Abstract
Targeting the mucosal immune system of the genital tract (GT) with subunit vaccines failed to induce potent and durable local CD8+ T cell immunity, crucial for protection against many sexually transmitted viral (STV) pathogens, including herpes simplex virus type 2 (HSV-2) that causes genital herpes. In this study, we aimed to investigate the potential of a novel lipopeptide/adenovirus type 5 (Lipo/rAdv5) prime/boost mucosal vaccine for induction of CD8+ T cell immunity to protect the female genital tract from herpes. The lipopeptide and the rAdv5 vaccine express the immunodominant HSV-2 CD8+ T cell epitope (gB498-505) and both were delivered intravaginally (IVAG) in the progesterone-induced B6 mouse model of genital herpes. Compared to its homologous lipopeptide/lipopeptide (Lipo/Lipo); the Lipo/rAdv5 prime/boost immunized mice: (i) developed potent and sustained HSV-specific CD8+ T cells, detected in both the GT draining nodes (GT-DLN) and in the vaginal mucosa (VM); (ii) had significantly lower virus titers; (iii) had decreased overt signs of genital herpes disease; and (iv) did not succumb to lethal infection (p < 0.005), following intravaginal HSV-2 challenge. Polyfunctional CD8+ T cells, producing IFN-γ, TNF-α and IL-2 and exhibiting cytotoxic activity, were associated with protection (p < 0.005). The protective CD8+ T cell response was significantly compromised in the absence of the adaptor myeloid differentiation factor 88 (MyD88) (p = 0.0001). Taken together, these findings indicate that targeting the VM with a Lipo/rAdv5 prime/boost vaccine elicits a potent, MyD88-dependent, and long-lasting mucosal CD8+ T cell protective immunity against sexually transmitted herpes infection and disease.
Keywords: Genital herpes, adenovirus, lipopeptide, prime/boost, vaccine, CD8+ T cells, and MyD88
INTRODUCTION
Developing a mucosal immunization approach that generates long-lasting local effector and memory CD8+ T cell populations, in the genital tract and its draining lymph nodes, is likely to be essential in achieving immediate and sustained protective immunity against many sexually transmitted viral (STV) pathogens that use the genital mucosa as a portal entry into their human hosts (1–4). Evidence from both animal models (5, 6) and humans (7) suggests that successful control of many STV infections, such as HIV-1 that causes AIDS and HSV-2 that causes genital herpes, is associated with the presence of sustained local CD8+ T cells within the genital tract draining lymph nodes (GT-DLN) and the vaginal mucosal (VM) tissues (4, 8–11). Increasing evidence demonstrates a substantial link between the epidemics of sexually transmitted HIV-1 and HSV-2 infection (12). However, no subunit vaccine strategy delivered parenterally has generated demonstrable high-level and sustainable protective CD8+ T cell immunity against either infection in clinical trials (3, 13, 14). A successful immunization through the mucosal route, such as intravaginally (IVAG), appears critical but remains a roadblock due to the robust mucosal epithelial barrier and the inability to overcome mucosal tolerance by many sub-unit vaccines (4). Lipopeptides (peptide epitopes linked to a fatty acid) are promising mucosal vaccines that induce protective CD8+ T cells against many STV pathogens for which induction of cytotoxic CD8+ T-cells and IFN-γ signaling is critical (reviewed in (1)). We recently demonstrated that a prototype herpes lipopeptide vaccine expressing the immunodominant H2b-restricted CD8+ T cell epitope from HSV-2 gB (HSV-2 gB498-505) (15), delivered IVAG, induced HSV-specific protective CD8+ T cell responses (6). However, these CD8+ T cell responses were rather moderate and were only transiently protective against genital herpes (6). An emerging and promising mucosal immunization approach to elicit potent CD8+ T cell responses is to use recombinant viruses (16). Several studies have recently used replication-defective adenovirus vectors to induce potent local viral-specific CD8+ T-cells in GT-DLN and to mobilize them quickly into the VM tissues (8, 16, 17).
In the present study, we hypothesize that a prime-boost vaccination regimen, which consists of priming CD8+ T cell responses with a lipopeptide vaccine and boosting them with an adenovirus vector-based vaccine; would result in strong and long-lasting protective CD8+ T cell immunity against genital herpes. We used the replication-defective adenovirus serotype 5 (rAd5) vector based on its many attractive features: (i) its remarkable ability to significantly boost mucosal CD8+ T cell responses that are primed by a sub-unit vaccine (e.g. peptide and DNA) (reviewed in (18)); (ii) its ability to elicit a considerably potent and long-lasting pathogen-specific CD8+ T cell response in humans (19); and (iii) its natural tropism for mucosal tissues (20–22), which makes it an ideal antigen delivery system for IVAG vaccination.
Although some progress has been made in defining the cellular mechanisms of the immunogenicity of lipopeptide and rAdv-based vaccines, relatively little is known about the underlying innate molecular pathways. Mucosal delivery of lipopeptide and rAdv vaccines potentially recruits many of the thirteen known mammalian Toll-like receptor (TLR) pathways (6). Each TLR has Toll/Interleukin-1 receptor (TIR) domains that engage two main intracellular signaling pathways: (1) the myeloid differentiation factor 88 (MyD88) pathway; and (2) the TIR domain containing adaptor-inducing IFN-beta (TRIF) pathway (23). Since most TLRs recruit the MyD88 pathway (6, 24), the present study is focused on exploring whether the MyD88 pathway would be required for the generation of protective HSV-specific CD8+ T cell responses by the lipopeptide/rAdv5 vaccine (Lipo/rAdv5) following an IVAG immunization. The results show that: (i) priming with a lipopeptide and boosting with a rAd5 vector, both expressing the same HSV-2gB498-505 epitope and both delivered IVAG, induced a much more potent local mucosal HSV-specific IFN-γ-producing CD8+ T cells than the homologous lipopeptide/lipopeptide (Lipo/Lipo) vaccine (p ≤ 0.05); (ii) Lipo/rAdv5 prime/boost mucosal vaccine elicits long-lasting CD8+ T cells, with a faster kinetic of mobilization, than the Lipo/Lipo vaccine; (iii) the HSV-specific CD8+ T cell responses induced by the Lipo/rAdv5 prime/boost mucosal vaccine are associated with protection against genital herpes infection and disease; (iv) the HSV-specific CD8+ T cell responses induced by the Lipo/rAdv5 prime/boost mucosal vaccine persisted up to 8 months post-immunization; and (v) MyD88 pathway plays a pivotal role in the HSV-specific CD8+ T cell response and protection induced following IVAG immunization with the Lipo/rAdv5 vaccine. Altogether, these findings lay the foundation for an accessible and durable mucosal prime/boost T-cell based vaccine approach to reduce genital herpes, and presumably other sexually transmitted pathogens.
MATERIALS & METHODS
Mice
MyD88 deficient mice (MyD88(−/−) mice), 4–5 weeks old, were provided by Dr. Shizuo Akira (Osaka University, Osaka, Japan), and were on the C57BL/6 background. Female C57BL/6 (B6) mice, 4–5 weeks old, were purchased from the Jackson Laboratory (Bar Harbor, ME). Animal studies conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health.
Viruses and cell lines
Triple plaque-purified 333 HSV-2 was prepared, as we described previously (6). The live attenuated thymidine kinase-deficient HSV-2 (HSV-2 TK(−)), was provided by Dr. James R. Smiley and Dr. Lynda A. Morrison. Triple plaque-purified HSV-2 was prepared as we described previously (55). Rabbit skin (RS) cells, used to prepare virus stocks and to culture virus from vaginal swabs, were grown in Eagle’s minimum essential medium (EMEM) supplemented with 5% fetal calf serum (Invitrogen, Grand Island, NY). Heat-killed virus was made by heating virus solution at 100°C for 5 min. HSV inactivation was confirmed by the inability to produce plaques when tested on Vero cells, as we described (1). A recombinant adenovirus virus type 5 (rAdv5) expressing the H-2Kb-restricted, HSV-1/2-cross-reactive CTL recognition epitope, HSV glycoprotein B residues 498 to 505 (SSIEFARL) (gB498-505), was constructed. Briefly, the DNA sequence (PB1) encoding the H2Kb SSIEFARL CD8+ CTL target peptide (HSV-gB498-505) (30) was synthesized and cloned into pShuttle-CMV (Stratagene, Santa Clara, CA) vector between KpnI and XbaI sites. The transfer pShuttle-CMV-PB1 plasmid DNA was linearized with PmeI, gel-purified and then transformed into BJ5183-AD-1 bacterial cells (Stratagene, Santa Clara, CA) carrying the pAdEasy-1 plasmid by electroporation to generate recombinant adenovirus plasmid pAdEasy-1-PB1. The recombinant pAdEasy-1-PB1 plasmid DNA was linearized with PacI and transfected into 293 cells to produce recombinant adenovirus Ad5.CMV-PB1 that will express the HSV-gB498-505 CD8+ T cell epitopes by CMV promoter. The Ad5.CMV-PB1 recombinant adenovirus was plaque-purified and amplified to the titer of 108 PFU/ml. The virus stock was aliquoted and stored in −80°c.
Synthesis and assembly of lipopeptide vaccines
The H2Kb SSIEFARL CD8+ CTL target peptide (HSV-gB498-505) (56) and the [dA]K[Cha]VAAWTLKAA[dA][Ahx]C Pan DR peptide (PADRE) (30) were synthesized either individually or as PADRE-CTL chimeric epitopes using Fmoc (9-Fluorenylmethoxycarbonyl) chemistry, with PyBOP/HOBt (Benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluorophosphate/N-hydroxybenzotriazole) activation, as we described previously (30, 57). The parental peptides were washed three times with DMF (dimethylformamide) and treated twice with 2% hydrazine in DMF. After additional washings (x 6) with DMF, hydrazine acetic acid peptides were cleaved using TFA/TIS/H2O (trifluoroacetic acid/tri-isopropylsilane/water; 95:2.5:2.5) and the resultant peptides precipitated in cold ether. The peptides were then washed (x 3) with cold ether and analyzed by MS and HPLC. Purification of the peptides was performed using Gilson HPLCs, Vydac C18 columns (2.2 × 25 cm or for larger amounts of crude peptide, 5 × 25 cm). The analysis was performed using Vydac C18, 5um, 0.46 × 25 cm columns, with a gradient of 2% per minute of water, 0.1% TFA, 95% acetonitrile and 0.1% TFA. Once peptides were purified to over 95% purity rate, they were lyophilized. Mass spectrometric analysis was performed by MDS/Sciex QStar XL mass spectrometer equipped with a nanospray source. Final QC included collision-induced dissociation MSMS experiments using nitrogen as the collision gas, which confirm the number and nature of amino acids of the peptides.
The prototype Th-CTL chimeric lipopeptide vaccines were synthesized following, one, two or three N-terminal attachments of glyoxylyl lipid to the Th-CTL backbone using chemoselective ligation as previously described (6). This was achieved by adding one, two or three lysine residues whose side-chains were selectively protected with ivDde (1-(4,4-Dimethyl-2,6-dioxoxyxlohex-1-ylidene)-3-methylbutyl), a hydrazine-sensitive side-chain protecting group. In order to allow maximal attachment of the lipid moieties, the lysine residues were interspaced with alanines. The synthesis of the glyoxylyl derivative of palmitate and ligation of peptides were performed using a modification of chemoselective ligation (6). Briefly, dimethyl-2,3-O-isopropylidene tartrate was added to a polyethylene glycol amino resin using PyBOP activation. The second ester was then displaced via the addition of 1,3-diaminopropane. Finally palmitic acid was activated using PyBOP and used to acylate the amino terminus. Treatment with TFA followed by periodate oxidation generated the alpha-oxo-aldehyde moiety. Following lyophilization, the peptides were transferred in 50 mL round bottom flasks, fitted with septa and flushed with nitrogen. A minimal amount of degassed water was added until the peptides were solubilized and displayed as a gel. Stochiometric amounts of lipid were then added in 2-methyl-propan-2-ol drop-wise with stirring. The final ratio of water to organic solvent was 95:5. To add the 2nd and 3rd lipids, an aliquot equal to 120% of the concentration of the peptide was added, with 10–20 minutes of stirring in between each addition. The reactions were monitored using the QStar XL mass spectrometer (Fig. 1). The disappearance of the parent peptide was observed concomitantly with the appearance of the lipid-tailed peptide. In all cases, the parent peptide was not detectable at the end of the acylation process.
Figure 1. Potent and long-lasting CD8+ T cell responses detected in both GT-DLN and VM following IVAG immunization with Lipo/rAdv5 prime/boost vaccine.
