Abstract
Background
Resveratrol has been shown to reverse some of the detrimental affects of metabolic syndrome (MetS). We sought to define the impact of supplemental resveratrol on normal myocardium remote from an ischemic territory in a swine model of MetS and chronic myocardial ischemia.
Study Design
Yorkshire swine were fed either a normal diet (control), high cholesterol diet (HCD), or high cholesterol diet with orally supplemented resveratrol (HCD-R; 100mg/kg/day). Four weeks after diet modification myocardial ischemia was induced by ameroid constrictor placement. Seven weeks later myocardial tissue from a territory remote from the ischemia was harvested. Animals in the HCD and HCD-R groups underwent functional cardiac MRI before ischemia and prior to sacrifice. Tissue was harvested for protein expression analysis.
Results
After 7 weeks of ischemia, regional left ventricular systolic function was significantly increased in HCD-R as compared to HCD. During ventricular pacing the HCD group had significantly decreased flow (p=0.03), while perfusion in the HCD-R was preserved as compared to the control. There was no difference in microvascular relaxation. Expression of metabolic proteins Sirt-1 (p=0.002), AMPkinase (p=0.02), and Carnitine palmitoyltransferase-I (p=0.002) were upregulated in the HCD-R group. Levels of protein oxidative stress were significantly increased in the HCD and HCD-R groups, as compared to the control (p=0.003). Acitvated eNOS was increased in HCD-R (p=0.01). There was no difference in myocardial endothelial cell density between the groups, however dividing endothelial cells were decreased in the HCD and HCD-R groups (p=0.006).
Conclusions
Resveratrol supplementation improves regional left ventricular function and preserves perfusion to myocardium remote from an area of ischemia in an animal model of metabolic syndrome and chronic myocardial ischemia.
INTRODUCTION
The prevalence of obesity and metabolic syndrome (MetS) is increasing in the developed world.1 MetS is defined by the constellation of obesity, dyslipidemia, hypertension, insulin resistance, and increased serum pro-inflammatory markers.2 Each component of MetS increases the risk of symptomatic coronary artery disease (CAD), but the presence of all components in an individual patient is thought to further increase the risk. A recent prospective observational study of non-diabetic middle-aged men showed that obesity and MetS was associated with an increased risk of death from a cardiovascular event (myocardial infarction, stroke, or heart failure). Normal weight patients with MetS had a hazard ratio of 1.63, obese patients without MetS had a hazard ratio of 1.95, and obese patients with MetS demonstrated a 2.55 fold increase risk for death from cardiovascular events as compared to normal weight patients without MetS.3
Left ventricular systolic dysfunction in the setting of insulin resistance and diabetes leads to remodeling and potential heart failure, attributed in part to a shift in myocardial energy sources. Further, the diseases that comprise MetS lead to impaired myocardial angiogenesis through decreased expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF), and increased expression of anti-angiogenic factors such as angiostatin and endostatin.4, 5 Modifying the global burden of these diseases on the myocardium may decrease the risk of a cardiovascular event or improve the ability to tolerate ischemia.
Resveratrol is a polyphenol found in high concentrations and thought to impart some of the beneficial properties of red wine.6 It is thought that the higher concentration in red wine is due to the fermentation of the juice with the rest of the grape, as resveratrol is in highest concentration in the skin and seeds. This supplement has been shown in cultured cell and small animal models to reverse the detrimental effects of MetS, improve endothelial dysfunction, and myocardial function.7, 8
At this time little is known regarding the effects of resveratrol on normal myocardium remote from an ischemic territory. We sought to define the impact of supplemental resveratrol on myocardial perfusion and function, microvascular reactivity, myocardial metabolism, and the angiogenic potential in a swine model of MetS and chronic myocardial ischemia. We hypothesized that in a clinically relevant large animal model of MetS and chronic ischemia, high-dose resveratrol would improve the detrimental effects of MetS and preserve myocardial function and metabolism.
MATERIALS AND METHODS
Animal Model
Yorkshire miniswine (Parsons Research, Amherst, MA) were fed one of three diets throughout the 11 week experiment. Group 1 was given 500 grams of a hypercholesterolemic diet daily (HCD, n=7) (2,248 kcal/day) composed of 4% cholesterol, 17.2% coconut oil, 2.3% corn oil, 1.5% sodium cholate, and 75% regular chow. Group 2 was fed the same hypercholesterolemic diet supplemented with 100mg/kg/day of resveratrol (HCD-R, n=7) (ChromaDex, Irvine, CA). Group 3was fed regular chow (control, n=7, 1,824 kcal/day). There have been concerns about the bioavailablity of orally delivered resveratrol, and some studies have shown low systemic concentrations9. Thus we chose a higher dose in an attempt to achieve adequate cardiac tissue levels.
After 4 weeks of dietary modification, all animals underwent ameroid constrictor placement on the proximal left circumflex coronary artery (LCx) using previously reported methods.10 Briefly, anesthesia was induced with ketamine (10mg/kg IM) and thiopental 2.5%, and maintained with a gas mixture of oxygen at 1.5–2 L/min and 3.0% isoflurane. The pericardium was opened through a left mini-thoracotomy, and a titanium ameroid constrictor (1.75mm internal diameter) was placed around the proximal LCx. This device is designed to produce myocardial ischemia without causing infarction.
Seven weeks after ameroid placement, swine were again anesthetized for a cardiac magnetic resonance imaging (CMR) study. The heart was then exposed through a median sternotomy and physiologic measurements were taken, followed by euthanasia. Myocardial tissue was harvested for study. The non-ischemic myocarcial tissue was defined as the one cubic centimeter of tissue surrounding the LAD at the mid-papillary level.
