Abstract
Objective
To determine whether endogenous sex hormones (estradiol (E2), testosterone (T), sex hormone binding globulin (SHBG), and follicle-stimulating hormone (FSH)) are longitudinally associated with progression of atherosclerosis among women at midlife.
Methods
249 Pre- or early peri-menopausal women (42–57 years) from the Study of Women’s Health Across the Nation (SWAN) were followed for up to 9 years (median=3.7 years) and had up to 5 repeated measures of common carotid intima-media thickness (IMT) and adventitial diameter (AD). Linear mixed models were used for statistical analysis. Final models included age at baseline, time since baseline, cycle day of blood draw, race, income, SBP, BMI, insulin resistance index, lipids, C-reactive protein and co-morbidity.
Results
In final models for IMT, each one log unit decrease in SHBG was associated with a 0.005 mm/year increase in IMT progression (P=0.003). E2, T, and FSH were not associated with level or progression of IMT. For AD, each one log unit decrease in E2 was associated with a 0.012 mm/year increase in AD progression (P=0.04) and each one log unit increase in FSH was associated with a 0.016 mm/year increase in AD progression (P=0.003). T and SHBG were not associated with progression or level of AD.
Conclusions
Independent of SBP, BMI, lipids and other covariates, lower E2 and SHBG, and higher FSH were associated with increased subclinical atherosclerosis progression in women at midlife.
Keywords: subclinical atherosclerosis, sex hormones, women
Introduction
After menopause women have greater morbidity and mortality from cardiovascular disease (CVD) than before menopause [1]. The menopausal transition [2–4], mainly the peri-menopausal stage [2,3], is associated with remarkable changes in lipid profiles [2–4] and subclinical measures of CVD [2,3]. Lipids have been reported to be exponentially higher around the final menstrual period than either before or after it [4]. The remarkable changes in the endogenous sex hormones (ESHs) milieu during the menopausal transition have been assumed to be responsible for these observations [5]. However, the role of ESHs in the development of CVD in postmenopausal women has not been conclusively established.
Epidemiological studies suggest that the replacement of ovarian hormones decreases the occurrence of CVD in postmenopausal women [6]. On the other hand, clinical trials have failed to document this protective effect among postmenopausal women treated with hormone therapy (HT) [7,8]. This unexpected result may be explained by the older age of the participants in these trials since they may already have considerable atherosclerosis [9].
Further, the association between ESHs and subclinical measures of atherosclerosis has not been well-established. Studies assessing this association have been mainly cross-sectional and/or have evaluated postmenopausal women [2,10–14] who were treated with HT [10]. To the best of our knowledge, no previous study has assessed the longitudinal associations between levels of ESHs and progression of subclinical atherosclerosis as measured by carotid artery intima-media thickness (IMT) and adventitial diameter (AD) in women at midlife who are not using HT. IMT is a well-established index for atherosclerosis that has been associated with an unfavorable cardiovascular risk profile and cardiovascular outcomes [15]. AD has been shown to be an informative measure of vascular remodeling among women. Larger AD is correlated with adverse cardiovascular risk factors [16,17], and is also associated with a higher risk of CHD [18–20]. A dilated artery cannot adapt as well to adverse conditions, which may make it more vulnerable to damage.
We have previously shown that both IMT and AD increase substantially starting at the late peri-menopause [3], a time when levels of ESHs are significantly altered. To better understand the role of ESHs in these changes in subclinical measures during the menopausal transition, we sought to determine whether the ESHs are longitudinally associated with progression of common carotid IMT and AD among women at midlife.
Methods
Study participants
SWAN is an ongoing, longitudinal, multi-ethnic study of the biological, physical, psychological, and social changes during the menopausal transition. The study design has been previously reported [21]. In brief, between 1996 and 1997, 3,302 participants aged 42–52 years were recruited from seven designated sites (Boston, MA; Detroit, MI; Oakland, CA; Los Angeles, CA; Pittsburgh, PA; Chicago, IL; and Newark, NJ). The eligibility criteria for the SWAN study were 1) An intact uterus and at least 1 ovary, 2) not pregnant or breastfeeding, 3) at least 1 menstrual period within the past 3 months, 4) no HT use within the past 3 months.
Participants of the current study were part of an ancillary study to SWAN at the Pittsburgh site. Enrollment began between the baseline and 3rd annual visit of the SWAN study (N=257). Participants were deemed eligible if they were not pregnant. Each participant had up to 5 carotid scans (815 total scans) over a 9-year follow-up period. For this analysis, data were censored when participants reported having a stroke and/or angina (n=3 observations), a hysterectomy (n=27 observations), or using HT (n=179 observations). The final analysis included 249 participants with a total of 606 scans (mean time in years between scans± SD=2.01±0.63).
The research protocols were approved by the institutional review board at each site and all participants provided written informed consent before enrollment.