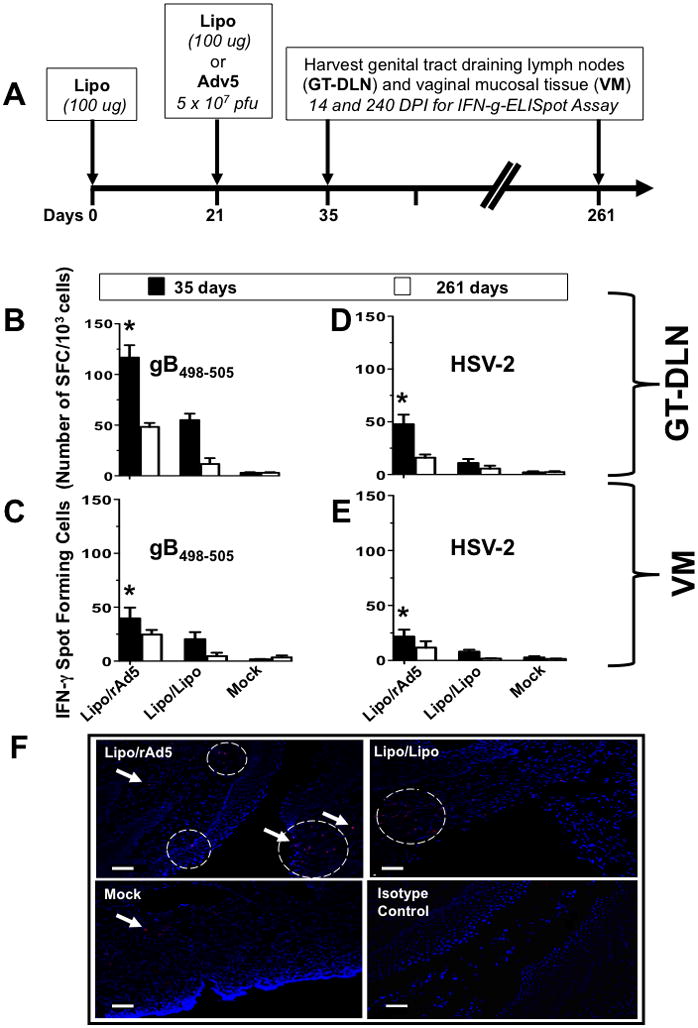
(A) Time course for immunization and CD8+ T cell response analysis. Four groups of B6 mice (n =10) were immunized IVAG with: 100 μg of lipopeptide in saline on days 0, and 21 (Lipo/Lipo), with lipopeptide on day 0 and then with 5 × 107 pfu of rAd5 in saline on days 21 (Lipo/rAdv5 prime/boost mucosal vaccine or Lipo/rAd5), or with the irrelevant OVA257-264 lipopeptide and an empty Ad5 vector in saline on days 0 and 21 (mock-immunized or Mock). (B–E) On day 35 and 261 (i.e. fourteen and 240 days post-immunization (DPI)), the iliac and inguinal lymph nodes draining the genital tract (GT-DLN) (B and D) as well as the vaginal mucosa (VM) (C and E) were harvested. GT-DLN and VM cell suspensions were assayed for gB498-505- (B and C) and HSV-2- (D and E) specific IFN-γ-producing CD8+ T cell responses using ELISpot assay, as described in Material & Methods. Values represent the mean of IFN-γ spot forming cells detected in an average of 5 mice. (*) Indicates the P values were ≤ 0.05 when HSV-2 or gB498-505-specific IFN-γ-secreting CD8+ T cell responses from the Lipo/rAdv5 vaccine group of mice was compared to the homologous Lipo/Lipo group or to the mock-immunized group (one-way ANOVA). (F) Vaginal mucosal tissue was collected 14 days after the second immunization from the progesterone-treated mice that were immunized with Lipo/rAdv5 (upper left), Lipo/Lipo (upper right) or mock-immunized (lower left) and CD8+ T cells were detected by immunofluorescence microscopy, as described in Material & Methods. Sections were stained with fluorescein isothiocyanate-conjugated anti-CD8 antibody, and the nuclei were visualized by staining with DAPI (blue). Arrowheads and circles indicate CD8+ T cells accumulating at the vaginal epithelium and stroma. Staining with an isotype control IgG is shown in the lower right picture. Scale bars = 50 υm. Results are representative of two independent experiments.
Immunizations and HSV-2 challenge
All immunizations were carried out with 100 ug of lipopeptide vaccine and 5 × 107 of the rAd5 vaccine, both delivered intravaginally (IVAG) in sterile PBS on day 0 and 21 (Figs. 1A, 2A, 3A, 4A, 5A and 6A). As a negative control mice were primed IVAG with the irrelevant OVA257-264 lipopeptide and boosted with an empty Ad5 vector (mock-immunized mice). As a positive control, mice were inoculated IVAG with 5×103 PFU of the HSV-2 TK(−) virus, as previously described (6, 44) (Figs. 3A, 4A, 5A and 6A). We previously found that IP injection of 0.5 mg of Depo-Provera 4 times, instead of one time 2 mg, was safer and better in the synchronizing of estrus cycle of mice. Ten days after the final immunization, each group of mice was IP injected daily with 4 doses of 0.5 mg of Depo-Provera in 100 uL sterile PBS. Mice were then challenged intravaginally, on day 14, either with 5 × 106 pfu (= 200 x LD50 for survival analysis) or with 5 × 104 pfu (for virus titers and disease analysis) of HSV-2 (strain 333).
Figure 2. Kenetics of gB498-505-specific CTL responses induced by the Lipo/rAdv5 prime/boost vs. Lipo/Lipo mucosal vaccines.
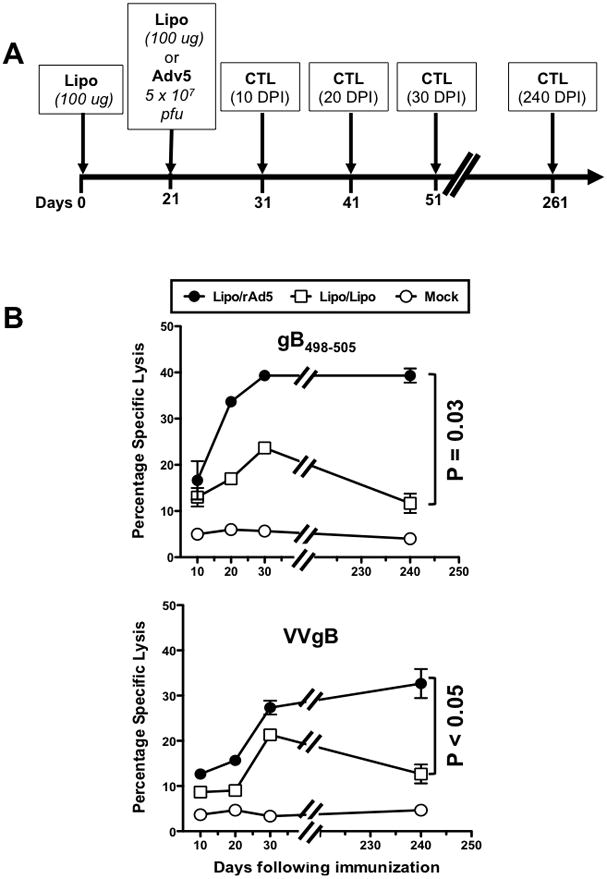
(A) Groups of B6 mice (n =40) were immunized IVAG with the Lipo/rAdv5 prime/boost mucosal vaccine in saline, or with the homologous Lipo/Lipo, or with the irrelevant OVA257-264 lipopeptide and empty Ad5 vector in PBS on days 0 and 21 (mock-immunized or Mock), similar to Fig. 1 above. On day 10, 20, 30 and 240 post-immunization 5 mice were euthanized per each time point/group, and CTL activity was assessed of GT-DLN derived effector CD8+ T-cells was determined in a CRA assay using as target cells autologous EL-4 (at E:T of 30) loaded with HSV-2 gB498-505 peptide (Fig. 2B upper panel) or transfected with VVgB, a vaccinia virus expressing gB (Fig. 2B lower panel). The data are representative of two independent experiments and the bars represent SD between the experiments. The p values show significance levels of differences in the overall amount of cytotoxic activity between Lipo/rAdv5 and Lipo/Lipo immunized mice (one way, ANOVA test).
Figure 3. Higher percentages and increased numbers of HSV-gB498-505-specific CD8+ T-cells induced by the Lipo/rAdv5 prime/boost compared to its Lipo/Lipo homologous mucosal vaccine.
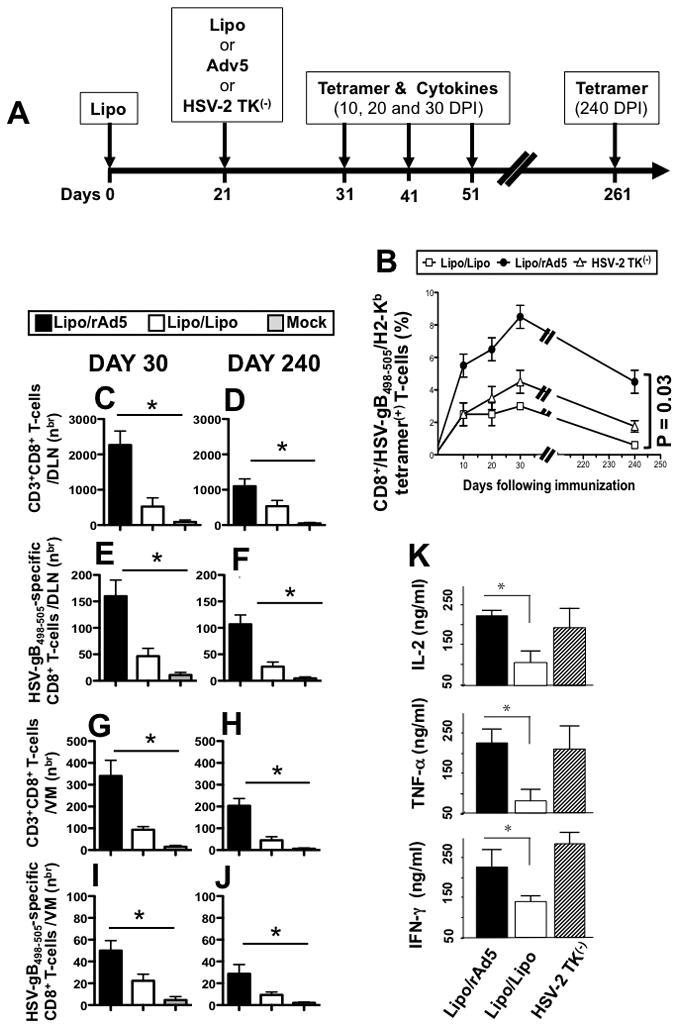
(A) Groups of female B6 mice (n =40) were immunized IVAG, with lipopeptide on day 0 and 5 × 107 pfu of rAd5 in saline on days 21 (Lipo/rAdv5 prime/boost mucosal vaccine or Lipo/rAd5, with 100 μg of lipopeptide in saline on days 0 and 21 (homologous Lipo/Lipo vaccine or Lipo/Lipo), or with the live attenuated HSV-2 TK(−) on day 21, as we previously described (6) (positive control). (B) On day 10, 20 30 and 240 post-immunization, GT-DLN were harvested (5 mice per each time point) and derived T cells were stimulated in vitro with UV-inactivated virus pulsed APCs for 5 days. Stimulated HSV-gB498-505- specific CD8+ T-cells were then stained with a PE-labeled anti-mouse CD8+ mAb followed by either an FITC labeled HSV-gB498-505/H2-Kb tetramer. Cells were then analyzed using a FACS Calibur with a total of 2 ×105 events collected for each point. The percentages of CD8+/Tetramer+ cells are determined for each time point. Shown is mean ± SD of the results obtained in 5 mice/group. Each bar is representative of the mean ± standard error of results from 5 mice. Data for each group were repeated twice and compared by analysis of variance (ANOVA test) and multiple comparison procedures (Tukey) to determine differences between groups, as we previously described (6). P value of 0.03 indicates significant differences between Lipo/Lipo and Lipo/rAd5 immunized groups (one-way ANOVA test). (C–D) Numbers of total CD3+CD8+ T cells and (E–F) HSV-gB498-505-specific CD8+ T cells detected in the GT-DLN on days 30 and 240 DPI. (G–H) Numbers of total CD3+CD8+ T cells and (I–J) HSV-gB498-505-specific CD8+ T cells detected in the VM on days 30 and 240 DPI. (K) Profile of HSV-gB498-505-specific cytokine produced by CD8+ T-cells following Lipo/Lipo and Lipo/rAd5 immunizations. GT-DLN were harvested 30 days after the second immunization and GT-DLN-derived CD8+ T-cells were stimulated in vitro with target gB498-505 peptide loaded H2b irradiated splenocytes for 72 days at γ, TNF-α and IL-2 secreted into the culture media were 37°C in 5% CO2. The amounts of IFN-determined in a specific sandwich ELISA, according to the manufacturer’s instructions. Shown is cytotoxic activity and profiles obtained in a group of 5 mice. The data are representative of two independent experiments and the bars represent SD between the experiments. The (*) indicate p values < 0.05 using one-way ANOVA test when comparing the amount of cytokine levels between Lipo/Lipo and Lipo/rAd5 groups.
