Summary
After an immune response, the expanded population of antigen-specific CD4+ T cells contract to steady-state levels. We have found that the contraction is neither cell-autonomous nor mediated by competition for generic trophic factors, but regulated by relatively rare subsets of neighboring CD4+ T cells not necessarily of a conventional T-regulatory lineage. These regulators, referred to as deletors, specifically limit the frequency of particular antigen-specific T cells even though they are not reactive to the same agonist as their targets. Instead, an isolated deletor T cell could outcompete the target T cell for recognition of a shared, non-stimulatory endogenous peptide-MHC ligand. This mechanism was sufficient to prevent even agonist-driven autoimmune disease in a lymphopenic environment. Such a targeted regulation of homeostasis within narrow colonies of T cells with related TCR specificities for sub-threshold ligands, can prevent the loss of unrelated TCRs during multiple responses, helping preserve the valuable diversity of the repertoire.
Introduction
The number of T cells in the peripheral immune system is tightly regulated during. In the steady state, homeostatic processes maintain a stable population of helper T cells, balancing thymic output with normal attrition (Freitas and Rocha, 2000). Infections trigger a dramatic expansion of otherwise rare antigen-specific T cells; but this is transient and the population density is restored soon after the pathogen is cleared. Furthermore, a separate set of processes ensure that T cells capable of reacting to self-antigens are eliminated from the population by clonal deletion (Gardner et al., 2008). These various elimination mechanisms must also be discriminating enough to ensure that a diverse set of T cell receptors (TCRs) are still retained in the peripheral repertoire in order to maintain defenses against as wide a variety of future infections as possible. Since each T cell response yields a large frequency of expanded pathogen-specific T cells, if the subsequent contraction was regulated by stochastic processes, it could also lead to a large loss of unrelated “bystander” T cells and therefore a progressive loss of repertoire diversity over multiple infections. The cellular mechanisms that ensure such a precise homeostatic control, especially for CD4+ T cells are not clear.
In the last two decades, reductionist approaches to study this complex problem have focused on understanding the regulation of T cell survival – since the frequency of particular T cells and the diversity of the repertoire can be influenced by how each T cell survives. These studies have coalesced around a conceptual framework based on competition for limiting trophic resources, keeping T cell subsets within certain population limits (Freitas and Rocha, 2000). Strong antigenic stimulation can allow the antigen-specific T cell numbers to exceed these limits but the population returns to competing for the limiting interactions after antigen clearance. The critical trophic factors that anchor this process can be segregated into two categories – public and cognate. The former are sensed by receptors not related to the TCR and therefore do not respect the antigen specifities of the T cells competing for them. These include cytokines - such as interleukin-2 (IL-2), IL-7, IL-15, thymic stromal lymphopoietin (TSLP) as well as nutrients, co-stimulatory molecules, etc. (Schluns and Lefrancois, 2003; Surh and Sprent, 2005; Takada and Jameson, 2009). The cognate factors, on the other hand, require sensing via the TCR – the stimulatory antigen being the best example (Obar et al., 2008; Smith et al., 2000).
Even within these models, the relative contribution of either category to T cell survival, especially in the context of CD4+ T cells, is far from clear. Early experiments suggested that TCR-major histocompatibility complex (MHC) interactions were quite critical for survival (Kirberg et al., 1997; Polic et al., 2001; Takeda et al., 1996; Tanchot et al., 1997). Subsequent experiments, however, controlling for factors such as cell proliferation and rejection, concluded that MHC-II recognition was not necessary for CD4+ T cell survival – and therefore could not be the critical determinant of their population control (Dorfman et al., 2000; Grandjean et al., 2003).
A second set of experiments critical to understand peripheral homeostasis, is the behavior of CD4+ T cells in lymphopenic models. Under these conditions, otherwise quiescent naïve T cells can proliferate and differentiate, even in the absence of their cognate antigen (Cho et al., 2000; Oehen and Brduscha-Riem, 1999). In fact, this behavior has severe clinical ramifications, where aggressive immunopathology results from the response of T cells in lymphopenic conditions generated during bone marrow transplants, HIV infections, etc. and even hampers conventional tolerance induction (Brown et al., 2006; Schietinger et al., 2012; Singh et al., 2006; Wu et al., 2004).
The common explanation for this lymphopenia-driven T cell proliferation is that it reflects a response to an overabundance of trophic factors that normally maintain peripheral homeostasis. It occurs even in MHC-II deficient environments (suggesting that the public factors alone are relevant)(Clarke and Rudensky, 2000; Grandjean et al., 2003); but it can only be blocked by packing the host with cells of the same clonotype (suggesting instead that cognate factors are critical) (Moses et al., 2003; Troy and Shen, 2003). This paradox has nevertheless led to the notion of “clonal competition” which suggests that long term population control in the peripheral CD4+ T cell compartment is achieved by narrow competition between identical clones of T cells (Hataye et al., 2006). However, it is very difficult to extrapolate such data from TCR transgenic model systems to a truly polyclonal scenario. The frequency of any particular clonotypic receptor in such a repertoire is likely to be exceedingly low - making it difficult (but not impossible) to mediate such potent effects (Quiel et al., 2011). In the absence of a high resolution functional dissection of natural polyclonal repertoires of T cells, our understanding of these control mechanisms remains very limited.
