Summary
Objective
Diminish interleukin-1β (IL-1β) signaling in a model of primary osteoarthritis by RNA interference-based transcript reduction or receptor blockade, and quantify changes incurred on transcript expression of additional mediators.
Methods
Knees of Hartley guinea pigs were collected at 120 and 180 days of age following injection with viral vectors (N=4/treatment group/date) at 60 days. Two groups received either adeno-associated viral serotype 5 vector containing a knockdown sequence (TV), or adenoviral vector encoding for IL-1 receptor antagonist protein (Ad-IRAP); treatments were contrasted with opposite knees administered corresponding vector controls. A third group evaluated TV relative to saline-only injected knees. Chondropathy and immunohistochemistry findings were compared to untreated guinea pigs. Transcript expression levels in cartilage were calculated using the comparative CT (2−ΔΔCT) method and analyzed by one-way ANOVA with pairwise comparisons using Tukey 95% confidence intervals.
Results
Vector transduction was confirmed at both harvest dates. TV and Ad-IRAP, relative to vector controls, significantly decreased IL-1β. Inflammatory mediators [tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), interferon-γ (IFN-γ)], and catabolic matrix metalloproteinase 13 (MMP13) were also decreased, while anabolic transforming growth factor-β1 (TGF-β1) was increased. IL-1β was also decreased by TV versus saline, with a decrease in MMP13 and increase TGF-β1; TNF-α, IL-8, and IFN-γ were transiently increased.
Conclusions
This work confirmed that a reduction in IL-1β signaling was accomplished by either method, resulting in decreased expression of three inflammatory mediators and one catabolic agent, and increased expression of an anabolic molecule. Thus, evidence is provided that IL-1β serves a role in vivo in spontaneous osteoarthritis and that these translational tools may provide beneficial disease modification.
Keywords: Osteoarthritis, Interleukin-1β, Cartilage, RNA interference, Viral vectors, Interleukin-1 Receptor Antagonist Protein, Guinea pig
INTRODUCTION
Osteoarthritis (OA) is the leading cause of physical disability in developed nations1. Unfortunately, multiple molecular and biomechanical factors contribute to the pathogenesis of this degenerative process and restorative treatment options continue to be elusive2. Interleukin-1β (IL-1β), a pro-inflammatory cytokine that stimulates joint tissue to produce several proteases involved in cartilage degradation, is implicated as a principal instigator of OA3. Inhibiting the biological activities of IL-1β through RNA interference (RNAi) or receptor antagonism may delineate the contribution of its signaling pathway to disease and offers promise as a translational therapeutic strategy. Efforts to reduce or block the effective concentration of IL-1β have been demonstrated using diacerein, a compound that inhibits IL-1β production from synovium and cartilage4–10, or interleukin-1 receptor antagonist protein (IRAP) in experimental OA11–15. Although these studies suggest that IL-1β is a viable target to modify development and succession of secondary joint deterioration, the majority of human cases of OA are idiopathic16 and work is warranted to evaluate the definitive role of IL-1β in the context of spontaneous disease3,17.
Recently, our laboratory provided a comprehensive immunohistochemical (IHC) map describing the temporal expression and tissue distribution of IL-1β through progression of OA in two strains of guinea pigs with varying propensity for spontaneous knee joint disease18. At 60 days of age, IL-1β was detected in cartilage, menisci, synovium, and subchondral bone in both strains. Persistent expression was found in these tissues in OA-prone Hartley animals at 120 and 180 days, while OA-resistant Strain 13 animals demonstrated a significant reduction in immunostaining. Enduring IL-1β levels in Hartley animals appeared to coincide with histologic onset of OA, and this aberrant expression may correlate to early incidence of disease. Although findings suggested, but did not confirm, a cause-and-effect relationship between IL-1β expression and OA, we demonstrated a window when targeted cytokine reduction and/or blockade may identify such a connection and point towards mechanistic components. As such, the primary aim of this study was to minimize IL-1β-mediated signaling via RNAi-based transcript reduction or receptor blockade and quantify changes incurred on expression of mediators implicated in pathogenesis. We accomplished these aims via independent administration of a validated adeno-associated viral serotype 5 (AAV5) vector containing a short hairpin (sh)RNA knockdown sequence19, or an adenoviral (Ad) vector encoding for recombinant human IRAP (hIRAP). These treatments were contrasted with opposite knees receiving corresponding vector controls with the goal of defining the contribution of IL-1β to spontaneous OA. A secondary aim was to challenge the efficacy and potential therapeutic viability of the AAV5 vector relative to saline-only injected control knees. Histologic chondropathy and IHC findings were compared to untreated guinea pigs.
MATERIALS & METHODS
This study was performed according to the Guide for the Care and Use of Laboratory Animals of the NIH; all procedures were approved by the Institutional Laboratory Animal Care and Use Committee at the host university. Male Hartley guinea-pigs (Charles River Laboratories, Wilmington, MA) were utilized in this study (N=32) starting at 60 days of age. Animals were housed individually in solid bottom cages and allowed ad libitum water and guinea-pig chow (Harlan Teklad 7006) until euthanasia. Body weight (grams) at time of harvest was recorded.
EXPERIMENTAL DESIGN
AAV5 vectors allowed simultaneous expression of shRNA via the U6 promoter and CMV-driven enhanced GFP expression. Plasmids containing the IL-1β specific shRNA knockdown sequence or non-targeting shRNA control sequence (Table 1A) were used to produce targeting knockdown vector (TV) and non-targeting control vector (NTV), respectively, by the Viral Vector Core at Nationwide Children’s Hospital19,20. Ad vectors contained coding regions for CMV-driven firefly luciferase (Ad-Luc) or hIRAP (Ad-hIRAP) and were propagated, as described12. Active protein production from Ad vectors was confirmed in vitro using bioluminescent luciferase detection or ELISA analyses for hIRAP (R&D Systems, Minneapolis, MN); results were positive for intended proteins, only. Animals were injected at 60 days of age (4 treatment groups; 4 animals/treatment group per collection date) and harvested at either 120 or 180 days of age. Vectors were aseptically administered into the medial aspect of the knee just distal to the femoral condyle using a 1/2cc 28g insulin syringe (Becton Dickinson, Franklin Lakes, NJ).
Table 1A.
Targeting knockdown and control shRNA sequences.
| Targeting knockdown IL-1β sequence | GCCAGGATATAATTGACTTCACGAATGAAGTCAATTATATCCTGGC |
| Non-targeting control sequence | GGATATATCCCGAACTAGACACGAATGTCTAGTTCGGGATATATCC |
Animals were assigned to one of two groups to investigate reduction/blockade of IL-1β versus respective vector controls: TV or NTV (1×1012DNase resistant particles) were injected into opposite knees (group 1); Ad-Luc or Ad-hIRAP (2×1011IFUs) were injected into opposite knees (group 2). Group 3 animals received TV or an equivalent volume of PBS in opposite knees to challenge the efficacy of TV in the absence of vector control. Group 4 animals did not receive injections in either knee to allow reference of the above treatment groups to untreated controls. A total volume of 100μl was administered; dosages and final injection volume were consistent with peer-reviewed manuscripts19,20.
For groups 1–3, RNA was extracted from weight-bearing cartilage taken from the lateral femoral condyle and lateral tibial plateau of opposite knees for gene expression analyses. DNA was collected from patellar cartilage to quantify viral particle numbers. The medial femoral condyle and medial tibial plateau were processed for IHC and histology. Whole knee joints from group 4 animals were processed for IHC and histology.