Figure 4. Intravaginal immunization with Lipo/rAd5 prime/boost vaccine confers protection against genital herpes infection and disease in mice.
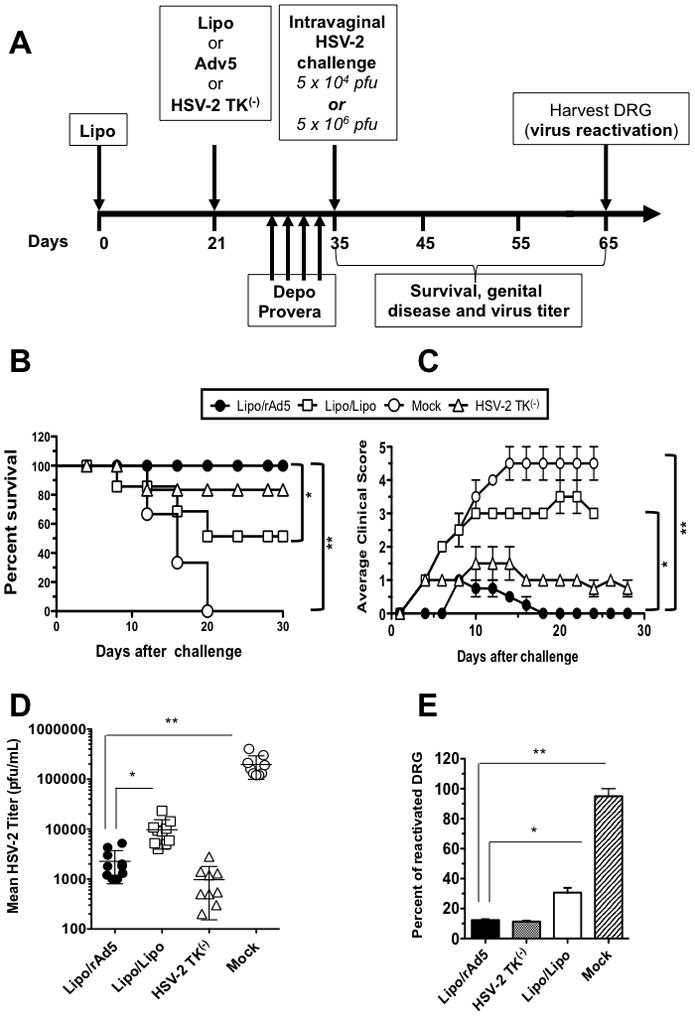
(A) Illustrates time course for immunization, challenge and protection analysis in B6 mice. Three groups of forty sex- and age-matched B6 female mice were immunized IVAG with the Lipo/rAdv5 prime/boost vaccine (Lipo/rAdv5); with the homologous Lipo/Lipo vaccine (Lipo/Lipo) or with the irrelevant OVA257-264 lipopeptide and empty vector Ad5 in PBS (Mock) on days 0 and 21. Ten days after the final immunization each group of mice was divided in two groups of 20 mice, treated daily with four doses of Depo-Provera® and were then challenged intravaginally, on day 14, either with 5 × 106 pfu (= 200 x LD50 for survival analysis) or with 5 × 104 pfu (for virus titers and disease analysis) of HSV-2 (strain 333), as described in Materials and Methods. (B) Mice were observed daily from day 0 to day 30 post-challenge for mortality. (C) Mice were also observed daily for genital disease and clinically scored from 0 to 5. (D) The presence of infectious virus in VM was monitored 5, 7, 9 and 11 post-infection. Data show average of titers in each group, detected on day 11 days post-infection. The data are expressed as mean + S.E.M. of virus load (plaque forming units (PFU)/sample). (E) Dorsal root ganglia (DRG) were harvested from mice that survived beyond day 30 post-infection and the presence of reactivated virus was monitored from explanted DRG for 10 days. The percentage of DRG that showed positive virus reactivation as determined by explant co-cultivation is calculated. The results are representative from two independent experiments.
Figure 5. Longevity and CD8+ T cell-dependence of protection induced by Lipo/rAd5 prime/boost mucosal vaccine.
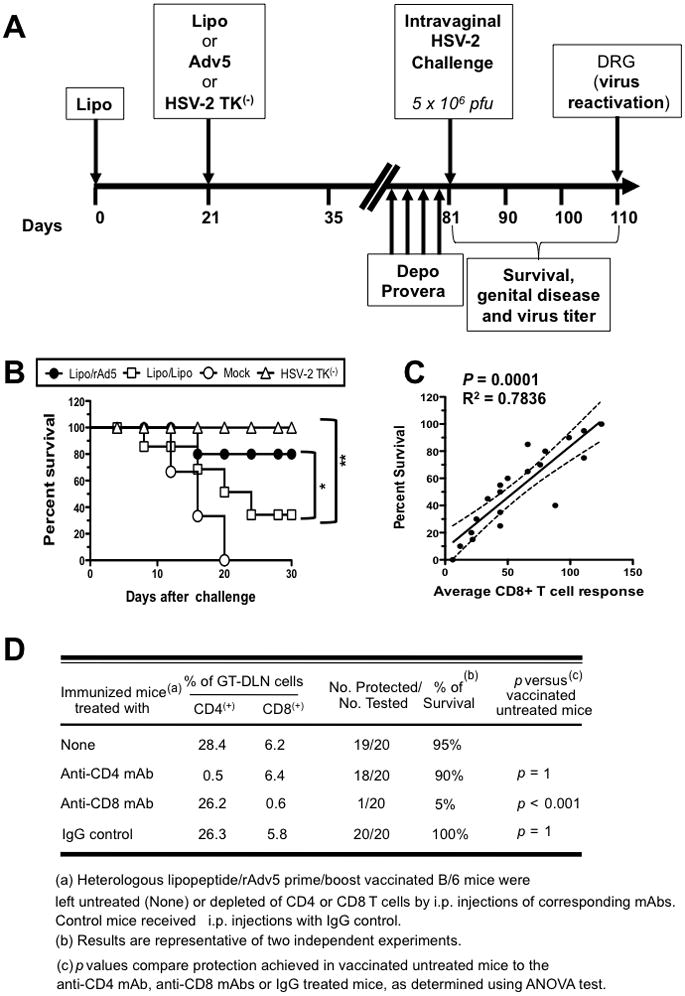
(A) Four groups of forty age-matched B6 female mice (n = 10 each) were immunized IVAG with the Lipo/rAdv5 prime/boost vaccine (Lipo/rAdv5); with the homologous Lipo/Lipo vaccine (Lipo/Lipo), with the irrelevant OVA257-264 lipopeptide and empty vector Ad5 in PBS (Mock), or with or with HSV-2 TK(−) (positive control) on days 0 and 21, as in Fig. 4A above. Ten days after the final immunization, all animals were treated daily with four doses of Depo-Provera® and were then challenged intravaginally, on day 60 with 5 × 106 pfu of HSV-2 (strain 333). (B) Immunized and infected mice were examined for survival in a window of 30 days post-challenge, as described in Material & Methods. (*) and (**) indicate P value of < 0.05 and 0.01, respectively, using one-way ANOVA test. (C) Scattergram and linear regression analysis of mouse survival (%) and HSV-specific CD8+ T cell responses in the VM after challenge with HSV-2. Correlation was performed using the Pearson test with two-tailed p-value analysis (R2 = 0.7836; p < 0.0001). R2 = correlation coefficient. (D) The protective immunity against genital herpes disease induced by the Lipo/rAdv5 prime/boost mucosal immunization is abrogated following depletion of CD8+ T cells, but not of CD4+ T cells. Three groups of female mice were immunized IVAG with the Lipo/rAdv5 prime/boost vaccine. Following the second dose of Lipo/rAdv5 immunization, and before challenge with HSV-2 (333), mice were injected i.p. with six doses of 100 uL of saline containing anti-CD4, anti-CD8 or isotype control mAbs. Flow cytometry analysis confirmed that after mAb treatment there was a decrease in spleen CD4+ and CD8+ T-cells in the treated mice to consistently less than 2%. The P values compare protection achieved in mAb treated versus untreated mice using the ANOVA test. Immunized, mAb treated, and infected mice were examined for survival in a window of 30 days post-challenge. Results are representative of two independent experiments.
Figure 6. MyD88 is required for induction of HSV-2 specific CD8+ T cell responses and protective immunity against genital herpes following IVAG immunization Lipo/rAdv5.
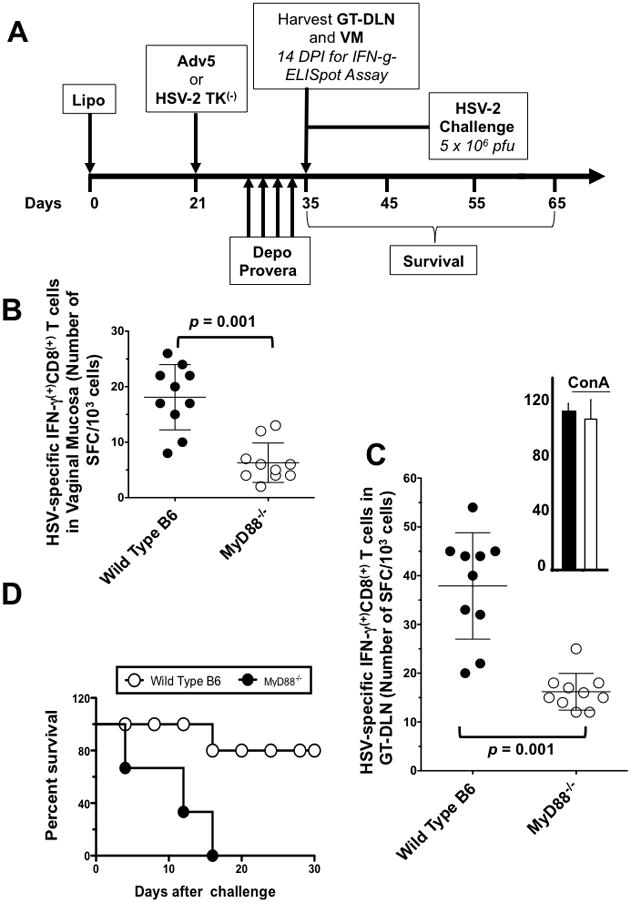
(A) Groups of wild-type B6 (n =10) and MyD88−/− deficient mice (n =10) were immunized IVAG with Lipo/rAdv5. 10 days after the second immunization; VM (B) and GT-DLN (C) were harvested from each group and cell suspensions were stimulated in vitro for 5 days with gB498-505-pulsed irradiated autologous H2b cells. The numbers of IFN-γ producing CD8+ T cells were measured by ELISpot assay, from VM and from GT-DLN derived from individual mice, as described in Material & Methods. (*) The upper right panel shows the GT-DLN-derived IFN-γ producing CD8+ T cells from wild type (black) and MyD88−/− mice (while) stimulated with Con A (positive control). p value = 0.001 when comparing the IFN-γ producing CD8+ T cell responses detected in wild type B6 mice to MyD88−/− mice using one way ANOVA test. (D) Survival. Two groups of age-matched B6 female wild type B6 mice (n =10) and MyD88−/− mice (n = 10) were immunized IVAG with the Lipo/rAdv5 prime/boost vaccine on days 0 and 21, as above. Ten days after the final immunization each group of mice was injected daily four times with 0.5 mg of Depo-Provera, as described in Materials and Methods. Four days later, all animals were challenged intravaginally with 5 × 106 pfu of HSV-2 (strain 333) in 10-uL sterile saline. Immunized and infected mice were examined for survival in a window of 30 days post-challenge, as described in Material & Methods. (*) Indicates p < 0.005; when comparing the survival in wild type B6 mice to MyD88−/− mice using one-way ANOVA test. The results are representative for two independent experiments.
Immunohistochemistry
Mice were euthanized and the vaginal mucosal tissues were collected fixed with 2% paraformaldehyde. After overnight fixation the VM samples were cut into small longitudinal bands. Then samples were blocked with anti-FcRg, antibody (US biological, MA) at a dilution of 1:100 and in goat serum/PBS overnight. The anti-CD8 antibody conjugated to FITC at a dilution of 1:100 and 14.3 mM DAPI (Molecular probes, Invitrogen, CA) were applied overnight at 4°C. Then samples were mounted in 50% glycerol/PBS. Confocal microscopy was performed with a laser confocal and multiphoton microscope system with a conventional laser confocal microscope (Zeiss LSM 510 META, Jena, Germany) equipped with a Femtosecond titanium laser (Chameleon, Coherent, CA).
