Abstract
Background
Our previous research with the GABA reuptake inhibitor tiagabine suggested the involvement GABA in the interoceptive effects of Δ9-THC. The aim of the present study was to determine the potential involvement of the GABAB receptor subtype by assessing the separate and combined effects of the GABAB-selective agonist baclofen and Δ9-THC using pharmacologically specific drug-discrimination procedures.
Methods
Eight cannabis users learned to discriminate 30 mg oral Δ9-THC from placebo and then received baclofen (25 and 50 mg), Δ9-THC (5, 15 and 30 mg) and placebo, alone and in combination. Self-report, task performance and physiological measures were also collected.
Results
Δ9-THC functioned as a discriminative stimulus, produced subjective effects typically associated with cannabinoids (e.g., High, Stoned, Like Drug), elevated heart rate and impaired rate and accuracy on a psychomotor performance task. Baclofen alone (50 mg) substituted for the Δ9-THC discriminative stimulus, and both baclofen doses shifted the discriminative-stimulus effects of Δ9-THC leftward/upward. Similar results were observed on other cannabinoid-sensitive outcomes, although baclofen generally did not engender Δ9-THC-like subjective responses when administered alone.
Conclusions
These results suggest that the GABAB receptor subtype is involved in the abuse-related effects of Δ9-THC, and that GABAB receptors were responsible, at least in part, for the effects of tiagabine-induced elevated GABA on cannabinoid-related behaviors in our previous study. Future research should test GABAergic compounds selective for other GABA receptor subtypes (i.e., GABAA) to determine the contribution of the different GABA receptors in the effects of Δ9-THC, and by extension cannabis, in humans.
Keywords: drug-discrimination, marijuana, subjective effects, repeated acquisition task, digit-symbol-substitution task, cardiovascular
1. Introduction
In a previous study we investigated the potential involvement of γ-aminobutyric acid (GABA) in the discriminative-stimulus effects of Δ9-tetrahydrocannabinol (Δ9-THC) in humans by administering the GABA reuptake inhibitor tiagabine in subjects trained to discriminate oral Δ9-THC (Lile et al., 2012). Tiagabine alone occasioned Δ9-THC-appropriate responding, and significantly enhanced drug-appropriate responding when combined with Δ9-THC. Similar potentiation was observed on self-report and performance measures. Those results were consistent with observations from another experiment in which the CB agonist nabilone was administered alone and in combination with Δ9-THC (Lile et al., 2011). The leftward/upward shift in the dose-effect curves across several cannabinoid-sensitive measures following tiagabine administration, and the similarity to the results obtained with nabilone, indicate that GABA is involved in the behavioral effects of cannabinoids in humans. Because tiagabine modulates GABA activity by blocking reuptake transporters, thereby producing global elevations in GABA levels, the contribution of specific receptor subtypes could not be determined in our previous study.
There are two well characterized GABA receptor subtypes, ionotropic GABAA and metabotropic GABAB receptors, and prior research has implicated both in the behavioral and physiological effects of cannabinoids. With respect to GABAB, a preclinical study found that Δ9-THC and baclofen both decreased open field locomotor activity in rodents, and the GABAB antagonist CGP 35348 reversed the locomotor-decreasing effects of both drugs (Romero et al., 1996). In addition, baclofen augmented the catalepsy produced by Δ9-THC in rodents (Pertwee et al., 1988). Preclinical studies that have tested cannabinoid and GABAB agonists separately have demonstrated overlap in their pharmacological profiles; specifically, these drugs are anxiolytic (Moreira and Wotjak, 2010; Pilc and Nowak, 2005), induce hypothermia (Frosini et al., 2004; Wenger and Moldrich, 2002), impair memory processes (Castellano et al., 2003; DeSousa et al., 1994), and produce peripheral antinociception (Dario et al., 2007; Hohmann, 2002).
GABA participation in the effects of cannabinoids in humans, particularly GABAB, has received less attention. There appears to be only a single clinical research study that has combined a GABAB agonist (baclofen) with a cannabinoid under controlled laboratory conditions (Haney et al., 2010). In that study, daily cannabis users were maintained on 60 and 90 mg/day of baclofen (20 and 30 mg, t.i.d.) and then received active (3.3% Δ9-THC) and placebo cannabis. Baclofen significantly decreased self-reported ratings of High and Want Marijuana, but did not impact cannabis self-administration in a relapse model of cannabis use. In addition, a case study of six male patients with cannabis dependence suggested that 40 mg/day baclofen treatment reduced the signs and symptoms of cannabis withdrawal and facilitated abstinence (Nanjayya et al., 2010). Further clinical research with GABAB compounds is warranted given the important objective of identifying medications to manage cannabis-use disorders (Vandrey and Haney, 2009).
The purpose of this experiment was therefore to determine the potential involvement of the GABAB receptor subtype in the clinical effects of Δ9-THC by assessing the separate and combined effects of Δ9-THC and the preferential GABAB agonist baclofen using drug-discrimination procedures. The discriminative-stimulus effects of a drug are concordant with the central actions of a drug at the receptor level, and are therefore pharmacologically specific (Holtzman and Locke, 1988). For example, in previous studies in which human subjects learned to discriminate Δ9-THC, the cannabinoid agonist nabilone, but not methylphenidate, triazolam or hydromorphone, occasioned drug-appropriate responding, whereas each of these drugs increased positive drug-effect questionnaire ratings (Lile et al., 2009, 2010). In the present study, findings from the drug discrimination task were supplemented by testing the subject-rated, psychomotor performance, cardiovascular and thermoregulatory effects of baclofen and Δ9-THC separately and in combination.
2. Methods
2.1. Subjects
Adults with a history of cannabis use were recruited from the local community. Potential subjects completed demographic, drug-use history and medical history questionnaires, as well as medical screens. Individuals with current or past histories of Axis I or DSM-IV psychiatric disorder, including substance dependence disorders other than nicotine, were excluded from participating. Substance abuse was not an exclusion criterion. The Institutional Review Board of the University of Kentucky Medical Center approved the study and the informed consent document.
Eight potential subjects (6 white males, 1 white female and 1 female of mixed race) were screened for participation, and all completed the protocol. They ranged in age from 18 to 30 years (median = 22 years), in education from 12 to 17 years (median = 15), and in weight from 59 to 106 kg (median = 69 kg). All reported cannabis use (range of 4 to 7 days/week, mean = 6; 4-13 “puffs” per day, mean = 6). Seven subjects reported consuming alcohol-containing beverages 2 to 4 days per week (mean = 2). Five subjects reported daily tobacco cigarettes use; one subject smoked one pack per day and the remaining four subjects used between 1 to 5 tobacco cigarettes per day (mean = 4). Other lifetime non-medical drug use included benzodiazepines (2 subjects, no reported use in the month prior to screening), hallucinogens (three subjects, no reported use in the month prior to screening), opioids (three subjects, no reported use in the month prior to screening) and stimulants (three subjects, with one reporting amphetamine use as a study aid on 6 occasions in the month prior to participation). All subjects provided a urine sample negative for substances other than cannabinoids.