QUANTIFICATION OF CMV COPY NUMBER
DNA was isolated via the QIAamp® DNA Mini kit (Qiagen, Valencia, CA) according to manufacturer’s protocol. DNA quality and concentration were determined using the BioMate 3 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Absolute quantification of initial CMV copy number per 1μg starting DNA was performed in triplicate using SYBR® Green PCR Master Mix (Applied Biosystems, Warrington, UK) and primers specific for the CVM promoter (Table 1B) on the ABI Prism® 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). A standard curve ranging from 107 to 101 starting particles was prepared using the TV plasmid. If amplification was not detected, negative results were verified by repeating reactions with up to 2μg starting DNA.
Table 1B.
Specific primers used for real-time qRT-PCR.
| Transcript of Interest | Primer Sequences (5′ to 3′) |
|---|---|
| 18S ribosomal RNA | F: TGCATGGCCGTTCTTAGTTG R: AGTTAGCATGCCAGAGTCTCGTT |
| GAPDH | F: GTATCGTGGAAGGACTCATGACC R: GTTGAAGTCACAGGACACAACCT |
| GFP | F: CATGATATAGACGTTGTGGCTGTTG R: AAGCTGACCCTGAAGTTCATCTGC |
| Firefly luciferasea | F: GCCTGAAGTCTCTGATTAAGT R: ACACCTGCGTCGAAGT |
| CVM promoter | F: GGTCTATATAAGCAGAGCTG R: GTGGTATGGCTGATTATGATCAG |
| IL-1β | F: ACGCCTGGTGTTGTCTGAC R: GGGAACTGAGCGGATTC |
| IL-8 | F: GGCAGCCTTCCTGCTCTCT R: CAGCTCCGAGACCAACTTTGT |
| Human IRAPb | F: TGGCTAACTAGAGAACCCACTGCT R: TTCTGAAGGCTTGCATCTTGCTGG |
| Collagenase 3 (MMP13) | F: TTCTGGCACATGCTTTTCCTC R: GGTTGGGGTCTTCATCTCCTG |
| TGF-β1 | F: CATCGATATGGAGCTGGTGAAG R: GCCGTAATTTGGACAGGATCTG |
| TNF-α | F: CCTACCTGCTTCTCACCCATACC R: TTGATGGCAGAGAGAAGGTTGA |
| INF-γ | F: ATTTCGGTCAATGACGAGCAT R: GTTTCCTCTGGTTCGGTGACA |
Fan X, Roy E, Zhu L, Murphy TC, Kozlowski M, Nanes MS, Rubin J. Nitric oxide donors inhibit luciferase expression in a promoter-independent fashion. J Biol Chem. 2003;278(12):10232-8.
Jia X, Cheng K, Mahato RI. Co-expression of Vascular Endothelial Growth Factor and Interleukin-1 Receptor Antagonist for Improved Human Islet Survival and Function. Mol Pharm. 2007;4(2):199–207.
RELATIVE REAL-TIME (Q)RT-PCR
Total RNA was isolated via TRIzol® (Invitrogen, Carlsbad, CA), followed by DNase I treatment (Invitrogen, Carlsbad, CA), according to manufacturer’s protocol. Complementary DNA was made using Taqman® Reverse Transcription Reagents (Applied Biosystems, Foster City, CA) and relative qRT-PCR, complete with dissociation curve, was performed in triplicate for each transcript of interest using Power SYBR® Green PCR Master Mix (Applied Biosystems, Warrington, UK) and specific primers (Table 1B) on the ABI Prism® 7000. 18s ribosomal RNA and GAPDH were used as separate endogenous controls to which each transcript was normalized. Results were consistent between housekeeping genes; ΔCT values using GAPDH are represented. If amplification was not detected, reactions were repeated using a higher starting amount of cDNA to confirm negative findings. ΔCT values for GFP transcripts in groups 1 and 3 at 120 and 180 days were reported. Fold differences and subsequent percent gene expression levels relative to designated control groups were calculated for each transcript using the comparative CT (2−ΔΔCT) method. For all groups, comparative CT values for each treatment group were compared to opposite control knees.
LUCIFERASE EXPRESSION
Immediately prior to euthanasia, in vivo bioluminescent imaging was performed to ensure luciferase activity in knees receiving Ad-Luc. Group 2 animals at 120 and 180 days were induced and maintained under general anesthesia using 2% isofluorane and received an intra-peritoneal injection of D-luciferin (Caliper Life Sciences, Hopkinton, MA) at a dose of 150mg/kg. Fifteen minutes post-injection, animals were placed in a cooled charge-coupled device camera system (IVIS Imaging System-100; Xenogen/Caliper Life Sciences, Alameda, CA) for data acquisition according to recommendations21.
JOINT PROCESSING & ANALYSES
Knee joints were fixed in 10% neutral buffered formalin and prepared for histological analysis18,22–25. Paraffin sections (5μm) were taken from the center of the medial tibial plateau in each joint and either stained with toluidine blue or subjected to IHC. Four sections from each joint were examined using each scoring technique.
IHC was performed on the Benchmark XT (Ventana, Tucson, AZ) using the Ultraview Universal Red AP System and a rabbit polyclonal antibody for IL-1β (sc-7884; Santa Cruz Biotechnology, Santa Cruz, CA) at a dilution of 1:800. Sections were counterstained with hematoxylin. The percentage of chondrocytes staining positive for IL-1β were assessed by two independent, blinded reviewers (KSS/GN) and assigned a total IHC (based on the percentage and intensity of chondrocytes staining positive for IL-1β) ranging from 0–818. Intra- and inter-observer variability was negligible (within one numeric score in all cases).
Two independent, blinded observers (KSS/ALB) performed histological grading of serial coronal sections of each knee, using adapted Mankin criteria based upon species-specific features of OA24,25. Chondropathy was scored for the medial femorae and tibial plateaus, providing a total possible medial joint index ranging from 0–28. Intra- and inter-observer variability was negligible (within one numeric score in all cases).
STATISTICAL ANALYSES
Individual samples harvested from each animal were considered independent observations; for each gene or transcript of interest, this resulted in 4 independent observations per treatment group per harvest date. ΔCT values for each gene or transcript of interest (triplicate values for each independent observation) passed Gaussian distribution and homogeneous variance using the Kolmogorov-Smirnov normality test. Statistical differences were not detected between animals within the same treatment group and harvest date. Weight, viral particle numbers, and relative percent expression qRT-PCR data (directly converted from fold changes) were expressed as mean±95% confidence interval and analyzed by one-way ANOVA followed by pairwise comparisons using Tukey 95% confidence intervals. Total indices for OA and IHC (median and range provided) were analyzed using the one-way ANOVA, Kruskal-Wallis test, followed by Dunn’s Multiple Comparison post-hoc test18. All analyses were performed using the Minitab statistical software program (State College, PA) with a statistical significance of P<0.05. Throughout the manuscript, indicated “increases” and “decreases” in transcript expression refer to significant statistical differences in presented data.