Isolation of GT mucosal lymphocytes
Lymphocytes were isolated from the female genital tract (GT) mucosal tissues and treated with calcium- and magnesium-free phosphate-buffered saline for whole-body perfusion prior to tissue harvest, as previously described (29). The female GT included the ovaries, fallopian tubes, uterus, and vagina (5, 6). GT tissues were digested following a 2 hrs treatment with 2.5 mg/mL of collagenase type A (Roche catalogue number 1088 785) and 5 units/mL of DNase I (Roche catalogue number 104 159) suspended in RPMI-1640 with 5% fetal bovine serum, penicillin-streptomycin, HEPES. Mucosal GT tissues were pooled from five-mice/immunization group to provide sufficient cells to perform replicates of each experiment (IFN-γ ELISpot) and to allow for accurate measurement of immune responses in each immunization group. The average yield of cells per mouse was 4 × 106 cells/female reproductive tract.
Cytokine assays
Two weeks after the second immunization, mice were euthanized, the iliac and inguinal lymph nodes (5, 6) draining the GT (GT-DLN) were removed and single cell suspensions of GT-DLN cells placed into ice-cold serum free HL-1 medium supplemented with 15 mM HEPES, 5 × 10−5 M β-mercaptoethanol, 2 mM glutamine, 50 IU of penicillin and 50ug of streptomycin (GIBCO-BRL, Grand Island, NY) (referred as complete medium, or CM) (6). The cells were cultured in 6-well plates at 5 × 106 cells/well in CM with the target HSV-gB498-505 alone; with UV inactivated HSV infected stimulator cells at MOI of 3, or with Con-A (positive control). 72 hours later, the supernatants were collected and the concentrations of IFN-γ, TNF-α and IL-2 were determined in a specific sandwich ELISA according to the manufactured instructions (BD PharMingen, San Diego, CA).
IFN-γ ELISpot assay
GT-DLN or GT mucosal cells were cultured in 24-well plates for 5 days in a humidified 5% CO2 atmosphere with HSV-gB498-505 peptide alone (10ug/ml), the irrelevant OVA257-264 CD8+ T-cell peptide (10ug/ml) or autologous HSV-2 infected stimulator cells, and subsequently analyzed in an IFN-γ-ELISpot assay. Functional T-cell recognition IFN-γ-ELISpot assays were performed with the mouse IFN-γ-ELISpot mAb pair (BD PharMingen, San Diego, CA). Briefly, on day 4, 96 well multi-screen-IP plates were coated over night at 4 °C with 100 μl (1:250) of anti- IFN-γ capture mAb. Plates were then blocked with RPMI-1640 medium supplemented with 10% FCS for 2h at room temperature. 5 × 104 cells/well were added in triplicate to mAb coated plates and incubated for 18–24 hrs at 37°C, 5% CO2. Plates were then washed with PBS, supplemented with a detection peroxidase-labeled antibody followed by a substrate according to the manufacturer’s instructions. The developed spots were counted under a light microscope. Controls included cells cultured in medium in the absence of peptide stimulation. The frequency of IFN-γ SFCs in control wells (typically <10 IFN-γ SFCs/106 cells) was subtracted from the frequency of IFN-γ SFCs detected in the peptide-stimulated cells for calculation of antigen-specific IFN-γ responses.
Flow cytometry
Standard flow cytometry was employed, as we previously described (6, 34) to assess surface expression of various markers using the following mAbs directly conjugated with either PE or FITC: FITC-CD4, FITC-CD8, PE HSV-gB tetramer (PharMingen, San Diego, CA). IgG isotype-matched control Abs were used in all experiments. After staining, cells were washed and fixed in 1% buffered paraformaldehyde before being acquired on a Becton Dickinson FACSCalibur® (Mountain View, CA). Gating was on large granular cells, and for each sample, 20,000 events were acquired on a FACSCalibur® and analyzed with CellQuest® software, on an integrated Macintosh G4 (Becton Dickinson, San Jose, CA).
Tetramer assay
gB498-505 tetramers were prepared and used as described (6). A total of 0.1 to 0.2 μg of phycoerythrin-labeled gB498-505 tetramer complexes with allophycocyanin (APC)-labeled anti-mouse CD8 (Ly-2; Caltag, South San Francisco, Calif.) monoclonal antibody was used to identify gB498-505-specific CD8+ T cells. Samples were analyzed with tetrameric gB498-505 tetramer complexes for the percentage of CD8+ T cells by two-color flow cytometry with a FACSCalibur (Becton Dickinson, Mountain View, Calif.) system.
CTL activity
GT-DLN derived immune CD8+ T-cells (5 × 106) were re-stimulated in vitro with HSV-gB498-505 target peptide (5ug/ml)-pulsed syngeneic irradiated T cell depleted 1 × 106 splenocytes (2500 rad from a 137Cs source) and irradiated 1 × 106 EL-4 cells (3000 rad from a 137Cs source), as we previously described (6). CTL activity was assessed by a standard 4-h 51 Cr release assay against EL4 target cells loaded with 10 μM of HSV-gB498-505 target peptide or infected with heat-inactivated HSV-2 (MOI = 3), as previously described (6). After 5 days of culture, effector CD8+ cells were mixed at 1, 3, 10, 30, or 100 effector: target (E:T) ratios, with 51Cr-labeled EL4 cells for 4 h. Maximum release of 51Cr was determined by adding 5% Triton X-100 to 51Cr-labeled EL4 cells. Spontaneous release (<10% of total release) was determined by incubating target EL4 cells with medium alone. The percentage of specific lysis was calculated as follows: 100x ((experimental release − spontaneous release)/(maximum release − spontaneous release)).
Monitoring virus replication in vaginal tissue
Two weeks after the final immunization, mice were treated with progesterone (Depo-Provera®), to synchronize the ovarian cycle and increase susceptibility to herpes infection, and then received an IVAG HSV-2 challenge. The infection of the genital tract in the progesterone-treated mouse model appears to be similar to the initial infection in humans, the main difference being that the susceptible epithelial cells in mice are present in both vagina and cervix whereas they are mainly in the cervix of humans (58). An inoculum of 5 × 104 pfu of HSV-2 (333) or 5 × 106 pfu (= 200 x LD50 for survival analysis) in 10μl tissue culture medium was placed into the vaginal canal of immunized and control mice. To quantify vaginal HSV-2, the vaginal canal of immunized and control mice were swabbed once daily (days 1 to 10 post infection) with a Dacron swab and each swab placed in a 75-mm culture tube containing 0.5 ml of media. 100μl aliquots of 10 fold serial dilutions were placed on confluent monolayer of RS cells in 6-well plates, incubated at 37°C for 1 hr and overlaid with medium containing 1% methylcellulose. The plates were incubated at 37°C for 3 days, stained with 1% crystal violet, and the viral plaques were counted.
Detection of latent virus in dorsal root ganglia
Equal numbers of mice in each group surviving 30 days post-infection were euthanized; their dorsal root ganglia (DRG) removed and individually explanted onto RS cell monolayer in RPMI medium. The culture was monitored for 10 days for the presence of infectious virus.
CD8+ depletion in vitro
CD8+ lymphocytes were depleted from GT-DLN and mucosal samples with magnetically activated cell sorting CD8α (Ly-2) MicroBeads (Miltenyi, Auburn, CA), following the protocol provided with the MicroBeads. Briefly, single-cell suspensions were incubated with MicroBeads for 20 min at 4°C. The cells were then washed with 5 mL of phosphate-buffered saline, 0.5% FBS, and 2 mM EDTA. The pellet was then re-suspended in 500 μL of wash buffer and placed onto a pre-wetted MS+ selection column (Miltenyi) in the separator. Following the separation, the column was washed three times, and the negatively selected cells were washed and pelleted before being counted and adjusted to a proper concentration for the ELISpot assay.
Statistical analysis
Data for each assay were compared by analysis of variance (ANOVA) and Student’s t test using Graph Pad Prism 5 software (San Diego, CA). Differences between the groups were identified by ANOVA, multiple comparison procedures, as we previously described (6). Data are expressed as the mean ± SD. Results were considered to be statistically significant at p < 0.05.
RESULTS
Lipo/rAdv5 prime/boost vaccine induced potent and long-lasting HSV-specific IFN-γ-producing CD8+ T-cells detected in both the GT draining lymph nodes (GT-DLN) and in the vaginal mucosa (VM)
We first performed a dose-response study in B6 mice of the lipopeptide vaccine using 50, 100 or 200μg as well as of the rAd5 vaccine using 107, 5 × 107 or 108 pfu, both delivered intravaginally (IVAG) in adjuvant-free saline on days 0 and 21. All 3 doses induced a similar magnitude of T cell responses in GT-DLN (data not shown). There were no obvious vaccine-related severe side effects, with either the lipopeptide or the rAdv5 vaccine, at any of the three doses tested, as evaluated by weight loss or vaginal lesions (not shown). Accordingly, all subsequent experiments were carried out using the middle dose of 100 μg for the lipopeptide vaccine and 5 × 107 pfu for the rAd5 vaccine.
Three groups of female B6 mice (n =10) were immunized IVAG with: (i) the lipopeptide in adjuvant-free saline on day 0 and 5 × 107 pfu of rAd5 in saline on day 21 (Lipo/rAdv5 prime/boost mucosal vaccine); or (ii) 100 μg of the lipopeptide in saline on days 0 and 21 (homologous Lipo/Lipo vaccine); or (iii) with the irrelevant OVA257-264 lipopeptide on day 0 and 5 × 107 pfu of an empty Ad5 vector in saline on day 21 (mock-vaccine). An illustration of the immunization scheme and subsequent immunological experiment timeline is shown in Fig. 1A. The iliac and inguinal lymph nodes draining the genital tract (5, 6) (designated as GT-DLN) and the vaginal mucosa (VM) were harvested on day 35 and day 261 (i.e. about 8 months after the final immunization). The induced IFN-γ-producing CD8+ T cells against autologous H2b EL4 cells loaded with the HSV-gB498-505 target peptide (Fig. 1B and C) or EL4 cells infected with HSV-2 (Fig. 1D and E) were evaluated in GT-DLN and VM by a standard ELISpot assay, as we previously described (6). Both the Lipo/rAdv5 prime/boost and the Lipo/Lipo vaccines induced significant HSV- and gB498-505-specific IFN-γ-producing CD8+ T cell responses, as compared to mock-vaccine detected, 35 days post-immunization, in both GT-DLN and VM (p < 0.005, Fig. 1B–E). However, CD8+ T cell responses induced by the Lipo/Lipo vaccine were rather moderate, as compared to those induced by the Lipo/rAdv5. Immunoassaying of vaginal mucosal tissue from progesterone-treated immunized mice confirmed an abundance of CD8+ T cells in the vaginal epithelium of the Lipo/rAdv5 group compared to the Lipo/Lipo group (Fig. 1F). When assessed on day 261, only the Lipo/rAdv5 vaccinated mice had significant HSV- and gB498-505-specific IFN-γ-producing CD8+ T cell responses compared to mock-immunized mice (p < 0.05). However, no significant HSV- and gB498-505-specific IFN-γ-producing CD8+ T cell responses were detected in the Lipo/Lipo vaccinated mice (p > 0.05). As expected, no significant T cell responses were seen against H2b EL4 cells loaded with the irrelevant OVA257-264 target peptide in either the Lipo/rAdv5 prime/boost or the Lipo/Lipo-vaccinated mice, demonstrating the specificity of CD8+ T cell responses induced by either type of vaccine (not shown). The CD8+ T cell responses induced by either vaccine were abrogated following blockage with anti-CD8 mAb, but not with anti-CD4 mAb (not shown). Altogether, these results suggest that Lipo/rAdv5 prime/boost vaccine induced potent and long-lasting HSV-specific IFN-γ-producing CD8+ T-cells in both GT-DLN and VM.
