Abstract
Abuse of a dangerous street drug called mephedrone (4-methylmethcathinone) has become commonplace in the United States. Mephedrone is hypothesized to possess abuse liability, share pharmacological properties with psychostimulants, and display toxicity that has been linked to fatalities and non-fatal overdoses. Knowledge about the pharmacology of mephedrone has been obtained primarily from surveys of drug abusers and emergency room visits rather than experimental studies. The present study used motor activity and conditioned place preference (CPP) assays to investigate behavioral effects of mephedrone. Acute mephedrone (3, 5, 10, 30 mg/kg, ip) administration increased ambulatory activity in rats. Mephedrone (5 mg/kg, ip)-induced ambulation was inhibited by pretreatment with a dopamine D1 receptor antagonist (SCH 23390) (0.5, 1, 2 mg/kg, ip) and enhanced by pretreatment with a dopamine D2 receptor antagonist (sulpiride) (2 mg/kg, ip). Rats injected for 5 days with low dose mephedrone (0.5 mg/kg, ip) and then challenged with mephedrone (0.5 mg/kg, ip) following 10 days of abstinence displayed sensitization of ambulatory activity. In CPP experiments, mephedrone (30 mg/kg, ip) conditioning elicited a preference shift in both rats and mice. The CPP and dopamine-sensitive motor activation produced by mephedrone is suggestive of abuse liability and indicates commonalities between the neuropharmacological profiles of mephedrone and established drugs of abuse.
Keywords: mephedrone, dopamine, locomotor, conditioned place preference, SCH 23390, sulpiride, bath salts
1. Introduction
Mephedrone (4-methylmethcathinone), also called drone, meph, MCAT, and plant feeder, is a synthetic stimulant that shares structural similarities with amphetamine and cathinone. It is one of several substances contained in psychoactive bath salts (PABS; Wood et al. 2010). Mephedrone use is increasing in the United States, where the American Association of Poison Control Centers reported almost a 20-fold rise in mephedrone exposures from 2010 to 2011. In the United Kingdome, mephedrone was the sixth most frequently used drug among experienced drug users (Winstock et al., 2011). The popularity of mephedrone can be attributed to its high degree of purity, ease of purchase through extensive web-based marketing, versatile administration routes (oral, intranasal), favorable quality of high with the absence of a hangover, and high end-product yields from simple synthetic processes that utilize inexpensive precursors (Schifano and Corkery, 2008; Newcombe, 2009). Chronic mephedrone use results in tolerance and an abstinence syndrome characterized by withdrawal and intense craving (Carhart-Harris et al., 2011; Ross et al., 2011). Health risks include sympathetic stimulation (tachycardia, hypertension, hyperthermia, seizures), altered mental status (panic attacks, agitation, paranoia, hallucinations, self-mutilation, suicide attempts, homicidal activity), and even death (Al Motarreb et al., 2010; Stepens, 2008).
The neuropharmacological profile of mephedrone remains incomplete because only a few experimental studies have investigated its effects in laboratory animals (Kehr et al., 2011; Hadlock et al., 2011; Martinez-Clemente et al., 2011; Motbey et al., 2012). Results from those studies indicate that mephedrone enhances extracellular dopamine and serotonin in the nucleus accumbens, is self-administered under a fixed-ratio schedule of reinforcement, and produces locomotor activation at doses that tend to reduce social preference. In vitro release studies using rat brain synaptosomes indicate mephedrone is a nonselective substrate for plasma membrane monoamine transporters that is similar to MDMA in potency and selectivity but different from methamphetamine, which is a potent and selective substrate for the norepinephrine transporter (NET) and dopamine transporter (DAT; Baumann et al., 2012). Mephedrone also evokes transporter-mediated monoamine release through reversal of normal transporter flux (Baumann et al., 2012). At the cellular level, mephedrone produces patterns of Fos expression that demonstrate its capacity to activate mesolimbic substrates and resemble a combination of those observed with methamphetamine and MDMA (McGregor et al., 2012). For example, mephedrone causes strong Fos expression in the cortex, striatum, and ventral tegmental area, which is typical of both methamphetamine and MDMA, and in the supraoptic nucleus, which is typical of MDMA. Pharmacokinetic studies in rats and humans suggest mephedrone is metabolized in a manner similar to ring-substituted amphetamines (Meyer et al, 2010), thus raising the possibility that bioactive metabolites might be formed in vivo. To provide insight into the behavioral effects of mephedrone, the present study tested the hypotheses that: 1) acute mephedrone exposure produces motor activation that is sensitive to dopamine D1 and D2 receptor activity in rats and 2) repeated mephedrone exposure elicits a conditioned place preference (CPP) in rats and mice.