Study measures
Ultrasound measures
A B-mode ultrasound (Toshiba American Medical Systems, Tustin, CA) SSA-270A scanner was used to measure IMT and AD of the right and left carotid arteries. For IMT, images were obtained from 8 locations – 4 each from the left and right carotid arteries: the near and far walls of the distal common carotid artery (1 cm proximal to the carotid bulb), the far walls of the carotid bulb, and the internal carotid artery (from the flow divider to 1cm distal to this point). IMT measures were obtained by electronically tracing the lumen-intima interface and the media-adventitia interface across a 1-cm segment; one measurement was generated for each pixel over the area, for a total of approximately 140 measures for each segment. For analyses, the average value of the average readings at all 8 locations was used. For AD, the distance from the adventitial-medial interface on the near wall to the medial-adventitial interface on the far wall at end-diastole was measured for both right and left common carotid artery. For analyses, the average value of the readings at both sides was used. All readings were conducted at the University of Pittsburgh Ultrasound Research Laboratory. Readers were recertified annually against the same set of scans to guard against reader drift. Replicate readings were performed on 20 scans, with an intra-class correlation of 0.98 for IMT values and 0.99 for AD.
Blood assays
A fasting blood sample was drawn during the early follicular-phase of the menstrual cycle (day 2–5) if women were still menstruating. If a timed sample could not be obtained, a random fasting sample was taken within the 90-day period of recruitment.
ESHs were measured at the University of Michigan Endocrine Laboratory using the Automated Chemilumisence System −180 automated analyzer (Bayer Diagnostics Corp., Norwood, MA). Estradiol (E2) was measured using a modified, off-line Automated Chemilumisence System: 180 (E2-6). The lower limit of detection (LLD) was between 1–7 pg/mL. The inter- and intra-assay coefficients of variation averaged were 10.6% and 6.4%, respectively. Follicle-stimulating hormone (FSH) was measured by a modification of a manual assay kit (Bayer Diagnostics) utilizing two monoclonal antibodies directed to different regions on the beta subunit. The LLD was between 0.4–1.0 mIU/mL. The inter-and intra-assay coefficients of variation were 11.4% and 3.8%, respectively. Serum testosterone (T) concentration was evaluated with the Automated Chemilumisence System: 180 total T assay modified to increase precision in the low ranges. The LLD was between 2–2.2 ng/dL. The inter- and intra-assay coefficients of variation were 10.5% and 8.5%, respectively. Sex hormone binding globulin (SHBG) was measured with a two-site chemiluminescent immunoassay. The LLD was between1.9–3.2 nM. The inter- and intra-assay coefficients of variation were 9.9% and 6.1%, respectively. Only E2 assays were conducted in duplicate. The arithmetic mean for the duplicate measures was calculated and reported (coefficients of variation of 3% to 12%). Hormone values below the LLD were replaced with a random value between zero and the LLD. Cycle day of blood draw was reported as days 2 to 5 (for regularly menstruating women) or outside of that period (for irregularly and non-menstruating women).
Lipids, glucose, insulin and high sensitivity C-reactive protein (hs-CRP) were measured at the Medical Research Laboratories (Lexington, KY). Total cholesterol and triglyceride levels were analyzed using enzymatic methods on a Hitachi 747 analyzer (Boehringer Mannheim Diagnostics, Indianapolis, IN), and high-density lipoprotein cholesterol (HDL-C) was isolated using heparin-manganese. Low-density lipoprotein cholesterol (LDL-C) was calculated using the Friedewald equation [22]. Serum insulin was measured by a radioimmunoassay (DPC Coat-a-count) and glucose was measured with a hexokinase-coupled reaction (Boehringer Mannheim Diagnostics). The HOMA-index was calculated from fasting insulin and glucose as (insulin (mU/Liter)* glucose (mmoles/Liter))/22.5 [23]. hs-CRP was quantified using an ultra-sensitive rate immunonephelemetric method (hs-CRP on BN 100, Dade-Behring, Marburg, Germany).
Study covariates
Weight and height were measured annually. Body mass index (BMI) was calculated as weight/height2. Blood pressure was averaged after 2 sequential measures in the right arm with the participant seated after at least 5 minutes of rest.
Race/ethnicity and educational level were derived from SWAN screening interviews. Age and smoking status were derived from questionnaires and interviews at each annual visit. Hypertension and CVD medication use (heart medication, an anticoagulant, or antihypertensive in the past year) were combined into a single co-morbidity variable.
Menopausal status was based on bleeding data as follows: 1) premenopausal: menses in the last 3 months with no change in regularity in the last 12 months; 2) early peri-menopausal: menses in the last 3 months with some change in regularity during the prior 12 months; 3) late peri-menopausal: no menses within the last 3 months, but some menstrual bleeding over the prior 12 months; 4) postmenopausal: no menses within the last12 months.
Statistical Analysis
The distribution of AD was normal, whereas that of IMT was slightly skewed. Consequently, IMT was studied with and without a log-transformation. Because results were comparable, results from models using the original values of IMT were presented for simplicity of interpretation. ESHs, triglycerides, glucose, insulin, HOMA-index, and hs-CRP were highly skewed and were log-transformed for analyses.