Lipo/rAdv5 prime/boost mucosal vaccine elicits long-lasting CD8+ CTL with fast kinetics of mobilization than the Lipo/Lipo vaccine
Three groups of female B6 mice (n =40) were immunized IVAG with: (i) the lipopeptide in adjuvant-free saline on day 0 and 5 × 107 pfu of rAd5 in saline on day 21 (Lipo/rAdv5 prime/boost mucosal vaccine); or (ii) 100 μg of the lipopeptide in saline on days 0 and 21 (homologous Lipo/Lipo vaccine); or (iii) with the irrelevant OVA257-264 lipopeptide day 0 and 5 × 107 pfu of an empty Ad5 vector in saline on day 21 (mock-vaccine). An illustration of the immunization scheme and subsequent CTL experiment timeline is shown in Fig. 2A. To better analyze the kinetics of CD8+ T cell responses induced by each vaccine; time points were expanded by harvesting GT-DLN on days 31, 41, 51 and 261 (i.e. 10, 20, 30 and 240 days post-immunization (DPI)). Five mice were used per time point in each group. The cell suspensions were re-stimulated in vitro with UV-inactivated HSV-2 for 5 days and CD8+ CTL responses were evaluated in a standard Cr51 assay against autologous H2b EL4 cells loaded with the HSV-gB498-505 target peptide (Fig. 2B, upper panel) or against target cells transfected with VVgB, a vaccinia virus expressing gB (Fig. 2B, lower panel). VVgB was used to ascertain that the induced CD8+ T cells were able to recognize the native epitope processed endogenously, not just the artificial synthetic gB498-505 peptide epitope, Significantly higher CTL responses were detected against HSV-gB498-505 on days 20, 30 and 240 post-immunization in the Lipo/rAdv5 prime/boost vaccine group, compared to Lipo/Lipo group (Fig. 2B, upper panel, p = 0.03, one-way ANOVA test). However, higher CTL responses were detected against target cells transfected with VVgB in the Lipo/rAdv5 prime/boost vaccine group only later days 30 and 240 post-immunization (p = 0.05, one-way ANOVA test, Fig. 2B, lower panel). As expected no CTL responses were detected in the mock-immunized control group in any time point. The difference in the CTL responses between the Lipo/rAdv5, the Lipo/Lipo was even more significant on day 240, suggesting that the Lipo/rAdv5 prime-boost mucosal vaccine may induce more sustainable memory CD8+ CTL responses than the homologous Lipo/Lipo. The gB498-505- and HSV-specific CTL cell responses were abrogated following blockage with anti-CD8 mAb, but not with anti-CD4 mAb (not shown). All together these results: (i) indicate a clear difference in the kinetics with which CD8+ CTL are mobilized following Lipo/Lipo vs. Lipo/rAdv5 prime/boost mucosal vaccination, with the later mobilizing CD8+ T cells faster than the former; (ii) confirm the sustainability of the CD8+ T cell responses induced by the Lipo/rAdv5 prime/boost vaccine that last 8 months after the final immunization; (iii) suggest that the Lipo/rAdv5 prime-boost mucosal vaccine may induce memory CD8+ T cells that are polyfunctional (i.e. produce IFN-γ and have a cytotoxic activity).
High and sustained frequency of HSV-specific CD8+ T cells induced by Lipo/rAdv5 prime/boost mucosal vaccine
Three groups of female B6 mice (n =40) were immunized IVAG with: (i) the lipopeptide in adjuvant-free saline on day 0 and 5 × 107 pfu of rAd5 in saline on day 21 (Lipo/rAdv5 prime/boost mucosal vaccine); or (ii) 100 μg of the lipopeptide in saline on days 0 and 21 (homologous Lipo/Lipo vaccine); or (iii) with the live attenuated HSV-2 TK(−) on day 21, as we previously described (6) (positive control), as illustrated in Fig. 3A. GT-DLNs were harvested on days 10, 20, 30 and 240 post-immunization from Lipo/rAdv5, Lipo/Lipo, and HSV-2 TK(−) immunized mice (n = 5, for each time point) and cell suspensions were re-stimulated in vitro with UV-inactivated HSV-2 and then stained with HSV-gB498-505/H2-Kb tetramer followed by a mAb specific to mouse CD8. To obtain an objective enumeration of HSV-gB498-505-specific CD8+ T-cells induced by the Lipo/rAdv5 prime/boost versus the homologous Lipo/Lipo vaccine, we used the MHC tetramer-staining assay, which provides a quantitative measure of the frequency of gB498-505-specific CD8+ T-cells. The specificity of the HSV-gB498-505 epitope in combination with H2-Kb MHC heavy chain modified for tetramerformation has been established (25). As shown in Fig. 3B, a significantly higher percentage of CD8+ b /HSV-gB498-505/H2-K tetramer(+) T-cells were detected on days 10, 20, 30 and 240 post-immunization in Lipo/rAdv5 prime/boost vaccine group, compared to Lipo/Lipo group (p = 0.03, one-way ANOVA test). The difference in the percentage of CD8+/HSV-gB498-505/H2-Kb tetramer(+) T-cells detected between the Lipo/rAdv5 and the Lipo/Lipo groups was highest on days 30 and 240. Significantly increased number of CD3+CD8+ T cells (Fig. 3C–D) and more HSV-gB498-505-specific CD8+ T cells (Fig. 3E–F) were detected in the GT-DLN of Lipo/rAdv5 immunized mice compared to Lipo/Lipo mice, both on 30 and 240 DPI. Similarly, significantly higher numbers of CD3+CD8+ T cells (Fig. 3G–H) and of HSV-gB498-505-specific CD8+ T cells (Figs. 3I–J) were detected in the VM of the Lipo/rAdv5 group (p < 0.05, one-way ANOVA test). As expected the thymidine kinase mutant HSV-2 (HSV-2 TK(−) also induced a high number and percentage of CD8+/HSV-gB498-505/H2-Kb tetramer(+) T-cells. Altogether, these results: (i) confirm that the Lipo/rAdv5 prime/boost mucosal vaccine induced highest number of HSV-specific CD8+ cells with different kinetics of mobilization; (ii) suggest that the Lipo/rAdv prime/boost mucosal vaccine induced stronger and more sustained memory CD8+ T-cells in both GT-DLN and VM than its homologous Lipo/Lipo vaccine.
Next, we compared the profile of cytokines produced by CD8+ T cells induced by the Lipo/rAdv5 vaccine versus the homologous Lipo/Lipo vaccine. Thirty days after the second immunization GT-DLN-derived CD8+ T-cells were isolated from 5 mice in each group and re-stimulated in vitro with gB498-505 peptide-pulsed irradiated autologous spleen cells for 72 hrs. The amount of IFN-γ, TNF-α and IL-2 released in culture media, was determined in a specific sandwich ELISA. The Lipo/rAdv5 vaccine induced significantly higher amounts of IFN-γ, TNF-α and IL-2 compared to Lipo/Lipo vaccine (p < 0.05, one-way ANOVA test, Fig. 3K). A similar level of cytokines were detected in the Lipo/rAdv5 group and the HSV-2 TK(−)positive control group. Altogether, these data (i) indicate that IVAG immunization with Lipo/rAdv5 prime/boost vaccine induced a higher and sustained frequency of HSV-specific CD8+ T cells than homologous Lipo/Lipo; and (ii) suggest that the Lipo/rAdv5 prime/boost mucosal vaccine induced a polyfunctionality of CD8+ T cell responses, with a type 1 pattern of cytokines and a cytotoxic function.
The Lipo/rAdv5 prime/boost mucosal vaccine induced better protection against genital herpes than its homologous Lipo/Lipo vaccine
Since immunization with the Lipo/rAdv5 prime/boost mucosal vaccine induced stronger HSV-specific CD8+ T cell responses than the homologous Lipo/Lipo vaccine, it was of interest to determine whether it could also better protect against genital herpes. Four groups of twenty age-matched B6 mice were immunized IVAG with the Lipo/rAdv5 prime/boost vaccine, with the homologous Lipo/Lipo, with the irrelevant OVA257-264 lipopeptide/”empty” Adv (mock-immunized negative control) or with HSV-2 TK(−), positive control (Fig. 4A). Ten days after the final immunization, all mice were treated daily with four doses of Depo-Provera®, to synchronize the ovarian cycle and increase susceptibility to herpes infection. The mice were divided into two groups of ten, were then challenged intravaginally, on day 35 (i.e. 14 days post-immunization, DPI), either with 5 × 106 pfu (200 x LD50 for survival analysis) or with 5 × 104 pfu (for virus titers and disease analysis) of HSV-2 (strain 333), as we previously described (6). Mice immunized with the Lipo/rAdv5 prime/boost vaccine showed 100% survival compared to mice immunized with the homologous Lipo/Lipo (only 50% of mice survived, 30 days post-challenge; p < 0.005, one-way ANOVA test), and to mock-vaccinated mice (0% survived, 20 days post-challenge; p < 0.005, one-way ANOVA test) (Fig. 4B). As expected, mice immunized with HSV-2 TK(−) vaccine also showed little mortality with 80% of mice surviving 30 days post-challenge (positive control). The pathology scores observed in the Lipo/rAdv5 prime/boost group were also much lower than all other 3 groups (p <0.005 for all, one-way ANOVA test) (Fig. 4C). Additionally, viral titers measured in the vaginal washes from days 5, 7, 9 and 11 post-infection showed that the Lipo/rAdv5 prime/boost vaccine group had significantly lower viral loads than the other groups (Fig. 4D, <0.005 for all, t-test test only day 11 showed). Virus clearance in the VM was within 5–7 days in the Lipo/rAdv5 prime/boost mucosal vaccine groups as compared to 9 and 11 days in the homologous Lipo/Lipo group and to the mock-vaccinated group, respectively (not shown).
The effect of these vaccinations on virus reactivation from latency was examined by ex vivo explant cultivation of dorsal root ganglia (DRG) harvested on day 30 post-infection (Fig. 4E). The presence of reactivated virus was monitored from explanted DRG for 10 days. Since it is unlikely that vaccination prior to initial infection would suppress detection of virus reactivating from explanted DRG, these types of assays are assumed to produce an estimate of the relative ability of the vaccines to inhibit establishment of latency. Virus reactivation was detected from a significantly fewer DRG of Lipo/rAdv5 immunized mice compared to the Lipo/Lipo group Fig. 4E, p < 0.004, t-test test). Thus, Lipo/rAdv5 prime/boost mucosal vaccination appeared to significantly reduce the amount of virus that reactivated from DRG.
Altogether, the above results indicate that following intravaginal HSV-2 challenge, Lipo/rAdv5 immunized mice had significantly lower virus titers at both the genital tract (the site of viral entry), and DRG (the site of latency). Lipo/rAdv5 immunization also decreased overt signs of genital herpes disease. Finally, Lipo/rAdv5 immunized mice did not succumb to lethal infection compared to Lipo/Lipo immunized mice (p < 0.005).
The long-lasting CD8+ T cell dependent protective immunity induced by the Lipo/rAdv5 prime/boost mucosal vaccine
To assess the protective efficacy of Lipo/rAdv5 vaccine during the memory phase, 60 days after the final immunization (i.e. on day 81), ten mice in each of the four groups above were challenged intravaginally with 5 × 106 pfu of HSV-2, (Fig. 5A). While all the HSV-2 TK(−) immunized mice survived lethal infection (positive control); 90% of the Lipo/rAdv5-immunized mice had less death rate, while only 40% of Lipo/Lipo immunized mice survived (Fig. 5B). Linear regression analysis of all experimental groups indicated that high IFN-γ–producing CD8+ T cell responses correlated positively with the survival (Fig. 5C). The linear regression was also found indicating the correlation between CD8+ T cell response in the VM and survival (R2 = 0.7836; p < 0.0001). To investigate the roles of CD4+ and CD8+ T-cell subsets in the protection, Lipo/rAdv5-immunized mice were depleted for either CD4+ or CD8+ T-cell subsets before intravaginal HSV challenge, as we previously described (6) (Fig. 5D). CD8+ T-cell depleted mice lost their protection against death as compared to non-depleted mice. In contrast, depletion of CD4+ T-cells had little effect on survival in Lipo/rAdv5-immunized mice (Fig. 5D). Sixty days after immunization ten additional mice in each of the four groups above were challenged intravaginally with 5 × 104 pfu of HSV-2 and the both the virus replication and disease followed during the memory phase of the responses (i.e. days 81 to 110). The pathology scores were 0 to 1 in both the Lipo/rAdv5 prime/boost group and the HSV-2 TK(−) group, compared to score of 2 to 3 in the Lipo/Lipo and to score of 2 to 5 in the Mock group. As expected, viral titers measured in the vaginal washes on day 71 post-infection were significantly lower in the Lipo/rAdv5 prime/boost vaccine group as compared to the other groups (p <0.005 for all, t-test test). Additionally, when DRG were harvested on day 110, there were less than 10% of reactivated ganglions in the Lipo/rAdv5 and TK(−)groups compared to 25–30% in the Lipo/Lipo and to 75–90% Mock group (p <0.005 for all, one-way ANOVA test). Altogether, these results suggest that: (i) in the Lipo/rAdv5 prime/boost mucosal vaccine system, virus-specific CD8+ T cells were involved in protective immunity to genital herpes; (ii) both long lasting CD8+ T cell responses and better protections were observed in mice immunized with Lipo/rAdv5 vaccine, suggesting that the combination of lipopeptide and viral vectors is beneficial for the induction of an effective long-term protective immunity against genital herpes.