2. Methods
2.1. Animals and chemicals
Male Sprague-Dawley rats (225–275 g) (ACE Animals, Boyertown, PA) and CD-1 mice (25–30 g; Charles River Laboratories, Raleigh, NC) mice were housed 2 and 4 per cage, respectively. Animals were maintained on a 12-hour light-dark cycle and provided food and water ad libitum. Animal use procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. (R,S)-mephedrone was obtained from Fox Chase Chemical Diversity Center, Inc. (Doylestown, PA, USA). SCH 23390 hydrochloride ((R)-(+)-7-Chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride), a dopamine D1 receptor antagonist, and sulpiride, a dopamine D2 receptor antagonist, were purchased from Tocris Bioscience (St. Louis, MO, USA). All drugs were dissolved in physiological saline and injected intraperitoneally (ip).
2.2. Motor activity experiments
Activity was measured using a Digiscan DMicro system. The activity monitors consisted of transparent plastic boxes (45 cm × 20 cm × 20 cm) set inside metal frames equipped with 16 infrared light emitters and detectors. Ambulation was recorded as consecutive beam breaks resulting from horizontal movement. All animals were placed into activity chambers for 60 min prior to drug administration. For acute mephedrone experiments, rats were injected with mephedrone (0.5, 1, 3, 5, 10, 30 mg/kg) or saline and activity was measured for 60 min. For combination experiments, rats were pretreated with SCH 23390 (0.5 mg/kg), sulpiride (40 mg/kg), or saline. Thirty min later rats were injected with mephedrone (5 mg/kg) or saline and activity was measured for 60 min. For repeated mephedrone administration, rats were injected with mephedrone (0.5 mg/kg) or saline once daily for 5 days. Following 10 days of forced abstinence, rats were placed into activity monitors and injected with mephedrone (0.5 mg/kg) after the 60-min habituation period. Activity was measured for 60 min following mephedrone administration. One-way ANOVA was used to identify main effects followed by a Bonferroni’s test to identify group differences. Criterion for significance was p < 0.05. Doses of SCH 23390 and sulpiride were based on prior in vivo studies in rats (Vezina and Stewart, 1989; Garrett and Holtzman, 1994; Vanattou-Saïfoudine et al., 2010).
2.3. CPP experiments
CPP chambers (45 × 20 × 20 cm) used in the rat experiments consisted of two compartments separated by a removable door. One compartment had black walls with a sandpaper floor. The other compartment had white walls with black vertical stripes and a smooth floor. A counterbalanced, biased design was used. Each rat’s preference for one side of the conditioning chamber was assessed during preconditioning, and the drug-paired side was designated as the non-preferred side. During pre-conditioning rats were allowed access to the entire chamber for 30 min, and the time spent in each compartment was recorded. A rat was considered to be in a compartment if its forelimbs were inside the compartment. The conditioning phase began the day after preconditioning and at the same time of day for each animal. Animals received two conditioning sessions per day, one with an injection of mephedrone (3, 10, 30 mg/kg) and the other with an injection of saline. Following mephedrone or saline administration, rats were respectively confined to the non-preferred or preferred compartment for 30 min. The order of drug injection was randomized each day, and sessions were conducted 4 h apart. Rats in a control group received saline injections during both daily sessions. Rats underwent CPP acquisition trials for 4 consecutive days. Testing occurred the following day during which rats were allowed to freely explore both sides of the chamber in a drug-free state for 30 minutes, and the time spent on each side was recorded. A preference score was determined by subtracting time spent on the non-preferred side prior to mephedrone conditioning from time spent on the non-preferred side after conditioning.
CPP experiments with a dose of 30 mg/kg of mephedrone were conducted in mice for comparison. The design followed that described for rats with some modifications (Huang et al., 2003). A 2-compartment chamber was used (12.7 × 34.7 × 12.7 cm). One compartment was covered with checkered paper on the three walls and floor with a blue light bulb (5-watt) hung at the top, while the other one was covered with white paper and a red light bulb (5-watt) hung at the top. A counterbalanced, unbiased design was used. Mice were handled and weighed for three days for habituation before experiments. On preconditioning day (pre-test), mice were allowed free access to both compartments for 15 min. The time spent in each compartment was recorded and mice spending over 540 s (60% of total time) in either compartment were excluded. Mice then received two conditioning sessions per day for 6 days. Mephedrone (30 mg/kg) treatment was paired with one compartment and in the other session, saline was paired with the opposite compartment. On test day (post-test), the time that each mouse spent in each compartment was recorded for 15 min. A Student’s t-test was used to assess significance. p < 0.05 was considered statistically significant.
