Abstract
Dihydroartemisinin (DHA) is an important derivative of an herb medicine Artemisia annua L., used in ancient China. DHA is currently used world-wide to treat malaria by killing malaria-causing parasites. In addition to this prominent effect, DHA is suggested to regulate cellular functions, such as angiogenesis, tumor cell growth and immunity. Nonetheless, how DHA affects T cell function remains poorly understood. We found that DHA potently suppressed Th cell differentiation in vitro. Unexpectedly however, DHA greatly promoted Treg cell generation, in a manner dependent on TGF-βR:Smad signal. In addition, DHA treatment effectively reduced EAE onset and ameliorated ongoing EAE in mice. Administration of DHA significantly decreased Th but increased Treg cells in EAE-inflicted mice without apparent global immune suppression. Moreover, DHA modulated mTOR pathway, because mTOR signal was attenuated in T cells upon DHA treatment. Importantly, enhanced Akt activity neutralized DHA-mediated effects on T cells in an mTOR dependent fashion. This study therefore reveals a novel immune regulatory function of DHA to reciprocally regulate Th and Treg cell generation through modulating mTOR pathway. It addresses how DHA regulates immune function and suggests a new type of drug for treating diseases where mTOR activity to be tempered.
Keywords: Dihydroartemisinin, Th-Treg, experimental autoimmune encephalomyelitis, Akt, mTOR
Introduction
Artemisinin is a sesquiterpene lactone isolated from a Chinese plant Artemisia annua (commonly known as Qinghaosu or sweet wormwood) and has been used as a Chinese herbal medicine for the last 2000 years to treat more than 20 different diseases including fever (1). Since the 1970s, Artemisinin and its derivatives have been used extensively as an anti-malaria drug by effectively killing multi-drug resistant strains of malarial parasites (2) through specific and selective inhibition on the SERCA of P. falciparum(3). Dihydro-artemisinin (DHA) is the active metabolite of all artemisinin compounds (artemisinin, artesunate, artemether, etc.) and is also an important derivative available as a drug itself. Apart from its prominent anti-malaria effect, DHA impacts cellular functions including tumor cell growth (4), angiogenesis (5) and immune regulation.
Aberrant function of immune system leads to the development of inflammatory and autoimmune diseases. Immune suppressive agents are therefore needed to treat such diseases. There have been limited amounts of research directed at the identification and characterization of natural ingredients in plants to be used to treat inflammatory disease, although natural plant compounds are potential sources of new classes of therapeutic agents to control inflammation with reduced side effects. One such promising phytochemical is Artemisinin and its derivatives. Increasing evidence suggest that artemisinin and its derivatives possess immune suppressive function. Artemisinin was reported to effectively relieve the symptoms of lupus nephritis in mice (6). In addition, artemisinin-derivatives were shown to suppress delayed-type hypersensitivity response (7) and collagen-induced arthritis (8). These findings suggest that the derivatives of artemisinin are promising agents to modulate immune response and to treat inflammatory disease. Yet, the question of how artemisinin or its derivative, such as DHA, impacts T cell function remains poorly understood. More importantly, the molecular mechanisms underlying immune regulatory function of artemisinin and its derivatives remain to be revealed. In this report, we investigated these questions.
We found that DHA treatment modestly inhibited the proliferation of activated T cells. DHA suppressed helper T (Th) cell function. Particularly, DHA treatment virtually abolished Th17 differentiation. Unexpectedly however, DHA greatly promoted Treg cell generation in a manner dependent on TGF-βR:Smad signal. These findings prompted us to test whether DHA can be used to treat autoimmune disease. We found that DHA treatment effectively reduced EAE onset and ameliorated ongoing EAE in mice. Administration of DHA significantly decreased Th but increased Treg cells in EAE-inflicted mice without apparent global immune suppression. We further investigated the molecular mechanisms underlying DHA mediated effects on T cells and found that DHA attenuated mTOR signal in T cells. Importantly, we found that enhanced Akt activity neutralized DHA-mediated effects on T cells in an mTOR dependent fashion. This study therefore reveals a novel immune regulatory function of DHA to reciprocally regulate Th17 and Treg cell generation through modulating mTOR pathway. It addresses how DHA regulates immune function and suggests a new type of drug for treating diseases where mTOR activity to be tempered.
Material and Methods
Mice and DHA
Wild-type (WT) and CD4cre:Smad2fl/fl mice are on C57BL/6 background and kept under specific pathogen-free conditions in the animal care facility at the University of North Carolina at Chapel Hill. All mouse experiments were approved by Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill. DHA was purchased from Chengdu Okay Plant & Chemical Co., Ltd or TCI America with the purity of ≥98%. For in vitro experiments, DHA was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich). For in vivo injection, DHA was dissolved in the mixtures of DMSO and polysorbate 80 (Alfa Aesar) at 6/4 (v/v) ratio.
Flow-cytometric analysis
Cells were stained per manufactures’ protocols with fluorescence-conjugated antibodies for CD4, CD8, CD44, IFN-γ, IL-4, CD62L, Foxp3 and IL-17A purchased from eBioscience. For cytokines staining, cells were stimulated with 50ng/ml phorobol 12-myristate 13-acetate (PMA) (Sigma-Aldrich) and 1μM of Ionomycin (Sigma-Aldrich) for 3–4 hours in the presence of 2μg/ml Brefeldin A (Sigma-Aldrich) in Bruff’s medium under cell culturing conditions (37°C, 5% CO2). Flow-cytometric analysis of antibody labeled cells was performed on LSRII (Becton Dickinson) or CyAn (Dako Cytomation, Beckman Coulter). The numbers of various cell populations were calculated by multiplying the total cell number with the percentages of each cell population determined by flow-cytometry.
In vitro T cell differentiation assay
CD4+ T cells were purified by positive selection with CD4-conjugated magnetic beads (Miltyeni Biotec). CD4+ T cells were activated by plate bound anti-CD3 and anti-CD28 in the presence of 0.4μg/ml DHA or Mock (DMSO). TGF-β receptor I (Alk5) inhibitor SB525334 (5mM) or mTOR inhibitor Rapamycin (5nM) was added in specific experiments as indicated. Th cell differentiation conditions were as following. Th1: 40ng/ml rIL-12 (Peprotech) and 40μg/ml anti-IL-4 (11B11); Th2: 40ng/ml rIL-4 (Peprotech) and 10μg/ml anti-IFN-γ (XMG); Th17: 1ng/ml rTGF-β (Preprotech), 40ng/ml rIL-6 (R&D), 40μg/ml anti-IL-4, and 10μg/ml anti-IFN-γ; Treg: 1ng/ml rTGF-β and 2ng/ml rIL-2 (R&D).