To address these issues, we designed a series of cellular experiments exploiting the contrasting behavior of T cells in lymphopenic or intact environments. After exhaustively eliminating competition for public or conventional cognate factors as the primary regulators of T cell frequency in these models, we took apart a polyclonal population in order to isolate the regulatory component ab initio. In an unbiased in vivo screen, we identified a specific T cell that was sufficient on its own, to constrain the numbers of the self-reactive T cell and prevent it’s pathogenicity, even in a lymphopenic environment. This regulatory T cell – termed as a “deletor”, specifically controlled the self-reactive T cell even in the absence of agonistic antigen, by recognizing a shared sub-threshold self-ligand. These data reveal a unifying mechanism that controls peripheral T cell frequencies during the steady state, but surprisingly, also during pathological responses to a strong agonist. It suggests that peripheral CD4+ T cells are functionally organized into relatively small cliques or colonies, due to the communal recognition of specific sub-threshold ligands. The control of population dynamics primarily at the level of such colonies, might be the key to reducing the risk of broad bystander repertoire loss during each immune response and preservation of the valuable diversity of peripheral T cells.
Results
1. Neighboring CD4+ T cells limit the pathogenic potential of auto-reactive T cells
A dramatic illustration of the consequences of perturbing homeostatic processes in the peripheral immune system is the behavior of T cells in clinical or experimentally-induced lymphopenic environments. A model for dissecting these sequelae involves adoptively transferring antigen-specific T cells to mice expressing the target antigen. In experiments using the 5C.C7 TCR-transgenic T cells responding to a self-antigen (transgenically expressed pigeon cytochrome C (PCC) under an MHC-I promoter) in mice that are T-cell deficient (PCC+, Cd3e−/−) or intact (PCC+, with endogenous T cells), we have shown that autoimmune arthritis develops only in the lymphopenic host (Singh et al., 2006). The absence of disease in the T-cell-intact hosts correlated with a slow “deletion” of the self-reactive T cells that was not observed in the T-cell-deficient hosts. This suggested that host T cells are critical for effective control of the antigen-specific response and can help decide between “disease” and “tolerance” in this context. We therefore designed a series of experiments aimed at identifying the activity within a polyclonal host T cell repertoire - which we will refer to as “deletor” activity - that elicits a phenotype akin to “clonal deletion” in self-reactive T cells.
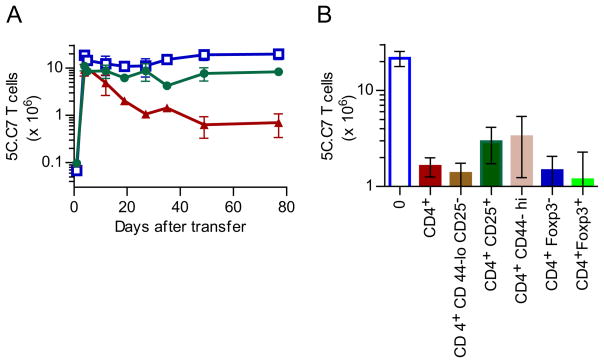
Such deletors are primarily contained within the mature peripheral T cell population because self-reactive T cells in a PCC+, Cd3e −/− host could be controlled by introducing new polyclonal T cells, but did not require continuous thymic output (Figure S1 A & B). Deletors were also absent in PCC+, Tcra−/− mice implying that they are αβ T cells rather than γδ T cells (Figure S1C). In fact, simply transferring a million flow cytometry-sorted CD4+ polyclonal αβ T cells, a day before the 5C.C7 transfer, was sufficient to trigger a 95% contraction, while CD8+ T cells had only a minimal effect (Figure 1A). However, the deletor activity could not be further fractionated within the CD4+ pool based on the expression of CD44, CD25 or FOXP3 (Figure 1B). This suggested that simply the presence of an abundance of neighboring CD4+ T cells might be sufficient to control the population dynamics of antigen-specific T cells.
Figure 1. Neighbors regulate the frequency of auto-reactive T cells.
A. Kinetics of expansion and contraction of PCC reactive 5C.C7, Rag2−/− T cells in pooled lymph nodes & spleen of lymphopenic PCC+, Cd3e−/− mice (open squares) compared to that in similar recipients who received 106 polyclonal CD4+ (red triangles) or CD8+ (green triangles) T cells 1 day earlier. Data pooled from 2 experiments; n=4 per time point.
B. The number of 5C.C7 T cells in pooled lymph nodes & spleen of PCC+, Cd3e −/− mice that were first injected with 0.5 (Foxp3-gfp+/−) or 1×106 cells (all other groups) of flow cytometry sorted CD4+ polyclonal T cell subsets (as labeled on the X-axis) and harvested 26 or 40 days later. In a 1way ANOVA analysis, the control (0) group was significantly (p<0.001) different from all other groups; but the differences between subsets were not significant (p >0.05). Data are from 2 separate experiments with n=3 or 4 per group.
