Abstract
Misfolded immunoglobulin light chain proteins (LC) in light chain amyloidosis (AL) are toxic to vascular tissues. We tested the hypothesis that chaperone protein clusterin preserves endothelial function and cell survival during LC exposure.
Methods
LC (20 μg/mL) were given to human aortic endothelial cells (EC) for 24-hours and clusterin protein/gene expression and secretion were measured. DNA fragmentation was measured with/without recombinant clusterin (Clu, 300 ng/mL). Adipose arterioles (non-AL subjects) were tested for dilator responses to acetylcholine/papaverine at baseline and after 1-hour of LC±Clu.
Results
LC reduced EC clusterin secretion, protein and gene expression while increasing DNA fragmentation. Clu attenuated LC-induced DNA fragmentation and restored dilator response to acetylcholine (logEC50: control −7.05±0.2, LC+Clu −6.53±0.4, LC −4.28±0.7, p<0.05 vs. control, LC+Clu).
Conclusions
LC induced endothelial cell death and dysfunction while reducing clusterin protein/gene expression and secretion. Exogenous clusterin attenuated LC toxicity. This represents a new pathobiologic mechanism and therapeutic target for AL amyloidosis.
Keywords: amyloid, endothelial function, chaperone protein
Chaperone proteins protect the cell against misfolded proteins and are promising novel agents against intractable amyloid diseases. Yet the beneficial versus pathologic role of clusterin, a chaperone glycoprotein, in Alzheimer’s disease (AD) or light chain amyloidosis (AL) remains controversial1–3. AL involves overproduction of amyloidogenic immunoglobulin light chain proteins (LC) that cause toxicity to blood vessels4, cardiac and other tissues with high mortality and morbidity rates to this day. Endothelial dysfunction and vascular injury occur early in AL4–7 leading to ischemic tissue damage8, 9. We tested the hypothesis that clusterin plays an important role in maintaining human arteriole endothelial function and endothelial cell survival in the setting of LC exposure. Following exposure to LC, we measured human endothelial cell clusterin protein and gene expression, clusterin secretion and cell death, as well as human arteriole endothelial function. We also tested whether exogenous clusterin co-treatment provides protection against LC vascular toxicity.
Methods
LC purification
Human subjects provided informed consent for urine/tissue collection and the study was approved and supervised by the Institutional Review Boards of the Phoenix Veterans Affairs Medical Center and Medical College of Wisconsin. LC (all lambda type) were purified from the urine of 4 biopsy-proven AL subjects (1 female, 58±6 years, all with cardiac involvement) as per previous method4. Briefly, the urine underwent dialysis, size exclusion and lyophilization. LC was verified using antibody to human lambda light chains (Abcam, Cambridge MA) by Western blot (WB) or enzyme-linked immunosorbent assay (ELISA) (Biovendor, Candler, NC).
Endothelial cells
Human aortic endothelial cells (EC) (Lonza, Hopkinton MA) were exposed to vehicle or LC (20 μg/mL) for 24 hours. Clusterin (Clu) protein level was detected by WB using human clusterin antibody (Santa Cruz Biotech, CA) or ELISA (R&D Systems, Minneapolis MN). Clusterin gene expression was detected using real-time polymerase chain reaction (PCR). Secretion was quantified by measuring clusterin in cell media following 24-hour exposure to LC (50 μg/mL) or vehicle. Cell death was measured using cellular DNA fragmentation ELISA (Roche, Indianapolis IN) in LC (50 μg/mL)-treated EC with or without recombinant human clusterin (Origene, Rockville MD, 300 ng/mL, ~3.6 times mean plasma concentration10) for 1 hour followed by washing/3-hour standing.
Reverse Transcription and PCR Analysis
Total RNA was purified from treated cells using Aurum total RNA kits (Bio-Rad, Hercules CA) and then subjected to first-strand cDNA synthesis using iScript kits (Bio-Rad), both according to the recommendations of the manufacturer. Total clusterin and 18S cDNAs were detected using IQ SYBR green Supermix with a quantitative PCR (qPCR) iQ5 instrument (Bio-Rad). The primer sets used to amplify cDNAs were purchased from Integrated DNA Technologies. Total clusterin primer sequences are as follows: FWD: tggaaga tgctcaacac ctc and REV: cgagtcagaagtgtgggaag. Resultant (Ct) values were analyzed using the Ct comparative method with amplification of 18S rRNA serving as a loading control. Values are reported as fold change against vehicle controls.