The study was approved by the Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee. Animals were cared for in compliance with the Harvard Medical Area Institutional Animal Care and Use Committee and in accordance with the ‘Principles of Laboratory Animal Care’ formulated by the National Society for Medical Research and the ‘Guide for the Care and Use of Laboratory Animals’ (NIH publication no. 5377-3 1996).
Myocardial Perfusion analysis
Myocardial perfusion was determined during each procedure with isotope-labeled microspheres, 15 µm diameter (BioPAL Worcester, MA) using previously reported methods.4 Briefly, 1.5×107 gold-labeled microspheres were injected during temporary LCx occlusion at the time of ameroid placement to identify the area at risk. Labeled microspheres were also injected at the final procedure during rest and ventricular pace (150 beats/min) conditions. Following euthanasia, 10 transmural left ventricular (LV) sections were collected for assay. The samples were exposed to neutron beams and microsphere densities were measured using a gamma counter.
Cardiac Magnetic Resonance (CMR) Imaging
Animals in the HCD and HCD-R groups underwent CMR study using a 1.5T Philips Achieva scanner (Philips Healthcare, Best, Netherlands) with a five-element cardiac phased-array receiver coil before placement of the ameroid (pre) and prior to sacrifice (post) 7 weeks later. LV function was evaluated by free-breathing cine short axis steady-state free processing (SSFP) cine images covering the entire LV and a free-breathing phase contrast velocity map in the ascending aorta. CMR tagging was performed to evaluate dyssynchrony and local LV myocardial function using three short axis slices with a spiral complementary spatial modulation of magnetization sequence.11 Images were analyzed using a MATLAB (The Mathworks, Natick, MA) program. Each LV slice was divided into 6 segments and the circumferential strain of each segment was analyzed. The values obtained represent the amount of regional myocardial contraction in the mid-papillary anterior region contributing to the overall LV ejection fraction. Comparisons of the anterior segment myocardial function were made between the HCD and HCD-R groups prior to induction of ischemia and again after 7 weeks of ischemia.
X-ray coronary angiography
X-ray coronary angiography was performed prior to each animal sacrifice in order to ensure occlusion of the LCx and assess collateral formation. Images were interpreted by an interventional cardiologist blinded to the treatment groups.
Microvascular Responses
After cardiac harvest, coronary arterioles (80–180µm in diameter) from the left anterior descending (LAD) territory were placed in isolated microvessel chambers. Vessels were preconstricted by 25–50% of the baseline diameter with the thromboxane A2 analog, U46619 (10−6 M). The microvascular responses to sodium nitroprusside (SNP, 10−9 to 10−4 mol/L, an endothelium-independent vasodilator), substance P (SubP, 10−12 to 10−7 mol/L, an endothelium-dependent vasodilator), adenosine diphosphate (ADP, 10−12 to 10−7 mol/L, an endothelium-dependent vasodilator), and vascular endothelial growth factor (VEGF, 10−15 to 10−10 mol/L, an endothelium-dependent vasodilator) were evaluated. All reagents were obtained from Sigma-Aldrich (St Louis, MO).
Immunoblotting Studies
Whole-cell lysates were isolated from homogenized myocardial samples from the normal myocardium with radio-immunoprecipitation assay buffer (Boston Bioproducts, Worcester, MA). Sixty micrograms of total protein were fractionated by SDS-PAGE (Invitrogen, San Diego, CA) and transferred to PVFD membranes (Millipore, Bedford, MA). Each membrane was incubated with specific primary antibodies (Cell Signaling, Beverly, MA). Immune complexes were visualized with enhanced chemiluminescence (Amersham, Piscataway, NJ). Bands were quantified by densitometry of autoradiograph films. Ponceau staining was used to ensure equal protein loading.
Protein oxidative stress
Dinitrophenylhydrazine-derivatized myocardial tissue homogenates samples were fractionated by 10% PAGE and transferred to PVFD membranes (Chemicon International, Inc. Temecula, CA). Membranes were incubated with specific primary antibody, followed by incubation with secondary antibody using the OxyBlot protein oxidation detection kit (Millipore, Billerica, MA). Immune complexes were visualized with the enhanced chemiluminescence detection system (Amersham, Piscataway, NJ, USA).
Immunoflorescence
Frozen sections of non-ischemic myocardium were fixed in acetone and blocked. Anti-CD31 and Ki67 primary antibodies were applied (Epitomics, Burlingame, CA). Slides were washed and secondary antibody applied (Jackson Immunoresearch). Slides were mounted with 4',6-diamidino-2-phenylindole (DAPI) and viewed with a confocal microscope. Photomicrographs were taken with a Zeiss Axiolab microscope (Carl Zeiss Inc, Thornwood, NY) equipped with a digital camera (Photodoc, Upland, CA).
Data Analysis
All data are presented as mean ± standard error of the mean (SEM). Microvessel responses are expressed as percent relaxation of the preconstricted diameter and were analyzed using a two-way, repeated-measures analysis of variance (ANOVA) with Bonferroni correction, which was applied to interactions of treatment and dose. CMR data are compared using two-tailed Student’s t test. Western blots were analyzed after digitalization of x-ray films using a flatbed scanner (ScanJet 4c; Hewlett-Packard, Palo Alto, CA) and NIH ImageJ 1.40g software (National Institute of Health, Bethesda, MD). Levels of phosphorylated proteins were normalized to total levels. Comparisons between groups were analyzed by one-way ANOVA with a Newman-Keuls Multiple Comparison post-hoc test.