2. The frequency of auto-reactive T cells is unaffected by public or clonal competition
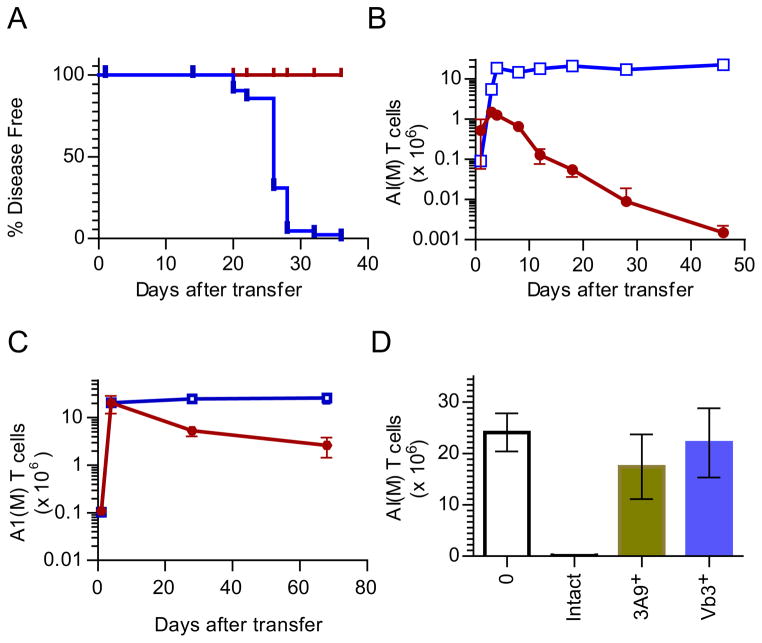
The ability of host CD4+ αβ T cells to limit the frequency of the pathogenic CD4+ 5C.C7 T cells appeared to conform to existing ideas regarding homeostasis of naive and memory T cells - i.e., a result of trophic competition between T cells of the same lineage. In order to test this we replaced the complete lymphopenia of a Cd3e−/− host with selective populations of CD4+ T cells of known specificity. A1(M) T cells (specific for the Dby, male-specific antigen) serve as the best example of this, since they are restricted by the same MHC as 5C.C7 (IEk) and can therefore compete for all the public niches, except the cognate antigen. They could also be examined under different contexts - with ~15 million naive T cells occupying the CD4+ niche (in Rag2−/− TCR transgenics that are PCC+ or male) or by transferring them to male, PCC+, Cd3e−/− mice, where both A1(M) and 5C.C7 would have cognate antigenic stimulation. Surprisingly, even with an abundance of the other T cells in the naive (Figure 2A & 2B) or activated (Figure 2C & 2D) state, neither the 5C.C7 response to PCC (Figure 2A & 2C) nor the A1(M) response to Dby (Figure 2B & 2D) was affected. This was not unique to the A1(M)-5C.C7 pair (Figure S2A), since three other TCR transgenics (3A9, 3.L2 and Marilyn) also failed to demonstrate any deletor activity against 5C.C7 (Figure S2). These data suggest that generic competition for cytokines or nutrients are unlikely to be the sole mechanism for the deletor activity.
Figure 2. Neither public nor cognate competition between T cells is sufficient to control T cell density.
A. The kinetics of expansion of 5C.C7, Ly5.1+ T cells in pooled lymph nodes & spleen of Rag2−/−,PCC+ animals (empty blue squares) compared to A1(M), RAG2−/−,PCC+ mice (red circles) (n=1 per time point; 1 of 4 similar experiments)
B. The reverse experiment of (A), following the fate of A1(M) T cells in male Rag2−/− mice (open green squares) or in 5C.C7 TCR+, Rag2−/− mice (blue circles).(n=1 per time point; 1 of 4 similar experiments)
C. & D. 5C.C7 T cells transferred to male PCC+, Cd3e−/− mice either alone (open blue squares) or together with A1(M) T cells (red circles). The expansion of the 5C.C7 T cells in pooled LN & spleen are plotted in C and the data for A1(M) T cells (alone –open green squares or together – blue circles) in the same experiment are plotted in (D)(n=1 per time point; 1 of 3 similar experiments). Also see Figure S2A.
E. & F. Chimeric mice generated to have a PCC+, H-2a bone marrow derived cells in a H-2b body were used to adoptively transfer another PCC-specific T cell AND (H-2b) together with the 5C.C7 T cell (H-2a). The peripheral T cell populations were recovered and analyzed 40 days after transfer (flow cytometry profiles in (E) and cell enumeration in (F)). (1 of 3 similar experiments; t tests, 5C.C7 alone vs 5C.C7 in mix p=0.054; AND alone vs AND in mix p=0.6564)
In the absence of public competition, it was possible that the deletor may simply be competing in a cognate-antigen-specific fashion. In the PCC model, ubiquitous expression of the antigen is known to eliminate PCC-specific T cells in the polyclonal repertoire during thymic development (Oehen et al., 1996). Therefore, high affinity TCRs specific for PCC should not be present in the periphery in numbers sufficient to mediate potent antigen-specific competition. Nevertheless, we tested the AND TCR transgenic, which is also specific for PCC, for its ability to delete 5C.C7. Using chimeric mice generated to have a PCC+, H-2a bone marrow in a H-2b body, we found that AND and 5C.C7 T cells reached the same plateau irrespective of whether the other cell was present (Figure 2E & 2F). Finally, it has been proposed that the long term survival of naive and memory T cells are regulated by clonal abundance - the competition between identical clonotypes of T cells (Hataye et al., 2006). Clonal competition, however, did not seem to be the basis for the differential behavior of 5C.C7 T cells in intact and lymphopenic mice in the context of chronic antigen stimulation (Singh et al., 2006)(and Figure S2D & S2E). Taken together, this extensive dataset is a dramatic demonstration of the idea that most of the popular conceptions of trophic factors regulating T cell survival and density in vivo do not apply – at least to the context of these autoimmune responses. Rather, this suggested that some other property encompassed within the polyclonal T cell repertoire underlies the critical regulatory unit limiting the overtly aggressive and often pathological T cell responses revealed during lymphopenic situations.