Associations between progression rates of AD and IMT and ESHs were evaluated using linear mixed-effect model with random intercept. Repeated measures of AD and IMT were modeled as a function of: time since baseline in years, repeated measures of ESHs (separate model for each hormone with each outcome), and their interaction with time since baseline. The progression rates of AD and IMT associated with one log unit change in ESHs were estimated from the regression coefficient associated with the interaction terms of ESHs X time since baseline [24]. Since none of the study covariates were found to be associated with annual changes of each of IMT and AD over time in longitudinal analyses, univariate analyses (Supplemental Table 1) were performed between each of IMT, AD and study covariates at baseline to determine potential covariates to be adjusted for in the multivariable analyses. All covariates that were found to be associated with subclinical measures at p-value ≤0.1 were considered. For variables that were highly correlated (e.g. glucose, insulin, HOMA) models were tested with each of these variables separately and the model which showed a better fit was selected. Hormones were first modeled unadjusted for any factor. Model 1 was adjusted for age at baseline, race, cycle day of blood draw, and co-morbidity variable. Model 2 was adjusted for model 1 covariates plus BMI, SBP, and HOMA-index. The final model was adjusted for model 1 and 2 covariates plus HDL-C and triglycerides (for AD outcome, based on univariate analyses) or LDL-C, triglycerides, and hs-CRP (for IMT outcome, based on univariate analyses). All included covariates were time-dependent except for age and race. Covariates were included one at a time in models to determine how each covariate affected the tested association. Educational level (time-independent), smoking status (time-dependent), and lipid lowering medications (time-dependent) (only 14 participants were reported using cholesterol lowering medication at certain time point of the study follow-up) were not associated with AD or IMT and including them in the multivariable model did not change the results. Therefore these variables were not included in final models. Akaike’s information criterion was used for model selections (smaller criterion indicates better fit).
To clearly present the significant interactions of ESHs with time since baseline (intended that changes in subclinical measures over time vary by level of ESHs), predicted means of IMT and AD were estimated from multivariable mixed-effect models at selected time points (baseline, 50th, 75th, and 95th percentile of time since baseline) for 5 percentiles (5th, 25th,50th, 75th, and 95th) of ESHs. Predicted means of subclinical measures were plotted against the selected time points by ESHs percentiles [25]. These analyses revealed thresholds for both E2 and FSH in relation to increasing width of AD. To better understand the combined effect of E2 and FSH on AD, we dichotomized both E2 and FSH at the threshold levels (defined as < 25th percentile for E2=23.45 pg/ml (low E2) and >75th percentile for FSH=54.80 mIU/ml (high FSH)) and modeled these two dichotomous variables with their interaction with time in a three-way interaction (Time* E2 dichotomous*FSH dichotomous) to test progression rate of AD by E2 and FSH levels above and below the defined thresholds.
Residual analyses were conducted to verify model assumptions. Analyses were performed with SAS v9.2 (SAS Institute, Cary, NC). All tests were 2-sided at alpha=0.05.
Results
Table 1 presents participants characteristics and summary statistics for study measures.
Table 1.
Baseline characteristics of the study population
| Variable | Total N=249 |
|---|---|
| Mean ± SD/Median (Q1, Q3) Or N (%) | |
| Age, Y | 46.2±2.8 |
| BMI, Kg/m2 | 28.2±6.3 |
| BP, mm Hg | 113.3±15.9 |
| HDL-C, mg/dL | 55.3±12.3 |
| LDL-C, mg/dL | 122.4±31.2 |
| Triglycerides, mg/dL | 89.0(68.0,124.0) |
| Glucose, mg/dL | 89.0(84.0,95.0) |
| Insulin, uIu/Ml | 7.8(5.9,11.3) |
| HOMA-index | 1.7 (1.3,2.6) |
| hs-CRP, mg/DL | 1.4(0.8,4.1) |
|
| |
| Race | |
| White | 176 (70.7%) |
| Black | 73 (29.3%) |
| Menopausal Status | |
| Premenopausal | 122 (49.0%) |
| Early Peri-menopausal | 115 (46.2%) |
| Late Peri-menopausal | 4 (1.6%) |
| Postmenopausal | 8 (3.2%) |
| Co-morbidity * | |
| Yes | 42 (17.1%) |
| No | 204 (82.9%) |
| Current Smoker | |
| Yes | 37 (15.0%) |
| No | 209 (85.0%) |
|
| |
| AD, mm | 6.7±0.57 |
| IMT, mm | 0.68±0.07 |
|
| |
| E2, pg/ml | 56.7(33.8, 92.7) |
| FSH, mIU/ml | 16.1(11.3,27.8) |
| SHBG, nM | 39.5(27.7,56.3) |
| T, ng/dL | 37.1(26.6,51.1) |
AD: adventitial diameter; IMT: intima-media thickness; E2: estradiol; FSH: follicle stimulating hormone; SHBG: sex hormone binding globulin; T: testosterone; BMI: body mass index; BP: systolic blood pressure; HDL-C: high density lipoprotein cholesterol; LDL-C: low density lipoprotein cholesterol; hs-CRP: high sensitivity C-reactive protein.
Composite variable for high blood pressure/antihypertensive, and medication for heart, and anticoagulant.
ESHs and IMT progression
In final model, each one log unit decrease in SHBG was associated with a 0.005 mm/year increase in IMT progression (P=0.002), Table 2. Interestingly, a similar significant effect of SHBG on IMT progression was found even after adjusting for T levels, HDL-C or baseline AD in separate models (Data not shown). E2, T, and FSH were not associated with level or progression of IMT.
Table 2.