RESULTS
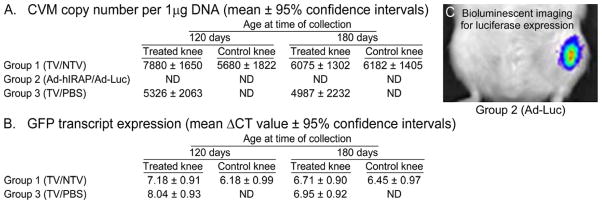
Successful transduction of patellar cartilage by TV and NTV was confirmed via detectable amplification of the CMV promoter at 120 and 180 days (Figure 1A); significant statistical differences between days 120 and 180 or between groups 1 and 3 were not present. The CMV promoter was not detected in patellar cartilage harvested from group 2 animals injected with either Ad vector.
Figure 1.
In vivo detection of the CMV promoter (A) in 1 μg starting DNA and expression of reporter genes for adeno-associated serotype 5 vectors (AAV5) (B) and adenoviral (Ad) vectors (C) were confirmed using PCR, qRT-PCR, and Xenogen IVIS System, respectively. DNA containing the CMV promoter was not detected (ND) in patellar cartilage collected from group 2 Ad-injected animals at either 120 or 180 days of age. A significant difference for this promoter between groups 1 and 3 was not present at either harvest date. Statistical differences in GFP expression were not noted in cartilage at 120 or 180 days of age in groups 1 and 3, as determined using one-way ANOVA followed by pairwise comparisons using Tukey’s 95% confidence intervals. Luciferase (Luc) expression was detected in all Ad-Luc injected knees immediately prior to harvest on both collection ages (C). TV = AAV5-shRNA-TV, targeting knockdown vector; NTV = AAV5-shRNA-NTV, non-targeting control vector; Ad-Luc, vector containing a CMV-driven coding region for firefly luciferase (Luc); Ad-hIRAP, vector containing a CMV-driven coding region for human interleukin-1 receptor antagonist protein (hIRAP).
GFP expression was detected via qRT-PCR in knees receiving either TV or NTV. Significant statistical differences in ΔCT values were not present between days 120 and 180 or between groups 1 and 3 (Figure 1B).
Luciferase expression in knees receiving Ad-Luc displayed positive bioluminescence immediately prior to collection, indicating successful transduction in joint tissue throughout the duration of the study (Figure 1C). Neither luciferase nor hIRAP transcripts was detected in cartilage via qRT-PCR, confirming that, along with lack of detection of CMV promoter, Ad-Luc and Ad-IRAP vectors transduced joint structures other than the examined cartilage.
IL-1β reduction/blockade versus respective vector controls
TV relative to NTV, and Ad-hIRAP relative to Ad-Luc, demonstrated decreases in IL-1β expression in cartilage at 120 days (Table 2A). IL-1β was below detection limits collected from both groups at 180 days. In general, TV and Ad-hIRAP decreased expression of tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8), and interferon-γ (IFN-γ) relative to respective vector controls (Table 2B). TNF-α and IL-8 were decreased by TV compared to NTV at both harvest ages. Significant statistical differences in IFN-γ were not detected at 120 or 180 days. TNF-α was increased by Ad-hIRAP versus Ad-Luc at 120 days. This expression, however, was decreased relative to Ad-Luc by 180 days, revealing a significant statistical difference in Ad-hIRAP treated knees between 120 and 180 days (Supplemental Table I). IL-8 and IFN-γ were decreased by Ad-hIRAP at both collection times. Overall, anabolic transforming growth factor-β1 (TGF-β1) was increased by TV and Ad-hIRAP relative to vector controls at 120 days (Table 2C). This increase in group 1 continued at 180 days; however, TGF-β was decreased in group 2 at this time. Catabolic collagenase III/matrix metalloproteinase 13 (MMP13) was decreased by either method used to manipulate IL-1β at 120 days (Table 2D). At 180 days, the decrease in MMP13 incited by TV relative to NTV persisted, while Ad-hIRAP increased expression relative to Ad-Luc.
Table 2.
Relative percent expression (mean ± 95% confidence intervals) of (A) interleukin-1β (IL-1β), (B) inflammatory cytokines of interest, and (C) anabolic and (D) catabolic agents in cartilage of viral vector treated knees versus vector control knees (groups 1 and 2) at specified ages.
| Group 1 | Group 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| A. | IL-1β | |||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (84.1,115.9) | 5.5 (3.9,7.1) | < 0.001 | 120 days | 100.0 (90.5,109.5) | ND | < 0.001 | |
| 180 days | ND | ND | NA | 180 days | ND | ND | NA | |
| B. | TNF-α | |||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (80.9,119.1) | 15.3 (7.3,23.3) | < 0.001 | 120 days | 100.0 (82.5,117.5) | 720.6 (584.1,1357.0) | < 0.001 | |
| 180 days | 100.0 (87.3,112.7) | 23.8 (7.9,39.7) | < 0.001 | 180 days | 100.0 (85.7,114.3) | 33.0 (14.7,80.7) | 0.01 | |
| IL-8 | ||||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (84.1,115.9) | ND | < 0.001 | 120 days | 100.0 (94.5,105.4) | 35.4 (15.5,55.3) | < 0.001 | |
| 180 days | 100.0 (85.7,114.3) | 21.0 (5.1,36.9) | < 0.001 | 180 days | 100.0 (83.7,117.4) | 32.2 (17.6, 51.9) | < 0.001 | |
| IFN-γ | ||||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (94.1,105.9) | 120.0 (65.8,239.1) | > 0.05 | 120 days | 100.0 (90.4,109.5) | 5 (1.8,8.2) | < 0.001 | |
| 180 days | 100.0 (86.8,113.4) | 75.0 (44.3,194.3) | > 0.05 | 180 days | 100.0 (87.2,113.7) | 42.1 (18.2,65.9) | < 0.001 | |
| C. | TGF-β1 | |||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (92.0,108.0) | 643.0 (324.8,961.2) | < 0.001 | 120 days | 100.0 (90.5,109.5) | 873.2 (236.7,1510.0) | < 0.001 | |
| 180 days | 100.0 (92.4,108.0) | 412.6 (223.9,1049.0) | < 0.001 | 180 days | 100.0 (84.1,115.9) | 32.5 (6.8,64.3) | 0.008 | |
| D. | MMP13 | |||||||
| NTV | TV | P value | Ad-Luc | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (94.1,105.9) | ND | < 0.001 | 120 days | 100.0 (93.4,107.5) | ND | < 0.001 | |
| 180 days | 100.0 (85.6,114.3) | ND | < 0.001 | 180 days | 100.0 (87.3,112.7) | 200.0 (144.3,255.7) | < 0.001 | |
ND = not detected; NA = not applicable.
Reduction of IL-1β by shRNA-TV relative to PBS control (group 3)
To stringently evaluate efficacy of novel TV in the context of primary OA, this vector was compared to opposite control knees receiving PBS, only (group 3). Successful in vivo transduction of patellar cartilage by TV was confirmed via detectable, and similar, amplification of the CMV promoter at both 120 and 180 days (Figure 1A) and discernible, and comparable, ΔCT values for GFP between days 120 and 180 (Figure 1B). At 120 and 180 days, the IL-1β transcript was decreased relative to PBS control (Table 3A). At 120 days, TNF-α, IL-8, and IFN- γ were increased by TV relative to PBS (Table 3B). TNF-α and IL-8, however, were decreased by 180 days, while IFN-γ remained increased. TGF-β1 was increased by TV relative to PBS at 120 and 180 days, with impressive expression found at the latter time point (Table 3C). MMP13 was decreased by TV at both harvest dates (Table 3D).
Table 3.