RESULTS
Experimental model
The ameroid led to complete (100%) occlusion of the LCx in all animals. There was no gross evidence of myocardial infarction in any of the animals. There was one death in the control and one in the HCD-R groups. All groups had similar body mass indices at the time of the ameroid placement (p= 0.11). Immediately prior to sacrifice animals in the HCD group were significantly larger (control 28 ± 1.2 kg/m2, HCD 36 ± 1.3 kg/m2, HCD-R 31 ± 1.4 kg/m2, p= 0.001). The HCD group had significantly higher levels of total cholesterol as compared to the control and HCD-R groups (control 79.9 ± 0.1 mg/dL, HCD 390 ± 53 mg/dL, HCD-R 261 ± 54 mg/dL, p< 0.001). Thirty minutes post-dextrose infusion, the HCD group had blood glucose levels significantly higher than the control and HCD-R groups (control 108 ± 7 mg/dL, HCD 171 ± 6 mg/dL, HCD-R 132 ± 14 mg/dL, p= 0.002). There was no statistical difference in blood glucose levels between the HCD-R group and the control groups.
Cardiovascular Magnetic Resonance (CMR) Imaging
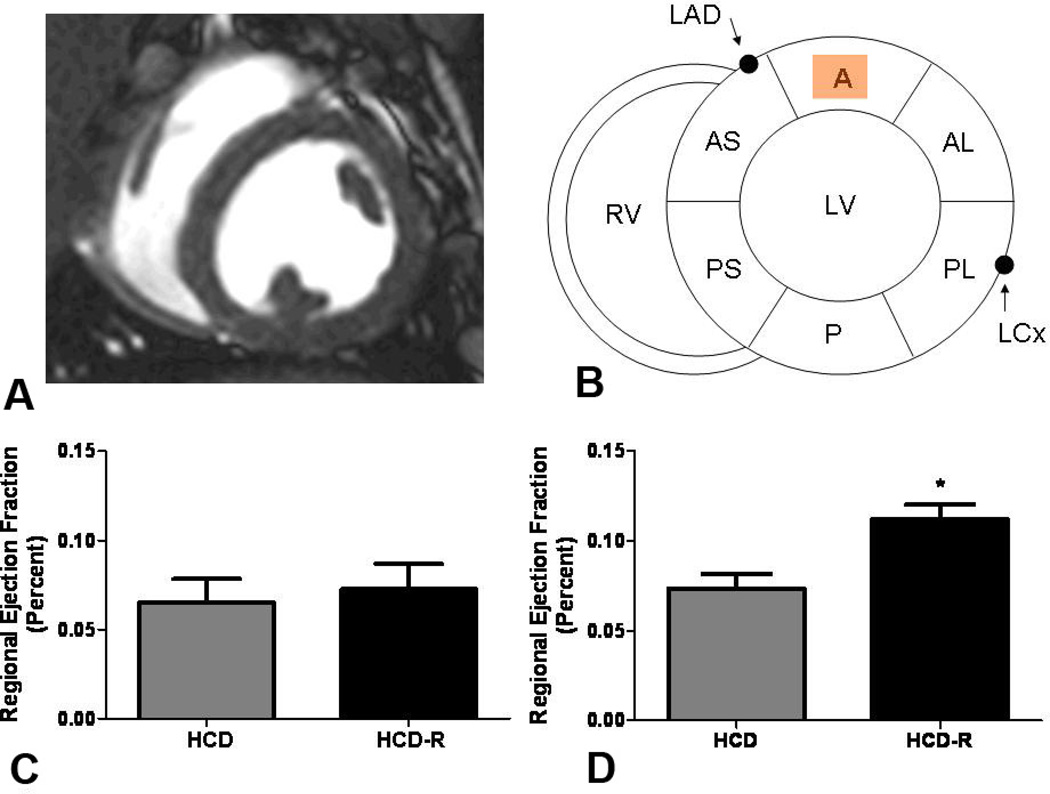
Global LV systolic function was similar between groups at the conclusion of the study (HCD 45.9 ± 4.4%, HCD-R 48.5 ± 6.9%, p= 0.6). Comparisons of regional function were made by analyzing the differences in the amount of muscle contraction in each region before placement of the ameroid with the amount of contraction at the end of the experiment in the HCD and HCD-R groups. Regional wall motion analysis using CMR circumferential strain (Figure 1A) demonstrated no change in anterior regional function (region of measurement shown in Figure 1B) from baseline (pre) to 7 weeks of chronic ischemia (post) in HCD swine in the mid-anterior myocardial slice (6.5 ± 1.3% to 7.4 ± 1.0%). In the HCD-R group, regional function in the remote non-ischemic myocardium demonstrated a significant increase in contractility at the conclusion of the experiment (7.3 ± 1.4% to 11.2 ± 1.0%) (Figure 1C and D). Similar improvements in segmental function were observed in the basal-anterior (HCD pre 13 ± 1%, HCD post 12 ±0.4%, p= 0.33; HCD-R pre 11 ± 1.8%, HCD-R 15 ± 1.0, p= 0.04) and mid-posteroseptal segments of the HCD-R group (HCD pre 11 ± 1%, HCD post 9 ± 2%, p= 0.58; HCD-R pre 9 ± 0.7%, HCD-R post 12 ± 0.5%, p=0.006).
Figure 1. Cardiac magnetic resonance (CMR) imaging.
(A) Axial mid-papillary CMR image. (B) Schematic cross-sectional representation of the left ventricular (LV) short axis depicting the left anterior descending coronary artery (LAD) and relationship to the anterior (A) region of the LV. RV, Right ventricle; AS, anterior septum; A, anterior; AL, anterolateral; PL, posterolateral; P, posterior; PS, posteroseptal; LCx, Left circumflex coronary artery. (C) Regional wall motion analysis using CMR circumferential strain demonstrates no difference in anterior wall function between the HCD and HCD-R groups at baseline (pre-ischemia, p= 0.7) (D) After 7 weeks of ischemia, the anterior wall function is significantly increased in the HCD-R group as compared to the HCD (*p= 0.01). HCD, high cholesterol diet; HCD-R, high cholesterol diet with orally supplemented resveratrol.