Mixed models for longitudinal associations between ESHs and progression rate of IMT
| Hormones * | Unadjusted | Model 1 ‡ | Model 2 § | Model 3 || | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| IMT progression rate/Year (SE) † | P | IMT progression rate/Year (SE) † | P | IMT progression rate/Year (SE) † | P | IMT progression rate/Year (SE) † | P | |
|
| ||||||||
| E2, pg/ml | 0.0004 (0.001) | 0.7 | 0.0002 (0.001) | 0.9 | −0.0003(0.001) | 0.8 | −0.0005 (0.001) | 0.7 |
| FSH, mIU/mL | 0.001 (0.001) | 0.2 | 0.001 (0.001) | 0.2 | 0.001 (0.001) | 0.2 | 0.001 (0.001) | 0.2 |
| SHBG, nM | −0.004 (0.001) | 0.02 | −0.004 (0.002) | 0.02 | −0.004 (0.002) | 0.007 | −0.005 (0.001) | 0.002 |
| T, ng/dl | 0.001 (0.002) | 0.5 | 0.001 (0.002) | 0.5 | 0.001 (0.002) | 0.6 | 0.002 (0.003) | 0.4 |
ESHs: endogenous sex hormones; IMT: intima-media thickness; E2: estradiol; FSH: follicle stimulating hormone; SHBG: sex hormone binding globulin; T: testosterone
Separate models. Hormones were Log transformed
Intima-media thickness progression rate (mm/year) per unit change in log transformed sex hormones
Adjusted for age at baseline, race, cycle day of blood draw, and composite variable for blood pressure/antihypertensive, heart and anticoagulant medication
Adjusted for covariates in model 1 plus BMI, SBP, and HOMA-index
Adjusted for covariates in model 2 plus LDL-C, triglycerides, and hs-CRP
Predicted means of IMT overtime by percentiles of SHBG is shown in supplemental Figure 1. At any follow-up time point lower levels of SHBG were associated with greater IMT thickening.
ESHs and AD progression
In the fully-adjusted model, each one log unit decrease in E2 was associated with a 0.012 mm/year increase in AD progression (P=0.04), whereas each one log unit increase in FSH was associated with a 0.016 mm/year increase in AD progression (P=0.003), Table 3. Interestingly, the associations between higher FSH and greater AD progression remained significant even after adjusting for E2 (Data not shown). T and SHBG were not associated with progression or level of AD in the final models.
Table 3.
Mixed models for longitudinal associations between ESHs and progression rate of AD
| Hormones * | Unadjusted | Model 1 ‡ | Model 2 § | Model 3 || | ||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| AD progression rate/Year (SE) † | P | AD progression rate/Year (SE) † | P | AD progression rate/Year (SE) † | P | AD progression rate/Year (SE) † | P | |
|
| ||||||||
| E2, pg/ml | −0.009 (0.005) | 0.07 | −0.008 (0.005) | 0.09 | −0.011(0.006) | 0.05 | −0.012 (0.006) | 0.04 |
| FSH, mIU/mL | 0.012 (0.005) | 0.01 | 0.012 (0.005) | 0.02 | 0.016 (0.005) | 0.004 | 0.016 (0.005) | 0.003 |
| SHBG, nM | −0.007 (0.004) | 0.1 | −0.005 (0.004) | 0. 2 | −0.002 (0.005) | 0.8 | −0.002 (0.005) | 0.8 |
| T, ng/dl | −0.005 (0.008) | 0.6 | −0.005 (0.008) | 0.5 | −0.004 (0.010) | 0.7 | −0.004 (0.010) | 0.7 |
ESHs: endogenous sex hormones; AD: adventitial diameter; E2: estradiol; FSH: follicle stimulating hormone; SHBG: sex hormone binding globulin; T: testosterone
Separate models. Hormones were Log transformed
Adventitial diameter progression rate (mm/year) per unit change in log transformed sex hormones
Adjusted for age at baseline, race, cycle day of blood draw, and composite variable for blood pressure/antihypertensive, heart and anticoagulant medication
Adjusted for covariates in model 1 plus BMI, SBP, and HOMA-index
Adjusted for covariates in model 2 plus HDL-C, and triglycerides
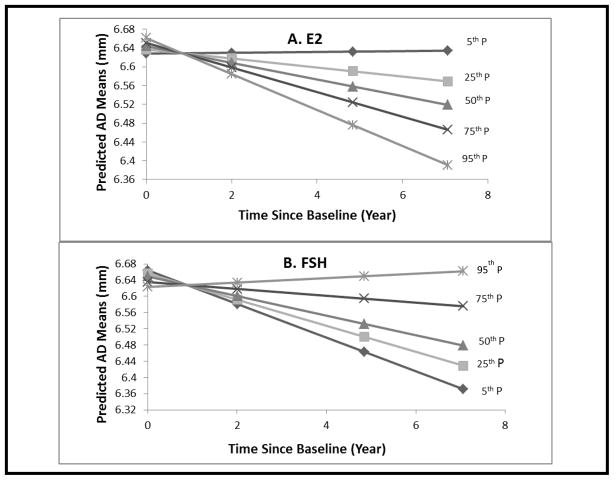
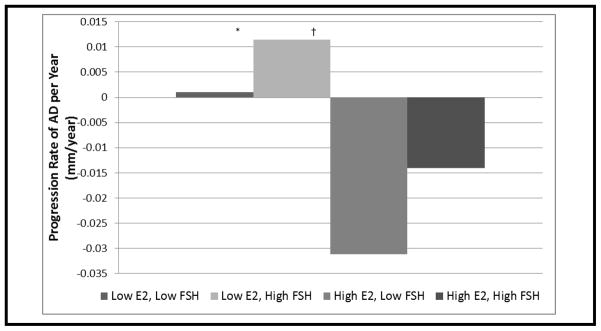
The predicted means of AD overtime by E2 percentiles is shown in Figure 1-A, and of FSH percentiles in Figure 1-B. At an E2 level <23.45 pg/ml (25th percentile) (low level), AD increases overtime, whereas for E2 levels higher than this threshold (high level), AD substantially decreases over time. In a similar fashion, at an FSH level > 54.80 mlU/ml (75th percentile) (high level), AD increases over time, whereas at an FSH ≤of 54.8 mIU/ml (low level), AD substantially decreases over time. AD increased only when E2 was low, regardless of FSH level. Similarly, AD decreased only when E2 was high, regardless of FSH level. However, maximum rates of AD increase occurred in the Low E2/High FSH group and maximum rates of AD decrease occurred in the High E2/Low FSH Group (Figure 2). The threshold for E2 of 23.45 pg/ml and that for FSH of 54.80 mIU/ml are very close to the median E2 and FSH levels among late peri-menopausal women in the current study.