3. Results
3.1. Acute mephedrone exposure elicits dopamine-sensitive ambulatory activation
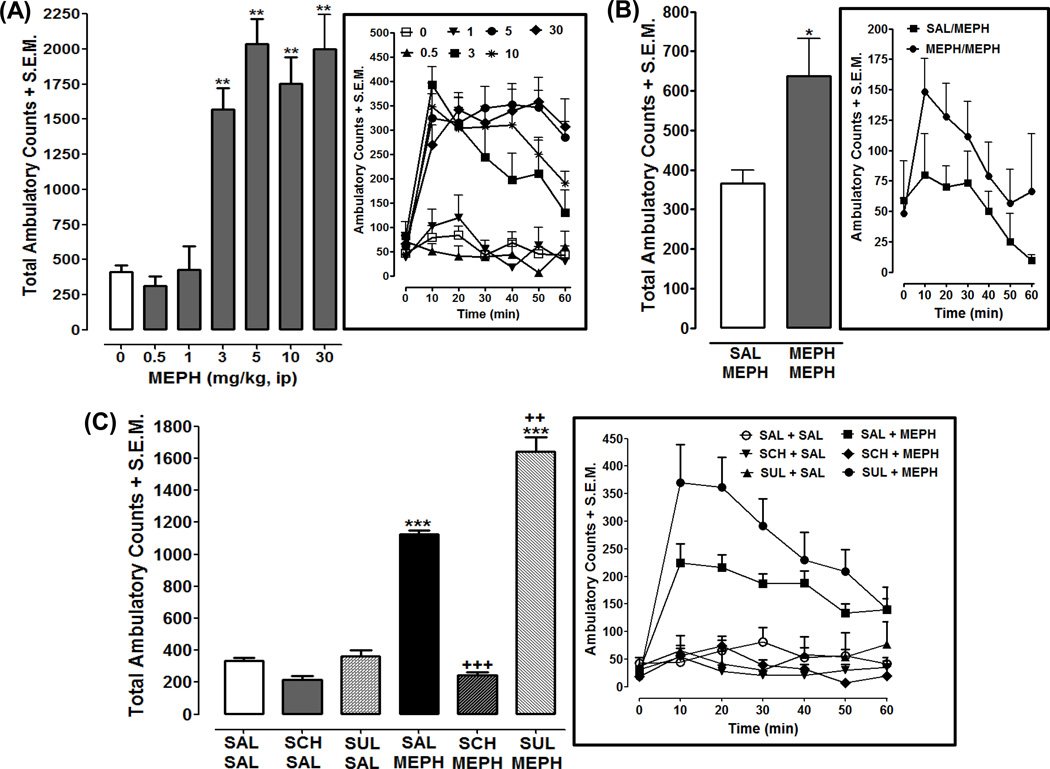
Effects of progressively increasing doses of mephedrone on ambulatory activity are presented in Fig. 1A. One-way ANOVA indicated a significant effect of mephedrone (0–30 mg) on cumulative ambulatory activity [F(6, 52) = 29.81, p < 0.0001] (Fig. 1A). Rats injected with mephedrone (3, 5, 10, 30 mg/kg) displayed enhanced ambulatory activity compared to saline-treated rats (p < 0.01) (Fig. 1A). Effects of repeated low dose administration of mephedrone (0.5 mg/kg) on ambulatory activity are presented in Fig. 1B. Rats exposed for 5 days to mephedrone (0.5 mg/kg) and then challenged with the same dose of mephedrone following 10 days of forced abstinence displayed a sensitized ambulatory response (i.e., mephedrone produced greater ambulatory activity in rats with prior mephedrone experience than in rats previously naïve to mephedrone) (p < 0.05, Student’s t-test) (Fig 1B).
Fig. 1. Acute mephedrone (MEPH) exposure produces a dopamine-sensitive increase in ambulatory activity and repeated exposure to a low dose of MEPH produces sensitization of ambulatory activity.
For each panel (A–C), data are presented as cumulative activity (ambulatory counts + S.E.M. over a 60-min interval) and as a time course (ambulatory counts + S.E.M in 10-min bins). Panel A) Acute effects of different doses of mephedrone. N=8 rats (MEPH groups) or 14 rats for control group (saline). **p < 0.01 compared to saline control (white bar). Panel B) Effects of repeated exposure to a low dose (0.5 mg/kg) of mephedrone: N= 6 rats per group. *p < 0.05 compared to SAL/MEPH group. Panel C) Effects of dopamine D1 and D2 receptor antagonists on acute ambulatory activity produced by 5 mg/kg of mephedrone (MEPH). Rats were pretreated with SCH 23390 (SCH) (0.5 mg/kg), sulpiride (SUL) (40 mg/kg), or saline (SAL) and then injected with MEPH (5 mg/kg) or SAL. N=8 rats per group except for control groups [SAL + SAL (N=14) and SAL + MEPH (N=16)]. ***p < 0.001 compared to SAL + SAL group (white bar) and +++p < 0.001 compared to SAL + MEPH group (black bar).