Myocardial Perfusion
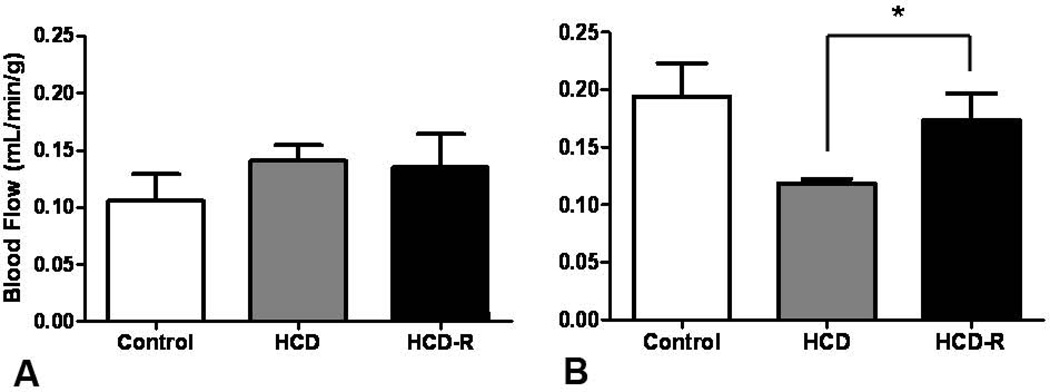
Blood flow to the non-ischemic myocardium was similar between the groups at rest (control 0.11 ± 0.02 ml/min/g, HCD 0.14 ± 0.04 ml/min/g, HCD-R 0.14 ± 0.03) (Figure 2A). During ventricular pacing, both the control and HCD-R groups had increased flow, while the HCD group did not (control 0.19 ± 0.03 ml/min/g, HCD 0.12 ± 0.003 ml/min/g, HCD-R 0.17 ± 0.02 ml/min/g) (Figure 2B). There was no significant difference between the control and HCD-R groups.
Figure 2. Myocardial perfusion.
(A) There was no difference in blood flow to the non-ischemic myocardium at rest at the completion of the experiment. (B) During stress (ventricular pacing), blood flow to the non-ischemic myocardium was significantly increased in the HCD-R group (*p= 0.03). HCD, high cholesterol diet; HCD-R, high cholesterol diet with orally supplemented resveratrol.
Microvascular Reactivity
Microvessels were isolated from fresh non-ischemic myocardium and subjected to increasing concentrations of vasorelaxing agents in vitro. The baseline vessel diameters were not different between the groups (control 159.2 ± 10.4µm, HCD 142.5 ± 10.5µm, HCD-R 138.4 ± 8.5µm, p= 0.35). The preconstricted vessel diameters were not different between groups (control 94.0 ± 3.7µm, HCD 88.8 ± 8.4µm, HCD-R 78.4 ± 7.0µm, p= 0.31). There was no significant difference between the groups in relaxation responses to SNP (control 94 ± 6%, HCD 96 ± 4%, HCD-R 97 ± 3%, p= 0.99), ADP (control 91 ± 5%, HCD 84 ± 8%, HCD-R 80 ± 4% at 10−4; p= 0.9), substance P (control 75 ± 2%, HCD 82 ± 5%, HCD-R 73 ± 5% at 10−7; p= 0.6), or VEGF (control 74 ± 4%, HCD 61 ± 6%, HCD-R 75 ± 6%; p=0.2) (not shown).
Oxidative Stress
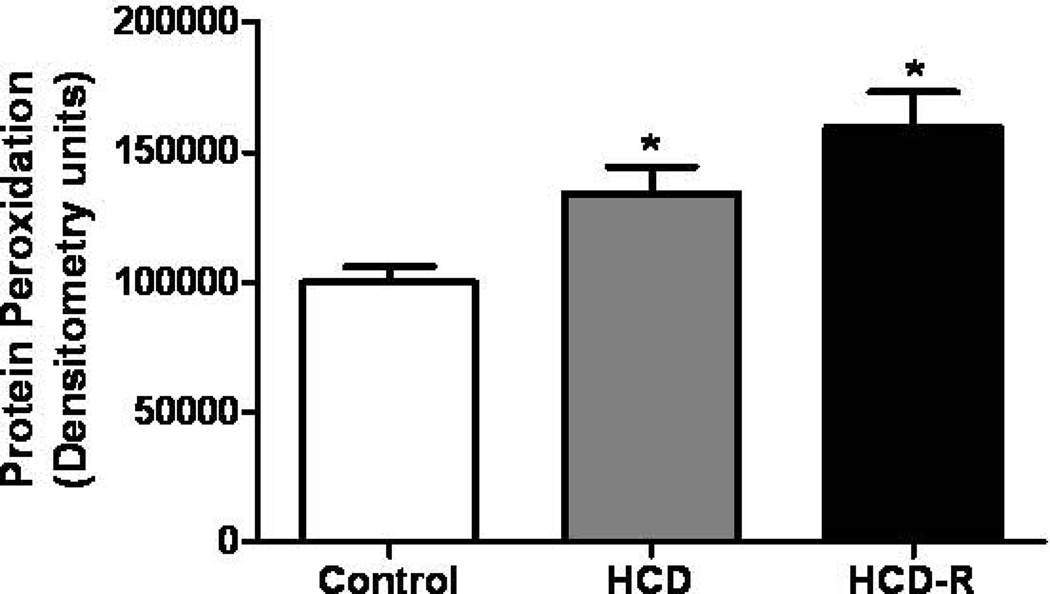
Protein oxidative stress, as measured by the levels of carbonyl groups introduced into proteins by oxidative reactions, was assessed. Levels of oxidation were significantly increased 1.3 fold in the HCD as compared to the control. In the HCD-R group the amount of oxidized protein was 1.6 fold increased as compared to the control group. When comparing HCD to HCD-R, the HCD-R group was 1.2 fold higher, but this difference was not statistically significant (Figure 3).