3. A subset of neighboring CD4+ T cells regulates the frequency of auto-reactive T cells
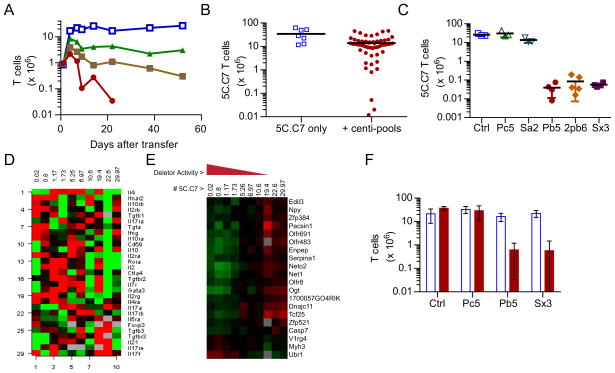
To isolate this property, we fractionated a truly polyclonal T cell population in an unbiased fashion and subjected it to a functional screen for deletor activity, in vivo. In order to facilitate downstream processing of the data (i.e., cloning TCRs etc.) we first examined if populations with limited diversity that are nevertheless polyclonal can mediate the contraction of 5C.C7 T cells. Thus, we first compared the activity of PCC+,3A9 (not RAG deficient) and PCC+, Vβ3-transgenic T cells (Figure 3A). Although both models retain endogenous TCR rearrangement machinery, the TCR transgenes force the expression of an α + β chain (3A9) or just a β chain (Vβ3) in all the T cells. As a result, while the latter is expected to have a fairly complex repertoire due to the necessary random generation of α chains, the former is most likely to express the 3A9 TCR, with a modest complexity due to leaky allelic exclusion. Interestingly, the contraction of 5C.C7 T cells showed a good correlation with the diversity of the repertoire - with the PCC+, Vβ3+ hosts showing slightly less activity relative to an intact PCC+ host and the PCC+,3A9 hosts performed worst, although slightly better than a completely lymphopenic PCC+, Cd3e ε−/− host (Figure 3A). We therefore used T cells from the PCC+, Vβ3+ hosts (additionally on a Tcra+/− background to limit each T cell to a single alpha chain) to devise an in vivo screening strategy to further fractionate the deletor activity within the polyclonal CD4+ T cell population (Figure S3).
Figure 3. An unbiased in vivo screen identifies T cells regulating antigen-specific T cell numbers.
A. Kinetics of the 5C.C7 T cell response in pooled LN & spleen of PCC+ Cd3e −/− hosts (blue squares), compared to PCC+ mice with an intact polyclonal repertoire (red circles), Vβ3-transgenic mice that have randomly rearranged α chains (brown squares) and 3A9 αβTCR+ mice with additional endogenous TCR rearrangements (green triangles). (n=2 per time point, 1 of 2 similar experiments)
B. Pools of 100 polyclonal T cells (centi-pools) transferred to PCC+, Cd3e −/− mice assayed for “deletor” activity by assaying “deletion” of 5C.C7 T cells in pooled LN & spleen, 40–60 days after transfer to mice with an individual centi-pool (red circles) compared to T cell-deficient controls (open blue squares).
C. Centi-pools recovered from pooled LN & spleen of mice that “deleted” 5C.C7 T cells (closed symbols on right) re-tested for their ability to restrict 5C.C7 numbers in fresh PCC+, Cd3e −/− hosts. The number of 5C.C7 T cells recovered 48 days after transfer to T cell-deficient (open blue squares) or control centi-pool containing (open triangles) mice are compared. 1 way ANOVA between Ctrl and PC5 or SA2 were NS while Ctrl and PB5, 2PB6 and SX3 were (p <0.0001).
D. Normalized microarray data from selected centi-pools was filtered on select gene signatures found in various regulatory T cell lineages and the relative expression values plotted against the deletor activity of each centi-pool. The most active pool is to the left and the least active in the right most column.
E. A quantitative trait analysis (QTA) on unfiltered microarray data showing the genes whose expression profile correlate significantly with the deletor activity observed in each centi-pool.
F. The specificity of the “deletor” pools isolated against 5C.C7 T cells (PC5, PB5 or SX3) were re-tested using a mixed transfer experiment with 5C.C7 and A1(M) together with each centi-pool. The number of 5C.C7 T cells remaining in lymph nodes & spleen, 40 days after transfer (red bars) is compared to those of A1(M) T cells (open blue bars). (n=3 per group, 1 of 2 experiments). 5C.C7 numbers in PB5 and SX3 were significantly lower than CTRL or PC5 (p < 0.001).