Arterioles
Human subcutaneous abdominal adipose arterioles were isolated from 16 patients (1 female, 53.7±3.6 years) without known AL, vascular disease or diabetes during routine surgery. The arterioles were cannulated and pressurized to 60 mm Hg as per previous protocol4. Baseline (control) dilator response was measured following preconstriction (~60% of baseline diameter) with endothelin-1 followed by acetylcholine (10−9-10−4M) and papaverine (10−4M). After washing, arterioles were given LC (20 μg/mL) ± Clu (300 ng/mL) ± NG-nitro-L-arginine methyl ester (L-NAME, 5 mmol, Sigma Aldrich, a nitric oxide synthase inhibitor) intraluminally for 1-hour and a second dilator response was measured.
Unpressurized arterioles were exposed to LC (20 μg/mL) ± Clu (300 ng/mL) ± L-NAME (5 mmol) and nitric oxide (NO) was measured from baseline to 30 minutes by vessel fluorescence using 5 μM 4,5-diaminofluorescein diacetate (Calbiochem, Gibbstown NJ) and fluorescence microscope4. Peroxynitrite was measured using dihydrorhodamine-123 (5 μM) fluorescence (Invitrogen, Eugene OR)4. Fluorescence signal was measured using Image J software (Bethesda MD) as per previous protocol4.
Data Analyses
Data are expressed as means±standard error of means and significant p value was set at <0.05. For multiple group analyses, analysis of variance with Bonferroni post-test (parametric) or Friedman’s test with Dunn’s post-test (nonparametric) were used. For paired analyses, paired t-test (parametric) or Wilcoxon matched pairs sign rank test (nonparametric) were used. Overall arteriole dilator response to acetylcholine was analyzed by calculating log effective concentration 50% (logEC50) using nonlinear regression and variable slope (4 parameters) and least squares fit (GraphPad Prism 5.0, San Diego CA)4.
Results
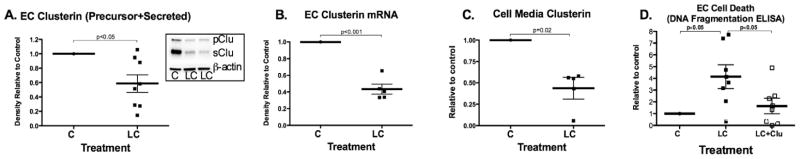
Light chain proteins reduced endothelial cell clusterin protein and mRNA expression (Figure 1A–B). They also caused reduced clusterin secretion (Figure 1C) and increased cell death (Figure 1D). Exogenous clusterin co-treatment attenuated DNA fragmentation.
Figure 1.
Endothelial cell clusterin. A. LC reduced precursor/secreted clusterin production (insert is Western blot), clusterin mRNA production (B) and secreted clusterin (C). D. LC increased EC cell death that was attenuated with clusterin co-treatment.
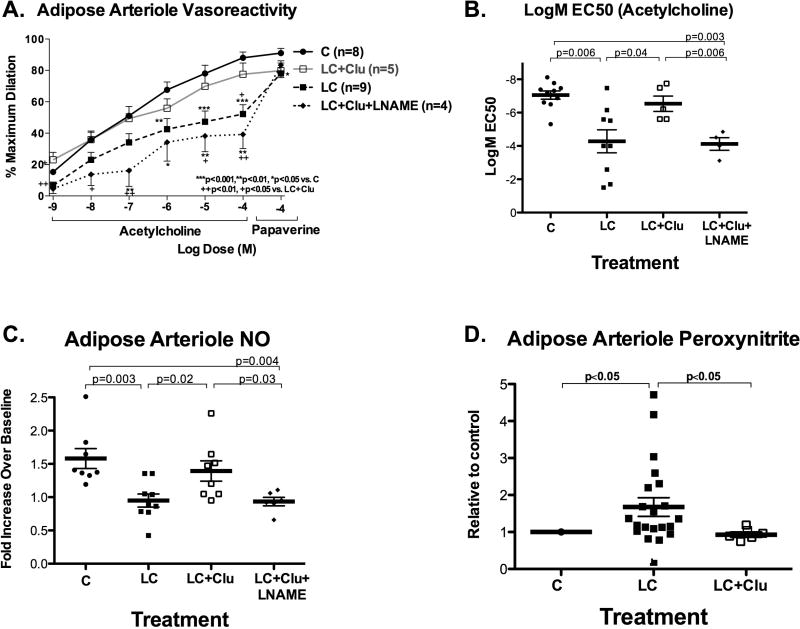
Light chain exposure reduced arteriole dilator response to acetylcholine and, to a modest degree, papaverine (Figure 2A–B), with reduced NO and increased peroxynitrite production (Figure 2C–D). The impaired acetylcholine dilator response to LC in this study (logEC50 −4.28±0.7) was comparable to the impaired response to LC (3 kappa and 1 lambda) from 4 other AL subjects (logEC50 −4.5±0.4) as part of our previously published study4. Clusterin co-treatment preserved overall response to acetylcholine (logEC50), restored NO and prevented increased peroxynitrite (Figure 2). L-NAME blunted the vasoprotective effect of clusterin.