Figure 3.
Oxidative stress. Levels of protein oxidative stress were increased in both the HCD and HCD-R groups as compared to the control groups after four weeks of chronic myocardial ischemia (*p= 0.003). HCD, high cholesterol diet; HCD-R, high cholesterol diet with orally supplemented resveratrol.
Protein Expression
Metabolic proteins
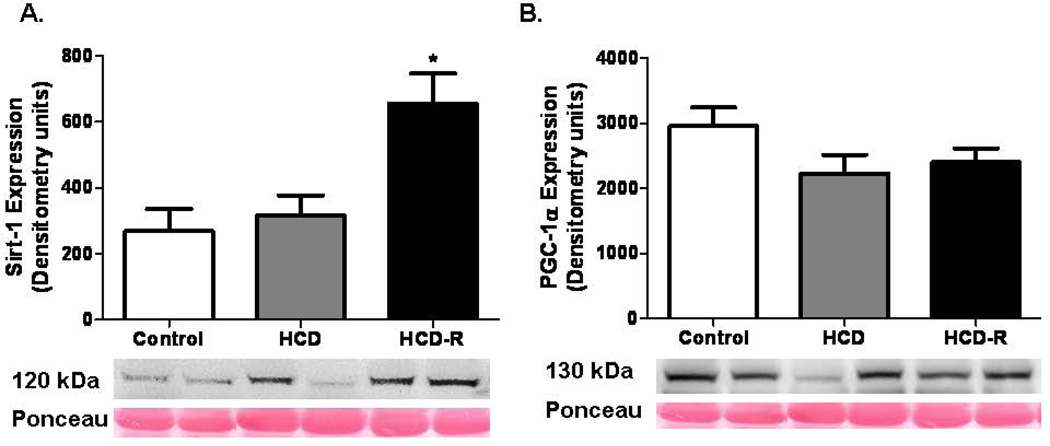
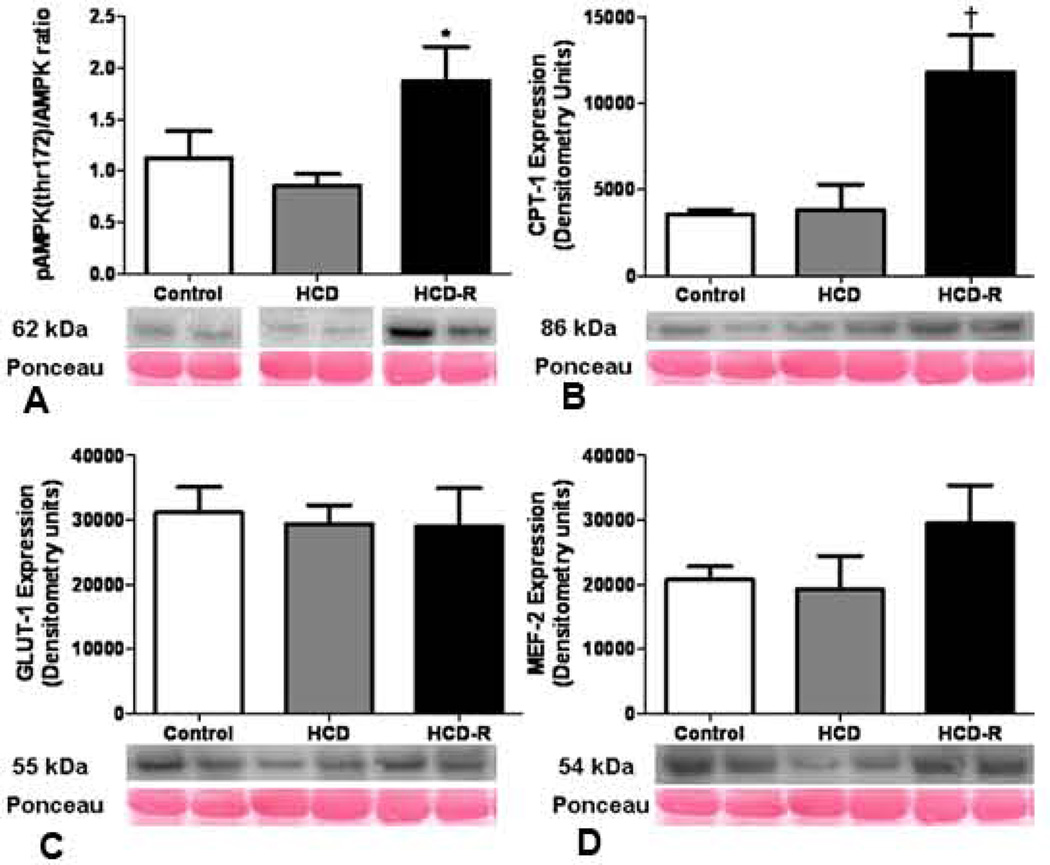
Sirt-1, a histone deacetylase and a primary target for resveratrol, was upregulated in the HCD-R group; 2.4 fold v. control and 2.1 fold v. HCD. There was no significant difference in the expression levels of Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) between the groups (Figure 4). Activated AMP-activated protein kinase (AMP kinase) was upregulated in the resveratrol group as compared to the control (1.7 fold) and HCD (2.2 fold). Carnitine palmitoyltransferase I (CPT-1) expression was increased in the HCD-R group as compared to the control (3.3 fold) and HCD (3.1 fold) groups. Glucose transporter-1 (GLUT-1) expression was not significantly different between the groups. Myocyte enhancer factor-2 (MEF2) levels were not significantly different between the groups (Figure 5A–D). There was no significant difference between the groups in the expression levels of GLUT-4 (p= 0.34) (not shown).