Pools of 100 flow cytometry-sorted CD4+ polyclonal T cells (centi-pools) were expanded in vitro as described in the methods, to generate ~ 2 million daughter cells. The progeny of each centi-pool was transferred to two PCC+, Cd3e −/− mice. A week later, congenically marked 5C.C7 T cells were infused and their number enumerated 40–60 days afterward as evidence of deletor activity (Figure 3B). After discarding 3 pools with discordant duplicate mice, a total of 54 centi-pools were screened. Since in our original experiments even a million polyclonal T cells reduced the 5C.C7 T cell density to <2 million, we assigned this as a cutoff for scoring deletor positive centi-pools. By those criteria 7 centi-pools were positive. However, three of the centi-pools (PB5, 2PB6 and SX3) were effective to a much greater degree, dramatically limiting 5C.C7 T cell numbers to ~10,000. Based on this, the most potent “deletor” activity (in this model) can be calculated to occur every 1 in 1800 T cells. This calculation however, is only an estimate and could be influenced by losses during in vitro culture or adoptive transfer – especially if these procedures selectively affected the deletor T cell subsets.
The populations of PB5, 2PB6 and SX3 centi-pools remaining in the recipient mice at the time of analysis were re-expanded in vitro and re-tested in vivo to confirm that these cells indeed retained the ability to restrain 5C.C7 numbers (Figure 3C). Compared to control populations (PC5 and SA2), mice that were infused with 100,000 PB5, 2PB6 or SX3 reduced the number of 5C.C7 T cells within 34 days (Figure 3C). The potent activity of the centi-pools might have been due to the presence of specific lineages of T cells within this population that can homeostatically regulate autoreactive lymphocytes. However, there was no enrichment of canonical regulatory or lineage signatures within these centi-pools, compared to non-deletor controls even in a genome wide expression profiling (Figure 3D). There is a very limited set of genes, whose modest expression changes (<3 fold) do correlate with deletor activity when subjected to a quantitative trait analysis (only 7 genes with an FDR < 0.2 and 20 with a parametric p value < 0.001) but these also do not assign to any canonical T cell functions (Figure 3E).
It was still formally possible that the active centi-pools were enriched for T cell phenotypes, albeit of a hitherto undescribed genetic phenotype, that were potent inhibitors of the survival of CD4+ T cells in general. Therefore, we tested this possibility by transferring A1(M) T cells into male PCC+, Cd3e −/− mice that received the active or control centi-pools (Figure 3F). Interestingly, neither centi-pool that was active against 5C.C7 affected A1(M) T cells. These data demonstrate that, surprisingly, the deletor T cells active against one T cell (5C.C7) do not globally regulate all lymphocyte homeostasis, but instead precisely modulate a particular antigen-specific response in a targeted fashion.
4. A single clonal TCR in the polyclonal population is sufficient to eliminate the consequences of lymphopenia
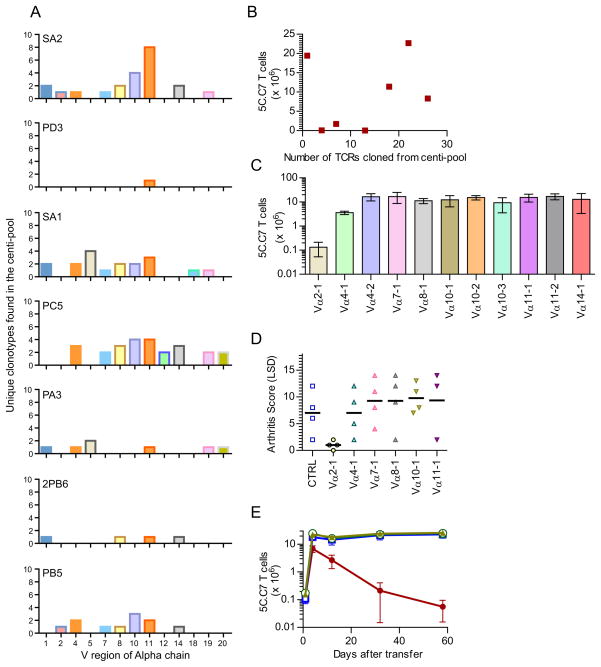
The ability of the deletor pools to discriminate between 5C.C7 and A1(M) suggested that the TCR-specificity of the centi-pools might be a critical component of their activity. We therefore dissected the TCR repertoires in the centi-pools by cloning and sequencing individual TCRα chain cDNAs from selected active and control centi-pools. Since this analysis was done after recovering the T cells from the in vivo screen (Figure 3B), and re-expansion in vitro, they were unlikely to retain the maximal possible complexity of 100 receptors. We therefore deemed a centi-pool as being “completely” sequenced if no new sequences were obtained on repeated rounds of 96 colony sequencing. By this criteria 7 centi-pools (marked in Figure S4A) including two of the deletor centi-pools - 2PB6 and PB5, were exhaustively analyzed and grouped according to their unique V, J and junctional amino acid sequences (Figure 4A). Some others, including the remaining deletor positive centi-pool (SX3) were partially analyzed (data not shown). The receptor complexity observed by this criterion within a given centi-pool did not correlate with greater deletor activity (Figure 4B) suggesting that individual specificities, rather than a synergistic repertoire might be critical to the activity of a centi-pool. Furthermore, there was no unique receptor that was common to the deletor-positive centi-pools, which would have predicted a suitable candidate for further analysis (Figure S4B).