For combination experiments with dopamine receptor antagonists, one-way ANOVA indicated a significant main effect [F(5, 56) = 232, p < 0.0001] (Fig. 1C). SCH 23390 (0.5 mg/kg) or sulpiride (40 mg/kg) by itself did not affect ambulatory activity compared to saline-treated rats (p > 05), although a trend toward a decrease was observed with SCH 23390. Ambulatory activity elicited by acute mephedrone (5 mg/kg) exposure was significantly less in rats pretreated with SCH 23390 (0.5 mg/kg) than in saline-pretreated rats (p < 0.001). Higher doses of SCH 23390 (1, 2 mg/kg) inhibited mephedrone (5 mg/kg)-induced ambulatory activity but also reduced basal ambulatory activity (not shown). Conversely, mephedrone (5 mg/kg) induced greater ambulatory activity in rats pretreated with sulpiride (40 mg/kg) than in saline-pretreated rats (p < 0.01). Lower doses of sulpiride (10, 20 mg/kg) did not significantly affect ambulatory activity produced by mephedrone (5 mg/kg) (not shown).
3.2. Mephedrone causes CPP
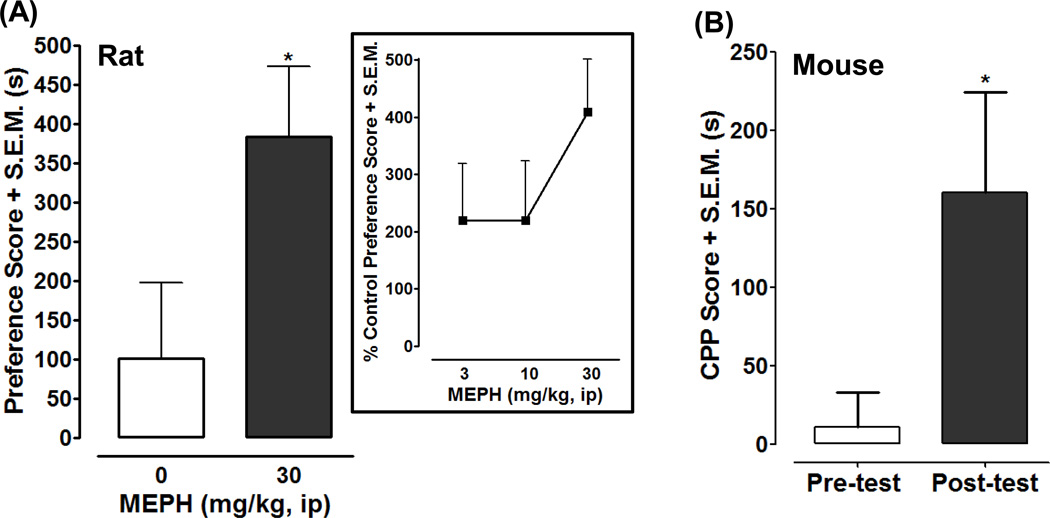
Rats conditioned with mephedrone (30 mg/kg) displayed a greater preference shift compared to saline-treated controls (p < 0.05) (Fig. 3A). A within-subjects comparison for the mephedrone-conditioned group revealed that rats spent a significantly greater amount of time in the non-preferred environment following 4 days of mephedrone conditioning (994 ± 88 s for post-test versus 610 ± 52 s for pre-test; p < 0.01). For saline-treated rats a within-subjects comparison for the time spent by rats in the non-preferred environment before and after conditioning not significantly different (738 ± 84 s for post-test versus 637 ± 45 s for pre-test; p > 0.05). Lower doses of mephedrone (3, 10 mg/kg) caused a greater preference shift compared to saline-treated controls but the effect did not attain statistical significance (p > 0.05; Figure 3A, box). For the mouse experiments, 23 mice which had no bias (spent no more than 9 min of 15 min in one side) for either compartment in the pre-test were used. Figure 3B shows that in mice mephedrone (30 mg/kg once/day for 6 days) induced a significant CPP with a score of 160 ± 64 s (time spent in the drug-paired compartment minus the time in saline-paired compartment) in the post-test, compared with the score of 11 ± 22 s in the pre-test (p < 0.05). Mice were tested several times after CPP was acquired and mephedrone-induced CPP persisted for at least 3 weeks (not shown).