T cell Proliferation and Apoptosis assays
For proliferation assay, CD4+ or CD8+ T cells were purified by MACS beads (Miltenyi Biotec) and labeled with carboxyl fluorescent succinimidyl ester (CFSE, AnaSpec). Labeled cells were stimulated with plate-bound anti-CD3 and anti-CD28 in the presence of 2ng/ml rIL-2. After 96-hour culture, cell proliferation was assessed by CFSE dilution using flow-cytometry. For apoptosis assay, CD4+ or CD8+ T cells were activated for indicated time. Cells were then stained with AnnexinV-APC and 7-AAD per manufacture’s protocols (BD Biosciences) and analyzed by flow-cytometry.
Quantitative RT-PCR and Immunoblotting assays
RNA was extracted using TRIsure reagent (Bioline) and cDNA was synthesized by Tetro cDNA Synthesis Kit per manufacturer’s protocols (Bioline). Quantitative PCR was performed on the ABI 7900HT Real-time PCR system using SensiMix™ II Probe Kit per manufacturer’s protocols (Bioline) and primer/probe sets obtained from Applied Biosystems specific for Rorc, IL-17A, IL-21, IL-23R, AhR, IL-22, IFNγ, T-bet, IL-4, GATA-3 and HPRT. Antibodies against p-STAT3, STAT3, p-S6, S6, p-S6K, S6K, p-Smad2, Smad2/3, p-STAT5, STAT5 (Cell Signaling Technology), RORγt (eBioscience) and β-actin (Santa Cruz Biotechnology) were used for immunoblotting per manufacturer’s protocols.
EAE induction, prevention and treatment
Mice (12 to 16 weeks old) were immunized subcutaneously (s.c.) with 50μg of MOG35–55peptide (MEVGWYRSPFSRVVHLYRNGK, AnaSpec) and 500μg of M. tuberculosis (Difco) emulsified in IFA (Difco). In addition, the animals received 200ng of Pertussis Toxin (List Biological Laboratories) intra-peritoneally (i.p.) on days 0 and 2. The severity of EAE was monitored and graded on a clinical score of 0 to 5: 0 = no clinical signs; 1 = Limp tail; 2 = Para-paresis (weakness, incomplete paralysis of one or two hind limbs); 3 = Paraplegia (complete paralysis of two hind limbs); 4 = Paraplegia with forelimb weakness or paralysis; 5 = Moribund or death. To prevent EAE development, DHA was prepared at 10mg/ml in the 6:4 mixture of DMSO and polysorbate 80, and injected i.p. at a dose of 25mg/kg on day 0, and continued for 7 days. To treat ongoing EAE, DHA was injected when mice showed clinical signs of EAE and then continued for 9 days.
Retrovirus mediated ectopic gene expression in T cells
Retroviral construct expressing a constitutive active form of AKT (caAkt) with linked Thy1.1 marker (MSCV-IRES-Thy1.1, abbreviated as MIT) were kindly provided by Dr. Hongbo Chi (St. Jude Children’s Research Hospital). 293 packaging cells were transfected with retroviral constructs using FuGENE® HD transfection reagent (Roche). The recombinant retroviruses were collected 48 and 72 h after transfection. MACS purified CD4+ T cells were activated for 48h and then transduced with recombinant retroviruses. Cells were cultured for additional 3 days and harvested for flow-cytometric analysis.
Statistical analysis
Data from at least three sets of samples were used for statistical analysis. Statistical significance was calculated by Student’s t-test. A P value of less than 0.05 was considered significant.
Results
DHA treatment suppressed T cell proliferation
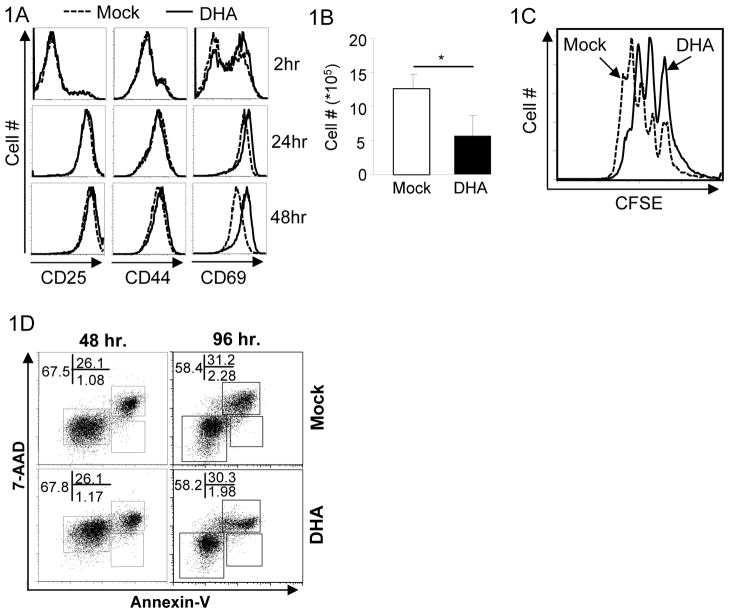
To study how DHA influences T cell function, we first investigated whether DHA treatment affected T cell activation, proliferation and survival. Under culturing conditions, DHA showed minimal toxicity to T cells at the dose of 0.4μg/ml, although it was toxic to T cells at high doses (>1.0μg/ml). Therefore, we used DHA at 0.4μg/ml for studies on cultured T cells. Upon anti-CD3 and anti-CD28 stimulation, DHA did not affect T cell activation because T cell activation markers, such as CD25, CD44 and CD69, were up-regulated normally in DHA-treated CD4+ T cells (Fig. 1A). Nonetheless, the numbers of live CD4+ T cells recovered from DHA-treated samples were less than those from Mock-treated samples (Fig. 1B). To further investigate the reason for reduced T cell numbers, we assessed how DHA treatment impacted T cell proliferation and survival. DHA treatment moderately inhibited the proliferation of activated CD4+ T cells (Fig. 1C) without apparently affecting cell survival (Fig. 1D). Similarly, DHA treatment marginally suppressed CD8+ T cell proliferation but not their activation or survival (Supplementary Fig. S1). These findings demonstrated that DHA exerts moderate inhibition on T cell proliferation without apparent effect on T cell activation and survival.