Figure 1. Predicted AD Means at Selected Time Points* by E2 and FSH Percentiles.
* Baseline, 50th, 75th, 95th percentiles of time since baseline. Adjusted for age at baseline, time since baseline, BMI, SBP, HOMA-index, HDL-C, and triglycerides
Figure 2. Progression Rates of AD by E2 and FSH Levels.
Low E2 < 23.45 pg/ml, High E2 ≥ 23.45 pg/ml; Low FSH ≤ 54.80 mIU/ml, High FSH > 54.80 mIU/ml
* Low E2, Low FSH significantly > High E2, Low FSH, P=0.04
† Low E2, High FSH significantly > High E2, Low FSH, P=0.003
Discussion
In this longitudinal study, we reported significant effects of ESHs on progression of early measures of atherosclerosis in women at midlife. Adjusting for age at baseline and traditional CVD risk factors, lower levels of SHBG were associated with an increase in the thickening of the carotid artery intima-media layer independent of baseline AD. In addition, lower E2 levels and higher FSH levels, two critical hormonal changes accompanying the menopausal transition, were associated with an increase in AD of the carotid artery, an informative measure of vascular vulnerability.
Effects of E2 and FSH on progression of Atherosclerosis
Our finding of a significant association between E2 levels and AD supports a previous cross-sectional analysis from the SWAN Heart ancillary study [2]. We extend those findings by providing longitudinal data showing a significant impact of lower E2 on the AD progression rate. We previously reported that the IMT and AD progression rates in the late peri-menopausal stage were substantially greater than in the premenopausal stage; irrespective of age at baseline and ethnicity [3]. The current results confirm our previous findings by documenting vascular changes in only AD at hormonal levels similar to those during the late peri-menopausal stage. Threshold levels of E2 (<23.45 pg/ml) and FSH (> 54.80 mlU/ml) have been found to be associated with the increase in AD in the current study. The absence of an estrogen effect on IMT in the current analysis may be because changes in IMT could be driven by changes in AD. We have previously shown that adjusting for baseline IMT did not impact the independent effect of menopausal status on AD while adjusting for baseline AD attenuated the independent effect of menopausal status on IMT [3]. Arterial dilation may occur first in response to lower E2 levels (marker of the menopausal transition) which is then followed by wall thickening as a mechanism to normalize tensile stress that would be increased when the artery dilates [26]. Our results highlight late peri-menopause as a time when early intervention strategies targeting CVD might have the greatest benefit. Previous studies have reported a reduced risk of clinical coronary events and coronary artery calcification among women treated with equine estrogens at midlife age (50–59 years) [27,28]. In addition, cross-sectional analysis from the Women on the Move through Activity and Nutrition (WOMAN) cohort has shown that early postmenopausal women on HT had significantly smaller AD compared to women not on HT, which suggests an acute vascular benefit of HT in women at midlife [29].
Estrogen has been hypothesized to have a cardioprotective role. Both direct and indirect effects on the vasculature via genomic (slow) and nongenomic (rapid) pathways have been suggested. The effects of estrogen are mediated through specific receptors found in the reproductive tract, liver, bone, brain, and cardiovascular tissue. Direct effects of estrogen can occur rapidly, such as the activation of nitric oxide synthase, which can lead to vascular smooth muscle relaxation. Long term direct effects include increased expression of genes for vasodilatory enzymes and the acceleration of endothelial cell growth that lead to increased vascular endothelial growth factors. On the other hand, the main indirect effects of E2 on the cardiovascular system are mediated via effects on serum lipids, coagulation, fibrinolytic, and antioxidant factors [30]. The reported effects of E2 on AD progression rate in the current study can be explained by estrogen’s effect on the sympathetic nervous system (a loss of vascular tone) or the degeneration of connective tissue within the arterial wall. Connective tissue has been found to decrease with menopause and increase with HT [31].
We reported a significant positive association between higher FSH and greater enlargement in AD that was independent of E2 level. This finding suggests an independent effect of FSH on the vasculature. Although we are not aware of any study that assessed the presence of FSH receptors in the vascular system, FSH receptors have been reported to be selectively expressed on the surface of the blood vessels of a wide range of tumors [32]. The findings that AD increased only when E2 was low and FSH was high or when E2 was low and FSH was low suggest a stronger effect of reduction in E2 than of increase in FSH on AD progression.