4. Discussion
Mephedrone elicited dopamine-sensitive ambulatory activation following acute exposure, sensitization of ambulatory activity after repeated exposure, and place preference following conditioning. Mephedrone doses that produced locomotor activation here also enhance extracellular dopamine levels in nucleus accumbens of rats (Kehr et al., 2011; Baumann et al., 2012). Evidence that mephedrone increases motor activity and extracellular dopamine suggests that increased dopamine transmission underlies its motor-stimulant properties. We investigated that possibility and demonstrated that pretreatment with a dopamine D1 receptor antagonist (SCH 23390) inhibited mephedrone-evoked ambulation. Identification of dopamine D1 receptor activation as a mechanism through which mephedrone produces ambulation is consistent with active D1 receptors contributing to the motor-stimulant properties of amphetamine, MDMA, and cocaine (Benturquia et al., 2008; Cabib et al., 1991; Gold et al., 1989).
Effects of sulpiride, a dopamine D2 receptor antagonist, on mephedrone-evoked ambulatory activity were different than SCH 23390. Lower doses of sulpiride were ineffective whereas the highest dose (40 mg/kg) enhanced the mephedrone response. The results with sulpiride are more difficult to interpret because prior studies investigating effects of sulpiride on the motor-activating properties of established drugs of abuse are not entirely consistent. Amphetamine-evoked locomotor activation in rats is either slightly enhanced, or unaffected, by doses of 25–30 mg/kg of sulpiride (Vezina and Stewart, 1989; Garrett and Holtzman, 1994). In vivo microdialysis data indicate that amphetamine-evoked extracellular dopamine in the rat striatum is enhanced by 50 mg/kg of sulpiride (Jaworski et al., 2001). Dopamine is known to inhibit its own release through dopamine D2 autoreceptor activation resulting in a reduction of extracellular dopamine (Benoit-Marand et al. 2001; Cragg and Greenfield 1997). Thus, in the case in which sulpiride was co-administered with mephedrone, dopamine D2 receptor antagonism may have caused an increase in dopamine release, and subsequent elevation in extracellular dopamine, that produced further dopamine D1 receptor activation resulting in an augmentation in mephedrone-induced ambulation. Sulpiride has also been tested against MDMA and cocaine. MDMA-evoked hyperactivity in rats is enhanced by sulpiride (100 mg/kg; Vanattou-Saïfoudine et al., 2010) but attenuated by eticlopride, an antagonist of dopamine D2 and D3 receptors (Ball et al., 2003; Bubar et al., 2004). Cocaine produces motor stimulant effects in rats that are enhanced by low doses of sulpiride and decreased by high doses whereas mice lacking dopamine D2 receptors display augmented motor responses to cocaine (Ushijima et al., 1995; Bello et al., 2011). Future studies will use knockout mice and different receptor antagonists to better discern a role for dopamine receptor subtypes in the motor-activating properties of mephedrone.
For repeated mephedrone exposure, rats that were repeatedly injected with mephedrone and then reintroduced to mephedrone following a period of abstinence displayed greater ambulatory activity than rats exposed to mephedrone for the first time. The augmentation in motor activation following repeated, intermittent exposure is called behavioral sensitization and is a common preclinical feature of addictive substances (Vanderschuren and Pierce, 2010; Robinson and Berridge, 2008), although the nature, degree, and consistency of the phenomenon display drug-specific variability. For example, cocaine and amphetamine consistently produce behavioral sensitization across different experimental paradigms whereas MDMA can produce sensitization, tolerance, or neither (Steketee and Kalivas, 2011; Pierce et al., 1996; Ball et al., 2011; Degenhardt et al., 2010; Cole and Sumnall, 2003). Future studies that vary factors such as dose, dosing frequency, and forced abstinence interval are needed to further assess the motor activating properties of mephedrone.
The present experiments provide the first evidence that mephedrone produces CPP in rats and mice. The preference shift detected following mephedrone conditioning suggests the drug displays rewarding properties that are consistent with a risk of abuse liability. Although more extensive experiments are required to compare hedonic effects of mephedrone and established drugs of abuse, the current results do provide insight into the reward profile of mephedrone. For example, mephedrone produced CPP following four conditioning trials, and CPP can also be detected following a similar number of trials with cocaine, amphetamine, and methamphetamine (Soderman and Unterwald, 2009; Leri and Franklin, 2000). One difference between mephedrone and established psychostimulants may be the threshold dose required to elicit CPP. Mephedrone only produced a significant preference shift in rats following conditioning with the highest dose of 30 mg/kg. A preference shift in rats was observed following conditioning with lower doses of 3 and 10 mg/kg, but the effects did not reach statistical significance. In contrast, CPP can be elicited by much lower doses of cocaine, amphetamine, and methamphetamine, and MDMA (Sanchis-Segura and Spanagel, 2006). For instance, MDMA can produce CPP in rats at a dose range of about 6 – 10 mg/kg, with the preference shift beginning to wane at about 20 mg/kg (Marona-Lewika et al., 1996; Diller et al., 2007; Bilsky and Reid, 1991). Methamphetamine produces biphasic effects, with low doses producing reward and high doses producing aversion (Cunningham and Noble, 1992).