Figure 1. The effects of DHA on T cell activation and proliferation.
1A. Purified CD4+ T cells were activated with anti-CD3 and anti-CD28 under the treatment of DHA (solid line) and mock (dashed line) for indicated time. The expression of activation markers CD25, CD44 and CD69 were detected by flow-cytometry. Results are representative of at least three experiments.
1B. The numbers of CD4+ T cells recovered 4 days after activation in the presence (solid bar) or absence (open bar) of DHA. Means ± SD of three experiments are shown. (*P<0.05)
1C. CFSE-labeled CD4+ T cells were activated with the treatment of mock (dashed line) or DHA (solid line) for 4 days. Cell proliferation was monitored by assessing CFSE dilution by flow-cytometry. Representative results of three experiments are shown.
1D. Purified CD4+ T cells were activated with plate bound anti-CD3 and anti-CD28 with the treatment of Mock and DHA for 48 and 96 hours. Cells were stained with Annexin-V and 7-AAD. The percentages of each population were detected by flow-cytometry and indicated. Results are representation of at least three experiments.
DHA inhibited helper CD4+ T cell differentiation
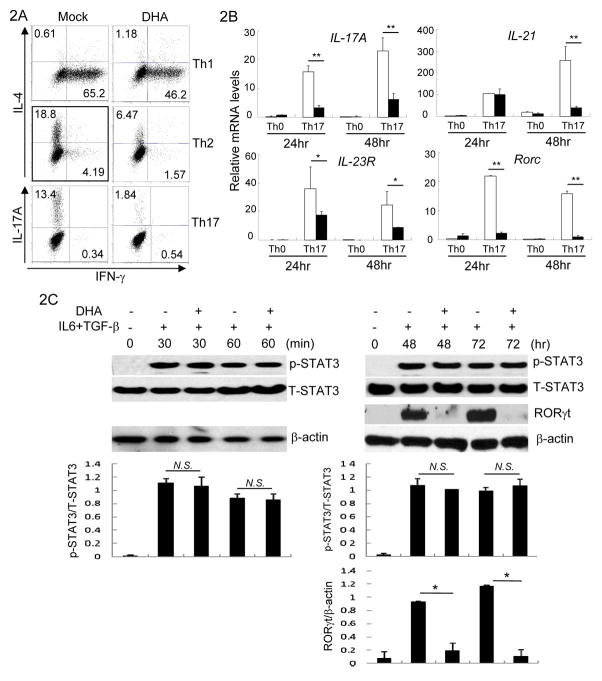
CD4+ T cells are pivotal in controlling immune responses in large part by differentiating into different types of Th cells to direct various types of immune response (9). IFN-γ producing Th1, IL-4 producing Th2 (10–12) and IL-17A producing Th17 cells (13–15) are the three major types of Th cells studied extensively. To test whether DHA influences Th differentiation, CD4+ T cells were purified and cultured under Th1, Th2 or Th17 cell skewing cytokines in the presence of DHA. We found that DHA treatment reduced IFN-γ and IL-4 production by Th1 and Th2 cells respectively (Fig. 2A, supplementary Fig. S2A). DHA treatment however virtually abrogated IL-17A production by differentiating Th17 cells (Fig. 2A).
Figure 2. DHA inhibited helper T cell differentiation.
2A. Purified CD4+ T cells were cultured under Th1, Th2, and Th17 cell polarizing conditions with Mock or DHA treatment. IFN-γ, IL-4 and IL-17A production in CD4+ T cells were assessed by intracellular staining and flow-cytometry. Results are representative of at least three experiments.
2B. CD4+ T cells were cultured under Th17 cell polarizing conditions for indicated time in the presence (solid bar) or absence (open bar) of DHA. The mRNA expression of IL-17A, IL-21, IL-23R and Rorc was detected by qRT-PCR. Means ± SD of triplicates done in one experiment representative of three are shown. (*P<0.05, **P<0.01)
2C. CD4+ T cells were activated under Th17 polarizing conditions in the presence (+) or absence (−) of DHA for indicated time. The protein amounts of phosphorylated-STAT3 (p-STAT3), STAT3, RORγt and β-actin were detected by immunoblotting. Results are representative of three experiments. Densitometry analysis of immuno-blotting was also shown. (N.S. Not Significant; *P<0.05)
Further comprehensive analysis revealed that the expression of IL-21, another Th17 promoting cytokine (16) was suppressed by DHA. In addition, the expression of IL-23R, which promotes Th17 cells (17), was inhibited by DHA. Moreover, DHA abrogated the expression of transcription factor retinoic-acid-related orphan receptor γ (RORγt), a master regulator controlling Th17 differentiation (18) (Figure 2B and 2C). Nevertheless, DHA-mediated effects appeared to be gene specific, because the expression of certain Th17-related genes, such as IL-22 (19) and AhR (20), were not impaired by DHA treatment (Supplementary Fig. S2B). In addition, DHA treatment had unnoticeable effects on phosphorylation of STAT-3, which is activated by IL-6 and critical for Th17 differentiation (21) (Figure 2C). These findings suggest that DHA potently suppresses Th17 cell differentiation by interfering with specific molecular programs.