Relative percent expression (mean ± 95% confidence intervals) of (A) interleukin-1β (IL-1β), (B) inflammatory cytokines of interest, and (C) anabolic and (D) catabolic agents in cartilage of AAV5 vector-exposed guinea pigs compared to PBS-treated knees (group 3) at specified ages.
| Group 3 | ||||
|---|---|---|---|---|
| A. | IL-1β | |||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (87.2,112.7) | 64.8 (45.7,82.9) | 0.03 | |
| 180 days | 100.0 (88.8,111.1) | 42.0 (18.1,65.8) | < 0.001 | |
| B. | TNF-α | |||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (93.6,106.4) | 266.0 (126.9,425.1) | < 0.001 | |
| 180 days | 100.0 (92.0,108.0) | 25.0 (4.8,64.7) | 0.005 | |
| IL-8 | ||||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (87.2,112.7) | 179.0 (123.3,234.7) | < 0.001 | |
| 180 days | 100.0 (88.6,111.4) | 70.0 (49.3,90.7) | 0.04 | |
| IFN-γ | ||||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (82.7,117.2) | 608.0 (448.9,767.1) | < 0.001 | |
| 180 days | 100.0 (90.6,110.4) | 1087.0 (768.8,1405.0) | < 0.001 | |
| C. | TGF-β1 | |||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (93.6,106.4) | 156.0 (116.4,235.6) | 0.009 | |
| 180 days | 100.0 (92.0,108.0) | 542.7 (304.0,781.4) | < 0.001 | |
| D. | MMP13 | |||
| PBS | TV | P value | ||
|
|
||||
| 120 days | 100.0 (87.3,112.7) | 30.0 (16.1,53.8) | 0.003 | |
| 180 days | 100.0 (88.6,111.1) | 10.0 (2.0,17.9) | < 0.001 | |
Comparison of Ad-Luc and Ad-hIRAP (group 2) to PBS control knees (group 3)
Comparison of Ad-Luc or Ad-hIRAP versus PBS was performed across animals. At 120 days, Ad-Luc-treated knees demonstrated increased IL-1β relative to PBS control knees (Table 4A). IL-1β was below detection in Ad-hIRAP-treated knees at either time point; although detected at 120 days, IL-1β was not present in Ad-Luc treated knees at 180 days. All inflammatory cytokines of interest were increased by Ad-Luc and Ad-hIRAP relative to PBS control knees at 120 days (Table 4B). TNF-α was increased compared to PBS exposed cartilage at 180 days and, in Ad-Luc treated knees, this expression was increased relative to the earlier time point (Table 4B; Supplemental Table I). IL-8 and IFN-γ were decreased at 180 days relative to 120 days within knees exposed to Ad vectors (Supplemental Table 1). At 180 days, only Ad-Luc demonstrated an increase in IL-8 relative to PBS-exposed cartilage and both IL-8 and INF-γ were decreased in Ad-hIRAP treated cartilage relative to PBS knees by this harvest date. At 120 days, TGF-β1 was increased in cartilage treated with either Ad vector relative to PBS treated knees (Table 4C). By 180 days, however, transcript expression was decreased in Ad-hIRAP treated knees. MMP13 was decreased by both Ad vectors relative to PBS control cartilage at 120 days, but increased at the latter harvest age (Table 4D).
Table 4.
Relative percent expression (mean ± 95% confidence intervals) of (A) interleukin-1β (IL-1β), (C) inflammatory cytokines of interest, and (D) anabolic and (E) catabolic agents in cartilage at specified ages in Ad vector treated animals versus PBS-treated knees.
| A. | IL-1β | |||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (87.2,112.7) | 362.0 (123.3,600.7) | < 0.001 | 120 days | 100.0 (87.2,112.7) | ND | < 0.001 | |
| 180 days | 100.0 (88.8,111.1) | ND | < 0.001 | 180 days | 100.0 (88.8,111.1) | ND | < 0.001 | |
| B. | TNF-α | |||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (93.6,106.4) | 1420.0 (1261.0,1579.0) | < 0.001 | 120 days | 100.0 (93.6,106.4) | 2181.0 (1544.0,2817.0.0) | < 0.001 | |
| 180 days | 100.0 (92.0,108.0) | 4440.0 (1258.0,7622.0) | < 0.001 | 180 days | 100.0 (92.0,108.0) | 1465.0 (1306.0,1624.0) | < 0.001 | |
| IL-8 | ||||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (87.3,113.1) | 1389.0 (672.9,2105.0) | < 0.001 | 120 days | 100.0 (87.3,113.1) | 492.0 (332.9,651.1) | < 0.001 | |
| 180 days | 100.0 (88.8,111.1) | 173.0 (119.3,252.6) | < 0.009 | 180 days | 100.0 (88.8,111.1) | 55.8 (23.9, 87.6) | 0.04 | |
| IFN-γ | ||||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (90.4,109.5) | 3335.0 (1744.0,4926.0) | < 0.001 | 120 days | 100.0 (90.4,109.5) | 166.8 (121.1,222.4) | < 0.001 | |
| 180 days | 100.0 (87.7,112.7) | 84.0 (52.1,115.8) | > 0.05 | 180 days | 100.0 (87.7,112.7) | 35.3 (19.4,51.2) | 0.006 | |
| C. | TGF-β1 | |||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (93.6,106.3) | 251.0 (127.8,394.2) | < 0.001 | 120 days | 100.0 (93.6,106.3) | 2.1×105 (1.9×105, 2.5×105) | < 0.001 | |
| 180 days | 100.0 (84.1,115.9) | 222.0 (174.3,269.7) | < 0.001 | 180 days | 100.0 (84.1,115.9) | 39.6 (25.3,53.9) | 0.01 | |
| D. | MMP13 | |||||||
| PBS | Ad-Luc | P value | PBS | Ad-hIRAP | P value | |||
|
|
|
|||||||
| 120 days | 100.0 (87.3,112.7) | 50.0 (10.2,79. 8) | 0.005 | 120 days | 100.0 (87.3,112.7) | ND | < 0.001 | |
| 180 days | 100.0 (89.6,111.1) | 179.0 (139.2,218.8) | < 0.001 | 180 days | 100.0 (89.6,111.1) | 358.0 (198.9,517.1) | < 0.001 | |
ND = not detected.