Absence of myeloid differentiation factor 88 (MyD88) abolishes HSV-specific CD8+ T cell response and protection induced by the Lipo/rAdv5 prime/boost mucosal vaccine
To examine the contribution of MyD88, a critical adaptor protein shared by most of TLRs (23), in the immunogenicity of the Lipo/rAdv5 vaccine delivered intravaginally, groups of age-matched female MyD88−/− mice (n = 20) and wild type parental B6 mice (n = 20) were immunized intravaginally with the Lipo/rAdv5 vaccine or with HSV-2 TK(−) (positive control) (Fig. 6A). Fourteen days after the second immunization, VM tissues (Fig. 6B) and GT-DLN (Fig. 6C) were harvested from ten mice and their cell suspension analyzed for HSV-specific IFN-γ-producing CD8+ T cell responses by ELISpot assay. The other ten mice were treated daily, starting 10 DPI, with four doses of Depo-Provera®, to synchronize the ovarian cycle and increase susceptibility to herpes infection, and were then challenged intravaginally, 14 DPI, with 5 × 106 pfu of HSV-2 as above. B6 mice developed significantly more HSV-specific IFN-γ-producing CD8+ T cells than MyD88−/− mice, as detected in VM (Fig. 6B) and GT-DLN (Fig. 6C), (p = 0.001 for both VM and GT-DLN, one-way ANOVA test). As expected, the apparent similar trend in HSV-2 TK(−) immunized mice did not reach significance suggesting that immunity induced by HSV-2 TK(−) was independent of MyD88 (not shown). These results are consistent with the role of MyD88−/− pathway in the downstream signaling triggered by TLRs Lipo/rAdv5 vaccine interactions intravaginally (26). Finally, MyD88−/− mice immunized with the Lipo/rAdv5 vaccine had increased death rate compared to wt B6 mice (Fig. 6D). Thus absence of MyD88 significantly abolished both the HSV-specific CD8+ T cell response and protection induced following IVAG immunization with the Lipo/rAdv5 vaccine (p = 0.001). As expected, wt and MyD88−/− mice immunized with HSV-2 TK(−) had similar reduced virus titers and survivals (not shown). Together, these results indicate a pivotal role of the MyD88 pathway in the immunogenicity of Lipo/rAdv5 vaccine following intravaginal immunization.
DISCUSSION
A mucosal immunization regimen that induces potent CD8+ T cells in the GT and GT-DLN is a perquisite for protection against STV pathogens, such as HSV-2. Surprisingly, compared to other mucosal tissues, induction of local CD8+ T cell immunity to protect the genital mucosa from STV infection and pathogenicity has received much less attention. In the present study, we describe, for the first time, a Lipo/rAdv5 prime/boost mucosal immunization strategy that induced a robust HSV-specific CD8+ T cell-dependent protective immunity against genital herpes. Viral replication in the GT was significantly lower in the Lipo/rAd5 vaccine group compared to Lipo/Lipo vaccine group. Moreover, following the HSV-2 challenge, mice immunized IVAG with the Lipo/rAdv5 prime/boost mucosal vaccine showed less overt signs of genital herpes disease and did not succumb to lethal infection compared to mice immunized with the homologous Lipo/Lipo vaccine. The CD8+ T-cell responses elicited by the Lipo/rAd5 vaccine were completely abolished in MyD88-deficient mice. The induced long-lasting memory CD8+ T cell responses persisted in the GT-DLN for up to 8 months post immunization. These long-lasting CD8+ memory T cells may play a critical role in immune surveillance and could rapidly respond and expand after sensing the virus to prevent genital herpes. The findings underscore the potential of Lipo/rAdv5 prime/boost mucosal immunization, as an efficient vaccination strategy, for inducing genital CD8+ T cell immunity and should impact the development of vaccines against other STV diseases.
Understanding which innate molecular pathways are involved in the induction and maintenance of mucosal CD8+ T cell immunity is key to future successful clinical vaccines against STV infections (19). TLRs have TIR intracellular domains that engage two main signaling pathways, via the TIR-containing adaptors: MyD88 and TRIF (23). Most of the thirteen known mammalian TLRs recruit MyD88, with the exception of TLR3, which recruits TRIF, and of TLR4, which recruits both MyD88 and TRIF (23). One of the goals of the present study was to examine the role of MyD88 pathway in the immunogenicity and protective efficacy of the Lipo/rAdv5 prime/boost mucosal vaccine. Compared to wild-type B6 mice, MyD88−/− mice developed significantly lower HSV-specific CD8+ T cell responses following IVAG immunization with the Lipo/rAdv5 prime/boost mucosal vaccine. This result suggests an MyD88-dependent mechanism in the T cell protective immunity at the female genital tract. Not every mucosal immunization strategy results in MyD88-dependent protective immunity against genital herpes. Harandi recently reported that intranasal immunization with HSV-2 gD in combination with alpha-GalCer mucosal adjuvant elicited MyD88-independent T cell immunity that protected from an otherwise lethal vaginal HSV-2 challenge (27). Although the role of MyD88 pathway in the mucosal CD8+ T cell immunogenicity of lipopeptides (6) and rAd5 vectors has been previously reported (23), its role in the T cell immunity at the female genital tract following a Lipo/rAdv5 prime/boost IVAG administration has never been reported. Thus, the present study is first to demonstrate that CD8+ T-cell-mediated protective immunity, elicited by a Lipo/rAd5 prime/boost IVAG vaccine, against genital herpes was completely abolished in MyD88−/− mice. It is likely that stimulation of MyD88-dependent transcriptional program contributes to the success of the Lipo/rAdv5 prime/boost mucosal vaccine. However, our findings do not exclude the involvement of other intracellular signaling pathways in the immunogenicity and protective efficacy of the Lipo/rAdv5 prime/boost mucosal vaccine. The TRIF pathway may be particularly involved, since TLR3, which exclusively engages TRIF (23), has been recently reported as important in inducing HSV-specific CD8+ T cells (28). Thus, investigation of other intracellular pathways underlying TLRs signaling, following IVAG immunization with the Lipo/rAdv5, is currently being pursued in our laboratory. Since MyD88 is a critical adaptor protein shared by at least six TLRs (i.e. TLR2, TLR4, TLR5, TLR6, TLR7 and TLR9) (23), it remains to determine whether the potent immunogenicity and protective efficacy induced by the Lipo/rAdv5 prime/boost mucosal vaccine is abrogated in TLR2−/−, TLR4−/−, TLR5−/−, TLR6−/−, TLR7−/−, and TLR9−/−deficient mice (23). These studies will be the subject of future reports.
The Lipo/rAdv5 prime/boost mucosal vaccine delivered IVAG stimulated potent and sustained HSV-specific CD8+ T cell responses, detected not only in the GT-DLN but also in the VM. Clear differences were apparent in the kinetics with which CD8+ T cells are mobilized following Lipo/Lipo vs. Lipo/rAdv5 prime/boost mucosal vaccination. The success Lipo/rAdv5 prime/boost mucosal vaccine is also highlighted by its ability to induce and facilitate the mobilization and establishment of local effector memory CD8+ T cells in the genital mucosal tissue (VM), by apparently promoting their migration. We found a cluster of IFN-γ-producing CD8+ T cells in the VM of Lipo/rAdv5 vaccinated mice, suggesting an establishment of memory cell foci in the genital mucosa (not shown). These clusters of CD8+ T cells appear to migrate from the GT-DLN to virginal mucosa, since the vaginal sub-mucosa does not contain mucosal-associated lymphoid tissue (MALT) in in the steady state (29). Several explanations are possible for the difference in mobilization and kinetics of CD8+ T cells by Lipo/Lipo vs. Lipo/rAdv5 prime/boost mucosal vaccines. Generally, effector memory T cells circulate throughout the peripheral tissues, such as the VM, whereas central memory T cells reside in the secondary lymphoid tissues, such as GT-DLN. Thus, regardless of the site of antigen encounter, HSV-specific memory CD8+ T cells must be found in various tissues, including the VM and GT-DLN. However, peripheral tissue distribution of memory CD8+ T cells occurs mainly after immunization with live replicating vectors, such as rAdv5. The mucosa of vaginal canal is drained by several lymph nodes, including the common iliac, interiliac, external iliac and inguinal femoral lymph nodes (in descending order, designated in this report as GT-DLN) (reviewed in (4)). It is likely that the IVAG immunogenicity of Lipo/rAdv5 occurs through vaginal sub-mucosal dendritic cells (DC) efficiently take up the rAdv5 vaccine and migrate to the GT-DLN, where they present the gB498-505 peptide to cognate CD8+ T cells. The proportion of CD8+ T cells after lipopeptide priming is greater and that these expand more prominently following heterologous rAdv5 boost. Since all the mechanism above are not mutually exclusive, it is possible that they all play a role in our immunization scheme, but additional experiments will be needed to assess both the relative proportion of each mechanism in the observed immunogenicity.
We recently demonstrated the requirement of CD4+ T cell help for efficient priming and maintenance of HSV-specific CD8+ T cells (6, 30). Others have shown that CD4+ T cells are required to pave the way for efficient migration of memory CD8+ T cells into restricted tissues, such as the VM (31). Thus, epitope vaccines based on the generation of only systemic CD8+ T cell immunity are likely to fail because helpless CD8+ T cells may not be self-sufficient for entry into the infected VM mucosal tissue. For these reasons, we designed our lipopeptide mucosal prime vaccine to include both the HSV-gB498-505 CD8+ T cell epitope and the Pan DR peptide (PADRE) that expresses a universal CD4+ T helper epitope (6, 30). Besides the HSV-gB498-505-specificCD8+ T cell response, the rAdv5 also boosted substantial CD4+ T cells that likely help in the maintenance and migration of effector CD8+ T cells into infected tissue (17, 32, 33). Future studies will determine whether Lipo/rAdv5 vaccine-induced CD4+ T cells respond to local chemokines, enter the VM tissue and induce a subsequent wave of chemokines that would enable HSV-specific CD8+ memory T cell populations to enter the VM tissue, as has been shown in other systems (31), and the results will be the subject of future reports.
HSV-2 is one of the most common STV infections (6). After rectal, vaginal, or penile exposure to HSV-2, the virus replicates in the mucosal epithelial cells that leads eventually to rectal or genital herpes lesions (34). The global prevalence of seropositive individuals, fifteen years and older, is estimated to be at least 60 million within the United States, and well over 540 million worldwide (35, 36) with a greater frequency of infection in women (6). Despite the availability of many intervention strategies, such as sexual behavior education, barrier methods, and the costly guanine nucleoside anti-viral drug therapies (e.g. Acyclovir and derivatives), controlling the spread of genital herpes remains a challenge (1, 6, 36). The current medical opinion is that an effective clinical vaccine would constitute the best approach to protect the human population from genital herpes (35, 36). Such a vaccine would likely have the greatest impact in both developed and under-developed regions of the world (35, 36). To date, however, no clinical vaccine for the prevention or treatment of genital herpes is available (22). Direct experiments in animal models (5, 6, 37) and indirect evidence in humans (7) suggests that successful control of herpes infection is associated to induction of robust and polyfunctional CD8+ T cells within the vaginal sub-mucosal tissues (9–11). Our current inability to efficiently deliver Ags to stimulate strong local mucosal HSV-specific CD8+ T cell responses remains a critical roadblock in the development of an effective vaccine. The present study is the first to show that a combination of the two vaccine formulation (i.e. a lipopeptide and an adenovirus) in a prime-boost Lipo/rAdv5 mucosal vaccine strategy, administered IVAG, induced robust, sustained and polyfunctional CD8+ T cells locally in the GT-DLN and VM, characterized by cytotoxic activity and coproduction of IFN-γ, TNF-α and IL-2. Both migrating and tissue-resident memory T cells have been implicated in long-term peripheral protective immunity, especially at the non-lymphoid muco-cutaneous tissues, such as the vaginal mucosa (VM) that serves as virus entry points into the body (38). In the present study a correlation was found between the numbers of VM-resident HSV-specific CD8+ T cells and protection against vaginal infections. A linear regression analysis also indicated that high numbers of VM-resident IFN-γ-producing CD8+ T cell responses correlated positively with the survival (R2 = 0.7836; p < 0.0001, Fig. 5C). These results are in agreement with recent reports that tissue-resident memory (TRM) CD8+ T cells, those that survived in non-lymphoid tissues, may provide a more potent protective immunity against HSV than circulating memory (TCM) CD8+ T cells (9–11). Various anti-herpes TRM subsets may make distinct contributions to the observed protective immunity at the inflamed VM, as has been reported in other systems (38). Whether a unique subset of anti-herpes TRM CD8+ T cells (e.g. the non-migratory CD103+CD8+ TRM cell subset) induced by the heterologous Lipo/rAdv5 immunization or following the acute HSV-2 challenge, persisted for an extended period of time in the VM tissue and/or in the latently infected sensory dorsal root ganglia (DRG), will be determined in future phenotypic and functional kinetic studies.