DHA promoted Treg cell generation induced by TGF-β
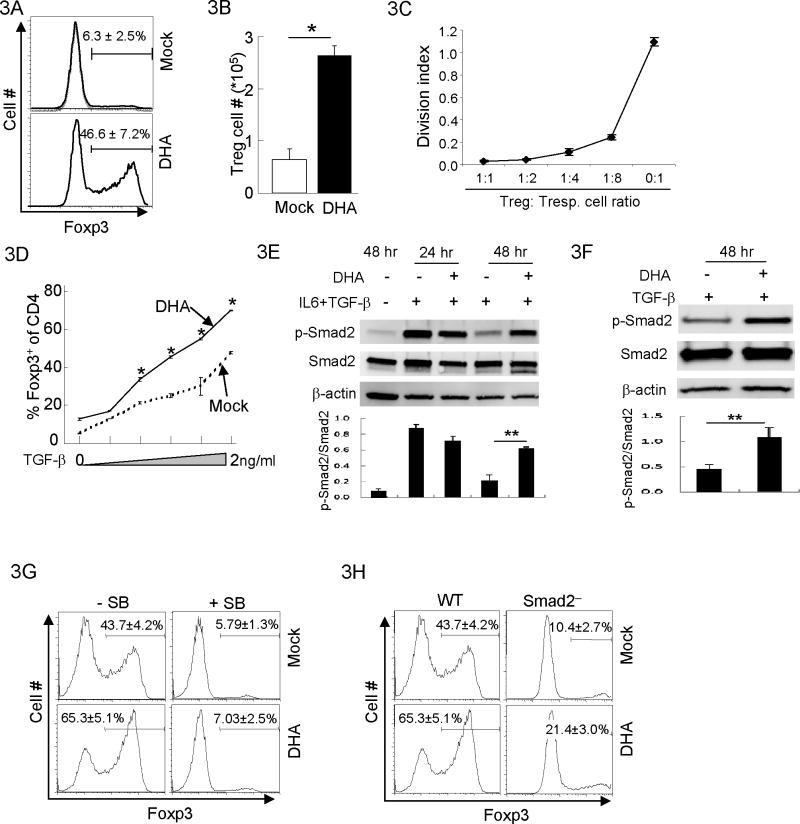
Foxp3-expressing regulatory T (Treg) is a subset of CD4+ T cells critical to suppress immune response (22, 23). The differentiation programs of Treg and Th17 cells antagonize each other (24, 25): while TGF-β promotes the generation of Treg cells (26, 27), the presence of IL-6 suppresses Treg cell generation but promotes Th17 cell generation (24, 28). Because DHA inhibited Th17 cell differentiation (Fig. 2), we investigated if DHA affected Treg cell generation under the same condition. Unexpectedly, the proportion of Treg cells was greatly increased in DHA-treated cells compared to Mock-treated cells (Fig. 3A). Such an increase was not merely due to reduced numbers of non-Treg cells (Supplementary Fig. S2C), because the numbers of Foxp3+ Treg cells were also substantially increased (Fig. 3B). Further investigation revealed that DHA-promoted Foxp3+ cells were indeed Treg cells, because DHA-promoted Foxp3+ cells displayed potent immune suppressive activity in vitro (Fig. 3C). Intrigued by this observation, we hypothesized that TGF-β-induced Treg cell generation is promoted by DHA. Indeed, DHA treatment notably enhanced Treg cell generation induced by TGF-β (Fig. 3D). Therefore, DHA exerted reciprocal effect on Th17 and Treg cells by inhibiting Th17 cell differentiation and promoting Treg cell generation.
Figure 3. DHA promoted Treg generation through TGF-βR:Smad signaling.
3A. Purified CD4+ T cells were activated under Th17 polarizing conditions for 4 days with DHA or Mock treatment. The fractions of Foxp3 expressing Treg cells were determined by Foxp3 intracellular staining and flow-cytometry. Means ± SD of three experiments are shown.
3B. The total numbers of Treg cells recovered from experiments described in (A) were compared. Means ± SD of three experiments are shown. (*P<0.05)
3C. Purified CD4+ T cells from Foxp3 reporter mice (27) were activated under Th17 polarizing conditions for 4 days with DHA treatment. Foxp3+ Treg cells were sorted and mixed with CFSE-labeled Foxp3− naïve CD4 responder T cells (Tresp.) at different ratios. Cell mixtures were activated with soluble anti-CD3 in the presence of irradiated APC. Four days post activation, the proliferative index of responder T cell was monitored by CFSE dilution and flow-cytometry. The division index of responder T cells was determined using FlowJo software to quantitate the degree of Treg cell-mediated suppression on the proliferation of responder T cells. Means ± SD of three experiments are shown.
3D. CD4+ T cells were activated in the presence of varying doses of TGF-β (0–2ng/ml) with the treatments of Mock (dashed line) or DHA (solid line) for 4 days. The percentages of Foxp3+ Treg cells were determined by intracellular staining and flow-cytometry. Means ± SD of triplicates done in one experiment representative of three are shown (*P<0.05).
3E. CD4+ T cells were activated under Th17 polarizing conditions with DHA (+) or Mock (−) treatment for indicated time. The protein amounts of phosphorylated-Smad2 (p-Smad2), Smad2 and β-actin protein were detected by immunoblotting. Results are representative of three experiments. Densitometry analysis of immuno-blotting was also shown. (**P<0.01)
3F. CD4+ T cells were activated in the presence of 1ng/ml TGF-β with the treatment of DHA (+) or Mock (−) for indicated time. The protein amounts of phosphorylated-Smad2 (p-Smad2), Smad2 and β-actin protein were detected by immunoblotting. Results are representative of three experiments. Densitometry analysis of immuno-blotting was also shown. (**P<0.01)
3G. CD4+ T cells were activated in the presence of 1ng/ml TGF-β with the treatment of DHA or Mock for 4 days. Cells were simultaneously treated with TGF-βRI (ALK5) inhibitor SB525334 (+) or remain untreated (−). The fractions of Foxp3+ Treg cells were detected by intracellular staining and flow-cytometry. Means ± SD of three experiments are shown.
3H. CD4+ T cells were purified from wild-type (WT) or CD4cre-Smad2fl/fl mice (Smad2−) in the presence of 1ng/ml TGF-β with the treatment of DHA or Mock for 4 days. The fractions of Foxp3+ Treg cells were detected by intracellular staining and flow-cytometry. Results are representative of at least three experiments. Means ± SD of three experiments are shown.
TGF-βR signal is critical for Treg cell generation (29, 30). We hypothesized that DHA regulates TGF-βR signal cascade to promote Treg cell generation. Indeed, TGF-β-induced Smad2 activation (31), measured by its phosphorylation, was prolonged in DHA-treated cells (Fig. 3E and 3F), although early Smad2 activation in DHA-treated cells appeared to be comparable to Mock-treated cells (Fig. 3E and supplementary Fig. S3A). In addition, DHA treatment did not result in a global alteration of cell signaling, because the activities of MAPK, such as p38 and ERK, appeared normal in DHA-treated cells (Supplementary Fig. S3B). These findings suggest that TGF-βR:Smad2 signaling cascade is important for DHA-mediate promotion of Treg cells. To address whether TGF-βR:Smad2 signaling is required for DHA-promoted Treg cell generation, we blocked TGF-βR function in T cells using a pharmacological inhibitor SB525334(32) and abrogated Smad2 function using T cells from CD4cre-Smad2fl/fl mice(33, 34), where Smad2 was deleted specifically in T cells. Disruption of TGF-βR signal led to greatly reduced Treg generation promoted by DHA (Fig. 3G and 3H) without Th differentiation (Supplementary Fig. S3C). These results suggest that DHA promotes Treg generation through mechanisms dependent on TGF-βR:Smad2 signal.