IHC and histology
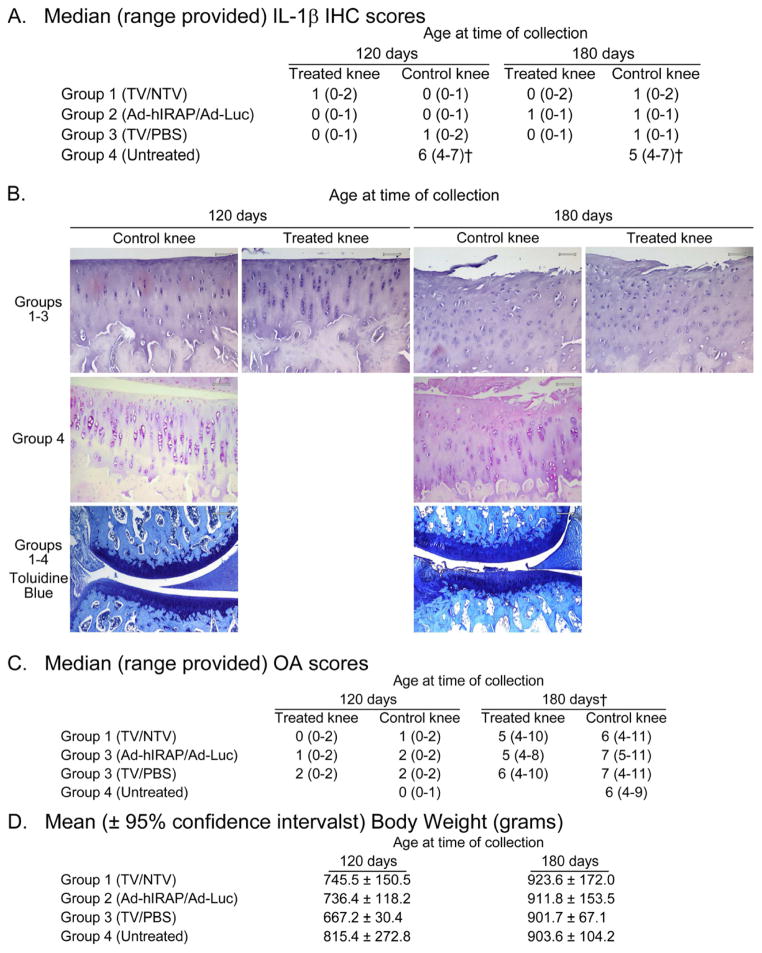
A final set of animals (group 4) did not receive injections in either knee to allow reference of IHC and chondropathy from treatment groups to untreated controls. IHC to detect IL-1β was performed and graded to corroborate qRT-PCR findings. Scores for IL-1β at the protein level reflected qRT-PCR data, where IHC indices were lower in groups 1, 2, and 3 relative to untreated group 4 at both 120 and 180 days (Figure 2A,B). Total OA indices for the medial joint compartments increased from 120 to 180 days, similar to that previously reported18,22–25 (Figure 2B,C). Significant statistical differences in OA indices were not present among treatment groups within specified harvest dates. Significant statistical differences in weight were not detected among the treatment groups at either 120 or 180 days (Figure 2D).
Figure 2.
Median (range provided) immunohistochemistry (IHC) scoring (A) and osteoarthritis (OA) grading (C), as well as representative photomicrographs (B) (100X, final magnification), of medial tibial plateaus from treatment groups harvested at specified ages. Animals were injected with viral vectors (N=4 animals per treatment group per collection date) at 60 days of age and harvested at either 120 or 180 days of age. At time of treatment, animals were assigned to one of two groups to investigate reduction or blockade, respectively, of IL-1β versus respective vector controls: TV or NTV were injected into contralateral knees (group 1); Ad-Luc or Ad-hIRAP were injected into contralateral knees (group 2). A third set of animals (group 3) received TV or PBS in contralateral knees to challenge the efficacy of TV in the absence of vector control. A final set of animals (group 4) did not receive injections in either knee to allow reference of the above treatment groups to untreated controls. The percentage of chondrocytes staining positive for IL-1β were assigned a score of 0–5 and a scale of 1–3 was used to gauge the intensity of immunostaining (total ranging from 0–8). Histological grading of serial coronal sections of the medial weight-bearing compartment of each knee was performed using adapted Mankin criteria based upon characteristic features of OA in this species. The score for each structure ranged from 0 (normal) to 14 (severe structural damage and complete loss of toluidine blue staining), providing a total chondropathy index ranging from 0–28. Data were analyzed using the ANOVA, Kruskal-Wallis test, followed by Dunn’s Multiple Comparison post-hoc test. (Please see Figure 1 for full description of abbreviations. †P<0.05)
DISCUSSION
The goals of our study were to demonstrate effective intra-articular vector transduction aimed at reducing IL-1β expression and/or signaling in OA-prone cartilage, quantify ensuing chondrogenic mediator profiles in articular cartilage, and provide compelling molecular evidence for potential disease modification. Both TV and Ad-hIRAP were successful at decreasing IL-1β expression in OA-prone cartilage at both the transcript and protein levels. Notably, this IL-1β diminution directly affected several key players implicated in joint degeneration. Relative to respective vector controls, these anti-IL-1β treatments reduced TNF-α, IL-8, IFN-γ, and simultaneously increased anabolic TGF-β and decreased catabolic MMP1326,27. Importantly, these same patterns were elucidated when TV was compared to contralateral PBS-exposed cartilage; these findings can be anticipated to allow a joint environment less acquiescent to pathology. As such, our work supplied confirmation that treatments aimed at decreasing the effective concentration of IL-1 and/or its signaling may hold merit for amendment of disease-prone cartilage.
To date, intra-articular hIL-1Ra injections to treat symptomatic knee OA in people found no statistical improvement over placebo at one month28 and trials with diacerein did not show clinical, radiographic, or structure-modifying effects as anticipated29–31. Use of recombinant proteins and/or pharmaceutical compounds is limited not only by short half-lives and the requirement for repeat administration, but uncharacterized innate and adaptive responses to exogenous substances may also affect efficacy, including internalization or down-regulation of receptors. Although we demonstrated an ability to manipulate IL-1β signaling in cartilage such that correlative expression changes in other mediators could be detected, onset of chondropathy was detected after 120 days of treatment. Additional work would be required to determine if these treatments would alter the progression and/or severity of OA in this animal model at later dates. Of note, quantification of hIRAP, type II collagen, and aggrecan, as well as IL-1β signaling molecules and additional cytokine isoforms, in the entire joint organ would provide greater insight into global responses to the chosen therapies. As sequencing and annotation of the guinea pig genome is still underway, full-scale investigations remain limited without an exhaustive list of available reagents. As such, it is plausible that compensatory or supplanting biomechanical/molecular factors not targeted in our work could have contributed to initial pathogenesis. Residual IL-1β activity may have persisted from tissues other than the examined cartilage and compensatory cytokines, including IL-1α, could have superseded therapeutic advantages. In addition, work has demonstrated persistent and robust reporter gene expression via self-complementary (sc)AAV20, It is possible this vector could achieve earlier gene expression and greater effectiveness in the guinea pig model than single-stranded AAV; indeed, scAAV vector encoding hIRAP has been validated for intra-articular use32. Further, it may be necessary to combine interventional therapies with IL-1β reduction to achieve a more effective suppression of disease. Attempts to alter the effective concentration of IL-1β may be co-dependent on additional management – such as reduction of loading – at critical time points during disease development. Continued, and longer term, in vivo work is warranted to investigate therapeutic modifications that would compliment anti-IL-1β molecular therapy. Assessment of gait improvements and/or reduction in joint pain may also detect positive outcomes related to the suppression of inflammatory mediators4. Our findings reinforce the credence that timely identification of individuals at premature risk for OA, followed by immediate multi-modal treatment, is necessary to provide a setting that is conducive to disease suppression.
One unique finding was that IL-1β transcript and protein expression was reduced at 180 days in all animals that received either anti-IL-1β vector, including untreated knees, when compared to untreated group 4 animals. Interestingly, this is not a new or unexpected observation. Beneficial alterations in untreated contralateral limbs or regional joints has been demonstrated under a number of experimental conditions33–36, including animal models of rheumatoid arthritis37,38. Two explanations for this include translocation of vector to opposite limbs or migration of transduced or immune-modulated cells to outside the joint. In particular, migration of immunotolerant dendritic cells stimulated by anti-IL-1β therapy has been described37 and additional uncharacterized mechanisms of local or systemic cytokine suppression may also exist. Given the positive feedback loop that this pro-inflammatory molecule exerts on itself, as well as the synergism it has with other cytokines, it is also possible that even slight reduction/blockade of IL-1β may have far-reaching effects. Interestingly, diacerein is reported to have a carryover effect, providing more pain relief than placebo or NSAID for several weeks after treatment cessation39. Although additional corroboration is needed, these results could represent one of our more important findings.