Effects of SHBG on progression of Atherosclerosis
In contrast to the significant associations observed between E2 and FSH with progression of AD, SHBG was not associated with AD progression but was significantly associated with IMT progression. The reason for this discrepancy remains unclear. In contrast to the vasodilatory and sympathetic nervous system effects noted above for E2, our results suggest that SHBG may act on the intimal and medial layers, and may also be related to obesity and insulin resistance. The relationship between SHBG and IMT progression remains significant even after further adjustment for baseline AD, which suggests a direct effect of SHBG on intima and media layers irrespective of AD status. Increasing evidence suggests that SHBG may have biological functions beyond its well-known function as a transport glycoprotein [33]. Lower levels of SHBG were associated with higher risk of diabetes in postmenopausal women and in men [34]. A causal effect for SHBG in incident diabetes in postmenopausal women was suggested by a recent mendelian randomization analysis [35]. Finally, in vitro studies found SHBG receptors in certain human tissues. The binding of these receptors with an SHBG androgen/estrogen complex was associated with greater intracellular cyclic adenosine monophosphate [34], suggesting a cellular level mechanism of action for SHBG.
On the other hand, the P value for the association between SHBG and AD progression changed from 0.1 to 0.8 once BMI and insulin resistance were added to the model, although the same association with progression of IMT remained significant independent of these risk factors. SHBG has been shown to correlate strongly with insulin sensitivity, and lower SHBG levels are predictive of insulin resistance [36]. Thus, the relationship of SHBG to atherosclerosis progression may be also related to its role as a predictor of metabolic status than a sex hormone carrier protein. Arterial wall area includes both diameter and wall thickness components. Each of these two measures plays a different role in the arterial remodeling process that precedes atherosclerosis. In vulnerable arteries; diameter enlargement, as measured herein by adventitial diameter, could be an important indicator of arterial wall damage. Given the distinct roles of each of IMT and AD on atherosclerosis development, it is expected that their associations with sex hormones may not be the same for intima media thickness and for arterial wall diameter.
Our finding of a significant negative association between SHBG and IMT is in line with the Atherosclerosis Risk in Communities Study and the Coronary Artery Risk Development in Young Adults Study [37, 13] which reported similar association. We were able to extend this association to the longitudinal setting independent of risk factors including HDL-C. We reported that IMT would increase by 0.005mm/year for each 1 log unit (~2.71 nm) decrease in SHBG. Therefore, a 6 log units decrease in SHBG (~16.32 nm) would result in 0.03 mm/year increase in IMT. According to Hodis et al, similar increase in IMT per year was found to be associated with a relative risk of 2.2 (95% CI, 1.4–3.6) for nonfatal myocardial infarction and a relative risk of 3.1 (95% CI, 2.1–4.5) for any coronary event [38].
The main limitations of this study were: 1) Blood specimens for hormone assays were collected during the early follicular phase of the menstrual cycle, which might not be ideal for all hormones that were measured. Nevertheless, all analyses were adjusted for cycle day of blood draw. 2) Biologically active T was not measured directly. The main strength of this study is that it is the first longitudinal analysis to examine the relationships between ESHs and progression of two important early measures of atherosclerosis in a sample of midlife women who were free of CVD and not on HT.
In conclusion, this longitudinal study of changes in endogenous sex hormones during the menopausal transition and the impact on subclinical measures of CVD has shown that endogenous sex hormones clearly affect both wall thickening and measures of arterial geometry that are related to vascular health.
Supplementary Material
Highlights.
Adjusting for age at baseline and traditional CVD risk factors, lower levels of SHBG were associated with an increase in the thickening of the carotid artery intima-media layer in women transitioning through menopause.
Lower E2 levels and higher FSH levels, two critical hormonal changes accompanying the menopausal transition, were associated with an increase in the adventitial diameter of the carotid artery, an informative measure of vascular vulnerability.
These data may help to explain one mechanism through which hormone replacement therapy may be beneficial in the short term for younger women.
Acknowledgments
The authors acknowledge Nanette Santoro, MD, University of Colorado School of Medicine Anschutz Medical Campus; for scientific interpretation edits, and Janice Sabatine, PhD, Avanti Strategies, Cranberry Township PA, for editing assistance.
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). SWAN Heart is supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor – Siobán Harlow, PI 2011 – present, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA – Joel Finkelstein, PI 1999 – present; Robert Neer, PI 1994 – 1999; Rush University, Rush University Medical Center, Chicago, IL – Howard Kravitz, PI 2009 – present; Lynda Powell, PI 1994 – 2009; University of California, Davis/Kaiser – Ellen Gold, PI; University of California, Los Angeles – Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY – Carol Derby, PI 2011 – present, Rachel Wildman, PI 2010 – 2011; Nanette Santoro, PI 2004 – 2010; University of Medicine and Dentistry – New Jersey Medical School, Newark – Gerson Weiss, PI 1994 – 2004; and the University of Pittsburgh, Pittsburgh, PA – Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD – Winifred Rossi 2012; Sherry Sherman 1994 – 2012; Marcia Ory 1994 – 2001; National Institute of Nursing Research, Bethesda, MD – Program Officers.