Figure 4. Sirtuin and PGC-1α expression at the completion of the experiment.
(A) Sirt-1 expression was significantly increased in the HCD-R group as compared to the control and HCD groups after four weeks of ischemia and seven weeks of diet modification (*p= 0.002). (B) There was no difference in PGC-1α expression between the groups (p= 0.16).
Figure 5. Metabolic protein expression at the completion of the experiment.
(A) Levels of activated AMPK were increased in the HCD-R group as compared to the control and HCD groups after four weeks of ischemia and seven weeks of diet modification (*p= 0.02). (B) Carnitine palmitoyltransferase I (CPT1), a protein involved in fatty acid oxidation and under control of AMPK, was upregulated in the HCD-R group (†p= 0.002). (C) There was no difference in the expression of GLUT-1 between the groups (p= 0.9). (D) MEF2, an upstream regulator of GLUT-1 and -4 expression was not different between the groups (p= 0.3). HCD, high cholesterol diet; HCD-R, high cholesterol diet with orally supplemented resveratrol.
Angiogenic proteins
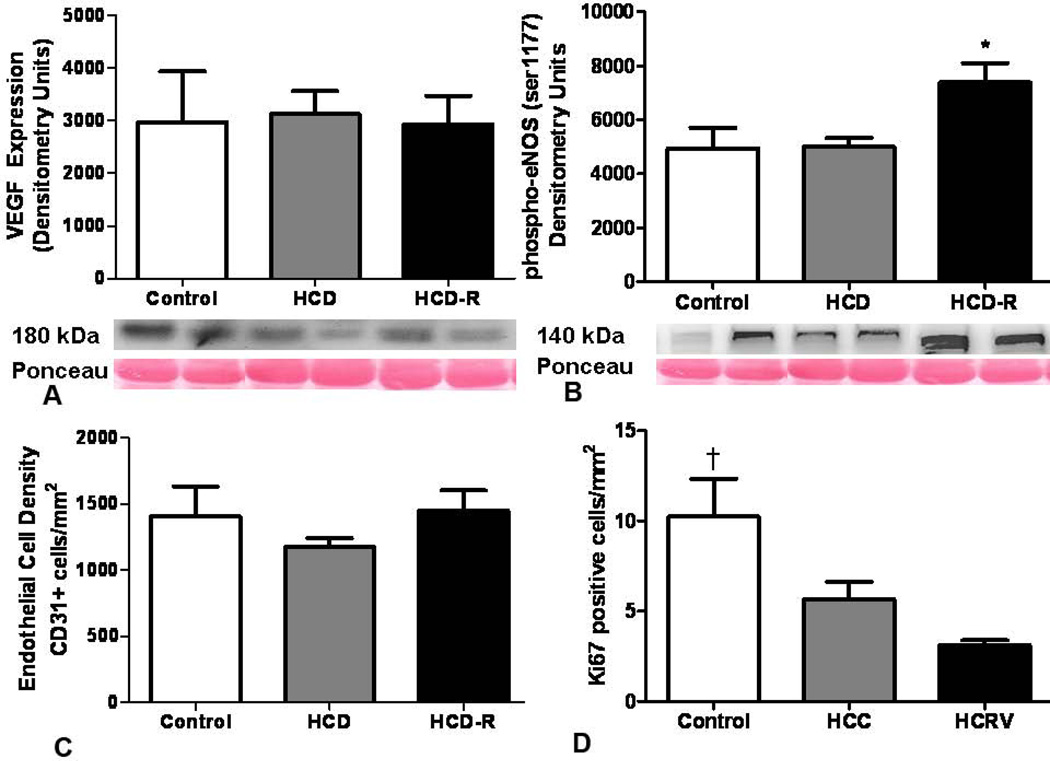
There was no significant difference in the expression levels of vascular endothelial growth factor between the groups. Levels of activated nitric oxide synthase (phospho-eNOS (ser1177)) were elevated 1.5 fold in the HCD-R group as compared to both the control and HCD groups (Figure 6A and B).
Figure 6. Angiogenic proteins.
(A) Expression of VEGF was not different between groups at the completion of the experiment (p= 0.9). (B) Activated eNOS was upregulated in the HCD-R group as compared to the control and HCD groups The number of dividing endothelial cells in the non-ischemic myocardium was significantly lower in the HCD and HCD-R groups as compared to the control in myocardium remote from the ischemic insult (*p= 0.01). (C) The density of endothelial cell in the non-ischemic myocardium was not different between the groups (p= 0.5). (D) The number of dividing endothelial cells in the non-ischemic myocardium was significantly lower in the HCD and HCD-R groups as compared to the control (†p= 0.006). HCD, high cholesterol diet; HCD-R, high cholesterol diet with orally supplemented resveratrol.
Vessel Density Measurements
Myocardium stained for CD31 demonstrated no differences in the density of endothelial cells between the groups. Myocardial sections co-stained for CD31 and Ki67 showed a significantly higher number of dividing endothelial cells in the control group (1.8 fold v. HCD and 3.2 fold v. HCD-R). There was no significant difference between the HCD and HCD-R groups (Figure 6C and D).