2.2. General Procedures
Subjects were enrolled as outpatients at the University of Kentucky Residential Research Facility. They completed two drug-free practice sessions to become familiarized with the procedures prior to completing between 22 and 25 (mean = 23) experimental sessions. Study participation lasted 5 to 11 weeks (mean = 7).
Subjects were informed that they would receive placebo, Δ9-THC and baclofen, alone or in combination, but were blind to the dose and order of administration. They were asked to abstain from illicit drugs other than cannabis, as well as over-the-counter medications, with the exception of non-steroidal anti-inflammatory analgesics, for the duration of the experiment, and any drug use on the day of experimental sessions to avoid potentially unsafe drug interactions. In addition, subjects were asked to refrain from food or caffeine intake for 4 hours prior to each experimental session, or alcohol for 12 hours prior to and following each experimental session. Subjects who smoked tobacco cigarettes were also asked to abstain from smoking the morning of each session, but were allowed to smoke a single tobacco cigarette upon arrival to the laboratory to avoid testing under conditions of nicotine withdrawal, but not again until the session had ended. There was no indication of nicotine withdrawal in these subjects.
Experimental sessions were conducted at a fixed time, Monday through Friday, and lasted approximately 6.5 h; subjects participated in 1 to 5 sessions per week. At the beginning of each session, breath (Alcolyzer, AK Solutions USA, Palisades Park, NJ) and urine tests to assess drug use (Integrated E-Z Split Cut, Acon Laboratories, San Diego, CA) and pregnancy (hCG Assay, Rapid Detect, Inc., Poteau, OK) were conducted. Urine samples were negative for substances other than cannabis metabolites (i.e., 11-nor-9-carboxy-Δ9-THC) and pregnancy throughout the study, with the following exception. Urine toxicology screening for one subject was positive for recent cocaine use; this subject was discharged from that session, but was permitted to continue study participation once a urine sample negative for all non-cannabinoid drugs was provided. At session intake, subjects also completed a modified version of the U.S. Department of Transportation Drug Evaluation and Classification Screening (walk and turn, timed one-leg balance or Romberg balance, time interval reproduction and the finger-to-nose tests; Toland and Green, 1991) and were observed by the research staff for signs of cannabis intoxication (e.g., bloodshot, glassy eyes); no cannabis intoxication was detected during intake throughout the study. Subjects were reassessed at the end of the session for possible intoxication and/or residual drug effects prior to release. In addition, subjects were required to report no further drug effects. If necessary, subjects were retained at the laboratory beyond the scheduled session time until residual drug effects dissipated.
Subjects consumed a low-fat snack approximately 20 minutes prior to drug administration. Because the peak plasma levels of Δ9-THC and baclofen were expected to occur at approximately the same time (i.e., 2 to 4 h) following oral administration (Hollister et al, 1981; baclofen Product Information, 2008), the drugs were administered together.
2.3. Drug-Discrimination Procedure
Well-established drug-discrimination procedures (e.g., Lile et al., 2009; Rush et al., 1998) were used to teach subjects to discriminate between a “Drug X” condition (i.e., 30 mg Δ9-THC) and a “Not Drug X” condition (i.e., placebo).
Sampling Phase
During two sampling sessions, subjects ingested four capsules that contained a total of 30 mg Δ9-THC. The capsules were identified by a letter code (e.g., Drug X; a unique letter code was used for each subject); subjects were not informed that the capsules contained Δ9-THC, but were instructed to associate drug effects with the letter code.
Control Phase
A control phase, lasting between 4 and 12 sessions, was conducted to determine whether subjects could discriminate 30 mg Δ9-THC from placebo. During this phase, subjects ingested capsules under double-blind conditions. The order of drug administration was random except that all subjects received each training condition, 30 mg Δ9-THC and placebo, at least twice every four sessions. Sessions were identical to the sampling phase, except subjects were not informed which drug condition (i.e., Drug X or Not Drug X) was administered until the end of the session. The criterion for having acquired the discrimination was ≥ 80% correct responding on the drug-discrimination task during the final 5-h assessment for four consecutive sessions. If subjects did not meet the control criteria within 12 sessions, they would have been dismissed from the study. Seven subjects correctly identified each of the two training conditions, 30 mg Δ9-THC and placebo, twice in the first four sessions. One subject required six sessions to meet the discrimination criteria.
Test Phase
A final test phase, lasting at least 16 sessions, was conducted to test placebo, Δ9-THC (5, 15 and 30 mg) and baclofen (25 and 50 mg), alone and in combination. Each drug dose and dose combination was administered once for a total of 12 sessions. The order of drug administration was random except that an active drug dose was never administered on more than three consecutive sessions, and the highest dose of Δ9-THC (30 mg) and baclofen (50 mg) were not administered together before a lower dose combination was tested.
Four control sessions (i.e., 30 mg Δ9-THC or placebo) were also randomly included in the test phase to monitor drug-discrimination performance and provide feedback to subjects regarding their performance. If a subject responded incorrectly on a control session, additional control sessions were scheduled until the subject accurately identified both of the training conditions once each across consecutive sessions. Only one subject incorrectly identified control sessions during the test phase, resulting in the addition of three more control sessions for that individual. Control sessions comprised an average of 26% of sessions during the test phase.
2.4. Outcome measures
Drug discrimination was the primary outcome measure, supplemented by self-report questionnaires, performance tasks and physiological assessments. Data were collected in fixed order, immediately prior to drug administration, and 1, 2, 3, 4 and 5 h after Δ9-THC administration, with the following exceptions. The drug-discrimination task was completed at only the 3 to 5 h time points because of the slow onset of the effects of Δ9-THC observed in our previous studies (Lile et al., 2009, 2010). A non-contingent Multiple-Choice Procedure was completed at the end of the 5-h assessment. Except for temperature assessments, data were collected on an Apple Macintosh computer (Apple Computer, Inc., Cupertino, CA).
Drug-Discrimination Task
Two circles labeled Drug X and Not Drug X and associated counters were displayed on a computer screen. Button presses increased the counter for a particular circle according to a fixed-interval 1-sec schedule for 60 s (no change-over delay). At the end of the final assessment, subjects were informed whether it was a control or a test session. During control sessions, points accumulated on the correct option were exchangeable for money at a rate of $0.28/point (up to approximately $50.00/session). During test sessions, when drugs and/or doses other than the control conditions were administered, subjects earned the average from all previous sessions in which control conditions were tested. These monetary contingencies prompted subjects to acquire points on the counters based on the presence (or absence) of the training drug cue at the time of task performance during both control and test sessions. The dependent variable for this task was the percent responding on the drug-appropriate option at the 5-h time point.