Figure 2.
Light chain proteins and arterioles. A–B. LC impaired dilator response to acetylcholine and papaverine. Co-treatment with clusterin attenuated adverse effect of LC and restored acetylcholine EC50. L-NAME blunted the protective effect of clusterin. C–D. LC reduced 30-minute NO production and increased peroxynitrite production in arterioles; clusterin protected against these changes.
Discussion
We present two novel observations. First, brief exposure to AL-derived immunoglobulin light chain proteins reduced endothelial cell clusterin protein and gene expression and secretion. Second, exogenous clusterin prevented light chain-induced endothelial cell death and arteriole endothelial dysfunction. These findings point to the protective role of clusterin in mitigating light chain vascular toxicity and demonstrate a new pathobiologic mechanism and potential therapeutic target for AL amyloidosis.
AL amyloidosis remains an intractable disease associated with poor outcomes. Despite use of high dose chemotherapy/autologous stem cell transplantation to eradicate plasma cells, recent series still show 30–44% 12–18 month overall mortality11. The mechanisms of LC injury remain poorly understood although increasing evidence points to direct tissue toxicity of prefibrillar LC proteins4, 12. Endothelial dysfunction and ischemic vascular injury induced by LC occur prior to and following amyloid deposition4, 5, 7–9, 13 and represent a less well-known key early pathology of AL that if understood and treated may prevent the serious morbidity that occurs later in the disease.
A major function of clusterin is its chaperoning capacity for protein stabilization and clearance of damaged and unfolded proteins14. In AD, clusterin inhibited amyloid formation through Aβ-protein binding3 and enhanced amyloid clearance14. Because of these effects, it has been proposed that the strong association between clusterin levels and AD severity reflects a neuroprotective response, rather than what was previously thought to be etiopathologic effect2, although this issue remains controversial. Recently, clusterin was found in cardiac amyloid deposits, with serum clusterin reduced in AL patients1. This led to suggestions that a pathogenic role exists for clusterin in AL1. Our results of reduced clusterin protein/gene expression and secretion in EC are consistent with the observed reduction in serum clusterin in AL subjects. However, our findings point to the protective, rather than pathologic, role of clusterin in vascular tissue exposed to LC.
Our current results suggest that clusterin enhances NO bioavailability which may underlie the preservation of arteriole endothelial function, and reduces peroxynitrite which may underlie enhanced cell survival since peroxynitrite is one of the most potent mediators of DNA and protein damage. Future work will further elucidate the exact mechanisms by which clusterin protects against LC toxicity which, based on prior studies on Aβ proteins in AD, may include physico-chemical sequestration, enhanced intracellular LC clearance, as well as antiapoptotic effects14. In addition, the role of chaperone proteins in chronic LC exposure (AL disease) needs to be further studied. The study is limited by the small sample size and therefore the results should be considered preliminary and should be validated in a larger population.
In summary, the findings point to the important protective role of clusterin in mitigating the toxicity of LC to human vascular tissue.
Acknowledgments
We thank John Hatfield, Christe Solie and the Phoenix Veterans Affairs Surgery Service.
Sources of funding:
NIH (R21HL092344-01A1), Amyloidosis Foundation and Carl T. Hayden Medical Foundation. The study was supported by VA employment. The contents do not represent the views of the VA or the US government.