DHA treatment prevented the onset of EAE
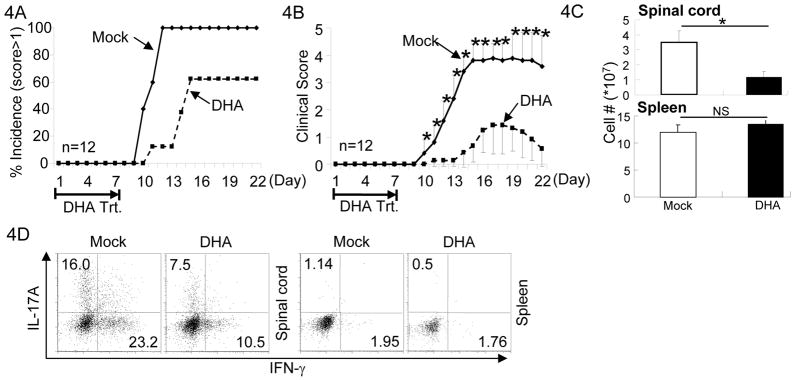
The findings that DHA reciprocally regulated Th and Treg cell function promoted us to investigate how DHA treatment may impact the development of inflammatory disease. Th17 and Th1 cells contributes to immune pathology in EAE induced by CFA-MOG injection (35, 36), a murine model for human Multiple Sclerosis. Treg cells, on the contrary, suppress EAE development (37). We therefore hypothesized that DHA treatment will be therapeutic against EAE. In order to perform studies in vivo, we identified a non-toxic dose of DHA in mice. While DHA showed noticeable toxicity at high doses (>100mg/kg), minimal toxicity was observed when mice were injected with 25mg/kg of DHA. This dose was therefore used for the studies performed in vivo. We first addressed whether DHA treatment was able to prevent or to reduce the onset of EAE. When administrated daily during the first 7 days of EAE elicitation, DHA reduced the incidence of EAE onset (Fig. 4A). In addition, the clinical scores of DHA-treated mice that developed EAE were much lower than those of Mock-treated mice (Fig. 4B), suggesting that administration of DHA effectively prevented and reduced onset of EAE in mice.
Figure 4. DHA treatment prevented the onset of EAE.
4A. EAE was elicited in mice with MOG/CFA injection. Mice were also treated with DHA (dashed line) or Mock (solid line) during the first 7 days of EAE elicitation. Incidence of the mice showed clinical scores higher than 1 were recorded. Results of 12 mice in one experiment of three are shown.
4B. As described in (A), mice were injected with MOG/CFA to elicit EAE and treated with DHA (dashed line) or Mock (solid line). Clinical scores were recorded every day thereafter. Mean clinical scores ± SEM of 12 mice in one experiment of three are shown (*P<0.05).
4C. As described in (A), mice were injected with MOG/CFA to elicit EAE and treated with DHA (solid bar) or Mock (open bar). The numbers of lymphocytes infiltrating in the spinal cord (upper panel) and total splenocytes (lower panel) in mice were counted after the treatment. Means ± SD of six mice are shown.
4D. As described in (A), mice were injected with MOG/CFA to elicit EAE and treated with DHA or Mock. Lymphocytes were isolated from the spinal cords and spleens of mice with clinical score of 2. The fractions of IL-17A and IFN-γ producing CD4+ T cells were determined by intracellular staining and flow-cytometry. Results are representative of at least three experiments.
Associating with aforementioned observation, the numbers of lymphocytes infiltrating into the spinal cord (Fig. 4C top panel) as well as Th17 and Th1 cells (Fig. 4D) were decreased in DHA-treat mice compared to Mock-treated mice. Of note, DHA administration did not cause systemic immune depletion or suppression, because similar numbers of splenocytes were recovered from DHA- and Mock-treated mice (Fig. 4C lower panel) and CD4+ and CD8+ T cells were activated equally well in DHA- and Mock-treated mice (Supplementary Fig. S4). Thus, these findings demonstrated that DHA was effective to prevent EAE onset in part through suppressing Th differentiation without apparently inhibiting immune function globally.
DHA ameliorated on-going EAE
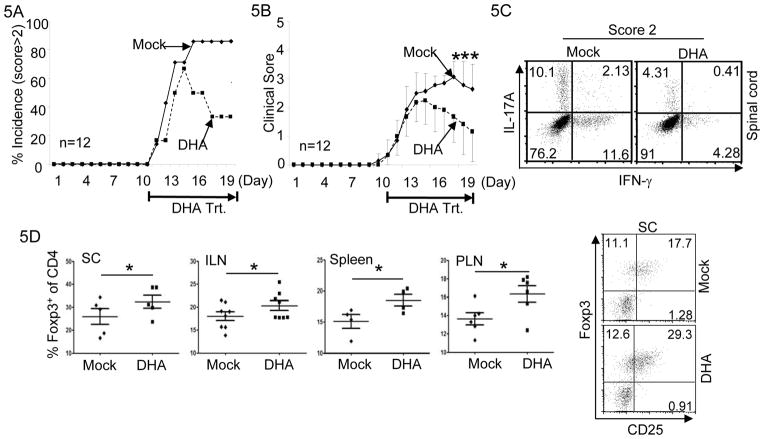
Encouraged by the above findings, we further investigated whether administration of DHA was able to treat on-going EAE in mice. To do this, EAE was elicited in mice. When mice developed clinical signs of EAE (limp tail), we treated them with either DHA or Mock. Over 80% of Mock-treated mice showed clinical scores above 2 and remained so (Fig. 5A and 5B). In contrast, while a large percentage of DHA-treated mice showed clinical scores above 2 during early time of treatment, majority of DHA-treated mice became less sick or disease free at the later time (Fig. 5A and 5B) without relapse after discontinuation of DHA treatment (data not shown). These findings suggest that DHA administration is able to ameliorate on-going EAE and can be used to treat mice inflicted with EAE.