Subject-Rated Questionnaires
Visual Analog Scale (VAS) Subject-Rated Drug-Effect Questionnaire
Subjects rated 20 items (see Lile et al., 2012) presented individually on the computer by marking a 100-unit line anchored on the extremes by “Not At All” and “Extremely.”
Multiple-Choice Procedure
This task provided a hypothetical assessment of the monetary value of each dose condition (Griffiths et al., 1993). Subjects made a series of nine discrete choices between the drug dose received during that session and ascending amounts of money. The dollar value increased across the choices ($0.10, 0.25, 0.50, 1.00, 2.00, 4.00, 8.00, 16.00 and 32.00). The dependent measure on the Multiple-Choice Procedure was the maximum dollar value at which subjects chose drug over money (i.e., “crossover point”).
Performance Tasks
These tasks were chosen because prior research has found them to be sensitive to the impairing effects of oral Δ9-THC (Hart et al., 2005; Kamien et al., 1994) and smoked cannabis (Heishman et al., 1989; Kelly et al., 1990, 1993; Wilson et al., 1994). Subjects did not receive additional compensation based on task performance.
Repeated Acquisition of Response Sequences Task (RA task)
During the initial acquisition component, subjects pressed 4 keys (1, 3, 7 and 9) on a numeric keypad to learn a new, randomly-determined 10-response sequence (a “chain”) for 180 s. When a correct key in the sequence was pressed, a “position” counter on the screen increased by 1. When the tenth and final key in the sequence was pressed, a “points” counter increased by one, and the position counter reset. A 60-s performance component of this task, in which the 10-response sequence remained the same across trials, followed the acquisition component. The primary dependent measures for this task were the number of chains completed (i.e., accuracy) and the total number of responses emitted (i.e., response rate).
Digit-Symbol-Substitution Test (DSST)
A modified version of the computerized DSST was used (McLeod et al., 1982). Briefly, subjects used a numeric keypad to enter the geometric pattern associated with one of nine patterns identified on a given trial for 90 s. The dependent measures were the number of patterns the subject entered correctly (i.e., trials correct; accuracy) and the total number of patterns entered (i.e., trials completed; response rate).
Time Reproduction Task
Four time periods, 3, 30, 60 and 180s were presented. Subjects responded to start a timer, and held down the response key until they believed that the interval had elapsed.
Physiological Indices
Heart Rate and Blood Pressure
Heart rate and blood pressure were recorded using an automated monitor (DINAMAP, Johnson and Johnson, Alexandria, TX).
Temperature
An infrared thermographic scanner (Derma-Temp, Exergen Corporation, Watertown, MA) was used to measure skin temperature on the tip of the index finger. An electronic thermometer was used to measure oral temperature.
2.5. Drug Administration
Doses were prepared by encapsulating commercially available capsules of dronabinol (Δ9-THC in sesame oil, Solvay Pharmaceuticals, Marietta, GA) and baclofen (Major Pharmaceuticals, Livonia, MI) in four opaque green size 00 capsules. Cornstarch was used to fill the remainder of all capsules. Placebo capsules contained only cornstarch.
For reference, the acute recommended Δ9-THC dosing range in adults for appetite stimulation and the prevention of nausea and vomiting is 2.5 to 20 mg (dronabinol product information). The recommended starting dose of baclofen for the treatment of muscle spasticity is 5 mg, taken three times a day, subsequently increased at 3-day intervals by 5 mg. The optimum dosage generally ranges from 30-75 mg daily (baclofen Product Information, 2008). Worth noting is that in the initial pharmacokinetic and efficacy studies with baclofen, single doses of 40 mg and 50 mg were administered under controlled clinical conditions (baclofen Product Information, 2008).
2.6. Data Analyses
Data from all eight subjects were analyzed statistically. Drug-discrimination data were analyzed as percent drug-appropriate responding using two-factor, repeated-measure analysis of variance (ANOVA; JMP, SAS Institute Inc., Cary, NC) with Δ9-THC and baclofen as the factors. For the 30 mg Δ9-THC and placebo conditions, data were averaged across the sessions in which these conditions were presented during the test phase. Raw data from the self-reported drug-effect questionnaires, performance tasks and physiological measures were analyzed for each drug as the peak-effect (i.e., the mean of the maximum or minimum value observed for each subject 1 to 5 h after drug administration) using two-factor, repeated-measure ANOVA. Crossover point data from the Multiple-Choice Procedure were first subjected to a square-root transformation because of violations in the assumptions of ANOVA (i.e., monetary increments across successive choices range from $0.15 to $16.00). For all measures, effects were considered significant for p ≤ 0.05. If a main effect of Δ9-THC attained statistical significance, contrast statements were used to compare active drug doses to placebo; if a main effect of baclofen, or an interaction of Δ9-THC and baclofen, attained statistical significance, each dose of Δ9-THC alone was compared to that dose of Δ9-THC administered in combination with baclofen.
3. Results
3.1. Drug-discrimination task
All subjects met the discrimination criterion, which took an average of 4.3 sessions (range = 4 to 6). During the final four sessions of the control phase, subjects reported an average of 0.0 (SEM = 0.0) percent Δ9-THC-appropriate responding on the drug-discrimination task during placebo sessions and 100.0 (SEM = 0.0) percent drug-appropriate responding during sessions when the training dose of Δ9-THC (i.e., 30 mg) was administered.