In conclusion, mephedrone displayed locomotor stimulant properties that were dependent on increased dopamine transmission and place conditioning effects that were suggestive of rewarding properties. Those behavioral findings correlate well with neurochemical studies demonstrating that mephedrone acts as a substrate for plasma membrane monoamine transporters, evokes transporter mediated-release of monoamines through reversal of normal transporter flux, and enhances extracellular levels of dopamine and serotonin in the rat nucleus accumbens (Baumann et al., 2012; Kehr et al., 2011). Because mephedrone exerts preferential effects on serotonin versus dopamine systems (e.g., serotonergic depletion from neuronal terminals, greater selectivity for serotonin transporters, and greater increase in extracellular serotonin levels), its neurochemical profile has been suggested to resemble MDMA more closely than methamphetamine (Angoa-Pérez et al., 2012). The present study did not directly compare the behavioral effects of mephedrone with methamphetamine and MDMA, but future studies incorporating self-administration and drug discrimination assays are planned to further investigate the potential abuse liability of mephedrone and better assess mechanistic similarities between mephedrone and prototypic drugs of abuse.
Fig. 2. Effects of mephedrone (MEPH) in the CPP assay in rats and mice. Panel A) Rat CPP.
Data are presented as preference score (post-test minus pre-test) (s) (+ S.E.M.) in rats conditioned with saline (0 mg/kg) (N=12) or MEPH (30 mg/kg) (N=10). *p < 0.05 compared to saline control. Box) Data from rat CPP experiments are presented as percentage of control (saline-treated) preference score (+ S.E.M.) for different doses of MEPH (3, 10, 30 mg/kg). N = 10–12 rats per group. Panel B) Mouse CPP: Data obtained from 23 mice are presented as CPP score (s) + S.E.M. (time spent in 30 mg/kg MEPH-paired compartment minus the time spent in saline-paired compartment). *p < 0.05 compared to the pre-test.
Acknowledgements
Role of Funding Source
The present study was supported by NIDA grants P30 DA013429 and T32 DA007237.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Disclosures
Contributors
Authors Scott M. Rawls, Lee-Yuan Liu-Chen, and Renata Lisek designed the studies. Author Allen B. Reitz synthesized mephedrone. Authors Renata Lisek and Ekaterina Yuvasheva conducted the motor activity studies (mephedrone dose response and effects of D1 and D2 antagonists on mephedrone activity). Author Renata Lisek conducted the conditioned place preference studies in rats and the mephedrone behavioral sensitization study. Authors Wei Xu and Yi-Ting Chiu conducted conditioned place preference studies in mice. Author Scott M. Rawls conducted the statistical analyses for the rat experiments and managed the literature searches and summaries of previous related work. Authors Lee-Yuan Liu-Chen, Wei Xu, and Yi-Ting Chiu conducted statistical analyses for the mouse experiments. Author Scott M. Rawls wrote drafts of the manuscript, which were subsequently circulated to all authors for their comments, critiques and suggestions. All authors contributed to and have approved the final manuscript.
Conflict of Interest
All authors declare that they have no conflicts of interest.
References
- Angoa-Pérez M, Kane MJ, Francescutti DM, Sykes KE, Shah MM, Mohammed AM, Thomas DM, Kuhn DM. Mephedrone, an abused psychoactive component of 'bath salts' and methamphetamine congener, does not cause neurotoxicity to dopamine nerve endings of the striatum. J. Neurochem. 2012;120:1097–1107. doi: 10.1111/j.1471-4159.2011.07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Motarreb A, Al-Habori M, Broadley KJ. Khat chewing, cardiovascular diseases and other internal medical problems: the current situation and directions for future research. J. Ethnopharmacol. 2010;132:540–548. doi: 10.1016/j.jep.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Ball KT, Budreau D, Rebec GV. Acute effects of 3,4-methylenedioxymethamphetamine on striatal single-unit activity and behavior in freely moving rats: differential involvement of dopamine D(1) and D(2) receptors. Brain Res. 2003;994:203–215. doi: 10.1016/j.brainres.2003.09.037. [DOI] [PubMed] [Google Scholar]