Figure 4. A single T-cell receptor can confer deletor activity against 5C.C7 and limit immunopathology.
A. The frequency of unique alpha chains cloned from 7 of the centi-pools (plotted with the least active centi-pool on top and the most active towards the bottom) represented using their TCR Alpha chain Variable region (TRAV) distribution.
B. The receptor diversity of 7 selected centi-pools (X-axis) plotted against the efficiency of each pool in limiting 5C.C7 numbers – as measured 40–60 days after cohabitation with the pool (Y-axis, data from Figure 3B). (A correlation analysis gives an insignificant Spearman R of 0.21, p=0.66)
C. The activity of individual receptors cloned from the pool PB5, against 5C.C7 T cells, as measured by the number of 5C.C7 T cells 40–55 days after transfer to retrogenic mice expressing individual receptors (and PCC).(n=4 per group)
D. The arthritic pathology elicited by 5C.C7 T cells (scored by limb swelling & deformity – LSD) in various retrogenic chimeric mice.
E. The Vα2, Vβ3 retrogenic mice (male, PCC+) specifically constrain the density of 5C.C7 T cells (red circles) and not A1(M) (open blue circles). A control Vα10, Vβ3 retrogenic constrains neither (open blue squares, 5C.C7; green triangles, A1(M)). (n=3 per group, 1 of 2 experiments).
We therefore further functionally dissected the most active centi-pool PB5, by separating out individual TCRs obtained from this cohort and retrogenically generating new T cells from 11 of the 13 receptors (Figure S4B). The retrogenic mice were also chimeric for PCC expressing bone marrow, but could not generate other T cells. This allowed us to transfer 5C.C7 T cells and track the autoreactive response in the presence of an additional monoclonal “endogenous” T cell. Of the 11 TCRs that were reconstructed and screened in this fashion, only one receptor - bearing the TRAV14 (Vα2) alpha chain - could singularly recapitulate the deletor potential of the entire polyclonal CD4+ population (Figure 4C). This TCR was unique to the PB5 centi-pool and was not observed even in receptors recovered from the other deletor-positive centi-pools 2PB6 and SX3.
In the PCC model, 5C.C7 T cells trigger a scorable arthritic pathology, but only in the absence of the endogenous polyclonal T cell repertoire (Singh et al., 2006). We therefore examined whether the Vα2 deletor T cell which restrains the density of 5C.C7 T cells, could also abrogate this lymphopenia-enhanced pathogenicity. Among retrogenic mice expressing 6 different TCRs, the presence of the monoclonal Vα2 T cells alone prevented immunopathology (Figure 4D). This was also evident in adoptive transfer experiments to the PCC+, Cd3e−/− host, where the Vα2 T cells and not a control Vα10 population was able to prevent the limb deformations triggered by 5C.C7 T cell-mediated autoimmune arthritis (Figure S4C).
We then asked whether the isolated monoclonal Vα2-bearing T cells could recapitulate the discriminatory characteristic of the original PB5 centi-pool towards 5C.C7 and A1(M) T cells. Indeed, transfers into male PCC+ retrogenic mice showed that only 5C.C7 and not A1(M) T cell numbers were targeted by the presence of the deletor Vα2 T cells (Figure 4F). In summary, using systematic dissection of a complex polyclonal repertoire, we isolated a unique T cell from it, that can recapitulate the deletor phenotype of the entire population.
5. The deletor T cell also operates specifically against its target, in the absence of cognate antigen
The specific activity of Vα2 against 5C.C7 (and not A1(M)) T cells is a key aspect of this form of T cell regulation. We therefore set out to examine the molecular basis for such specificity. The simplest possibility was that they are both reactive against the same antigen - PCC. However, in a variety of in vitro and in vivo assays, we could find no evidence for activation of the Vα2 T cell by PCC or for that matter by idiotopes from 5C.C7 TCR in the co-culture (Figure S5). We therefore considered if the presence of PCC was required at all for the ability of the Vα2 T cell to regulate 5C.C7 T cells.
In fact, there is an extensive body of literature (discussed earlier) examining the effect of lymphopenia on steady state T cell survival, in the absence of cognate antigen stimulation. Consistent with this, the lifespan of naive 5C.C7 T cells is also dramatically altered by the absence of neighboring T cells, and an abundance of a second TCR transgenic (A1(M)) was insufficient to restore it (Figure 5A). However, the concept of clonal competition did apply in this context, since the presence of >15 million 5C.C7 T cells could restore the limited life-span of a new 5C.C7 cohort (black triangles, Figure 5A). In addition, in lymphopenic environments, naive T cells also undergo a slow proliferative expansion - without the help of cognate antigen (Cho et al., 2000; Oehen and Brduscha-Riem, 1999). This lymphopenia induced proliferation (LIP) was also inhibited by clonal competition - but not an abundance of other monoclonal T cells (Figure S6).