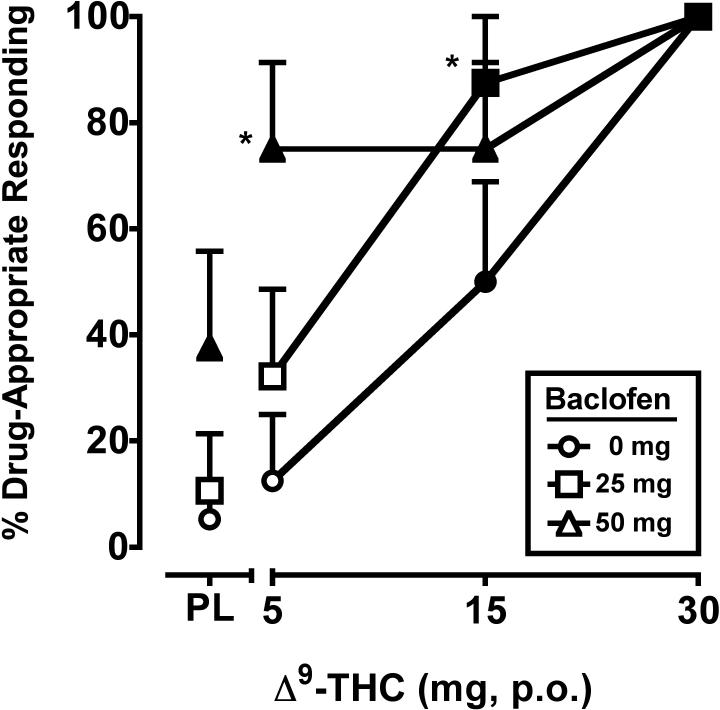
The two-factor, repeated-measure ANOVA revealed a significant main effect of Δ9-THC (F3,21 = 21.9, p < 0.001) and baclofen (F2,14 = 8.4, p < 0.01) on percentage of Δ9-THC-appropriate responding, with a trend (p = 0.07) towards an interaction of the two drugs. The separate and combined discriminative-stimulus effects of Δ9-THC alone and baclofen are shown in Figure 1. During the test phase, placebo and the training dose of Δ9-THC occasioned an average of 5.3 (SEM = 4.1) and 100.0 (SEM = 0.0) percent Δ9-THC-appropriate responding, respectively. Δ9-THC alone dose-dependently increased drug-appropriate responding on the drug-discrimination task. The 50 mg dose of baclofen alone also significantly increased drug-appropriate responding, and significantly enhanced the discriminative-stimulus effects of the 5 mg dose of Δ9-THC. The 25 mg baclofen dose alone did not differ from placebo, but significantly enhanced the discriminative-stimulus effects of the 15 mg dose of Δ9-THC.
Figure 1.
Separate and combined effects of Δ9-THC and baclofen on Δ9-THC-appropriate responding on the drug-discrimination task. Filled symbols indicate values that are significantly different from placebo. Asterisks indicate combinations of Δ9-THC and baclofen that are significantly different from that dose of Δ9-THC alone. The x-axis represents the Δ9-THC dose in mg; PL denotes placebo. Data points show means of 8 subjects. Uni-directional brackets indicate 1 SEM.
3.2. Subject Ratings
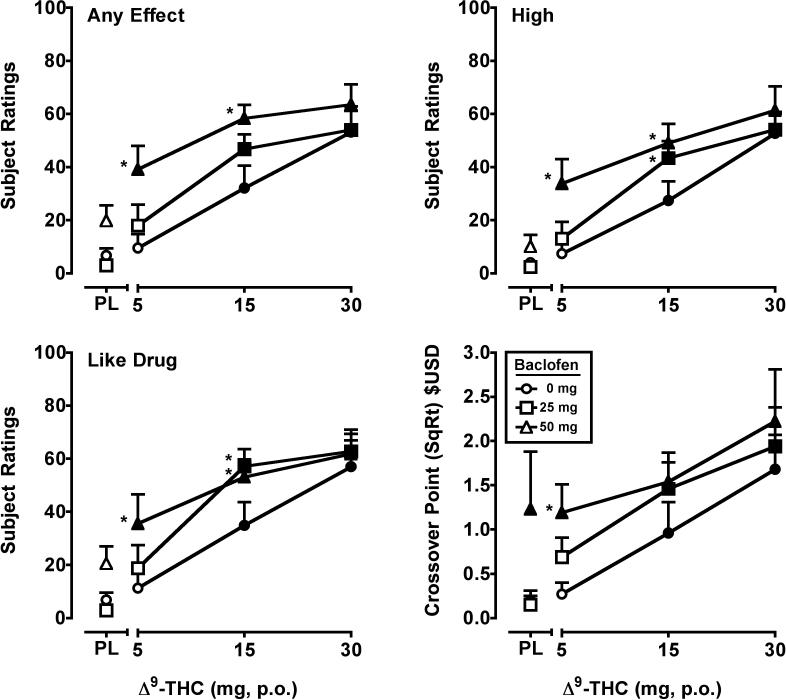
Significant main effects of Δ9-THC (F's3,21 = 3.0-26.5, p's ≤ 0.05) and baclofen (F's2,14 = 3.7- 52.4, p's ≤ 0.05) were detected for twelve VAS items: Any Effect*, Good Effects, High*, Anxious, Like Drug*, Stimulated, Take Again, Pay For, Shaky/Jittery, Stoned, Forgetful, Confused/Difficulty Concentrating. The data from VAS items marked with an asterisk* are presented in Figure 2. In general, 15 and 30 mg Δ9-THC increased ratings on these items in a dose-dependent manner, and the effects of the 5 mg or 5 and 15 mg doses of Δ9-THC were significantly enhanced by baclofen. An interaction of Δ9-THC and baclofen (F6,42 = 3.7, p < 0.01) was detected for Tired/Sedated. Responses to this item were of lower magnitude (maximum of approximately 20 out of 100 on a VAS) and not Δ9-THC-dose dependent. 50 mg of baclofen alone significantly increased subject ratings of Tired/Sedated; and both doses of baclofen significantly enhanced the effects of Δ9-THC, but also not in a dose-dependent fashion. For the VAS items Bad Effects, Hungry and Thirsty, significant main effects of Δ9-THC (F's3,21 = 4.0-8.2, p's < 0.05) only were found. Δ9-THC dose-dependently increased ratings on these VAS items.
Figure 2.
Peak (maximum value) Visual Analog Scale ratings for Δ9-THC and baclofen, alone and in combination, on the drug-effect questionnaire items Any Effect, High, Like Drug, and crossover point values (square root transformation) from a Multiple Choice Procedure. All other details are as in Figure 1.
3.3. Multiple-Choice Procedure
Significant main effects of Δ9-THC (F3,21 = 7.1, p < 0.01) and baclofen (F2,14 = 10.3, p < 0.01) were observed for crossover point. Δ9-THC increased crossover point relative to placebo at the 15 and 30 mg doses. The 50 mg dose of baclofen significantly increased crossover point alone, and when combined with Δ9-THC relative to Δ9-THC alone at the 5 mg dose (Figure 2).
3.4. Performance
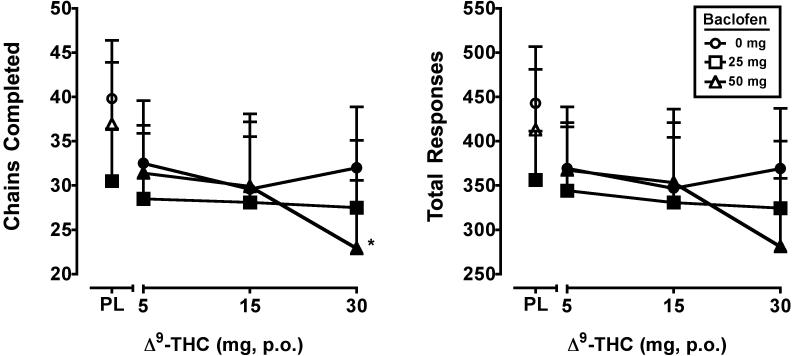
Main effects of Δ9-THC (F3,21 = 4.0, p < 0.05) and baclofen (F2,14 = 3.7, p = 0.05) were found for the number of chains completed on the acquisition component of the RA task. A main effect of Δ9-THC (F3,21 = 3.9, p < 0.05) was found for the total number of responses emitted on this task component, with a trend (p = 0.06) for baclofen. For these outcomes, performance was impaired by all active doses of Δ9-THC. The 25 mg dose of baclofen alone also reduced the number of chains relative to placebo and further reduced performance at the 30 mg Δ9-THC dose. The effects of Δ9-THC and baclofen on rate and accuracy on the acquisition component of the RA task are presented in Figure 3.