- Ball KT, Klein JE, Plocinski JA, Slack R. Behavioral sensitization to 3,4-methylenedioxymethamphetamine is long-lasting and modulated by the context of drug administration. Behav. Pharmacol. 2011;22:847–850. doi: 10.1097/FBP.0b013e32834d13b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. The Designer Methcathinone Analogs, Mephedrone and Methylone, are Substrates for Monoamine Transporters in Brain Tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noaín D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M. Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat. Neurosci. 2011;14:1033–1038. doi: 10.1038/nn.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit-Marand M, Borrelli E, Gonon F. Inhibition of dopamine release via presynaptic D2 receptors: time course and functional characteristics in vivo. J. Neurosci. 2001;21:9134–9141. doi: 10.1523/JNEUROSCI.21-23-09134.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benturquia N, Courtin C, Noble F, Marie-Claire C. Involvement of D1 dopamine receptor in MDMA-induced locomotor activity and striatal gene expression in mice. Brain Res. 2008;1211:1–5. doi: 10.1016/j.brainres.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Bilsky EJ, Reid LD. MDL72222, a serotonin 5-HT3 receptor antagonist, blocks MDMA's ability to establish a conditioned place preference. Pharmacol. Biochem. Behav. 1991;39:509–512. doi: 10.1016/0091-3057(91)90217-p. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Pack KM, Frankel PS, Cunningham KA. Effects of dopamine D1- or D2-like receptor antagonists on the hypermotive and discriminative stimulus effects of (+)- MDMA. Psychopharmacology. 2004;173:326–336. doi: 10.1007/s00213-004-1790-1. [DOI] [PubMed] [Google Scholar]
- Cabib S, Castellano C, Cestari V, Filibeck U, Puglisi-Allegra S. D1 and D2 receptor antagonists differently affect cocaine-induced locomotor hyperactivity in the mouse. Psychopharmacology. 1991;105:335–339. doi: 10.1007/BF02244427. [DOI] [PubMed] [Google Scholar]
- Cole JC, Sumnall HR. The pre-clinical behavioural pharmacology of 3,4- methylenedioxymethamphetamine (MDMA) Neurosci. Biobehav. Rev. 2003;27:199–217. doi: 10.1016/s0149-7634(03)00031-9. [DOI] [PubMed] [Google Scholar]
- Cragg SJ, Greenfield SA. Differential autoreceptor control of somatodendritic and axon terminal dopamine release in substantia nigra, ventral tegmental area, and striatum. J. Neurosci. 1997;17:5738–5746. doi: 10.1523/JNEUROSCI.17-15-05738.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Noble D. Methamphetamine-induced conditioned place preference or aversion depending on dose and presence of drug. AnnN. Y. Acad. Sci. 1992;654:431–433. doi: 10.1111/j.1749-6632.1992.tb25989.x. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Bruno R, Topp L. Is ecstasy a drug of dependence? Drug Alcohol Depend. 2010;107:1–10. doi: 10.1016/j.drugalcdep.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Diller AJ, Rocha A, Cardon AL, Valles R, Wellman PJ, Nation JR. The effects of concurrent administration of +/-3,4-methylenedioxymethamphetamine and cocaine on conditioned place preference in the adult male rat. Pharmacol. Biochem. Behav. 2007;88:165–170. doi: 10.1016/j.pbb.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett BE, Holtzman SG. D1 and D2 dopamine receptor antagonists block caffeineinduced stimulation of locomotor activity in rats. Pharmacol. Biochem. Behav. 1994;47:89–94. doi: 10.1016/0091-3057(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Gold LH, Geyer MA, Koob GF. Neurochemical mechanisms involved in behavioral effects of amphetamines and related designer drugs. NIDA Res. Monogr. 1989;94:101–126. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, Andrenyak DM, Vieira-Brock PL, German CL, Conrad KM, Hoonakker AJ, Gibb JW, Wilkins DG, Hanson GR, Fleckenstein AE. 4-Methylmethcathinone(mephedrone): neuropharmacological effects of a designer stimulant of abuse. J. Pharmacol. Exp. Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang EY, Liu TC, Tao PL. Co-administration of dextromethorphan with morphine attenuates morphine rewarding effect and related dopamine releases at the nucleus accumbens. Naunyn Schmiedebergs Arch. Pharmacol. 2003;368:386–392. doi: 10.1007/s00210-003-0803-7. [DOI] [PubMed] [Google Scholar]
- Jaworski JN, Gonzales RA, Randall PK. Effect of dopamine D2/D3 receptor antagonist sulpiride on amphetamine-induced changes in striatal extracellular dopamine. Eur. J. Pharmacol. 2001;418:201–206. doi: 10.1016/s0014-2999(01)00936-0. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. Mephedrone, compared to MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and serotonin levels in nucleus accumbens of awake rats. BrJ. Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Drugs of abuse: anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992;13:177–184. doi: 10.1016/0165-6147(92)90060-j. [DOI] [PubMed] [Google Scholar]