Figure 5. The activity of the deletor T cell does not require the presence of the cognate antigen.
A. The frequency of naïve 5C.C7 T cells (CFSE labeled) tracked in nodes and spleen of intact B10.A hosts (red squares - T½ = 11 days), Rag2−/− hosts (open squares - T½= indefinite), A1(M)(TCR-tg), RAG2−/− hosts (green triangles - T½ = indefinite) or congenic 5C.C7(TCR-tg), Rag2−/− hosts (black triangles, T½ ~ 11 days ) (n=1 per time point, 1 of 3 experiments)
B. The lymphopenia-induced proliferation of 5C.C7 T cells in Rag2−/− mice (top panel) or those bearing retrogenic Vα2+ T cells (middle panel) or control retrogenic Vα10+ T cells (bottom panel).
C. The number of 5C.C7 T cells (left group) 36 days after transfer to Vα2+, Rag2−/− mice (red bars) or Vα10+, Rag2−/− mice (open blue bars) compared to the recovery of transferred A1(M) (middle bars) or 3A9 (right bars) T cells (n=4 per group) In an unpaired t test, p<0.001 for the 5C.C7 T cell groups and NS for others.
In this context, we wondered if the Vα2 T cell could in fact mitigate the effects of lymphopenia on 5C.C7 T cells, in the absence of PCC. Intriguingly, 5C.C7 T cells transferred into retrogenic chimeras expressing the Vα2 TCR (and no PCC) were prevented from LIP in comparison to a control lymphopenic chimera or one expressing the Vα10 TCR (Figure 5B). Furthermore, over a 40 day period, about 80% fewer 5C.C7 T cells were recovered from the Vα2 retrogenic mice, relative to Vα10 retrogenics (Figure 5C). In striking reiteration of the Vα2 T cell’s specificity for 5C.C7 T cells, now even in the absence of PCC, this deletor T cell had no effect on 3A9 or A1(M) T cell numbers (Figure 5C). These data suggest that the mechanisms underlying homeostatic regulation of naive T cells in the steady state and those constraining the magnitude of a pathological autoimmune response, converge to a hitherto unappreciated degree.
6. The deletor and effector T cells recognize the same sub-threshold ligand
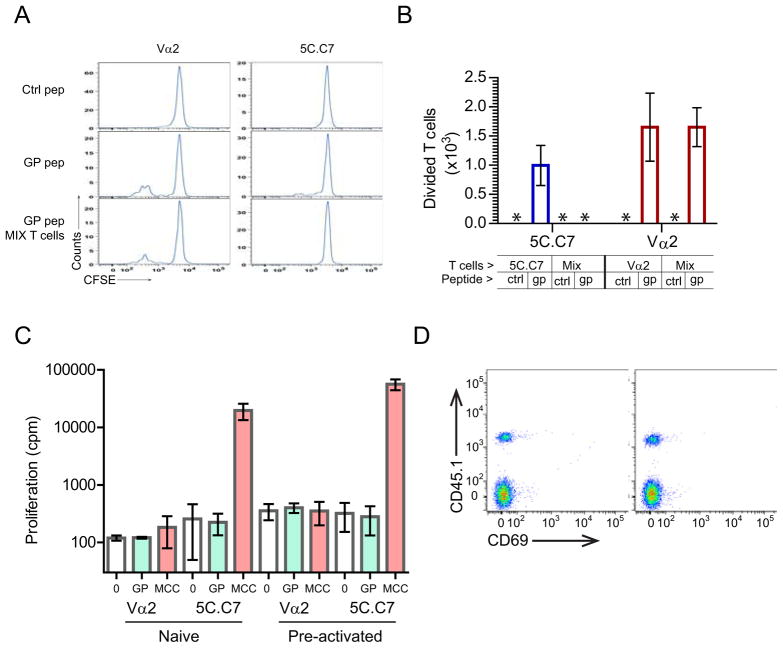
The ability of the Vα2 T cell to regulate 5C.C7 T cells in the absence of PCC prompted us to consider other elements of shared specificity. In addition to PCC, the 5C.C7 TCR has been reported to recognize an additional set of peptides (specifically one derived from an endogenous retrovirus - GP), that may be critical for positively selecting them during thymic development (Ebert et al., 2009). We decided to examine their role in the context of the LIP in a Cd3e −/− host that does not express PCC. Either 5C.C7 or Vα2 T cells individually transferred to such lymphopenic hosts do not initiate LIP for 7 days. We asked if this process could be accelerated by providing an excess of GP peptide. Interestingly repeated injection of high doses of GP peptide, every 2 days for 6 days, revealed a small but reproducible carboxyfluorescein diacetate succinimidyl ester (CFSE) dilution in the 5C.C7 T cells (Figure 6A). This effect was much more pronounced when the Vα2 T cells were stimulated similarly, suggesting that these T cells could engage GP peptide more efficiently. Most intriguingly, in a co-transfer experiment, the presence of the Vα2 T cell eliminated the small divided cohort of 5C.C7 T cells observed in response to multiple GP peptide injections (Figure 6B). The stimulatory activity of the GP peptide itself was not evident in most conventional assays. In fact, the GP peptide by itself was not able to activate either the 5C.C7 or Vα2 T cell in vitro to proliferate (Figure 6C) or express CD69 (Figure 6D). This suggests that this particular peptide delivers a TCR signal that is below the activation threshold for eliciting classical T cell responses in either T cell. Nevertheless, such a sub-threshold interaction provides the structural basis for a potent regulatory network involving non-clonal T cells that do not necessarily engage the same agonist.