DISCUSSION
In this swine model of chronic ischemia we examined the effects of resveratrol on the non-ischemic myocardium remote from an ischemic territory. The HCD animals developed significant clinical MetS which was mitigated with resveratrol supplementation. Regional LV systolic function was improved and blood flow during stress was increased in animals treated with resveratrol. Further, supplemental resveratrol impacted a number of molecular pathways that may help the myocardium adapt to and compensate for ischemic stress.
Sirt-1 has long been thought to be a primary target of resveratrol and the key to its many metabolic effects. In the current work Sirt-1 was significantly upregulated in the HCD-R group. Many studies have examined the effects of resveratrol, but few, if any have explored the effects in non-ischemic myocardium remote from an ischemic insult. It is thought that nearby normal myocardium provides support to the injured area. Indeed, regional LV systolic function was increased in the normal territory and a number of downstream metabolic signaling pathways thought to be influenced by Sirt-1 were upregulated to support an increase in energy demand.
An energy sensing protein, AMPK, described as a cellular “fuel gauge” in the myocardium, demonstrated increased levels of activity in the resveratrol treated group.12 Activated AMPK is upregulated during times of increased energy demand and results in ATP production. AMPK is thought to be a primary target of resveratrol and a recent study using mice with the enzyme knocked out showed none of the beneficial effects of the supplement.13 We demonstrated a similar increase in activated AMPK in ischemic myocardium in our swine model.14 This upregulation likely contributed to the improved regional LV systolic function. Further, a recent study using H9c2 muscle cells showed that resveratrol induced AMPK activation led to cellular protection from reactive oxygen species.15 Thus, increased AMPK may have provided protection to the cardiomyocytes in the setting of increased oxidative stress in the resveratrol treated group.
A downstream target of AMPK, CPT1, was also upregulated in the myocardium remote from the ischemic territory. This enzyme is involved with fatty acid metabolism and facilitates the entry of long-chain fatty acyl-CoA into mitochondria for enhanced β-oxidation and ATP generation. This is an important step to increase energy levels in non-ischemic myocardium in the setting of insulin resistance.16 In contrast, the ischemic myocardium is more dependent on glucose for energy production. Studies have shown that dysregulation of glucose metabolism during ischemia leads to greater cardiac dysfunction and possibly worse outcomes in patients.17, 18 In our study of the ischemic myocardium we observed a significant increase in GLUT-4, a protein important in myocardial glucose metabolism and also regulated by AMPK, in resveratrol treated animals.14 It is reasonable to assume that there is no pressure to enhance glucose uptake in the non-ischemic myocardium, and may explain why there was no increase in GLUT-1, GLUT-4, or the upstream regulator MEF2.
Expression of PGC-1α, a transcriptional co-activator of metabolic pathways, was not significantly different between the groups. This enzyme has similar activity to AMPK, and also is often upregulated during cellular stress and involved with mitochondrial biogenesis to meet increased energy needs.19 As there was no change in any of the groups it is possible that there is minimal stress on non-ischemic myocardium, and thus no drive to increase expression. However, the activity level of this enzyme appears to be heavily dependent on post-translational modifications such as phosphorylation, methylation, and deacetylation.20 The extent, influencing factors, and resultant changes in activity due to these modifications are not well understood. In this work we did not measure these post-translational changes, so it is not clear if the activity levels of PGC-1α were altered by MetS or by the addition of resveratrol.
In examining the angiogenic pathways we observed no difference in VEGF expression or endothelial cell density between the groups. This is not surprising as there is likely little need to form new vasculature in non-ischemic myocardium, and we have noted similar findings in previous work.21 We did note an increase in activated eNOS, a powerful vasorelaxer and pro-angiogenic protein. The significance of this is not clear as there was no increase in angiogenesis or microvascular relaxation responses to the drugs tested. Microvascular dysfunction is multifactorial and likely involves decreased levels of the vasodilator nitric oxide (NO), increased levels of reactive oxygen species, and impaired vascular responses to local vasoactive substances.23, 24 It is possible that vasorelaxation may have been occurring via pathways that were unmeasured. Further, in previous work by our group we demonstrated increased activated eNOS, but a lack of angiogenesis in the myocardium of our MetS swine model. The decrease in dividing endothelial cells was in agreement with our prior work. We have shown that both MetS and resveratrol increase the elaboration of antiangiogenic proteins such as endostatin.25 Without a clear pro-angiogenic signal and likely strong anti-angiogenic signals, it is not surprising that endothelial cell division is subdued. It is possible that in the resveratrol group there is less need to form new vessels in the setting of increased perfusion of the myocardium.
Limitations
There are several limitations of our study. First, it was performed in a porcine model of chronic myocardial ischemia. While in most situations, the porcine coronary circulation is similar to the physiology and pathophysiology of the human coronary circulation, this may not be the case for resveratrol supplementation. The number of animals in each group was relatively small, and due to logistical and financial constraints the control animals did not undergo functional MRI scans. In addition, the length of the study was limited to 11 weeks of diet modification and resveratrol supplementation. A longer study period may have allowed for further changes to take place, such as myocardial hypertrophy, ventricular remodeling, and changes in angiogenic signaling. Protein expression and microvascular reactivity were only measured at one time point, and a likely changing over time. Finally, the doses of resveratrol were much higher than found naturally in foods and wine. It is uncertain if the dose found in naturally occurring products would produce similar results.
Conclusion
In an animal model of MetS and chronic ischemia, resveratrol supplementation improves regional LV systolic function and stress related perfusion to the myocardium remote from an area of ischemia. A number of changes in cardiomyocyte metabolic proteins likely supported this improvement in function and blood flow. These findings suggest that resveratrol influences the remote normal territory and acts to compensate for the ischemic myocardium. A fuller understanding of how resveratrol impacts the ischemic and non-ischemic myocardium will be beneficial prior to large scale trials in humans.
