Abstract
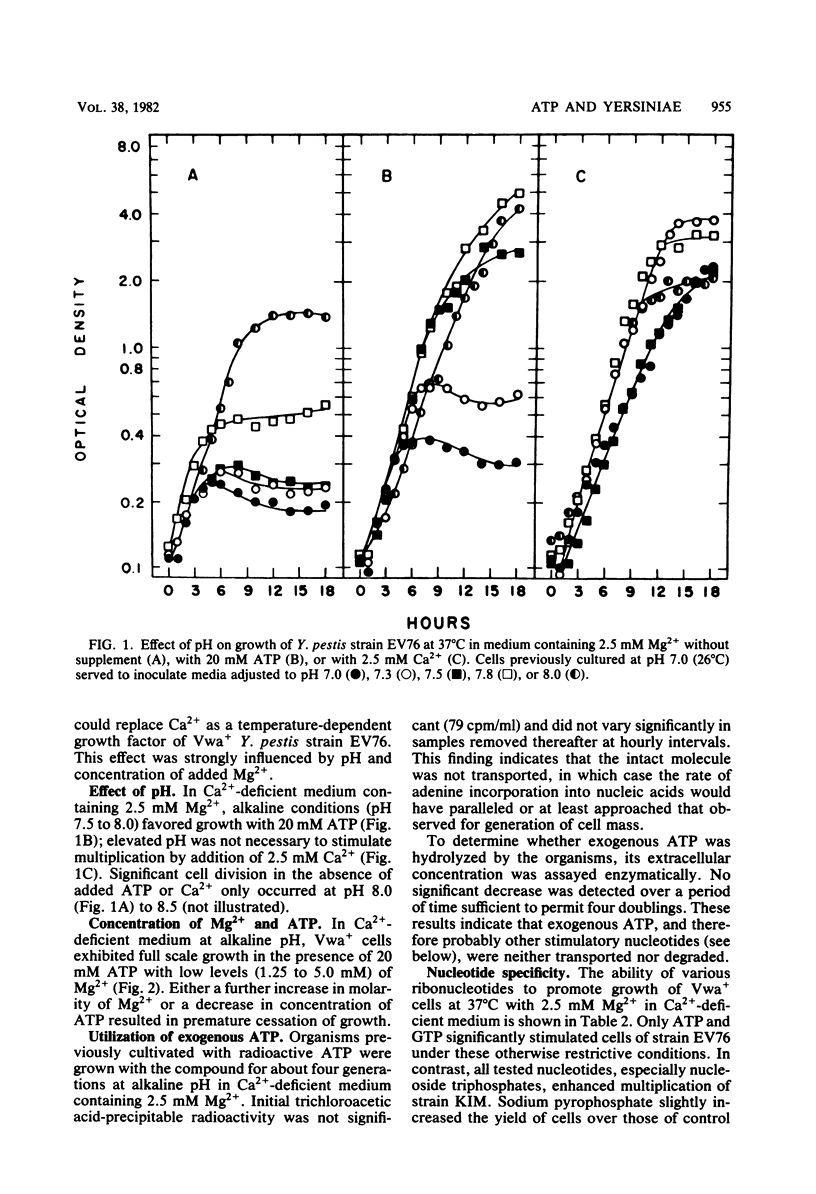
Cells of Yersinia pestis strain EV76 are known to cease growth after a shift from 26 to 37 degrees C in neutral Ca2+-deficient medium; this effect is potentiated by Mg2+. With 2.5 mM Mg2+ and no added Ca2+, restriction was relaxed at elevated pH at which maximum cell yields occurred in the presence of 20 mM exogenous ATP. This ATP-dependent growth was inhibited by Ca2+ or 20 mM Mg2+; the nucleotide was neither transported into the organism nor hydrolyzed extracellularly. With strain EV76, ATP could be replaced by GTP but not other nucleotides, nucleosides, free bases, or pyrophosphate. CTP and UTP also promoted growth of strain KIM, in which limited division also occurred with nucleoside di- and monophosphates. Intracellular V antigen was detected 1 h after temperature shift in Ca2+-deficient medium containing 20 mM Mg2+, a time corresponding to the earliest known events associated with restriction (shutoff of stable RNA synthesis and reduction of adenylate energy charge). Maximum yield of V was obtained 2 h later when cell division ceased; the titer of the antigen remained constant thereafter. The specific activity of V in cells grown with ATP was significantly reduced, especially at elevated pH. These results would be expected if exogenous nucleotides promote growth by sequestering sufficient Mg2+ to prevent restriction of cell division mediated by V antigen.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BACON G. A., BURROWS T. W. The basis of virulence in Pasteurella pestis: an antigen determining virulence. Br J Exp Pathol. 1956 Oct;37(5):481–493. [PMC free article] [PubMed] [Google Scholar]
- BRUBAKER R. R., SURGALLA M. J. THE EFFECT OF CA++ AND MG++ ON LYSIS, GROWTH, AND PRODUCTION OF VIRULENCE ANTIGENS BY PASTEURELLA PESTIS. J Infect Dis. 1964 Feb;114:13–25. doi: 10.1093/infdis/114.1.13. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., BACON G. A. V and W antigens in strains of Pasteurella pseudotuberculosis. Br J Exp Pathol. 1960 Feb;41:38–44. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The pigmentation of Pasteurella pestis on a defined medium containing haemin. Br J Exp Pathol. 1956 Dec;37(6):570–576. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- BURROWS T. W. VIRULENCE OF PASTEURELLA PESTIS AND IMMUNITY TO PLAGUE. Ergeb Mikrobiol Immunitatsforsch Exp Ther. 1963;37:59–113. doi: 10.1007/978-3-662-36742-1_2. [DOI] [PubMed] [Google Scholar]
- CAVANAUGH D. C., RANDALL R. The role of multiplication of Pasteurella pestis in mononuclear phagocytes in the pathogenesis of flea-borne plague. J Immunol. 1959 Oct;83:348–363. [PubMed] [Google Scholar]
- Carter P. B., Zahorchak R. J., Brubaker R. R. Plague virulence antigens from Yersinia enterocolitica. Infect Immun. 1980 May;28(2):638–640. doi: 10.1128/iai.28.2.638-640.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Brubaker R. R. RNA synthesis in Yersinia pestis during growth restriction in calcium-deficient medium. J Bacteriol. 1982 Mar;149(3):1089–1095. doi: 10.1128/jb.149.3.1089-1095.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanica-Gaignier M., Clement-Metral J., Kamen M. D. Adenine nucleotide levels and photopigment synthesis in a growing photosynthetic bacterium. Biochim Biophys Acta. 1971 Jan 12;226(1):135–143. doi: 10.1016/0005-2728(71)90185-x. [DOI] [PubMed] [Google Scholar]
- Gupta R. K., Yushok W. D. Noninvasive 31P NMR probes of free Mg2+, MgATP, and MgADP in intact Ehrlich ascites tumor cells. Proc Natl Acad Sci U S A. 1980 May;77(5):2487–2491. doi: 10.1073/pnas.77.5.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGUCHI K., KUPFERBERG L. L., SMITH J. L. Studies on the nutrition and physiology of Pasteurella pestis. III. Effects of calcium ions on the growth of virulent and avirulent strains of Pasteurella pestis. J Bacteriol. 1959 Mar;77(3):317–321. doi: 10.1128/jb.77.3.317-321.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. L., Tsai M. D. Does the magnesium(II) ion interact with the alpha-phosphate of adenosine triphosphate? An investigation by oxygen-17 nuclear magnetic resonance. Biochemistry. 1982 Mar 2;21(5):951–959. doi: 10.1021/bi00534a021. [DOI] [PubMed] [Google Scholar]