HSV-2 infects the GT and then establishes latency in sensory neurons of the dorsal root ganglia (DRG). While sub-optimal “inherent” CD8+ T cells are detected in sensory ganglia and appeared to provide an immune surveillance of the infected neurons, these cells cannot clear latent virus. It is generally assumed that prevention of HSV-2 reactivation from sensory ganglia requires a therapeutic vaccine that will boost a more vigorous and/or a different virus-specific CD8+ T cell response. This is because the quality and/or the magnitude of “inherent” CD8+ T cell responses resulting from natural infection are not sufficient enough to reduce virus reactivation and recurrent genital herpes in symptomatic individuals (1, 35, 36, 39, 40). Following HSV-2 genital challenge, our Lipo/rAdv5 prime/boost mucosal vaccination appeared to significantly reduce the amount of virus that reactivated ex vivo from DRG. Protection was mediated by CD8+ T cells, as determined by CD8-depletion studies and greater protection correlating with cytotoxic CD8+ IFN-γ-producing cells in both the GT-DLN and VM. As expected, mice immunized with thymidine kinase mutant HSV-2 TK(−), which is incapable of reactivation in the sacral DRG, also show little reactivation from the DRG (positive control). The cluster of IFN-γ-producing CD8+ T cells found in the VM of Lipo/rAdv5 vaccinated B6 mice is likely important for providing an immediate response following reactivation of HSV-2 from DRG. In humans, memory CD8+ T cells are rapidly recruited following reactivation of infection and persist adjacent to peripheral nerve endings at the dermal-epidermal junction for more than 60 days after reactivation and healing (41). Importantly, subsequent virus reactivation at the site where CD8+ T cells are present did not result in lesion formation, indicating that HSV-2-specific CD8+ T cells at the site of genital herpesvirus lesions control local viral replication. Therefore, localized mucosal memory T cell populations seem to provide superior control of viral infection compared with circulating memory T cells. Our hope is that a therapeutic genital herpes prime-boost Lipo/rAdv5 vaccine that would increase the number and the function of HSV-specific CD8+ T cells in sensory ganglia of latently infected hosts will significantly decrease HSV-2 spontaneous reactivation (as measured by shedding in GT) and recurrent genital herpetic disease. This will be the subject of future investigation in an HSV-2 latently infected guinea pigs model, which like humans, develops spontaneous reactivation that leads to recurrent genital herpetic disease (reviewed in (13)).
The morbidity and socioeconomic burden associated with genital herpes, as well as the alarming relationship between genital herpes and HIV susceptibility, transmission and acquisition, highlight the need for the development of an effective vaccine. Several vaccine strategies have been introduced in the last two decades (1, 36). A clinical vaccine trial using protein-in-adjuvant HSV-2 vaccine (gD) delivered parenterally in women from so-called discordant couples (in which one partner is infected and the other is not) have reported in 2002 a limited success (13, 20, 21). When delivered intra-muscularly that protein-in-adjuvant vaccine induced transient immunity against HSV-2 disease in women who were seronegative for both HSV-1 & HSV-2 (20–22). However, that vaccine failed to protect HSV-2 seropositive women or seropositive men, even though good neutralizing antibody responses were elicited (20, 21). A more recent clinical vaccine trial reported in 2012, based on the same glycoprotein gD vaccine, showed efficacy in preventing HSV-1, but not in preventing HSV-2, genital herpes (20, 21). The reason for this discrepancy remains to be determined. A common conclusion could be drawn from these clinical trials (20, 21), together with our recently reported pre-clinical studies using the established murine model of intravaginal immunization (6, 34), that T-cell based mucosal vaccines may be protective against genital herpes. However, despite intensive research, the progress toward a T-cell based vaccine still faces major hurdles including the lack of an efficient Ag delivery system that would safely induce potent and sustained mucosal T cell immunity in and around the GT mucosa. The present study is one of the first to demonstrate that IVAG immunization with a Lipo/rAdv5 prime/boost mucosal vaccine induced potent, sustained and polyfunctional HSV-specific CD8+ T-cells in the GT-DLN that were associated with a reduction in virus replication in the GT and protection from most overt signs of genital herpes disease.
CD8+ T cell cytotoxicity and IFN-γ are thought to be central effector functions in the control of genital herpes (6, 42–44), acting directly or indirectly on resident cells, including parenchymal cells, (45) or facilitating recruitment of effector CD8+ T cells into the inflamed vaginal tissue (46). In order to obtain the most accurate picture of CD8+ T cell responses elicited by the Lipo/rAd5 prime/boost vaccine, as compared to the HSV-2 TK(−) positive control vaccine, we employed multiple immunological assays including: (i) visualization of induced CD8+ T cells in inflamed VM, using immuno-staining assay; (ii) quantification of CD8+ T cell cytotoxic function, in Cr51 release assay; (iii) determination of the frequency of induced CD8+ T cells by tetramer/FACS assay; (iv) detection of multiple cytokines production by ELISA and IFN-γ-ELISpot assays. It is interesting that the high frequency of HSV-specific CD8+ T cells in the Lipo/rAd5 group did not translate into higher amounts of cytokines production, as compared to the HSV-2 TK(−) group. Nevertheless, it is not unusual that a vaccine may induced more T cell proliferation results in a high frequency of CD8+ T cells without exhibiting one of the immunological function above (e.g. cytokine production, cytotoxic activity etc…) (6, 34). The opposite is also true; a CD8+ T cell can produce cytokines or exhibit cytotoxic function without extensive proliferation.
The concept of using recombinant viral-vector vaccines to deliver an unrelated viral antigen was developed more than 25 years ago (39). Recombinant adenoviruses (rAd) are promising and safe vectors due to their capacity to elicit potent CD8+ T cell responses to unrelated T cell epitopes (reviewed in (8, 47)). rAd5 vector-based vaccines have been recently tested successfully in animal models and several clinical trials are currently ongoing in the US ((8, 47) and http://clinicaltrials.gov/). In this study, we show that IVAG priming with a lipopeptide and boosting with recombinant rAd5v expressing the same HSV-2 gB CD8+ T cell epitope significantly reduce HSV-2 replication in the GT. A critical roadblock in the development of an ocular herpes therapeutic vaccine is our current inability to safely and efficiently deliver the antigen to stimulate the ocular mucosal immune system and to boost protective HSV-specific CD8+ T cells in latently infected trigeminal ganglia (TG) that will then prevent or, at least, reduce attempts of virus reactivation before infectious viral particles reach the eye to cause potentially blinding ocular disease. The knowledge gained from the present study will be undoubtedly useful to guide the development of a future ocular mucosal herpes vaccine. Based on our and other recent reports, showing that intranasal immunizations efficiently induce T cell responses in remote HSV reactivation/replication sites (i.e. VM, eye and TG) (48–50); a logical extension of the present findings would be to ocularely or intranasally apply the Lipo/rAdv5 prime/boost vaccine and determine whether it would induce long-lasting protective CD8+ T cell responses in the eye and TG. However, we should keep in mind that the high frequency of human anti-Ad5 neutralizing antibodies in the developing world, which will likely limit the immunogenicity and clinical utility of Ad5-vector based vaccines (17, 32, 33). Thus, the ability of rAdv5 to boost potent HSV-specific CD8+ T cell responses induced in a mouse model by a lipopeptide demonstrated in this study may not be extrapolated to humans. Nevertheless, this study constitutes a first proof-of-principle showing a strong immunogenicity of a prime/boost mucosal vaccine strategy using a rAd vector. A future candidate Lipo/rAdv5 prime/boost clinical herpes vaccine will probably have to be constructed using a rAd vector derived from an adenovirus serotype that is rare in human populations and distinct from the Ad5 serotype, such as rAd26 or rAd35, which are serologically distinct from rAd5 and do not infect the same type of cells as Ad5 (13). Neither Ad35 nor Ad26 are affected by anti-Ad5 immunity and rAd35 is not affected by anti-Ad26 immunity (51).
A recent study, by a Pfizer group, showed weak correlation between IFN-γ-producing T cell responses induced by gB and/or gD protein-in-adjuvant vaccines and protection against genital herpes in mice and guinea pigs (52). In this context, we should emphasise our recent findings that most HSV proteins, including gB and gD, contain “asymptomatic” protective epitope but may also contain “symptomatic” non-protective T cell epitopes that rather exacerbate genital herpes disease (reviewed in (1) and (35)). “Asymptomatic” epitopes are those that are exclusively recognized by T cell from HSV-2 seropositive but asymptomatic individuals. An epitope-based vaccine that incorporates only “asymptomatic” CD8+ T cell epitopes, while excluding “symptomatic” epitopes, is logically expected be more protective and preferable to whole protein vaccines that contain “symptomatic” epitopes that may be a source of an exacerbating disease (6, 40, 53).
In conclusion, this study; (i) demonstrates the ability of a novel Lipo/rAdv5 mucosal vaccine regimen to induce higher CD8+ T cell responses, not only at a higher magnitude but also with an increase in quality level; (ii) Indicates that the protective efficacy of this formulation is associated with the induction of local and sustained polyfunctional CD8+ T cell responses. In the context of the limited success of recent antibody mediated herpes clinical vaccine trials using protein-in-adjuvant vaccines delivered parenterally, this is rather an interesting finding since T cell responses to HSV-2 antigens in the VM might be sub-optimal due to the unique immune vaginal environment (54). Thus the present findings are encouraging as they offer a needle-free and relatively low-cost mucosal route to provide a T-cell based vaccine for clinical trials. Which T cell epitopes will be protective in man have yet to determined. Nonetheless, the findings lay the ground for an accessible and durable mucosal prime/boost vaccine approach to reduce transmission of genital herpes, and presumably other sexually transmitted diseases.
Acknowledgments
The authors would like to thank Dr. Shizuo Akira for providing MyD88−/− deficient mice, Dr. James R. Smiley and Dr. Lynda A. Morrison for providing the live attenuated thymidine kinase-deficient HSV-2 (HSV-2 TK(−)), Dr. David M. Koelle for providing the gB vaccinia virus, and Dr. Alison Deckhut Augustine and Amy K. Stout from the NIH Tetramer Facility for providing the Tetramer, Drs. Scott Waniger and Mark Hirschel from the National Cell Culture Center for providing the mAbs used in this study, Dr. James V. Jester for the help with the confocal microscopy experiments.
Abbreviations
- STVs
Sexually Transmitted Viruses
- HSV
Herpes Simplex Virus
- GT
Genital Tract
- GT-DLN
Genital Tract Draining Lymph Nodes
- VM
Vaginal Mucosa
- DRG
Dorsal Root Ganglia
- Lipo
Lipopeptide
- rAd5
Recombinant Adenovirus Type 5
- SFC
Spot-Forming Cell
- PFU
Plaque-Forming Unit
- DPI
Days Post-Immunization
- MyD88
Myeloid Differentiation Factor 88
Footnotes
This work was supported by Public Health Service NIH grants EY14017, EY14900 and EY019896 to LBM, The Discovery Eye Foundation, The Henry L. Guenther Foundation and an unrestricted Research to Prevent Blindness Challenge grant.