Optimal viral vectors for intra-articular gene delivery for RNAi or protein production must be capable of efficient and sustained transgene expression within a reasonable vector particle dose. Our findings indicated that both AAV and Ad vectors are viable tools capable of these aims. Specifically, our results verified that AAV5 transduced weight-bearing and non-weight-bearing cartilage, which is consistent with previous work20,40, and are effective vehicles to induce RNAi machinery in situ41,42. Importantly, CMV copy numbers and GFP transcript expression were not significantly different between 120 and 180 days, indicating persistent expression over multiple months. As expected, evidence of transduction of articular cartilage by Ad was not demonstrated; however, luciferase expression from other articular structures was confirmed for at least 120 days in our study, which is longer than the two to three week period of transgene expression typically purported for Ad in most tissues of interest43.
Adverse cellular responses must be nominal in the presence of viral vectors and these delivery vehicles must not detrimentally influence or unseat the intended therapy. AAV vectors have been associated with minimal toxicity and immune responses43–45. In the current work, TNF-α, IL-8, and IFN-γ were statistically increased by TV relative to paired PBS-treated knees 60 days after injection. These relative increases in TNF-α and IL-8 expression levels induced by AAV vectors, however, appeared to be transitory43 and were statistically decreased 120 days post-injection. A persistent increase in IFN-γ was present in both TV and NTV knees, which is most likely related to vector use and not RNAi mechanisms. Our results support other peer-reviewed studies, which demonstrated cell-mediated immune responses to AAV capsid antigens46,47 and persistent, serotype-specific IFN-γ responses. Further, in vitro IFN-γ release assays have shown differences in species susceptibility to immunogens or mitogens, emphasizing the importance to scrutinize immune responses to AAV vectors in the context of individual animal models48 and tissue types. Continued work is necessary to examine the influence of innate and adaptive immune responses to AAV5 vectors on our results in the guinea pig, with emphasis on the significance of IFN-γ in cartilage.
Ad vector particles elicit strong immune responses, often in a dose-dependent manner, and systemic delivery has resulted in rapid physiological responses that include activation of intrinsic defense mechanisms, induction of inflammation, transient liver toxicity, and thrombocytopenia49. These vectors activate innate immunity through TLR-dependent and TLR-independent pathways, causing an upregulation of type I IFNs and inflammatory cytokines50. Adaptive responses against Ad may be directed against the capsid, double-stranded DNA genome, viral proteins expressed from the vector backbone, or incorporated transgenes43. In comparison to PBS-injected knees, Ad vector-exposed cartilage had a significant increase in inflammatory cytokine expression, specifically TNF-α, IL-8, and IFN-γ, especially at 120 days. Given the adaptive immune response anticipated in response to Ad, as well as the reversal of the potentially beneficial alterations in TGF-β and MMP13 expression between 120 and 180 days, it would be interesting to determine if there was evidence at the latter time point of host response to foreign antigens. Additional studies are needed to investigate immune responses specific to the joint environment and to ascertain if viral vector use usurped or prejudiced the beneficial effects of the investigated therapies.
There are theoretical advantages to using RNAi versus standard gene delivery of a therapeutic product. First, as selected shRNA sequences are reliably and constitutively transcribed from polymerase III promoters and efficiently processed by the RNA-induced silencing complex, RNAi does not face hurdles related to proper protein production. Second, the development of techniques to deliver long-lasting siRNAs to cells in the absence of a delivery vehicle or viral vector may eliminate negative consequences associated with vector/DNA administration. Finally, although it is unknown the extent to which dsRNA stimulates induction of innate cellular immune responses, this obstacle may be easier to overcome than conventional gene delivery due to the ability to identify highly potent RNAi sequences. Efficient small interfering RNA molecules decrease the likelihood of off-target effects, decrease the concentration of dsRNA molecules required, and may be better able to evade host responses. In the context of the current study, chondrocytes may also have different adaptive responses to a reduced IL-1β signaling at the transcript level compared to receptor antagonism of the IL-1 type I receptor by hIRAP. Further effort is required to determine which method pursued in this study may be more effective.
In summary, this work demonstrated that a reduction in IL-1β signaling by shRNA-mediated RNAi or receptor blockade by hIRAP was achieved in the Hartley guinea pig model of OA. Importantly, this manipulation was demonstrated over a 60-day period. Reducing the effective concentration of IL-1β decreased expression of three inflammatory mediators and one catabolic agent, while simultaneously increasing the number of anabolic TGF-β transcripts, providing biological evidence that IL-1β serves an important role in primary OA.
Supplementary Material
Acknowledgments
K.S. Santangelo was funded by a NIH NIAMS F32 (AR053805) throughout the majority of the work and is currently supported by a fellowship for residency training from GlaxoSmithKline organized by the American College of Veterinary Pathologists and Society of Toxicologic Pathology Coalition for Veterinary Pathology Fellows. The Comparative Orthopedics Laboratory also receives support through the Trueman Family Endowment. The automated instrument-reagent system and associated materials were kindly supplied by Ventana Medical Systems, Inc., with special thanks to Kathleen Sergott. The original plasmids used in this study were kindly provided by the Viral Vector Core at Nationwide Children’s Research Hospital. We thank Jeffrey Bartlett PhD and Stephen Weisbrode VMD PhD for their comments throughout this project. We would also like to acknowledge David Spencer Smith, Sarah Baker, Alan Flechtner, Marc Hardman, Holly Helbig, Amelia Kaeding, and Rebekah Sanchez-Hodge for technical assistance.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Contributions
Kelly S. Santangelo: design of the study, obtaining of funding, acquisition of the data, analysis and interpretation, drafting and revising of the article, and final approval. Responsibility for integrity of the work.
Gerard Nuovo: Acquisition of the data, analysis and interpretation, revising of the article, and final approval.