- Kight-Olliff L. C., Fitzgerald J. W. Inhibition of enzyme induction in Pseudomonas aeruginosa by exogenous nucleotides. Can J Microbiol. 1978 Jul;24(7):811–817. doi: 10.1139/m78-136. [DOI] [PubMed] [Google Scholar]
- LAWTON W. D., ERDMAN R. L., SURGALLA M. J. BIOSYNTHESIS AND PURIFICATION OF V AND W ANTIGEN IN PASTEURELLA PESTIS. J Immunol. 1963 Aug;91:179–184. doi: 10.21236/ad0299868. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morgan E. H. Iron exchange between transferrin molecules mediated by phosphate compounds and other cell metabolites. Biochim Biophys Acta. 1977 Aug 25;499(1):169–177. doi: 10.1016/0304-4165(77)90239-2. [DOI] [PubMed] [Google Scholar]
- OGG J. E., FRIEDMAN S. B., ANDREWS A. W., SURGALLA M. J. Factors influencing the loss of virulence in Pasteurella pestis. J Bacteriol. 1958 Aug;76(2):185–191. doi: 10.1128/jb.76.2.185-191.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portnoy D. A., Moseley S. L., Falkow S. Characterization of plasmids and plasmid-associated determinants of Yersinia enterocolitica pathogenesis. Infect Immun. 1981 Feb;31(2):775–782. doi: 10.1128/iai.31.2.775-782.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson M., Harkness T. K. Intracellular Pasteurella pseudotuberculosis: Multiplication in Cultured Spleen and Kidney Cells. Infect Immun. 1970 Nov;2(5):631–639. doi: 10.1128/iai.2.5.631-639.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose I. A. The state of magnesium in cells as estimated from the adenylate kinase equilibrium. Proc Natl Acad Sci U S A. 1968 Nov;61(3):1079–1086. doi: 10.1073/pnas.61.3.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Cytoplasmic and membrane proteins of yersiniae cultivated under conditions simulating mammalian intracellular environment. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1224–1228. doi: 10.1073/pnas.78.2.1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straley S. C., Brubaker R. R. Localization in Yersinia pestis of peptides associated with virulence. Infect Immun. 1982 Apr;36(1):129–135. doi: 10.1128/iai.36.1.129-135.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Une T. Studies on the pathogenicity of Yersinia enterocolitica. III. Comparative studies between Y. enterocolitica and Y. pseudotuberculosis. Microbiol Immunol. 1977;21(9):505–516. doi: 10.1111/j.1348-0421.1977.tb00316.x. [DOI] [PubMed] [Google Scholar]
- Weiss E. Growth and physiology of rickettsiae. Bacteriol Rev. 1973 Sep;37(3):259–283. doi: 10.1128/br.37.3.259-283.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler H. H. Rickettsial permeability. An ADP-ATP transport system. J Biol Chem. 1976 Jan 25;251(2):389–396. [PubMed] [Google Scholar]
- Yang G. C., Brubaker R. R. Effect of ca on the synthesis of deoxyribonucleic Acid in virulent and avirulent yersinia. Infect Immun. 1971 Jan;3(1):59–65. doi: 10.1128/iai.3.1.59-65.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahorchak R. J., Charnetzky W. T., Little R. V., Brubaker R. R. Consequences of Ca2+ deficiency on macromolecular synthesis and adenylate energy charge in Yersinia pestis. J Bacteriol. 1979 Sep;139(3):792–799. doi: 10.1128/jb.139.3.792-799.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilinsky J. W., Sojka G. A., Gest H. Energy charge regulation in photosynthetic bacteria. Biochem Biophys Res Commun. 1971 Mar 5;42(5):955–961. doi: 10.1016/0006-291x(71)90523-7. [DOI] [PubMed] [Google Scholar]