References
- 1.Chentoufi AA, Kritzer E, Yu DM, Nesburn AB, BenMohamed L. Towards a rational design of an asymptomatic clinical herpes vaccine: the old, the new, and the unknown. Clin Dev Immunol. 2012;2012:187585. doi: 10.1155/2012/187585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mestecky J, Russell MW, Elson CO. Perspectives on mucosal vaccines: is mucosal tolerance a barrier? J Immunol. 2007;179:5633–5638. doi: 10.4049/jimmunol.179.9.5633. [DOI] [PubMed] [Google Scholar]
- 3.Belyakov IM, Ahlers JD. What role does the route of immunization play in the generation of protective immunity against mucosal pathogens? J Immunol. 2009;183:6883–6892. doi: 10.4049/jimmunol.0901466. [DOI] [PubMed] [Google Scholar]
- 4.Iwasaki A. Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol. 2010;10:699–711. doi: 10.1038/nri2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.King NJ, Parr EL, Parr MB. Migration of lymphoid cells from vaginal epithelium to iliac lymph nodes in relation to vaginal infection by herpes simplex virus type 2. J Immunol. 1998;160:1173–1180. [PubMed] [Google Scholar]
- 6.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol. 2009;2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Posavad CM, Koelle DM, Shaughnessy MF, Corey L. Severe genital herpes infections in HIV-infected individuals with impaired herpes simplex virus-specific CD8+ cytotoxic T lymphocyte responses. Proc Natl Acad Sci U S A. 1997;94:10289–10294. doi: 10.1073/pnas.94.19.10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009 doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 10.Jiang X, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non-migratory memory CD8+ T(RM) cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackay LK, Stock AT, Ma JZ, Jones CM, Kent SJ, Mueller SN, Heath WR, Carbone FR, Gebhardt T. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc Natl Acad Sci U S A. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corey L, Wald A, Celum CL, Quinn TC. The Effects of Herpes Simplex Virus-2 on HIV-1 Acquisition and Transmission: A Review of Two Overlapping Epidemics. J Acquir Immune Defic Syndr. 2004;35:435–445. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 13.Dasgupta G, BenMohamed L. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine. 2011;29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert review of vaccines. 2009;8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blaney JE, Jr, Nobusawa E, Brehm MA, Bonneau RH, Mylin LM, Fu TM, Kawaoka Y, Tevethia SS. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J Virol. 1998;72:9567–9574. doi: 10.1128/jvi.72.12.9567-9574.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollier CS, Reyes-Sandoval A, Cottingham MG, Ewer K, Hill AV. Viral vectors as vaccine platforms: deployment in sight. Curr Opin Immunol. 2011;23:377–382. doi: 10.1016/j.coi.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Qureshi H, Ma ZM, Huang Y, Hodge G, Thomas MA, DiPasquale J, DeSilva V, Fritts L, Bett AJ, Casimiro DR, Shiver JW, Robert-Guroff M, Robertson MN, McChesney MB, Gilbert PB, Miller CJ. Low-dose penile SIVmac251 exposure of rhesus macaques infected with adenovirus type 5 (Ad5) and then immunized with a replication-defective Ad5-based SIV gag/pol/nef vaccine recapitulates the results of the phase IIb step trial of a similar HIV-1 vaccine. J Virol. 2012;86:2239–2250. doi: 10.1128/JVI.06175-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay RW, Darrah PA, Quinn KM, Wille-Reece U, Mattei LM, Iwasaki A, Kasturi SP, Pulendran B, Gall JG, Spies AG, Seder RA. CD8+ T cell responses following replication-defective adenovirus serotype 5 immunization are dependent on CD11c+ dendritic cells but show redundancy in their requirement of TLR and nucleotide-binding oligomerization domain-like receptor signaling. J Immunol. 2010;185:1513–1521. doi: 10.4049/jimmunol.1000338. [DOI] [PubMed] [Google Scholar]
- 20.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med. 2002;347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 22.Cohen J. Immunology. Painful failure of promising genital herpes vaccine. Science. 2010;330:304. doi: 10.1126/science.330.6002.304. [DOI] [PubMed] [Google Scholar]
- 23.Akira S, Takeda K, Kaisho T. Toll-like receptors: critical proteins linking innate and acquired immunity. Nat Immunol. 2001;2:675–680. doi: 10.1038/90609. [DOI] [PubMed] [Google Scholar]
- 24.Conrady CD, Zheng M, Fitzgerald KA, Liu C, Carr DJ. Resistance to HSV-1 infection in the epithelium resides with the novel innate sensor, IFI-16. Mucosal Immunol. 2012;5:173–183. doi: 10.1038/mi.2011.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khanna KM, Bonneau RH, Kinchington PR, Hendricks RL. Herpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory ganglia. Immunity. 2003;18:593–603. doi: 10.1016/s1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tengvall S, Harandi AM. Importance of myeloid differentiation factor 88 in innate and acquired immune protection against genital herpes infection in mice. J Reprod Immunol. 2008;78:49–57. doi: 10.1016/j.jri.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Lindqvist M, Persson J, Thorn K, Harandi AM. The mucosal adjuvant effect of alpha-galactosylceramide for induction of protective immunity to sexually transmitted viral infection. J Immunol. 2009;182:6435–6443. doi: 10.4049/jimmunol.0900136. [DOI] [PubMed] [Google Scholar]
- 28.Davey GM, Wojtasiak M, Proietto AI, Carbone FR, Heath WR, Bedoui S. Cutting edge: priming of CD8 T cell immunity to herpes simplex virus type 1 requires cognate TLR3 expression in vivo. J Immunol. 2010;184:2243–2246. doi: 10.4049/jimmunol.0903013. [DOI] [PubMed] [Google Scholar]
- 29.Iijima N, Linehan MM, Zamora M, Butkus D, Dunn R, Kehry MR, Laufer TM, Iwasaki A. Dendritic cells and B cells maximize mucosal Th1 memory response to herpes simplex virus. J Exp Med. 2008;205:3041–3052. doi: 10.1084/jem.20082039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol. 2005;79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8(+) T lymphocyte mobilization to virus-infected tissue requires CD4(+) T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bolton DL, Song K, Wilson RL, Kozlowski PA, Tomaras GD, Keele BF, Lovingood RV, Rao S, Roederer M. Comparison of systemic and mucosal vaccination: impact on intravenous and rectal SIV challenge. Mucosal immunology. 2012;5:41–52. doi: 10.1038/mi.2011.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilks AB, Christian EC, Seaman MS, Sircar P, Carville A, Gomez CE, Esteban M, Pantaleo G, Barouch DH, Letvin NL, Permar SR. Robust vaccine-elicited cellular immune responses in breast milk following systemic simian immunodeficiency virus DNA prime and live virus vector boost vaccination of lactating rhesus monkeys. J Immunol. 2010;185:7097–7106. doi: 10.4049/jimmunol.1002751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bettahi I, Zhang X, Afifi RE, BenMohamed L. Protective immunity to genital herpes simplex virus type 1 and type 2 provided by self-adjuvanting lipopeptides that drive dendritic cell maturation and elicit a polarized Th1 immune response. Viral Immunol. 2006;19:220–236. doi: 10.1089/vim.2006.19.220. [DOI] [PubMed] [Google Scholar]
- 35.Dervillez X, Wechsler S, Nesburn AB, BenMohamed L. Future of an “Asymptomatic” T-cell Epitope-Based Therapeutic Herpes Simplex Vaccine. Future Virology. 2012 doi: 10.2217/fvl.12.22. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dasgupta G, Chentoufi AA, Kalantari M, Falatoonzadeh P, Chun S, Lim CH, Felgner PL, Davies DH, BenMohamed L. Immunodominant “asymptomatic” herpes simplex virus 1 and 2 protein antigens identified by probing whole-ORFome microarrays with serum antibodies from seropositive asymptomatic versus symptomatic individuals. J Virol. 2012;86:4358–4369. doi: 10.1128/JVI.07107-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Deak E, Soderberg K, Linehan M, Spezzano D, Zhu J, Knipe DM, Iwasaki A. Vaginal submucosal dendritic cells, but not Langerhans cells, induce protective Th1 responses to herpes simplex virus-2. J Exp Med. 2003;197:153–162. doi: 10.1084/jem.20021109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, Carbone FR, Mueller SN. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature. 2011;477:216–219. doi: 10.1038/nature10339. [DOI] [PubMed] [Google Scholar]
- 39.Chentoufi AA, BenMohamed L. Future viral vectors for the delivery of asymptomatic herpes epitope-based immunotherapeutic vaccines. Future Virol. 2010;5:525–528. doi: 10.2217/fvl.10.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol. 2008;82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu J, Koelle DM, Cao J, Vazquez J, Huang ML, Hladik F, Wald A, Corey L. Virus-specific CD8+ T cells accumulate near sensory nerve endings in genital skin during subclinical HSV-2 reactivation. J Exp Med. 2007;204:595–603. doi: 10.1084/jem.20061792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milligan GN, Bernstein DI. Interferon-gamma enhances resolution of herpes simplex virus type 2 infection of the murine genital tract. Virology. 1997;229:259–268. doi: 10.1006/viro.1997.8441. [DOI] [PubMed] [Google Scholar]
- 43.Chan T, Barra NG, Lee AJ, Ashkar AA. Innate and adaptive immunity against herpes simplex virus type 2 in the genital mucosa. J Reprod Immunol. 2011;88:210–218. doi: 10.1016/j.jri.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 44.Nelson MH, Bird MD, Chu CF, Johnson AJ, Friedrich BM, Allman WR, Milligan GN. Rapid clearance of herpes simplex virus type 2 by CD8+ T cells requires high level expression of effector T cell functions. J Reprod Immunol. 2011;89:10–17. doi: 10.1016/j.jri.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bird MD, Chu CF, Johnson AJ, Milligan GN. Early resolution of herpes simplex virus type 2 infection of the murine genital tract involves stimulation of genital parenchymal cells by gamma interferon. J Virol. 2007;81:423–426. doi: 10.1128/JVI.01455-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parr MB, Parr EL. The role of gamma interferon in immune resistance to vaginal infection by herpes simplex virus type 2 in mice. Virology. 1999;258:282–294. doi: 10.1006/viro.1999.9739. [DOI] [PubMed] [Google Scholar]
- 47.Kaufman DR, Bivas-Benita M, Simmons NL, Miller D, Barouch DH. Route of adenovirus-based HIV-1 vaccine delivery impacts the phenotype and trafficking of vaccine-elicited CD8+ T lymphocytes. J Virol. 2010;84:5986–5996. doi: 10.1128/JVI.02563-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bettahi I, Nesburn AB, Yoon S, Zhang X, Mohebbi A, Sue V, Vanderberg A, Wechsler SL, BenMohamed L. Protective immunity against ocular herpes infection and disease induced by highly immunogenic self-adjuvanting glycoprotein D lipopeptide vaccines. Invest Ophthalmol Vis Sci. 2007;48:4643–4653. doi: 10.1167/iovs.07-0356. [DOI] [PubMed] [Google Scholar]
- 49.Chentoufi AA, Dasgupta G, Nesburn AB, Bettahi I, Binder NR, Choudhury ZS, Chamberlain WD, Wechsler SL, BenMohamed L. Nasolacrimal duct closure modulates ocular mucosal and systemic CD4(+) T-cell responses induced following topical ocular or intranasal immunization. Clin Vaccine Immunol. 2010;17:342–353. doi: 10.1128/CVI.00347-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Del Campo J, Lindqvist M, Cuello M, Backstrom M, Cabrerra O, Persson J, Perez O, Harandi AM. Intranasal immunization with a proteoliposome-derived cochleate containing recombinant gD protein confers protective immunity against genital herpes in mice. Vaccine. 2010;28:1193–1200. doi: 10.1016/j.vaccine.2009.11.035. [DOI] [PubMed] [Google Scholar]
- 51.Barouch DH, Kik SV, Weverling GJ, Dilan R, King SL, Maxfield LF, Clark S, Ng’ang’a D, Brandariz KL, Abbink P, Sinangil F, de Bruyn G, Gray GE, Roux S, Bekker LG, Dilraj A, Kibuuka H, Robb ML, Michael NL, Anzala O, Amornkul PN, Gilmour J, Hural J, Buchbinder SP, Seaman MS, Dolin R, Baden LR, Carville A, Mansfield KG, Pau MG, Goudsmit J. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–5209. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khodai T, Chappell D, Christy C, Cockle P, Eyles J, Hammond D, Gore K, McCluskie MJ, Evans DM, Lang S, Loudon PT, Townend T, Wright P, West K, Bright H. Single and combination herpes simplex virus type 2 glycoprotein vaccines adjuvanted with CpG oligodeoxynucleotides or monophosphoryl lipid A exhibit differential immunity that is not correlated to protection in animal models. Clin Vaccine Immunol. 2011;18:1702–1709. doi: 10.1128/CVI.05071-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol. 2008;180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 54.Mestecky J, Fultz PN. Mucosal immune system of the human genital tract. J Infect Dis. 1999;179(Suppl 3):S470–474. doi: 10.1086/314806. [DOI] [PubMed] [Google Scholar]
- 55.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol. 2002;32:2274–2281. doi: 10.1002/1521-4141(200208)32:8<2274::AID-IMMU2274>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 56.Berzofsky JA, Ahlers JD, Derby MA, Pendleton CD, Arichi T, Belyakov IM. Approaches to improve engineered vaccines for human immunodeficiency virus and other viruses that cause chronic infections. Immunol Rev. 1999;170:151–172. doi: 10.1111/j.1600-065x.1999.tb01336.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. Lipopeptide Epitopes Extended by Ne-Palmitoyl Lysine Moiety Increases Uptake and Maturation of Dendritic Cell Through a Toll-Like Receptor 2 Pathway and Triggers a Th1- Dependent Protective Immunity. Eur J Immunol. 2004;34:1142–1149. doi: 10.1002/eji.200425166. [DOI] [PubMed] [Google Scholar]
- 58.Parr MB, Parr EL. Protective immunity against HSV-2 in the mouse vagina. J Reprod Immunol. 1997;36:77–92. doi: 10.1016/s0165-0378(97)00055-7. [DOI] [PubMed] [Google Scholar]