Alicia L. Bertone: design of the study, obtaining of funding, analysis and interpretation, revising of the article, and final approval. Responsibility for integrity of the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004;427(Suppl):S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 2.Trippel SB, Ghivizzani SC, Nixon AJ. Gene-based approaches for the repair of articular cartilage. Gene Ther. 2004;11(4):351–9. doi: 10.1038/sj.gt.3302201. [DOI] [PubMed] [Google Scholar]
- 3.Tortorella MD, Malfait AM. The usual suspects: verdict not guilty? Arthritis Rheum. 2003;48(12):3304–7. doi: 10.1002/art.11356. [DOI] [PubMed] [Google Scholar]
- 4.Felisaz N, Boumediene K, Ghayor C, Herrouin JF, Bogdanowicz P, Galerra P, Pujol JP. Stimulating effect of diacerein on TGF-beta1 and beta2 expression in articular chondrocytes cultured with and without interleukin-1. Osteoarthritis Cartilage. 1999;7(3):255–64. doi: 10.1053/joca.1998.0199. [DOI] [PubMed] [Google Scholar]
- 5.Tamura T, Kosaka N, Ishiwa J, Sato T, Nagase H, Ito A. Rhein, an active metabolite of diacerein, down-regulates the production of pro-matrix metalloproteinases-1, -3, -9 and -13 and up-regulates the production of tissue inhibitor of metalloproteinase-1 in cultured rabbit articular chondrocytes. Osteoarthritis Cartilage. 2001;9(3):257–63. doi: 10.1053/joca.2000.0383. [DOI] [PubMed] [Google Scholar]
- 6.Alvarez-Soria MA, Herrero-Beaumont G, Sánchez-Pernaute O, Bellido M, Largo R. Diacerein has a weak effect on the catabolic pathway of human osteoarthritis synovial fibroblast--comparison to its effects on osteoarthritic chondrocytes. Rheumatology (Oxford) 2008;47(5):627–33. doi: 10.1093/rheumatology/ken116. [DOI] [PubMed] [Google Scholar]
- 7.Mendes AF, Caramona MM, de Carvalho AP, Lopes MC. Diacerhein and rhein prevent interleukin-1beta-induced nuclear factor-kappaB activation by inhibiting the degradation of inhibitor kappaB-alpha. Pharmacol Toxicol. 2002;91(1):22–8. doi: 10.1034/j.1600-0773.2002.910104.x. [DOI] [PubMed] [Google Scholar]
- 8.Sanchez C, Mathy-Hartert M, Deberg MA, Ficheux H, Reginster JY, Henrotin YE. Effects of rhein on human articular chondrocytes in alginate beads. Biochem Pharmacol. 2003;65(3):377–88. doi: 10.1016/s0006-2952(02)01485-5. [DOI] [PubMed] [Google Scholar]
- 9.Yaron M, Shirazi I, Yaron I. Anti-interleukin-1 effects of diacerein and rhein in human osteoarthritic synovial tissue and cartilage cultures. Osteoarthritis Cartilage. 1999;7(3):272–80. doi: 10.1053/joca.1998.0201. [DOI] [PubMed] [Google Scholar]
- 10.Pujol JP, Felisaz N, Boumediene K, Ghayor C, Herrouin JF, Bogdanowicz P, Galera P. Effects of diacerein on biosynthesis activities of chondrocytes in culture. Biorheology. 2000;37(1–2):177–84. [PubMed] [Google Scholar]
- 11.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, Feige U, Poole AR. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–35. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 12.Nixon AJ, Haupt JL, Frisbie DD, Morisset SS, McIlwraith CW, Robbins PD, Evans CH, Ghivizzani S. Gene-mediated restoration of cartilage matrix by combination insulin-like growth factor-I/interleukin-1 receptor antagonist therapy. Gene Ther. 2005;12(2):177–86. doi: 10.1038/sj.gt.3302396. [DOI] [PubMed] [Google Scholar]
- 13.Haupt JL, Frisbie DD, McIlwraith CW, Robbins PD, Ghivizzani S, Evans CH, Nixon AJ. Dual transduction of insulin-like growth factor-I and interleukin-1 receptor antagonist protein controls cartilage degradation in an osteoarthritic culture model. J Orthop Res. 2005;23(1):118–26. doi: 10.1016/j.orthres.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 14.Domagala F, Martin G, Bogdanowicz P, Ficheux H, Pujol JP, Domagala F, Martin G, Bogdanowicz P, Ficheux H, Pujol JP. Inhibition of interleukin-1beta-induced activation of MEK/ERK pathway and DNA binding of NF-kappaB and AP-1: potential mechanism for Diacerein effects in osteoarthritis. Biorheology. 2006;43(3–4):577–87. [PubMed] [Google Scholar]
- 15.Caron JP, Fernandes JC, Martel-Pelletier J, Tardif G, Mineau F, Geng C, Pelletier JP. Chondroprotective effect of intraarticular injections of interleukin-1 receptor antagonist in experimental osteoarthritis. Suppression of collagenase-1 expression. Arthritis Rheum. 1996;39(9):1535–44. doi: 10.1002/art.1780390914. [DOI] [PubMed] [Google Scholar]
- 16.Martel-Pelletier J, Alaaeddine N, Pelletier JP. Cytokines and their role in the pathophysiology of osteoarthritis. Front Biosci. 1999;4:D694–703. doi: 10.2741/martel. [DOI] [PubMed] [Google Scholar]
- 17.Clements KM, Price JS, Chamber MG, Visco DM, Poole AR, Mason RM. Gene deletion of either interleukin-1beta, interleukin-1beta-converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partial medial meniscectomy. Arthritis Rheum. 2003;48(12):3452–63. doi: 10.1002/art.11355. [DOI] [PubMed] [Google Scholar]
- 18.Santangelo KS, Pieczarka EM, Nuovo GJ, Weisbrode SE, Bertone AL. Temporal expression and tissue distribution of interleukin-1β in two strains of guinea pigs with varying propensity for spontaneous knee osteoarthritis. Osteoarthritis Cartilage. 2011;19(4):439–48. doi: 10.1016/j.joca.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santangelo KS, Bertone AL. Effective reduction of the interleukin-1β transcript in osteoarthritis-prone guinea pig chondrocytes via short hairpin RNA mediated RNA interference influences gene expression of mediators implicated in disease pathogenesis. Osteoarthritis Cartilage. 2011;19(12):1449–57. doi: 10.1016/j.joca.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Santangelo KS, Baker SA, Nuovo G, Dyce J, Bartlett JS, Bertone AL. Detectable reporter gene expression following transduction of adenovirus and adeno-associated virus serotype 2 vectors within full-thickness osteoarthritic and unaffected canine cartilage in vitro and unaffected guinea pig cartilage in vivo. J Orthop Res. 2010;28(2):149–55. doi: 10.1002/jor.20975. [DOI] [PubMed] [Google Scholar]
- 21.Martin CK, Werbeck JL, Thudi NK, Lanigan LG, Wolfe TD, Toribio RE, Rosol TJ. Zoledronic acid reduces bone loss and tumor growth in an orthotopic xenograft model of osteolytic oral squamous cell carcinoma. Cancer Res. 2010;70(21):8607–16. doi: 10.1158/0008-5472.CAN-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huebner JL, Otterness IG, Freund EM, Caterson B, Kraus VB. Collagenase 1 and collagenase 3 expression in a guinea pig model of osteoarthritis. Arthritis Rheum. 1998;41(5):877–90. doi: 10.1002/1529-0131(199805)41:5<877::AID-ART16>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 23.Huebner JL, Hanes MA, Beekman B, TeKoppele JM, Kraus VB. A comparative analysis of bone and cartilage metabolism in two strains of guinea-pig with varying degrees of naturally occurring osteoarthritis. Osteoarthritis Cartilage. 2002;10(10):758–67. doi: 10.1053/joca.2002.0821. [DOI] [PubMed] [Google Scholar]