Figure 3.
Peak number of chains completed and total responses on the repeated acquisition task (minimum value) for Δ9-THC and baclofen, alone and in combination. All other details are as in Figure 1.
With respect to the performance component of the RA task, main effects of Δ9-THC (F3,21 = 3.3, p < 0.05) and baclofen (F2,14 = 8.7, p ≤ 0.01) were detected on the number of chains completed. Both Δ9-THC (F3,21 = 3.0, p = 0.05) and baclofen (F2,14 = 7.7, p ≤ 0.01) also significantly impacted the total number of responses emitted. For these outcomes, performance was impaired following administration of 30 mg Δ9-THC, alone and in combination with both active baclofen doses, and also the 15 mg Δ9-THC + 50 mg baclofen dose condition (data not shown).
For the DSST, only a significant main effect of baclofen (F2,14 = 11.9, p ≤ 0.001) on the number of correct trials was found. Trends towards significance were observed for Δ9-THC on the number of correct trials (p = 0.07) and for baclofen on the number of trials completed (p = 0.07). Relative to Δ9-THC alone, 25 mg baclofen impaired performance at the 30 mg Δ9-THC dose, and 50 mg baclofen impaired performance at the 15 and 30 mg Δ9-THC doses (data not shown).
Δ9-THC had minor, non-dose-dependent effects on the reproduction of time (data not shown). A main effect of Δ9-THC (F3,21 = 3.9, p < 0.05) was observed on the Time Reproduction task for only the 3-s interval. Administration of 5 mg Δ9-THC alone and in combination with 25 mg baclofen, and 15 mg Δ9-THC in combination with both active baclofen doses, resulted in under-reproduction of the 3-s interval (range = 0.30-0.36 s). Neither Δ9-THC or baclofen affected reproduction of the 30-, 60- or 180-s time intervals.
3.5. Heart Rate, Blood Pressure and Temperature
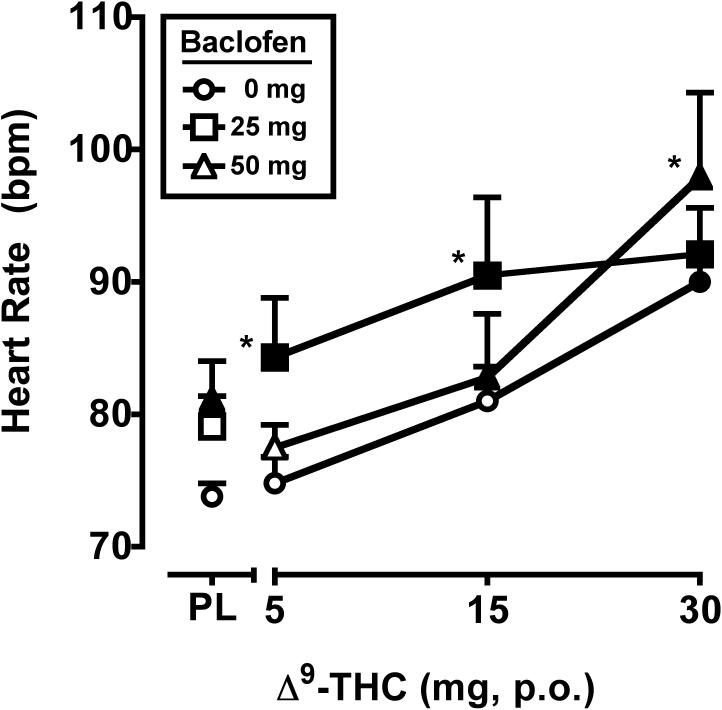
Significant main effects of Δ9-THC (F3,21 = 17.9, p < 0.001) and baclofen (F2,14 = 3.6, p = 0.05) were observed for heart rate, as illustrated in Figure 4. When administered separately, the 30 mg dose of Δ9-THC and the 50 mg dose of baclofen increased heart rate. When combined, the 50 mg baclofen significantly enhanced the effects of 30 mg Δ9-THC on heart rate, and the 25 mg baclofen dose enhanced the effects of the 5 and 15 mg doses of Δ9-THC. Blood pressure was not impacted by Δ9-THC or baclofen (data not shown).
Figure 4.
Peak heart rate (maximum value) for Δ9-THC and baclofen, alone and in combination. All other details are as in Figure 1.
Δ9-THC significantly reduced oral temperature (F3,21 = 3.4, p < 0.05). Compared to placebo, Δ9-THC decreased oral temperature following administration of the 5 mg dose alone, and both the 15 and 30 mg Δ9-THC doses decreased oral temperature when they were combined with 25 and 50 mg baclofen. The largest decrease in oral temperature (approximately 0.2 °C) occurred following administration of the 25 mg baclofen + 30 mg Δ9-THC condition. Index finger skin temperature was not influenced by Δ9-THC or baclofen (data not shown).
4. Discussion
The aim of this study was to assess the separate and combined effects of the preferential GABAB agonist baclofen and Δ9-THC using drug-discrimination procedures. Δ9-THC functioned as a discriminative stimulus and dose-dependently occasioned drug-appropriate responding. The larger baclofen dose alone occasioned Δ9-THC-appropriate responding, and when combined with Δ9-THC, both baclofen doses significantly enhanced drug-appropriate responding. Similar potentiation of baclofen and Δ9-THC dose combinations was observed on several cannabinoid-sensitive measures, including drug-effect ratings, a repeated acquisition task, heart rate and oral temperature. These results suggest that the GABAB receptor subtype is involved in some of the behavioral and physiological of Δ9-THC, and that GABAB receptors were responsible, at least in part, for the effects of tiagabine-induced elevated GABA on cannabinoid-related behaviors in our previous study (Lile et al., 2012).
4.1. Drug discrimination
As noted, substitution of the higher baclofen dose and potentiation of the discriminative-stimulus effects of Δ9-THC by concurrent administration with baclofen is concordant with results from our previous study with the GABA reuptake inhibitor tiagabine (Lile et al., 2012) and the cannabinoid agonist nabilone (Lile et al., 2011). The present results differ, however, from a prior study in rats trained to discriminate Δ9-THC, in which baclofen alone failed to occasion drug-appropriate responding (Browne and Weissman, 1981). The reason for the species-specific results is unknown, but could be due to differences in the drug-discrimination procedures (i.e., substitution versus drug combination; see below) or the use of a single baclofen dose in the rodent study.