ACKNOWLEDGMENT
The authors wish to acknowledge Shu-Hua Xu for assistance with molecular studies and Kraig V Kissinger for assistance in performing the CMR studies.
This work was supported by the National Health Institute, National Heart, Lung, and Blood Institute (NHLBI RO1HL46716 and RO1HL69024), NIH 5T32-HL0074 (M.P.R.), NIH 5T32-HL007734-19 (M.P.R), 5T32-HL094300 (L.M.C.) and the Irving Bard Memorial Fellowship (M.P.R., L.M.C.).
ABREVIATIONS
- ADP
adenosine diphosphate
- CMR
cardiac magnetic resonance imaging
- LAD
left anterior descending coronary artery
- LCx
left circumflex coronary artery
- LV
left ventricle
- MetS
metabolic syndrome
- NO
nitric oxide
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Information: Nothing to disclose.
Presented at the American College of Surgeons 97th Annual Clinical Congress, San Francisco, CA, October 2011.
REFERENCES
- 1.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 2.Grundy SM, Brewer HB, Jr, Cleeman JI, et al. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 3.Arnlov J, Ingelsson E, Sundstrom J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle-aged men. Circulation. 2010;121:230–236. doi: 10.1161/CIRCULATIONAHA.109.887521. [DOI] [PubMed] [Google Scholar]
- 4.Boodhwani M, Voisine P, Ruel M, et al. Comparison of vascular endothelial growth factor and fibroblast growth factor-2 in a swine model of endothelial dysfunction. Eur J Cardiothorac Surg. 2008;33:645–650. doi: 10.1016/j.ejcts.2007.12.016. discussion 251–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sodha NR, Clements RT, Boodhwani M, et al. Endostatin and angiostatin are increased in diabetic patients with coronary artery disease and associated with impaired coronary collateral formation. Am J Physiol Heart Circ Physiol. 2009;296:H428–H434. doi: 10.1152/ajpheart.00283.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cordova AC, Jackson LS, Berke-Schlessel DW, Sumpio BE. The cardiovascular protective effect of red wine. J Am Coll Surg. 2005;200:428–439. doi: 10.1016/j.jamcollsurg.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 7.Lavu S, Boss O, Elliott PJ, Lambert PD. Sirtuins--novel therapeutic targets to treat age-associated diseases. Nat Rev Drug Discov. 2008;7:841–853. doi: 10.1038/nrd2665. [DOI] [PubMed] [Google Scholar]
- 8.Penumathsa SV, Koneru S, Samuel SM, et al. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic Biol Med. 2008;45:1027–1034. doi: 10.1016/j.freeradbiomed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walle T, Hsieh F, DeLegge MH, et al. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 10.Robich MP, Matyal R, Chu LM, et al. Effects of neuropeptide Y on collateral development in a swine model of chronic myocardial ischemia. J Mol Cell Cardiol. 2010;49:1022–1030. doi: 10.1016/j.yjmcc.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ryf S, Kissinger KV, Spiegel MA, et al. Spiral MR myocardial tagging. Magn Reson Med. 2004;51:237–242. doi: 10.1002/mrm.10688. [DOI] [PubMed] [Google Scholar]
- 12.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase-deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59:554–563. doi: 10.2337/db09-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robich MP, Osipov RM, Chu LM, et al. Resveratrol modifies risk factors for coronary artery disease in swine with metabolic syndrome and myocardial ischemia. Eur J Pharmacol. 2011;664:45–53. doi: 10.1016/j.ejphar.2011.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hwang JT, Kwon DY, Park OJ, Kim MS. Resveratrol protects ROS-induced cell death by activating AMPK in H9c2 cardiac muscle cells. Genes Nutr. 2008;2:323–326. doi: 10.1007/s12263-007-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruce CR, Hoy AJ, Turner N, et al. Overexpression of carnitine palmitoyltransferase-1 in skeletal muscle is sufficient to enhance fatty acid oxidation and improve high-fat diet-induced insulin resistance. Diabetes. 2009;58:550–558. doi: 10.2337/db08-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–3223. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 18.Dutka DP, Pitt M, Pagano D, et al. Myocardial glucose transport and utilization in patients with type 2 diabetes mellitus, left ventricular dysfunction, and coronary artery disease. J Am Coll Cardiol. 2006;48:2225–2231. doi: 10.1016/j.jacc.2006.06.078. [DOI] [PubMed] [Google Scholar]
- 19.Arany Z, He H, Lin J, et al. Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 2005;1:259–271. doi: 10.1016/j.cmet.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Rodgers JT, Lerin C, Haas W, et al. Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 2005;434:113–118. doi: 10.1038/nature03354. [DOI] [PubMed] [Google Scholar]
- 21.Robich MP, Osipov RM, Chu LM, et al. Temporal and spatial changes in collateral formation and function during chronic myocardial ischemia. J Am Coll Surg. 2010;211:470–480. doi: 10.1016/j.jamcollsurg.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boodhwani M, Sodha NR, Mieno S, et al. Functional, cellular, and molecular characterization of the angiogenic response to chronic myocardial ischemia in diabetes. Circulation. 2007;116:I31–I37. doi: 10.1161/CIRCULATIONAHA.106.680157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchesi C, Ebrahimian T, Angulo O, et al. Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension. 2009;54:1384–1392. doi: 10.1161/HYPERTENSIONAHA.109.138305. [DOI] [PubMed] [Google Scholar]
- 24.Robich MPOR, Nezafat R, Feng J, et al. Resveratrol improves myocardial perfusion in a swine model of hypercholesterolemia and chronic myocardial ischemia. Circulation. 2010 doi: 10.1161/CIRCULATIONAHA.109.920132. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]