Figure 5. DHA treatment ameliorated on-going EAE.
5A. EAE was elicited in mice by MOG/CFA injection. When mice showed clinical signs of EAE, they were treated either with DHA (dashed line) or with Mock (solid line) for the following 9 days. Incidence of the mice showed clinical scores higher than 2 were recorded. Results of 12 mice in one experiment of three are shown.
5B. As described in (A), when mice showed clinical signs of EAE, they were treated either with DHA (dashed line) or with Mock (solid line) for the following 9 days. EAE Clinical scores were recorded every day. Mean clinical scores ± SEM of 12 mice in one experiment of three are shown (*P<0.05).
5C. As described in (A), when mice showed clinical signs of EAE, they were treated either with DHA or with Mock. Lymphocytes were isolated from the spinal cords of mice with clinical score of 2. The fractions of IL-17A and IFN-γ producing CD4+ T cells were determined by flow-cytometry. Results are representative of at least three experiments.
5D. At the end of experiments described in (A), lymphocytes were isolated from the spinal cords (SC), inguinal lymph-nodes (ILN), spleens and peripheral lymph-nodes (PLN) of mice. Treg cells were identified by Foxp3 and CD25 staining. Representative result is shown in right panel. The percentages of Foxp3+ Treg cells were determined and plotted in left panel. Mean ± SEM of multiple mice are shown. (*P<0.05).
We further investigated whether DHA treatment affected the distribution of Th and Treg cells in EAE-inflicted mice. The percentages of Th17 and Th1 cells were reduced in DHA-treated mice compared with Mock-treated mice (Fig. 5C). In contrast, the percentage of Treg cells was notably increased in DHA-treated mice compared with Mock-treated mice (Fig. 5D). Therefore, DHA treatment was effective in ameliorating ongoing EAE, associating with reduced Th differentiation but increased Treg generation.
DHA regulates T cell function through modulating mTOR pathway
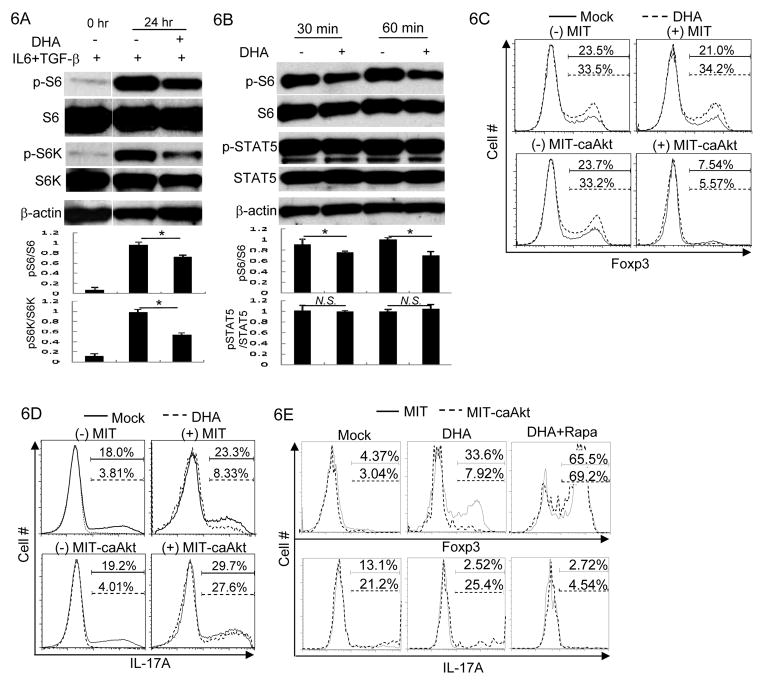
Aforementioned findings suggest that DHA is an agent effective in regulating Th and Treg cell function to suppress immune response. We sought to further understand the molecular mechanisms underlying DHA-mediated effects on T cells. mTOR pathway is important in promoting Th cell function and in suppressing Treg cell function (38, 39). Inhibition of mTOR pathway leads to defective Th cell differentiation and enhanced Treg generation, an observation strikingly similar to DHA-mediated effects. We thus hypothesized that DHA affected mTOR signal in T cells to reciprocally control Th and Treg cell generation. Indeed, by assessing the phosphorylation of p70S6K and S6, indicator for the activation of mTOR pathway (40), we found that DHA treatment reduced mTOR signaling in T cells (Fig. 6A). In addition, cytokine IL-2 promoted mTOR activities also decreased upon DHA treatment (Fig. 6B). Thus, DHA treatment attenuated mTOR signal.
Figure 6. DHA regulated T cell function by modulating mTOR pathway.
6A. CD4+ T cells were activated under Th17 cell polarizing conditions for indicated time with the treatment of DHA (+) or Mock (−). The protein amounts of phosphorylated S6 (p-S6), S6, phosphorylated p70 S6 kinase (p-S6K), S6K and β-actin were determined by immunoblotting. Results are representative of at least three experiments. Densitometry analysis of immuno-blotting was also shown. (*P<0.05)
6B. Effector CD4+ T cells were stimulated in the presence of 5ng/ml IL-2 for indicated time with the treatment of DHA (+) or Mock (−). The protein amounts of phosphorylated S6 (p-S6), S6, phosphorylated STAT5 (p-STAT5), STAT5 and β-actin were assessed by immunoblotting. Results are representative of at least three experiments. Densitometry analysis of immuno-blotting was also shown. (N.S. Not Significant; *P<0.05)
6C. CD4+ T cells were activated and then transduced with control recombinant virus MSCV-IRES-thy1.1 (MIT) or with recombinant virus expressing a constitutive active form of Akt (MIT-caAkt) in the presence of 1ng/ml of TGF-β under the treatment of DHA (dashed line) or Mock (solid line). Foxp3 expression in transduced (+) or un-transduced (−) cells were assessed by intracellular staining and flow-cytometric analysis. The percentages of Foxp3+ Treg cells were indicated above the brackets. Results are representative of three experiments.
6D. CD4+ T cells were activated and then transduced with control recombinant virus MSCV-IRES-thy1.1 (MIT) or with recombinant virus expressing a constitutive active form of Akt (MIT-caAkt) under Th17 cell polarizing conditions with DHA (dashed line) or Mock (solid line) treatment. IL-17A expression in transduced (+) or un-transduced (−) cells were assessed by intracellular staining and flow-cytometric analysis. The percentages of IL-17A+ cells were indicated above the brackets. Results are representative of three experiments.
