981. doi: 10.1080/10428190802353617. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini S, Nuccetelli M, Noguera NI, Bellincampi L, Lunghi P, Bonati A, et al. Role of GSTP1-1 in mediating the effect of As2O3 in the Acute Promyelocytic Leukemia cell line NB4. Ann Hematol. 2006;85:681–687. doi: 10.1007/s00277-006-0139-8. [DOI] [PubMed] [Google Scholar]
- 33.Chen GQ, Zhu J, Shi XG, Ni JH, Zhong HJ, Si GY, et al. In vitro studies on cellular and molecular mechanisms of arsenic trioxide (As2O3) in the treatment of acute promyelocytic leukemia: As2O3 induces NB4 cell apoptosis with downregulation of Bcl-2 expression and modulation of PML-RAR alpha/PML proteins. Blood. 1996;88:1052–1061. [PubMed] [Google Scholar]
- 34.Kinjo K, Kizaki M, Muto A, Fukuchi Y, Umezawa A, Yamato K, et al. Arsenic trioxide (As2O3)-induced apoptosis and differentiation in retinoic acid-resistant acute promyelocytic leukemia model in hGM-CSF-producing transgenic SCID mice. Leukemia. 2000;14:431–438. doi: 10.1038/sj.leu.2401646. [DOI] [PubMed] [Google Scholar]
- 35.Akao Y, Yamada H, Nakagawa Y. Arsenic-induced apoptosis in malignant cells in vitro. Leuk Lymphoma. 2000;37:53–63. doi: 10.3109/10428190009057628. [DOI] [PubMed] [Google Scholar]
- 36.Sahara N, Takeshita A, Kobayashi M, Shigeno K, Nakamura S, Shinjo K, et al. Phenylarsine oxide (PAO) more intensely induces apoptosis in acute promyelocytic leukemia and As2O3-resistant APL cell lines than As2O3 by activating the mitochondrial pathway. Leuk Lymphoma. 2004;45:987–995. doi: 10.1080/10428190310001617222. [DOI] [PubMed] [Google Scholar]
- 37.Wang ZG, Rivi R, Delva L, Konig A, Scheinberg DA, Gambacorti-Passerini C, et al. Arsenic trioxide and melarsoprol induce programmed cell death in myeloid leukemia cell lines and function in a PML and PML-RARalpha independent manner. Blood. 1998;92:1497–1504. [PubMed] [Google Scholar]
- 38.Cheng EH, Kirsch DG, Clem RJ, Ravi R, Kastan MB, Bedi A, et al. Conversion of Bcl-2 to a Bax-like death effector by caspases. Science. 1997;278:1966–1968. doi: 10.1126/science.278.5345.1966. [DOI] [PubMed] [Google Scholar]
- 39.Goussetis DJ, Platanias LC. Arsenic trioxide and the phosphoinositide 3-kinase/akt pathway in chronic lymphocytic leukemia. Clin Cancer Res. 2010;16:4311–4312. doi: 10.1158/1078-0432.CCR-10-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pende M, Um SH, Mieulet V, Sticker M, Goss VL, Mestan J, et al. S6K1(-/-)/S6K2(-/-) mice exhibit perinatal lethality and rapamycin-sensitive 5'-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol Cell Biol. 2004;24:3112–3124. doi: 10.1128/MCB.24.8.3112-3124.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huber S, Oelsner M, Decker T, zum Buschenfelde CM, Wagner M, Lutzny G, et al. Sorafenib induces cell death in chronic lymphocytic leukemia by translational downregulation of Mcl-1. Leukemia. 2011;25:838–847. doi: 10.1038/leu.2011.2. [DOI] [PubMed] [Google Scholar]
- 42.Rahmani M, Davis EM, Bauer C, Dent P, Grant S. Apoptosis induced by the kinase inhibitor BAY 43-9006 in human leukemia cells involves down-regulation of Mcl-1 through inhibition of translation. J Biol Chem. 2005;280:35217–35227. doi: 10.1074/jbc.M506551200. [DOI] [PubMed] [Google Scholar]
- 43.Katz SI, Zhou L, Chao G, Smith CD, Ferrara T, Wang W, et al. Sorafenib inhibits ERK1/2 and MCL-1(L) phosphorylation levels resulting in caspase-independent cell death in malignant pleural mesothelioma. Cancer Biol Ther. 2009;8:2406–2416. doi: 10.4161/cbt.8.24.10824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359:1–16. doi: 10.1042/0264-6021:3590001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramos AM, Fernandez C, Amran D, Esteban D, de Blas E, Palacios MA, et al. Pharmacologic inhibitors of extracellular signal-regulated kinase (ERKs) and c-Jun NH(2)-terminal kinase (JNK) decrease glutathione content and sensitize human promonocytic leukemia cells to arsenic trioxide-induced apoptosis. J Cell Physiol. 2006;209:1006–1015. doi: 10.1002/jcp.20806. [DOI] [PubMed] [Google Scholar]
- 47.Chiou JF, Tai CJ, Wang YH, Liu TZ, Jen YM, Shiau CY. Sorafenib induces preferential apoptotic killing of a drug- and radio-resistant Hep G2 cells through a mitochondria-dependent oxidative stress mechanism. Cancer Biol Ther. 2009;8:1904–1913. doi: 10.4161/cbt.8.20.9436. [DOI] [PubMed] [Google Scholar]
- 48.Chen D, Chan R, Waxman S, Jing Y. Buthionine sulfoximine enhancement of arsenic trioxide-induced apoptosis in leukemia and lymphoma cells is mediated via activation of c-Jun NH2-terminal kinase and up-regulation of death receptors. Cancer Res. 2006;66:11416–11423. doi: 10.1158/0008-5472.CAN-06-0409. [DOI] [PubMed] [Google Scholar]
- 49.Davison K, Cote S, Mader S, Miller WH. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia. 2003;17:931–940. doi: 10.1038/sj.leu.2402876. [DOI] [PubMed] [Google Scholar]
- 50.Iles KE, Liu RM. Mechanisms of glutamate cysteine ligase (GCL) induction by 4-hydroxynonenal. Free Radic Biol Med. 2005;38:547–556. doi: 10.1016/j.freeradbiomed.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 51.Pattillo CB, Pardue S, Shen X, Fang K, Langston W, Jourd'heuil D, et al. ICAM-1 cytoplasmic tail regulates endothelial glutathione synthesis through a NOX4/PI3-kinase-dependent pathway. Free Radic Biol Med. 2010;49:1119–1128. doi: 10.1016/j.freeradbiomed.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen ZH, Saito Y, Yoshida Y, Noguchi N, Niki E. Regulation of GCL activity and cellular glutathione through inhibition of ERK phosphorylation. Biofactors. 2008;33:1–11. doi: 10.1002/biof.5520330101. [DOI] [PubMed] [Google Scholar]
- 53.Jang JH, Surh YJ. Bcl-2 attenuation of oxidative cell death is associated with up-regulation of gamma-glutamylcysteine ligase via constitutive NF-kappaB activation. J Biol Chem. 2004;279:38779–38786. doi: 10.1074/jbc.M406371200. [DOI] [PubMed] [Google Scholar]
- 54.Hockenbery DM, Oltvai ZN, Yin XM, Milliman CL, Korsmeyer SJ. Bcl-2 functions in an antioxidant pathway to prevent apoptosis. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 55.Rollig C, Brandts C, Shaid S, Hentrich M, Kramer A, Junghanss C, et al. Survey and analysis of the efficacy and prescription pattern of sorafenib in patients with acute myeloid leukemia. Leuk Lymphoma. 2011 doi: 10.3109/10428194.2011.637210. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.