Footnotes
Disclosure: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Greene MJ, Sam F, Soo Hoo PT, Patel RS, Seldin DC, Connors LH. Evidence for a functional role of the molecular chaperone clusterin in amyloidotic cardiomyopathy. Am J Pathol. 178:61–68. doi: 10.1016/j.ajpath.2010.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrijvers EM, Koudstaal PJ, Hofman A, Breteler MM. Plasma clusterin and the risk of Alzheimer disease. JAMA. 305:1322–1326. doi: 10.1001/jama.2011.381. [DOI] [PubMed] [Google Scholar]
- 3.Yerbury JJ, Poon S, Meehan S, Thompson B, Kumita JR, Dobson CM, Wilson MR. The extracellular chaperone clusterin influences amyloid formation and toxicity by interacting with prefibrillar structures. FASEB J. 2007;21:2312–2322. doi: 10.1096/fj.06-7986com. [DOI] [PubMed] [Google Scholar]
- 4.Migrino RQ, Truran S, Gutterman DD, Franco DA, Bright M, Schlundt B, Timmons M, Motta A, Phillips SA, Hari P. Human microvascular dysfunction and apoptotic injury induced by AL amyloidosis light chain proteins. Am J Physiol Heart Circ Physiol. 2011;301:H2305–2312. doi: 10.1152/ajpheart.00503.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berghoff M, Kathpal M, Khan F, Skinner M, Falk R, Freeman R. Endothelial dysfunction precedes C-fiber abnormalities in primary (AL) amyloidosis. Ann Neurol. 2003;53:725–730. doi: 10.1002/ana.10552. [DOI] [PubMed] [Google Scholar]
- 6.Migrino RQ, Hari P, Gutterman DD, Bright M, Truran S, Schlundt B, Phillips SA. Systemic and microvascular oxidative stress induced by light chain amyloidosis. Int J Cardiol. 2010;145:67–68. doi: 10.1016/j.ijcard.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modesto KM, Dispenzieri A, Gertz M, Cauduro SA, Khandheria BK, Seward JB, Kyle R, Wood CM, Bailey KR, Tajik AJ, Miller FA, Pellikka PA, Abraham TP. Vascular abnormalities in primary amyloidosis. Eur Heart J. 2007;28:1019–1024. doi: 10.1093/eurheartj/ehm066. [DOI] [PubMed] [Google Scholar]
- 8.Ando Y, Nyhlin N, Suhr O, Holmgren G, Uchida K, el Sahly M, Yamashita T, Terasaki H, Nakamura M, Uchino M, Ando M. Oxidative stress is found in amyloid deposits in systemic amyloidosis. Biochem Biophys Res Commun. 1997;232:497–502. doi: 10.1006/bbrc.1996.5997. [DOI] [PubMed] [Google Scholar]
- 9.Miani D, Rocco M, Alberti E, Spedicato L, Fioretti PM. Amyloidosis of epicardial and intramural coronary arteries as an unusual cause of myocardial infarction and refractory angina pectoris. Ital Heart J. 2002;3:479–482. [PubMed] [Google Scholar]
- 10.Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, Guntert A, Proitsi P, Powell J, Causevic M, Killick R, Lunnon K, Lynham S, Broadstock M, Choudhry F, Howlett DR, Williams RJ, Sharp SI, Mitchelmore C, Tunnard C, Leung R, Foy C, O’Brien D, Breen G, Furney SJ, Ward M, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Hodges A, Murphy DG, Parkins S, Richardson JC, Resnick SM, Ferrucci L, Wong DF, Zhou Y, Muehlboeck S, Evans A, Francis PT, Spenger C, Lovestone S. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Arch Gen Psychiatry. 67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Migrino RQ, Mareedu RK, Eastwood D, Bowers M, Harmann L, Hari P. Left ventricular ejection time on echocardiography predicts long-term mortality in light chain amyloidosis. J Am Soc Echocardiogr. 2009;22:1396–1402. doi: 10.1016/j.echo.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Palladini G, Lavatelli F, Russo P, Perlini S, Perfetti V, Bosoni T, Obici L, Bradwell AR, D’Eril GM, Fogari R, Moratti R, Merlini G. Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL. Blood. 2006;107:3854–3858. doi: 10.1182/blood-2005-11-4385. [DOI] [PubMed] [Google Scholar]
- 13.Mueller PS, Edwards WD, Gertz MA. Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis. Am J Med. 2000;109:181–188. doi: 10.1016/s0002-9343(00)00471-x. [DOI] [PubMed] [Google Scholar]
- 14.Nuutinen T, Suuronen T, Kauppinen A, Salminen A. Clusterin: a forgotten player in Alzheimer’s disease. Brain Res Rev. 2009;61:89–104. doi: 10.1016/j.brainresrev.2009.05.007. [DOI] [PubMed] [Google Scholar]