6E. CD4+ T cells were activated under Th17 cell polarizing conditions with the treatment of Mock, DHA or DHA plus Rapamycin (DHA+Rapa). Cells were transduced with control recombinant virus MSCV-IRES-thy1.1 (MIT, solid line) or with recombinant virus expressing a constitutive active form of Akt (MIT-caAkt, dashed line). Foxp3 and IL-17A expression in transduced cells were assessed by intracellular staining and flow-cytometry analysis. The percentages of Foxp3+ and IL-17A+ cells among transduced T cells are shown above the brackets. Results are representative of three experiments.
We therefore were prompted to investigate whether mTOR signaling is functionally involved in DHA-mediated effect. To do so, we ectopically expressed a constitutively active form of Akt (caAkt) in T cells to enhance mTOR signal (41). The expression of caAkt suppressed DHA-promoted Treg generation (Fig. 6C). In addition, the expression of caAkt overcame DHA-mediated suppression of Th17 differentiation (Fig. 6D). Thus, caAkt expression neutralized DHA-mediated effects on T cells. More importantly, the neutralization effect of caAkt on DHA-treated T cells depended on mTOR activity, because addition of Rapamycin, a classic mTOR inhibitor (42), abolished caAkt-mediated effects on DHA-treated cells (Fig. 6E). These findings suggest that the reduction of mTOR signal upon DHA-treatment, albeit modest, is indeed functionally important for DHA-mediated effects on T cells.
Discussion
Widely used anti-malaria drugs, artemisinin and its derivatives showed immunoregulatory effect (6–8). Yet, the underlying mechanism remains poorly defined. In this study, we investigated the effect of DHA, the derivative and also major metabolite of artemisinin, on T cell function. We found that DHA suppressed Th differentiation but promoted Treg generation, suggesting that DHA could be therapeutic to treat inflammatory disease. Indeed, DHA treatment not only prevented the onset of EAE, but also ameliorated on-going EAE in mouse, associating with decreased Th cells but increased Treg cells. While another derivative of artemisinin SM933 was found suppressing EAE (8), it did not apparently affect T cell function. Thus, subtle difference in the molecular structures of artemisinin derivatives may result in substantial changes in their immune regulatory functions. Nonetheless, these findings suggest that artemisinin derivative is a new class of immune regulatory agent to suppress immune function. And DHA in particular is effective to dampen T cell function by reciprocally controlling Th and Treg generation and to treat T cell mediated autoimmune and inflammatory diseases.
Antigen specific and non-specific immune suppressive agents have been used to treat inflammatory diseases. Because natural compounds generally have low side effects, studies are needed to identify and characterize natural ingredients in plants to treat inflammatory disease. This study identifies DHA, a derivative of a natural compound artemisinin, as an agent that can be used for the development of antigen specific and non-specific immunosuppressant with reduced side effects. We have found that, at effective doses, DHA showed slight side effects on T cells in vitro and in vivo. DHA treatment only moderately inhibited T cell proliferation without discernable effects on T cell activation or survival in vitro. And DHA administration in vivo minimally affected animal physiology or T cell population. At appropriate doses, DHA affected specific functions of T cells: It inhibited Th differentiation but promoted Treg cell generation. While DHA abolished Th17 differentiation, it did not affect immediate signal of IL-6 or TGF-β and inhibited IL21/IL23-RORγt signal axis but not IL22-Ahr signal axis. The inability of DHA to affect STAT-3 activation suggests that DHA impacts signal downstream of STAT-3. Therefore, the effect of DHA is independent of STAT-3, but through modulating TGF-β/Smad and mTOR pathways. The inability of DHA to affect STAT-3 function, which is critical for initiating all Th17-associated molecular programs, could be one of the reasons for why DHA affects certain but not all molecular programs of Th17 cells. These findings suggest that DHA could be used as an agent to fine tune Th17 response. Therein, DHA can be used as an antigen non-specific drug against specific T cell function and inflammation with mild side effects. In addition, because Treg-based strategy is one of the important means to develop antigen specific immune suppressive therapies, agent able to promote the generation and function of Treg cells is desired for Treg-based therapy. We have found that DHA promoted Treg generation in vitro and in vivo without substantially affecting their survival or proliferation. It is therefore reasonable to believe that incorporation of DHA into current regimen will enhance the efficacies of Treg-based immune therapy. Thus further studies are warranted to explore such possibilities. Therefore, our findings suggest that DHA is a promising agent to facilitate the development of both antigen-specific and non-specific therapies against inflammatory diseases.
One of the limitations of using natural product derivative to treat disease is the lack of mechanistic understanding of their effect at the molecular levels, which contributes to the reluctant clinical use. It is important to elucidate the molecular pathways affected by DHA. We found that interfering with mTOR pathway is functionally critical for DHA mediated effects in T cells because DHA treatment attenuated mTOR signal and enhanced Akt activity corrected DHA mediated effects in T cells in a manner dependent on mTOR. We nonetheless believe that mTOR is not the only pathway affected by DHA. DHA treatment attenuated but not abrogated mTOR signal. In addition, our findings suggest that enhanced Smad pathway could be another important mechanism underlying DHA mediated effect on T cells; a mechanism that may or may not depends on mTOR signal. What additional signaling pathways affected by DHA in mTOR dependent or independent fashion warrants further investigation. The findings that DHA attenuated mTOR signal but enhanced Smad signal indicate a broad application of DHA to treat diseases beyond inflammatory diseases (e,g. cancer) where mTOR and Smad signals need to be adjusted.
While the findings in this study suggest that DHA may be beneficial to treat inflammatory disease, it suggests the potential undesirable effect for malaria treatment where immune suppression could be detrimental to the clearance of parasites. While DHA is effective in clearing malaria causing parasites, DHA resistant strain do emerge to contribute to the recrudescence of malaria. Given the observed immunosuppressive effect of DHA, the emergency of drug resistance may be due to weakened immune function of the host. Thus, to develop more effective malaria treatment, use of DHA in combination with immune-enhancing agents should be considered. On the other hand, DHA-promoted Treg cell function may benefit malaria treatment by reducing malaria-induced immune pathology, because it has been shown that enhanced Treg cell function paradoxically prevented experimental cerebral malaria via the function of CTLA-4 (43). Therefore, better understanding of the mechanism of the effect of DHA on immune function is critical in treating malaria more efficiently.