Central Laboratory: University of Michigan, Ann Arbor – Daniel McConnell (Central Ligand Assay Satellite Services).
Coordinating Center: University of Pittsburgh, Pittsburgh, PA – Kim Sutton-Tyrrell, Co-PI 2001 – present; Maria Mori Brooks Co-PI 2012; New England Research Institutes, Watertown, MA - Sonja McKinlay, PI 1995 – 2001.
Steering Committee: Susan Johnson, Current Chair, Chris Gallagher, Former Chair
We thank the study staff at each site and all the women who participated in SWAN.
Sources of Funding:
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants NR004061; AG012505, AG012535, AG012531, AG012539, AG012546, AG012553, AG012554, AG012495). SWAN Heart is supported by the National Heart, Lung, and Blood Institute (NHLBI) (Grants HL065581, HL065591). The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Footnotes
Disclosures: The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Samar R. El Khoudary, Email: elkhoudarys@edc.pitt.edu.
Rachel P. Wildman, Email: Rachel.wildman@einstein.yu.edu.
Karen Matthews, Email: matthewska@upmc.edu.
Rebecca C. Thurston, Email: thurstonrc@upmc.edu.
Joyce T. Bromberger, Email: brombergerjt@upmc.edu.
Kim Sutton-Tyrrell, Email: Tyrrell@edc.pitt.edu.
References
- 1.Lerner DJ, Kannel WB. Patterns of coronary heart disease morbidity and mortality in the sexes: a 26-year follow-up of the Framingham population. Am Heart J. 1986;111:383–390. doi: 10.1016/0002-8703(86)90155-9. [DOI] [PubMed] [Google Scholar]
- 2.Wildman RP, Colvin AB, Powell LH, et al. Associations of endogenous sex hormones with the vasculature in menopausal women: the Study of Women’s Health Across the Nation (SWAN) Menopause. 2008;15:414–421. doi: 10.1097/gme.0b013e318154b6f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton-Tyrrell Progression Rates of Carotid Intima-media Thickness and Adventitial Diameter during the Menopausal Transition. Menopause. 2012 doi: 10.1097/gme.0b013e3182611787. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matthews KA, Crawford SL, Chae CU, et al. Are changes in cardiovascular disease risk factors in midlife women due to chronological aging or to the menopausal transtion? J Am Coll Cardiol. 2009;54:2366–2373. doi: 10.1016/j.jacc.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guthrie JR, Dennerstein L, Taffe JR, Lehert P, Burger HG. The menopausal transition: a 9-year prospective population-based study. The Melbourne Women’s Midlife Health Project Climacteric. 2004;7:375–389. doi: 10.1080/13697130400012163. [DOI] [PubMed] [Google Scholar]
- 6.Stampfer MJ, Colditz GA, Willett WC, et al. Postmenopausal oestrogen therapy and cardiovascular disease. Ten year follow-up from The Nurse Health Study. N Engl J Med. 1991;325:756–762. doi: 10.1056/NEJM199109123251102. [DOI] [PubMed] [Google Scholar]
- 7.Hulley S, Grady D, Bush T, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/Progesterone Replacement Study (HERS) Research Group. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 8.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 9.Harman SM, Brinton EA, Clarkson T, et al. Is the WHI relevant to HRT started in the perimenopause? Endocrine. 2004;24:195–202. doi: 10.1385/ENDO:24:3:195. [DOI] [PubMed] [Google Scholar]
- 10.Karim R, Hodis HN, Stanczyk FZ, Lobo RA, Mack WJ. Relationship between serum levels of sex hormones and progression of subclinical atherosclerosis in postmenopausal women. J Clin Endocrinol Metab. 2008;93:131–138. doi: 10.1210/jc.2007-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ouyang P, Vaidya D, Dobs A, et al. Sex hormone levels and subclinical atherosclerosis in postmenopausal women: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2009;204:255–261. doi: 10.1016/j.atherosclerosis.2008.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michos ED, Vaidya D, Gapstur SM, et al. Sex hormones, sex hormone binding globulin, and abdominal aortic calcification in women and men in the multi-ethnic study of atherosclerosis (MESA) Atherosclerosis. 2008;200:432–438. doi: 10.1016/j.atherosclerosis.2007.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderon-Margalit R, Schwartz SM, Wellons MF, et al. Prospective association of serum androgens and sex hormone-binding globulin with subclinical cardiovascular disease in young adult women: the “Coronary Artery Risk Development in Young Adults” women’s study. J Clin Endocrinol Metab. 2010;95:4424–4431. doi: 10.1210/jc.2009-2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El Khoudary SR, Wildman RP, Matthews K, et al. Effect modification of obesity on associations between endogenous steroid sex hormones and arterial calcification in women at midlife. Menopause. 2011;18:906–914. doi: 10.1097/gme.0b013e3182099dd2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M. Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation. 2007;115(4):459–67. doi: 10.1161/CIRCULATIONAHA.106.628875. [DOI] [PubMed] [Google Scholar]