The drug discrimination procedure was more sensitive than self-report measures at detecting the interoceptive effects of baclofen in that 50 mg of baclofen occasioned significant drug-appropriate responding, but few subjective effects, when administered alone. In addition, the drug combination procedures used here appeared to permit greater sensitivity to detect the possible involvement of non-cannabinoid neurotransmitter systems in the effects of Δ9-THC compared to substitution procedures alone. Specifically, when combined with either active baclofen dose, the discriminative-stimulus effects of Δ9-THC were significantly increased compared to Δ9-THC alone. In contrast, when baclofen was administered alone, only the higher, 50-mg dose, substituted for the Δ9-THC discriminative stimulus. These findings are important for at least two reasons. First, low doses of GABAergic compounds could be used in future pharmacotherapeutic applications to modulate Δ9-THC effects, or low doses of each could be combined for therapeutic use, which might circumvent side effects of higher doses of the constituent drugs. Second, the sensitivity of the drug-discrimination procedures highlights their value for examining the effects of drugs used in combination.
4.2. Abuse potential of baclofen and baclofen-cannabinoid combinations in cannabis users
Based on decades of clinical use, baclofen appears to have little potential for abuse. By and large, baclofen alone did not impact ratings on “positive” items from the self-reported drug-effect questionnaire. The absence of abuse-related baclofen subjective effects is in agreement with previous clinical studies that have failed to detect significant subjective effects suggestive of abuse potential in cocaine-dependent individuals (10-30 mg, Lile et al., 2004; 30 or 60 mg/day for 7 days, Haney et al., 2006), heavy social drinkers (40 or 80 mg, Evans and Bisaga, 2009) and daily cannabis users (Haney et al., 2010). However, in the present study baclofen alone significantly increased the crossover point on a non-contingent Multiple-Choice Procedure, and enhanced the positive self-reported effects of Δ9-THC. These results are concordant with isolated case reports of baclofen misuse (e.g., May, 1983; Nasti and Brakoulias, 2011; Perry et al., 1998), increased subject ratings of Drug Liking, Relaxed and Mellow following baclofen administration during ad lib tobacco cigarette use in regular smokers (Cousins et al., 2001). In addition, a study in baboons demonstrated that at least one dose of baclofen maintained self-administration in every animal, though not as robustly as the benzodiazepines tested under the same conditions (Griffiths et al., 1991). Overall, though, the abuse potential of baclofen appears relatively low, and importantly, there appeared to be a ceiling on the combined effects of baclofen and Δ9-THC on the self-reported items and crossover point on the Multiple-Choice Procedure, which could limit the misuse of baclofen in combination with Δ9-THC in cannabis users.
4.3. Side effects of baclofen and baclofen-cannabinoid combinations
Baclofen significantly impaired psychomotor task performance consistent with deficits observed in previous laboratory studies in regular alcohol (Evans and Bisaga, 2009) and cannabis (Haney et al., 2010) users. Similarly, baclofen increased ratings on sedative-like drug-effect items in the present experiment and in the study by Evans and Bisaga (2009). When used therapeutically, ongoing baclofen treatment has been associated with cognitive disturbances and sedation, particularly at daily doses greater than 60 mg (e.g., Dario and Tomei, 2004; Dore et al., 2011), but overall its side effect profile has been found to be acceptable. Baclofen also significantly increased heart rate, in agreement with a previous laboratory study (Evans and Bisaga, 2009), although instances of bradycardia have been reported following therapeutic intrathecal baclofen administration (e.g., Rifici et al., 2011; Sill et al., 1986) and oral baclofen overdose (e.g., Peng et al., 1998; Perry et al., 1998; Sein Anand et al., 2005). Baclofen also potentiated the tachycardic effects of Δ9-THC; three of the eight subjects met criteria for tachycardia (>100 bpm) following administration of at least one of the combinations of baclofen and Δ9-THC, although no additional symptoms (e.g., blurry vision, sweating) indicative of a more serious cardiovascular problems were observed.
4.4. Limitations
Some limitations of the present study merit discussion. First, subjects were enrolled on an outpatient basis, so ongoing drug use could not be entirely monitored or controlled. Qualitative urine toxicology screening was conducted prior to the start of each experimental session to verify that subjects had not used non-cannabis illicit drugs prior to the session, which limited the likelihood of interactions with experimentally administered drugs, but subjects could have used drugs having short excretion half-lives between sessions (i.e., weekends). In addition, the amount of cannabis used during participation likely varied among subjects. However, because subjects were frequent cannabis users and cannabis metabolites can be measured in urine for days to weeks after the cessation of use, it would have been impractical to require subjects to remain abstinent throughout the enrollment period. Second, a negative control condition (i.e., an active drug such as methylphenidate expected to engender Not Drug X responding; Lile et al., 2009; 2010) was not included to ensure that subjects were not simply reporting any active drug dose as Drug X. Important to emphasize, however, is that subjects were instructed to respond on the Not Drug X option in the absence of drug effects as well as when they experienced drug effects that differed from those of Drug X. These methods have been used successfully in previous studies to teach human subjects a selective discrimination based on the pharmacology of the training drug (e.g., Lile et al., 2009, 2010; Rush et al., 1998).
4.5. Comparison of the results with baclofen and tiagabine
The results obtained with baclofen in the present study were comparable to tiagabine, suggesting that GABAB receptors were responsible, at least in part, for the effects of tiagabine-induced elevated GABA on cannabinoid-related behaviors. A few differences are worth pointing out, however. With respect to the drug-discrimination data, tiagabine fully (≥ 80% drug-appropriate responding) substituted for the Δ9-THC discriminative stimulus, whereas baclofen significantly, but only partially, substituted for Δ9-THC. Likewise, both doses of tiagabine (6 and 12 mg) significantly increased the discriminative-stimulus effects of 5 mg Δ9-THC, while only the larger dose of baclofen (50 mg) enhanced the low-dose Δ9-THC discriminative-stimulus effects. In addition, 12 mg of tiagabine alone engendered a response on many of the self-reported drug-effect items, but baclofen did not. One possible explanation for these differences is that relative doses of tiagabine were larger than baclofen. However, baclofen, but not tiagabine, augmented Δ9-THC effects on heart rate and oral temperature, which might not be expected if the differences were simply dose dependent. Another explanation is that activation of the GABAA receptor is also involved in the effects of cannabinoids in humans, and contributed to the responses observed when GABA was elevated by tiagabine. An ongoing study with the GABAA positive modulator diazepam (unpublished data) is assessing the contribution of that receptor subtype relative to GABAB, which will provide a better understanding of the neuropharmacology of Δ9-THC, and by extension cannabis, in humans.