Supplementary Material
Acknowledgments
We thank H. Chi (St. Jude Children’s Research Hospital) for providing MIG-caAkt plasmids; N. Fisher and J. Kalnitsky (University of North Carolina Flow-cytometry facility) for cell sorting; J. Ting, G. Matsushima, S. Lemon (University of North Carolina) for helpful discussions.
This study is supported by China Scholarship Council (Y.G.Z.), the NIH/NIAID, the Lupus Research Institute and the University Cancer Research Fund (Y.Y.W.).
References
- 1.Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985;228:1049–1055. doi: 10.1126/science.3887571. [DOI] [PubMed] [Google Scholar]
- 2.Kremsner PG, Krishna S. Antimalarial combinations. Lancet. 2004;364:285–294. doi: 10.1016/S0140-6736(04)16680-4. [DOI] [PubMed] [Google Scholar]
- 3.Eckstein-Ludwig U, Webb RJ, Van Goethem ID, East JM, Lee AG, Kimura M, O’Neill PM, Bray PG, Ward SA, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424:957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 4.Singh NP, Lai H. Selective toxicity of dihydroartemisinin and holotransferrin toward human breast cancer cells. Life Sci. 2001;70:49–56. doi: 10.1016/s0024-3205(01)01372-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen HH, Zhou HJ, Wang WQ, Wu GD. Antimalarial dihydroartemisinin also inhibits angiogenesis. Cancer Chemother Pharmacol. 2004;53:423–432. doi: 10.1007/s00280-003-0751-4. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Zhang W, Shi X, An P, Sun W, Wang Z. Therapeutic effect of artemisinin on lupus nephritis mice and its mechanisms. Acta biochimica et biophysica Sinica. 2010;42:916–923. doi: 10.1093/abbs/gmq101. [DOI] [PubMed] [Google Scholar]
- 7.Zhou WL, Wu JM, Wu QL, Wang JX, Zhou Y, Zhou R, He PL, Li XY, Yang YF, Zhang Y, Li Y, Zuo JP. A novel artemisinin derivative, 3-(12-beta-artemisininoxy) phenoxyl succinic acid (SM735), mediates immunosuppressive effects in vitro and in vivo. Acta pharmacologica Sinica. 2005;26:1352–1358. doi: 10.1111/j.1745-7254.2005.00232.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang JX, Tang W, Zhou R, Wan J, Shi LP, Zhang Y, Yang YF, Li Y, Zuo JP. The new water-soluble artemisinin derivative SM905 ameliorates collagen-induced arthritis by suppression of inflammatory and Th17 responses. British journal of pharmacology. 2008;153:1303–1310. doi: 10.1038/bjp.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coffman RL, Carty J. A T cell activity that enhances polyclonal IgE production and its inhibition by interferon-gamma. J Immunol. 1986;136:949–954. [PubMed] [Google Scholar]
- 11.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 12.Szabo SJ, Sullivan BM, Peng SL, Glimcher LH. Molecular mechanisms regulating Th1 immune responses. Annu Rev Immunol. 2003;21:713–758. doi: 10.1146/annurev.immunol.21.120601.140942. [DOI] [PubMed] [Google Scholar]
- 13.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 15.Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 16.Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, Dong C. Essential autocrine regulation by IL-21 in the generation of inflammatory T cells. Nature. 2007;448:480–483. doi: 10.1038/nature05969. [DOI] [PubMed] [Google Scholar]
- 17.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 19.Liang SC, Tan XY, Luxenberg DP, Karim R, Dunussi-Joannopoulos K, Collins M, Fouser LA. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 2008;453:106–109. doi: 10.1038/nature06881. [DOI] [PubMed] [Google Scholar]
- 21.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–458. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 23.Shevach EM. Regulatory T cells in autoimmmunity*. Annu Rev Immunol. 2000;18:423–449. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 24.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–1886. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Natl Acad Sci U S A. 2005;102:5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pasare C, Medzhitov R. Toll pathway-dependent blockade of CD4+CD25+ T cell-mediated suppression by dendritic cells. Science. 2003;299:1033–1036. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 29.Li MO, Sanjabi S, Flavell RA. Transforming growth factor-beta controls development, homeostasis, and tolerance of T cells by regulatory T cell-dependent and -independent mechanisms. Immunity. 2006;25:455–471. doi: 10.1016/j.immuni.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu Y, Zhang P, Li J, Kulkarni AB, Perruche S, Chen W. A critical function for TGF-beta signaling in the development of natural CD4+CD25+Foxp3+ regulatory T cells. Nat Immunol. 2008;9:632–640. doi: 10.1038/ni.1607. [DOI] [PubMed] [Google Scholar]
- 31.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. Embo J. 2000;19:1745–1754. doi: 10.1093/emboj/19.8.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grygielko ET, Martin WM, Tweed C, Thornton P, Harling J, Brooks DP, Laping NJ. Inhibition of gene markers of fibrosis with a novel inhibitor of transforming growth factor-beta type I receptor kinase in puromycin-induced nephritis. The Journal of pharmacology and experimental therapeutics. 2005;313:943–951. doi: 10.1124/jpet.104.082099. [DOI] [PubMed] [Google Scholar]
- 33.Dunn NR, Koonce CH, Anderson DC, Islam A, Bikoff EK, Robertson EJ. Mice exclusively expressing the short isoform of Smad2 develop normally and are viable and fertile. Genes Dev. 2005;19:152–163. doi: 10.1101/gad.1243205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, Perez-Melgosa M, Sweetser MT, Schlissel MS, Nguyen S. A Critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15:763–774. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 35.Jager A, Dardalhon V, Sobel RA, Bettelli E, Kuchroo VK. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J Immunol. 2009;183:7169–7177. doi: 10.4049/jimmunol.0901906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 37.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]
- 38.Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haxhinasto S, Mathis D, Benoist C. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J Exp Med. 2008;205:565–574. doi: 10.1084/jem.20071477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 41.Liu G, Burns S, Huang G, Boyd K, Proia RL, Flavell RA, Chi H. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat Immunol. 2009;10:769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- 43.Haque A, Best SE, Amante FH, Mustafah S, Desbarrieres L, de Labastida F, Sparwasser T, Hill GR, Engwerda CR. CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS pathogens. 2010;6:e1001221. doi: 10.1371/journal.ppat.1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.