- Leri F, Franklin KB. Effects of diazepam on conditioned place preference induced by morphine or amphetamine in the rat. Psychopharmacology. 2000;150:351–360. doi: 10.1007/s002130000448. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. Reinforcing effects of certain serotonin-releasing amphetamine derivatives. Pharmacol. Biochem. Behav. 1996;53:99–105. doi: 10.1016/0091-3057(95)00205-7. [DOI] [PubMed] [Google Scholar]
- Martínez-Clemente J, Escubedo E, Pubill D, Camarasa J. Interaction of mephedrone with dopamine and serotonin targets in rats. Eur. Neuropsychopharmacol. 2011;22:231–236. doi: 10.1016/j.euroneuro.2011.07.009. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Maurer HH. Metabolism of designer drugs of abuse: an updated review. Curr. Drug Metab. 2010;11:468–482. doi: 10.2174/138920010791526042. [DOI] [PubMed] [Google Scholar]
- Motbey CP, Hunt GE, Bowen MT, Artiss S, McGregor IS. Mephedrone (4-methylmethcathinone, 'meow'): acute behavioural effects and distribution of Fos expression in adolescent rats. Addict. Biol. 2012;17:409–422. doi: 10.1111/j.1369-1600.2011.00384.x. [DOI] [PubMed] [Google Scholar]
- Newcombe R. Mephedrone: Use of Mephedrone (M-Cat, Meow) in Middlesbrough. Manchester, UK: Lifeline Publications; 2009. [Google Scholar]
- Pierce RC, Bell K, Duffy P, Kalivas PW. Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. Neurosci. 1996;16:1550–1560. doi: 10.1523/JNEUROSCI.16-04-01550.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Psychonaut Web Mapping Research Group. Mephedrone Report. London, UK: Institute of Psychiatry, King’s College London; 2009. [Google Scholar]
- Robinson TE, Berridge KC. The incentive sensitization theory of addiction: some current issues. Philos. TransR. Soc. LondB. Biol. Sci. 2008;363:3137–3146. doi: 10.1098/rstb.2008.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Schifano F, Corkery J. Cocaine/crack cocaine consumption, treatment demand, seizures, related offences, prices, average purity levels and deaths in the UK (1990 – 2004) J. Psychopharmacol. 2008;22:71–79. doi: 10.1177/0269881107079170. [DOI] [PubMed] [Google Scholar]
- Soderman AR, Unterwald EM. Cocaine-induced mu opioid receptor occupancy within the striatum is mediated by dopamine D2 receptors. Brain Res. 2009;1296:63–71. doi: 10.1016/j.brainres.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steketee JD, Kalivas PW. Drug wanting: behavioral sensitization and relapse to drugseeking behavior. Pharmacol. Rev. 2011;63:348–365. doi: 10.1124/pr.109.001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepens A, Logina I, Liguts V, Aldins P, Eksteina I, Platkājis A, Mārtinsone I, Tērauds E, Rozentāle B, Donaghy M. A Parkinsonian syndrome in methcathinone users and the role of manganese. N. Engl. J. Med. 2008;358:1009–1017. doi: 10.1056/NEJMoa072488. [DOI] [PubMed] [Google Scholar]
- Ujike H, Onoue T, Akiyama K, Hamamura T, Otsuki S. Effects of selective D-1 and D-2 dopamine antagonists on development of methamphetamine-induced behavioral sensitization. Psychopharmacology. 1989;98:89–92. doi: 10.1007/BF00442011. [DOI] [PubMed] [Google Scholar]
- Ushijima I, Carino MA, Horita A. Involvement of D1 and D2 dopamine systems in the behavioral effects of cocaine in rats. Pharmacol. Biochem. Behav. 1995;52:737–741. doi: 10.1016/0091-3057(95)00167-u. [DOI] [PubMed] [Google Scholar]
- Vanattou-Saïfoudine N, McNamara R, Harkin A. Caffeine promotes dopamine D1 receptor-mediated body temperature, heart rate and behavioural responses to MDMA ('ecstasy') Psychopharmacology. 2010;211:15–25. doi: 10.1007/s00213-010-1864-1. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJ, Pierce RC. Sensitization processes in drug addiction. Curr. Top. Behav. Neurosci. 2010;3:179–195. doi: 10.1007/7854_2009_21. [DOI] [PubMed] [Google Scholar]
- Vezina P, Stewart J. The effect of dopamine receptor blockade on the development of sensitization to the locomotor activating effects of amphetamine and morphine. Brain Res. 1989;499:108–120. doi: 10.1016/0006-8993(89)91140-2. [DOI] [PubMed] [Google Scholar]
- Ward SJ, Rasmussen BA, Corley G, Henry C, Kim JK, Walker EA, Rawls SM. β-lactam antibiotic decreases acquisition of and motivation to respond for cocaine, but not sweet food, in C57Bl/6 mice. Behav. Pharmacol. 2011;22:370–373. doi: 10.1097/FBP.0b013e3283473c10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, Dargan PI. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin. Toxicol. 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]