4.6. Baclofen as a medication for cannabis-use disorders
Baclofen enhanced the discriminative-stimulus, self-reported, performance and physiological effects of Δ9-THC, and produced some overlapping effects when administered alone, consistent with a significant contribution of the GABAB receptor subtype in the behavioral and physiological effects of Δ9-THC. To the extent that drugs that enhance the effects of Δ9-THC or produce comparable effects alone could address some of the complaints associated with cannabis abstinence thought to contribute to continued use (i.e., craving, irritability, anxiety, depression, difficulty sleeping), baclofen should be further evaluated as a potential pharmacotherapy for cannabis-dependent patients. The existing data with baclofen are equivocal, with negative results from a laboratory model of relapse (Haney et al., 2010), but positive clinical findings from open-label case studies (Nanjayya et al., 2010). Given the need for effective medications for cannabis-use disorders, more research is warranted.
Acknowledgements
We appreciate the pharmacy services of Dr. Steve Sitzlar of the University of Kentucky Investigational Drug Service. We also thank Beth Eaves, Cleeve Emurian, Dustin Lee, Jillian O'Rourke and Glenn Robbins for expert technical assistance.
Role of Funding Source This research and the preparation of this manuscript were supported by grants from the National Institute on Drug Abuse (K02 DA031766 and R01 DA025605) awarded to Dr. Joshua Lile. These funding sources had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributors Drs. Lile, Kelly and Hays designed the study. Dr. Lile wrote the protocol; managed literature searches and summaries of previous related work; undertook the statistical analysis and graphical representation of the data; and wrote the first draft of the manuscript. Dr. Hays provided medical management and oversight. All authors contributed to and have approved the final manuscript.
Conflict of Interest There are no conflicts of interest to declare.
References
- Baclofen Product Information. Novartis Pharmaceuticals Limited; 2008. [Google Scholar]
- Browne RG, Weissman A. Discriminative stimulus properties of delta 9-tetrahydrocannabinol: mechanistic studies. J. Clin. Pharmacol. 1981;21:S227–34. doi: 10.1002/j.1552-4604.1981.tb02599.x. [DOI] [PubMed] [Google Scholar]
- Castellano C, Rossi-Arnaud C, Cestari V, Costanzi M. Cannabinoids and memory: animal studies. CNS Neurol. Disord. Drug Targets. 2003;2:389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat MS, de Wit H. Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob. Res. May. 2001;3:123–129. doi: 10.1080/14622200110042624. [DOI] [PubMed] [Google Scholar]
- Dario A, Pisani R, Sangiorgi S, Pessina F, Tomei G. Relationship between intrathecal baclofen and the central nervous system. Acta. Neurochir. Suppl. 2007;97:461–4. doi: 10.1007/978-3-211-33079-1_60. [DOI] [PubMed] [Google Scholar]
- Dario A, Tomei G. A benefit-risk assessment of baclofen in severe spinal spasticity. Drug Saf. 2004;27:799–818. doi: 10.2165/00002018-200427110-00004. [DOI] [PubMed] [Google Scholar]
- DeSousa NJ, Beninger RJ, Jhamandas K, Boegman RJ. Stimulation of GABAB receptors in the basal forebrain selectively impairs working memory of rats in the double Y-maze. Brain Res. 1994;641:29–38. doi: 10.1016/0006-8993(94)91811-2. [DOI] [PubMed] [Google Scholar]
- Dore GM, Lo K, Juckes L, Bezyan S, Latt N. Clinical experience with baclofen in the management of alcohol-dependent patients with psychiatric comorbidity: a selected case series. Alcohol Alcohol. 2011;46:714–20. doi: 10.1093/alcalc/agr131. [DOI] [PubMed] [Google Scholar]
- Dronabinol Product Information. Solvay Pharmaceuticals Inc.; 2006. [Google Scholar]
- Evans SM, Bisaga A. Acute interaction of baclofen in combination with alcohol in heavy social drinkers. Alcohol. Clin. Exp. Res. 2009;33:19–30. doi: 10.1111/j.1530-0277.2008.00805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frosini M, Valoti M, Sgaragli G. Changes in rectal temperature and ECoG spectral power of sensorimotor cortex elicited in conscious rabbits by i.c.v. injection of GABA, GABA(A) and GABA(B) agonists and antagonists. Br. J. Pharmacol. 2004;141:152–162. doi: 10.1038/sj.bjp.0705593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths RR, Lamb RJ, Sannerud CA, Ator NA. Self-injection of barbiturates, benzodiazepines and other sedative-anxiolytics in baboons. Psychopharmacology. 1991;103:154–161. doi: 10.1007/BF02244196. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Troisi JR, II, Silverman K, Mumford GK. Multiple-choice procedure: an efficient approach for investigating drug reinforcement in humans. Behav. Pharmacol. 1993;4:3–13. [PubMed] [Google Scholar]
- Haney M, Hart CL, Foltin RW. Effects of baclofen on cocaine self-administration: opioid- and nonopioid-dependent volunteers. Neuropsychopharmacology. 2006;31:1814–21. doi: 10.1038/sj.npp.1300999. [DOI] [PubMed] [Google Scholar]
- Haney M, Hart CL, Vosburg SK, Comer SD, Cooper ZD, Foltin RW. Effects of baclofen and mirtazapine on a laboratory model of marijuana withdrawl and relapse. Psychopharmacology. 2010;211:233–44. doi: 10.1007/s00213-010-1888-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart CL, Haney M, Vosburg SK, Comer SD, Foltin RW. Reinforcing effects of oral Delta9-THC in male marijuana smokers in a laboratory choice procedure. Psychopharmacology. 2005;181:237–243. doi: 10.1007/s00213-005-2234-2. [DOI] [PubMed] [Google Scholar]
- Heishman SJ, Stitzer ML, Yingling JE. Effects of tetrahydrocannabinol content on marijuana smoking behavior, subjective reports and performance. Pharmacol. Biochem. Behav. 1989;34:173–179. doi: 10.1016/0091-3057(89)90369-9. [DOI] [PubMed] [Google Scholar]
- Hohmann AG. Spinal and peripheral mechanisms of cannabinoid antinociception: behavioral, neurophysiological and neuroanatomical perspectives. Chem. Phys. Lipids. 2002;121:173–190. doi: 10.1016/s0009-3084(02)00154-8. [DOI] [PubMed] [Google Scholar]
- Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J. Clin. Pharmacol. 1981;21:171S–177S. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Holtzman SG, Locke KW. Neural mechanisms of drug stimuli: experimental approaches. Psychopharmacol. Ser. 1988;4:138–153. [PubMed] [Google Scholar]
- Kamien JB, Bickel WK, Higgins ST, Hughes JR. The effects of delta 9-tetrahydrocannabinol on repeated acquisition and performance of response sequences and on self-reports in humans. Behav. Pharmacol. 1994;5:71–78. doi: 10.1097/00008877-199402000-00008. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Emurian CS, Fischman MW. Performance-based testing for drugs of abuse: dose and time profiles of marijuana, amphetamine, alcohol and diazepam. J. Anal. Toxicol. 1993;17:264–272. doi: 10.1093/jat/17.5.264. [DOI] [PubMed] [Google Scholar]
- Kelly TH, Foltin RW, Fischman MW. Multidimensional behavioral effects of marijuana. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1990;14:885–902. doi: 10.1016/0278-5846(90)90075-r. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Substitution profile of the cannabinoid agonist nabilone in humans discriminating Δ9-THC. Clin. Neuropharmacol. 2010;33:235–242. doi: 10.1097/WNF.0b013e3181e77428. [DOI] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the cannabinoid agonists nabilone and Δ9-THC in humans discriminating Δ(9)-THC. Drug Alcohol Depend. 2011;116:86–92. doi: 10.1016/j.drugalcdep.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Hays LR. Separate and combined effects of the GABA reuptake inhibitor tiagabine and Δ9-THC in humans discriminating Δ(9)-THC. Drug Alcohol Depend. 2012;122:61–69. doi: 10.1016/j.drugalcdep.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Kelly TH, Pinsky DJ, Hays LR. Substitution profile of Δ9-tetrahydrocannabinol, triazolam, hydromorphone and methylphenidate in humans discriminating Δ9-tetrahydrocannabinol. Psychopharmacology. 2009;203:241–250. doi: 10.1007/s00213-008-1393-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Stoops WW, Allen TS, Glaser PE, Hays LR, Rush CR. Baclofen does not alter the reinforcing, subject-rated or cardiovascular effects of intranasal cocaine in humans. Psychopharmacoogy. 2004;171:441–449. doi: 10.1007/s00213-003-1598-4. [DOI] [PubMed] [Google Scholar]
- May CR. Baclofen overdose. Ann. Emerg. Med. 1983;12:171–173. doi: 10.1016/s0196-0644(83)80562-9. [DOI] [PubMed] [Google Scholar]
- McLeod DR, Griffiths RR, Bigelow GE, Yingling JE. An automated version of the Digit Symbol Substitution Test (DSST). Behav. Res. Methods Instrument. 1982;14:463–466. [Google Scholar]
- Moreira FA, Wotjak CT. Cannabinoids and anxiety. Curr. Top. Behav. Neurosci. 2010;2:429–450. doi: 10.1007/7854_2009_16. [DOI] [PubMed] [Google Scholar]
- Nanjayya SB, Shivappa M, Chand PK, Murthy P, Benegal V. Baclofen in cannabis dependence syndrome. Biol. Psychiatry. 2010;68:9–10. doi: 10.1016/j.biopsych.2010.03.033. [DOI] [PubMed] [Google Scholar]
- Nasti JJ, Brakoulias V. Chronic baclofen abuse and withdrawl delirium. Aust. N. Z. J. Psychiatry. 2011;45:86–87. doi: 10.3109/00048674.2010.524622. [DOI] [PubMed] [Google Scholar]
- Perry HE, Wright RO, Shannon MW, Woolf AD. Baclofen overdose: drug experimentation in a group of adolescents. Pediatrics. 1998;101:1045–1048. doi: 10.1542/peds.101.6.1045. [DOI] [PubMed] [Google Scholar]
- Peng CT, Ger J, Yang CC, Tsai WJ, Deng JF, Bullard MJ. Prolonged severe withdrawl symptoms after acute-on-chronic baclofen overdose. J. Toxicol. Clin. Toxicol. 1998;36:359–363. doi: 10.3109/15563659809028033. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Greentree SG, Swift PA. Drugs that stimulate or facilitate central GABAergic transmission interact synergistically with delta-9-tetrahydrocannabinol-induced to produce marked catalepsy in mice. Neuropharmacology. 1988;27:1265–1270. doi: 10.1016/0028-3908(88)90029-9. [DOI] [PubMed] [Google Scholar]
- Pilc A, Nowak G. GABAergic hypotheses of anxiety and depression: focus on GABA-B receptors. Drugs Today. 2005;41:755–66. doi: 10.1358/dot.2005.41.11.904728. [DOI] [PubMed] [Google Scholar]
- Rifici C, D'Aleo G, D'Aleo P, Bramanti P, Saltuari L, Kofler M. Cardiovascular alterations heralded by intrathecal baclofen bolus. Neuro. Rehab. 2011;28:389–393. doi: 10.3233/NRE-2011-0668. [DOI] [PubMed] [Google Scholar]
- Romero J, García-Palomero E, Fernández-Ruiz JJ, Ramos JA. Involvement of GABA(B) receptors in the motor inhibition produced by agonists of brain cannabinoid receptors. Behav. Pharmacol. 1996;7:299–302. doi: 10.1097/00008877-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Rush CR, Kollins SH, Pazzaglia PJ. Discriminative-stimulus and participant-rated effects of methylphenidate, bupropion and triazolam in d-amphetamine trained humans. Exp. Clin. Psychopharmacol. 1998;6:32–44. doi: 10.1037//1064-1297.6.1.32. [DOI] [PubMed] [Google Scholar]
- Sein Anand J, Chodorowski Z, Burda P. Selected clinical aspects of acute intoxication with baclofen. Przegl. Lek. 2005;62:462–464. [PubMed] [Google Scholar]
- Sill JC, Schumacher K, Southorn PA, Reuter J, Yaksh TL. Bradycardia and hypotension associated with baclofen used during general anesthesia. Anesthesiology. 1986;64:255–258. doi: 10.1097/00000542-198602000-00022. [DOI] [PubMed] [Google Scholar]
- Toland SL, Green W. DRE field testing of drug impaired drivers. In: Watts V, editor. The Effects of Drugs on Human Performance and Behavior: Drugs and Driving/Drugs in the Workplace. American Academy of Forensic Sciences; Colorado: 1991. [Google Scholar]
- Vandrey R, Haney M. Pharmacotherapy for cannabis dependence: how close are we? CNS Drugs. 2009;23:543–553. doi: 10.2165/00023210-200923070-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Moldrich G. The role of endocannabinoids in the hypothalamic regulation of visceral function. Prostaglandins Leukot. Essent. Fatty Acids. 2002;66:301–307. doi: 10.1054/plef.2001.0353. [DOI] [PubMed] [Google Scholar]
- Wilson WH, Ellinwood EH, Mathew RJ, Johnson K. Effects of marijuana on performance of a computerized cognitive-neuromotor test battery. Psychiatry Res. 1994;51:115–125. doi: 10.1016/0165-1781(94)90031-0. [DOI] [PubMed] [Google Scholar]