- 16.Jensen-Urstad K, Jensen-Urstad M, Johansson J. Carotid artery diameter correlates with risk factors for cardiovascular disease in a population of 55-year-old subjects. Stroke. 1999;30:1572–1576. doi: 10.1161/01.str.30.8.1572. [DOI] [PubMed] [Google Scholar]
- 17.Crouse JR, Goldbourt U, Evans G, et al. Risk factors and segment-specific carotid arterial enlargement in the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1996;27:69–75. doi: 10.1161/01.str.27.1.69. [DOI] [PubMed] [Google Scholar]
- 18.Bots ML, Grobbee DE, Hofman A, Witteman JC. Common carotid intima-media thickness and risk of acute myocardial infarction: the role of lumen diameter. Stroke. 2005;36(4):762–7. doi: 10.1161/01.STR.0000158924.71069.94. [DOI] [PubMed] [Google Scholar]
- 19.Eigenbrodt ML, Sukhija R, Rose KM, et al. Common carotid artery wall thickness and external diameter as predictors of prevalent and incident cardiac events in a large population study. Cardiovasc Ultrasound. 2007;5:11. doi: 10.1186/1476-7120-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leone N, Ducimetière P, Gariépy J, et al. Distension of the carotid artery and risk of coronary events: the three-city study. Arterioscler Thromb Vasc Biol. 2008;28(7):1392–7. doi: 10.1161/ATVBAHA.108.164582. [DOI] [PubMed] [Google Scholar]
- 21.Sowers M, Crawford S, Sternfed B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000. pp. 175–188. [Google Scholar]
- 22.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without the use of preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 23.Katsuki A, Sumida Y, Gabazza EC, et al. Homeostasis model assessment is a reliable indicator of insulin resistance during follow-up of patients with type 2 diabetes. Diabetes Care. 2001;24:362–365. doi: 10.2337/diacare.24.2.362. [DOI] [PubMed] [Google Scholar]
- 24.Hedeker D, Gibbons RD. Longitudinal data analysis. New Jersey: A John Wiley & Sons. INC; 2006. pp. 69–76. [Google Scholar]
- 25.Longitudinal data analysis with discrete and continuous responses course notes. SAS institute Inc; Cary, NC, USA: 2009. [Google Scholar]
- 26.Muscelli E, Kozàkovà M, Flyvbjerg A, et al. The effect of menopause on carotid artery remodeling, insulin sensitivity, and plasma adiponectin in healthy women. Am J Hypertens. 2009;22:364–370. doi: 10.1038/ajh.2009.16. [DOI] [PubMed] [Google Scholar]
- 27.Hsia J, Langer RD, Manson JE, et al. Conjugated equine estrogens and coronary heart disease: the Women’s Health Initiative. Arch Intern Med. 2006;166:357–65. doi: 10.1001/archinte.166.3.357. [DOI] [PubMed] [Google Scholar]
- 28.Manson JE, Allison MA, Rossouw JE, et al. Estrogen therapy and coronary-artery calcification. N Engl J Med. 2007;356:2591–602. doi: 10.1056/NEJMoa071513. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd K, Barinas-Mitchell E, Kuller L, et al. Hormone Therapy Affects Adventitial Diameter in Early Postmenopausal Women. Circulation. 2008;117:e284. [Google Scholar]
- 30.Mendelsohn ME. Protective effects of estrogen on the cardiovascular system. Am J Cardiol. 2002;89:12E–17E. doi: 10.1016/s0002-9149(02)02405-0. discussion 17E–18E. 36. [DOI] [PubMed] [Google Scholar]
- 31.Jayachandran M, Miller VM. Molecular and cellular mechanisms of estrogen’s action. In: Douglas PS, editor. Cardiovascular Health and Disease in Women. Section III: Hormones and heart disease. Philadelphia: WB Saunders; 2004. pp. 207–30. [Google Scholar]
- 32.Radu A, Pichon C, Camparo P, et al. Expression of follicle-stimulating hormone receptor in tumor blood vessels. N Engl J Med. 2010;363:1621–1630. doi: 10.1056/NEJMoa1001283. [DOI] [PubMed] [Google Scholar]
- 33.Rosner W. Plasma steroid-binding proteins. Endocrinol Metab Clin North Am. 1991;20:697–720. [PubMed] [Google Scholar]
- 34.Ding EL, Song Y, Manson JE, et al. Sex hormone-binding globulin and risk of type 2 diabetes in women and men. N Engl J Med. 2009;361:1152–1163. doi: 10.1056/NEJMoa0804381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalyani RR, Franco M, Dobs AS, et al. The association of endogenous sex hormones, adiposity, and insulin resistance with incident diabetes in postmenopausal women. J Clin Endocrinol Metab. 2009;94:4127–4135. doi: 10.1210/jc.2009-0910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sutton-Tyrrell K, Wildman RP, Matthews KA, et al. Sex hormone binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women’s Health Across the Nation (SWAN) Circulation. 2005;111:1242–1249. doi: 10.1161/01.CIR.0000157697.54255.CE. [DOI] [PubMed] [Google Scholar]
- 37.Golden SH, Maguire A, Ding J, et al. Endogenous postmenopausal hormones and carotid atherosclerosis: a case-control study of the atherosclerosis risk in communities cohort. Am J Epidemiol. 2002;155:437–445. doi: 10.1093/aje/155.5.437. [DOI] [PubMed] [Google Scholar]
- 38.Hodis HN, Mack WJ, LaBree L, et al. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128(4):262–9. doi: 10.7326/0003-4819-128-4-199802150-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.