- 24.Kraus VB, Huebner JL, DeGroot J, Bendele A. The OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the guinea pig. Osteoarthritis Cartilage. 2010;18(Suppl 3):S35–52. doi: 10.1016/j.joca.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huebner JL, Williams JM, Deberg M, Henrotin Y, Kraus VB. Collagen fibril disruption occurs early in primary guinea pig knee osteoarthritis. Osteoarthritis Cartilage. 2010;18(3):397–405. doi: 10.1016/j.joca.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finnson KW, Chi Y, Bou-Gharios G, Leask A, Philip A. TGF-b signaling in cartilage homeostasis and osteoarthritis. Front Biosci (Schol Ed) 2012;4:251–68. doi: 10.2741/S266. [DOI] [PubMed] [Google Scholar]
- 27.Pujol JP, Chadjichristos C, Legendre F, Bauge C, Beauchef G, Andriamanalijaona R, Galera P, Boumediene K. Interleukin-1 and transforming growth factor-beta 1 as crucial factors in osteoarthritic cartilage metabolism. Connect Tissue Res. 2008;49(3):293–7. doi: 10.1080/03008200802148355. [DOI] [PubMed] [Google Scholar]
- 28.Chevalier X, Goupille P, Beaulieu AD, Burch FX, Bensen WG, Conrozier T, et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61(3):344–52. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 29.Pham T, Le Henanff A, Ravaud P, Dieppe P, Paolozzi L, Dougados M. Evaluation of the symptomatic and structural efficacy of a new hyaluronic acid compound, NRD101, in comparison with diacerein and placebo in a 1 year randomised controlled study in symptomatic knee osteoarthritis. Ann Rheum Dis. 2004;63(12):1611–7. doi: 10.1136/ard.2003.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelletier JP, Martel-Pelletier J. DMOAD developments: present and future. Bull NYU Hosp Jt Dis. 2007;65(3):242–8. [PubMed] [Google Scholar]
- 31.Bartels EM, Bliddal H, Schøndorff PK, Altman RD, Zhang W, Christensen R. Symptomatic efficacy and safety of diacerein in the treatment of osteoarthritis: a meta-analysis of randomized placebo-controlled trials. Osteoarthritis Cartilage. 2010;18(3):289–96. doi: 10.1016/j.joca.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Kay JD, Gouze E, Oligino TJ, Gouze JN, Watson RS, Levings PP, Bush ML, Dacanay A, Nickerson DM, Robbins PD, Evans CH, Ghivizzani SC. Intra-articular gene delivery and expression of interleukin-1Ra mediated by self-complementary adeno-associated virus. J Gene Med. 2009;11(7):605–14. doi: 10.1002/jgm.1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wasserman A, Brahn E. Systemic sclerosis: bilateral improvement of Raynaud’s phenomenon with unilateral digital sympathectomy. Semin Arthritis Rheum. 2010;40(2):137–46. doi: 10.1016/j.semarthrit.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 34.Walker HC, Watts RL, Guthrie S, Wang D, Guthrie BL. Bilateral effects of unilateral subthalamic deep brain stimulation on Parkinson’s disease at 1 year. Neurosurgery. 2009;65(2):302–9. doi: 10.1227/01.NEU.0000349764.34211.74. discussion 309–10. [DOI] [PubMed] [Google Scholar]
- 35.Carroll TJ, Lee M, Hsu M, Sayde J. Unilateral practice of a ballistic movement causes bilateral increases in performance and corticospinal excitability. J Appl Physiol. 2008;104(6):1656–64. doi: 10.1152/japplphysiol.01351.2007. [DOI] [PubMed] [Google Scholar]
- 36.Carroll TJ, Lee M, Hsu M, Sayde J. Unilateral practice of a ballistic movement causes bilateral increases in performance and corticospinal excitability. Sports Med. 2007;37(1):1–14. doi: 10.1152/japplphysiol.01351.2007. [DOI] [PubMed] [Google Scholar]
- 37.Whalen JD, Thomson AW, Lu L, Robbins PD, Evans CH. Viral IL-10 gene transfer inhibits DTH responses to soluble antigens: evidence for involvement of genetically modified dendritic cells and macrophages. Mol Ther. 2001;4(6):543–50. doi: 10.1006/mthe.2001.0492. [DOI] [PubMed] [Google Scholar]
- 38.Schiffelers RM, Xu J, Storm G, Woodle MC, Scaria PV. Effects of treatment with small interfering RNA on joint inflammation in mice with collagen-induced arthritis. Arthritis Rheum. 2005;52(4):1314–8. doi: 10.1002/art.20975. [DOI] [PubMed] [Google Scholar]
- 39.Rintelen B, Neumann K, Leeb BF. A meta-analysis of controlled clinical studies with diacerein in the treatment of osteoarthritis. Arch Intern Med. 2006;166(17):1899–906. doi: 10.1001/archinte.166.17.1899. [DOI] [PubMed] [Google Scholar]
- 40.van de Loo FA, van den Berg WB. Gene therapy for rheumatoid arthritis. Lessons from animal models, including studies on interleukin-4, interleukin-10, and interleukin-1 receptor antagonist as potential disease modulators. Rheum Dis Clin North Am. 2002;28(1):127–49. 13. doi: 10.1016/s0889-857x(03)00073-5. [DOI] [PubMed] [Google Scholar]
- 41.Babcock AM, Standing D, Bullshields K, Schwartz E, Paden CM, Poulsen DJ. In vivo inhibition of hippocampal Ca2+/calmodulin-dependent protein kinase II by RNA interference. Mol Ther. 2005;11(6):899–905. doi: 10.1016/j.ymthe.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 42.Khoury M, Courties G, Fabre S, Bouffi C, Seemayer CA, Vervoordeldonk MJ, Tak PP, Jorgensen C, Apparailly F. Adeno-associated virus type 5-mediated intraarticular administration of tumor necrosis factor small interfering RNA improves collagen-induced arthritis. Arthritis Rheum. 2010;62(3):765–70. doi: 10.1002/art.27302. [DOI] [PubMed] [Google Scholar]
- 43.Nayak S, Herzog RW. Progress and prospects: immune responses to viral vectors. Gene Ther. 2010;17(3):295–304. doi: 10.1038/gt.2009.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol. 2002;76(9):4580–90. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Madry H, Cucchiarini M, Terwilliger EF, Trippel SB. Recombinant adeno-associated virus vectors efficiently and persistently transduce chondrocytes in normal and osteoarthritic human articular cartilage. Hum Gene Ther. 2003;14(4):393–402. doi: 10.1089/104303403321208998. [DOI] [PubMed] [Google Scholar]
- 46.Wang L, Figueredo J, Calcedo R, Lin J, Wilson JM. Cross-presentation of adeno-associated virus serotype 2 capsids activates cytotoxic T cells but does not render hepatocytes effective cytolytic targets. Hum Gene Ther. 2007;18(3):185–94. doi: 10.1089/hum.2007.001. [DOI] [PubMed] [Google Scholar]
- 47.Madsen D, Cantwell ER, O’Brien T, Johnson PA, Mahon BP. Adeno-associated virus serotype 2 induces cell-mediated immune responses directed against multiple epitopes of the capsid protein VP1. J Gen Virol. 2009;90(Pt 11):2622–33. doi: 10.1099/vir.0.014175-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yuasa K, Yoshimura M, Urasawa N, Ohshima S, Howell JM, Nakamura A, Hijikata T, Miyagoe-Suzuki Y, Takeda S. Injection of a recombinant AAV serotype 2 into canine skeletal muscles evokes strong immune responses against transgene products. Gene Ther. 2007;14(17):1249–60. doi: 10.1038/sj.gt.3302984. [DOI] [PubMed] [Google Scholar]
- 49.Seiler MP, Cerullo V, Lee B. Immune response to helper dependent adenoviral mediated liver gene therapy: challenges and prospects. Curr Gene Ther. 2007;7:297–305. doi: 10.2174/156652307782151452. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi T, Kawabata K, Koizumi N, Sakurai F, Nakashima K, Sakurai H, et al. Role of MyD88 and TLR9 in the innate immune response elicited by serotype 5 adenoviral vectors. Hum Gene Ther. 2007;18:753–762. doi: 10.1089/hum.2007.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.