Figure 6. Shared recognition of an endogenous peptide by the interacting T cells.
A. A million adoptively transferred Vα2+ T cells (left), 5C.C7 T cells (right) or a mixture of the two (bottom most panels) in B10.A, Cd3e −/− mice that do not express PCC, were challenged by three injections (150μg each, every other day) of control Dby peptide (top panels) or gp peptide (middle and bottom panels) before analyzing CFSE dilution 6 days after transfer.
B. Quantitation of the number of T cells that have diluted CFSE from the experiments shown in (A) with n=3 per group. The * indicates numbers below the limit of reliable detection. (1 of 3 similar experiments is shown).
C. Naïve (left 6 bars) or pre-activated (T cells activated with PMA, Ionomycin and αCD28 for 3 days and rested for 12 days with 10U/ml of IL-2) T cells were stimulated in the presence of irradiated B10.A, Cd3e −/− splenocytes and GP or MCC peptides (10μM each) for 48 hours before tritiated thymidine incorporation for an additional 20 hours.
D. Flow cytometry analysis of CD4+Vβ3+ T cells after the culture of a mixture of 5C.C7 (upper quadrants, CD45.1 +ve) and Vα2+ T cells (lower quadrant, CD45.1 −ve) with syngeneic APC and no peptide (left panel) or 10μM concentration of the GP peptide for 20 hours.
7. Deletors for different T cell specificities occur in distinct colonies
Our data have revealed a regulatory principle inherent in the polyclonal T cell repertoire, that targets 5C.C7 T cells during steady state homeostasis as well as in the context of an autoimmune response. By extension, it is likely that such a specific regulation operates on every antigen-specific T cell within the peripheral T cell repertoire. In order to confirm this hypothesis, we examined a second autoimmune response - mediated by A1(M) T cells responding to their natural self-antigen in male mice. A1(M) T cells in male, Cd3e −/− mice produce an autoimmune pathology marked by inflamed skin in the ear and snout (Figure S6C). In contrast, mice with an intact endogenous polyclonal repertoire did not develop this syndrome (Figure 7A). In striking similarity to the 5C.C7-PCC model, A1(M) T cells in male, Cd3e −/− mice not only expand more robustly than in intact male mice, but also maintain a high frequency of cells subsequently (Figure 7B). Indeed, the transfer of a single bolus of polyclonal CD4+ T cells could reduce the frequency of auto-reactive A1(M) T cells as well (Figure 7C) while the presence of “bystander” 5C.C7 T cells could not (Figure 2B). In the case of the PCC model, one of the earliest indications of a specific activity within the polyclonal repertoire that was crucial for controlling the frequency of 5C.C7 T cells, was the differential efficacy of the partially polyclonal repertoires generated in the presence of various TCR transgenes (Figure 3A). A similar experiment against A1(M) T cells yielded an interesting contrast (Figure 7D). Although a fully polyclonal repertoire in intact male mice (2nd bar, Figure 7D) could severely limit the A1(M) T cell frequency, repertoires with lesser complexity (such as the 3A9+, Rag2+ mice with an endogenous receptor rearrangement) show reduced efficacy. Importantly, the Vβ3-transgene repertoire, which previously was shown to restrain 5C.C7 T cells could not affect the A1(M) frequency. Such differences within the complex specificities of polyclonal repertoires, which can control individual T cell specificities, are reminiscent of the behavior of individual monoclonal T cell populations (Figure S6) or fractions of polyclonal T cells in preventing LIP (Leitao et al., 2009; Min et al., 2004). Taken together, these data suggest an intricate organization of the peripheral polyclonal T cell repertoire where a hierarchy of sub-threshold specificities controls individual T cell responses very precisely, rather than by limiting the generic availability of trophic factors alone (Figure S7).
Figure 7. Distinct polyclonal subsets regulate a different antigen-specific TCR.
A. The pathogenicity of A1(M) T cells in T-cell deficient (blue lines), but not intact (red) male mice. Sample of the inflammation is shown in Figure S6B. (n=52 each group, over 8 experiments).
B. The expansion of A1(M) T cells in male mice with an intact endogenous polyclonal repertoire of T cells (closed circles) compared to male T cell-deficient (Cd3e −/−) hosts (open blue squares). 1 of 2 similar experiments is shown with n=2–4 mice per time point (points without error bars have n=2).
C. The kinetics of expansion and contraction of A1(M) T cells in male B10.A, Cd3e −/− recipient mice (open blue squares) compared to recipients that previously received 1×106 polyclonal male T cells, a day before (closed red symbols). n=3–4 mice per time point.
D. The recovery of A1(M) T cells at 38 days from male mice that do not have endogenous T cells (0), compared to those that bear the Vβ3 or 3A9(αβ) transgenes (in the presence of RAG genes that allow for varying degrees of endogenous TCR rearrangement and development as well)(n=5 for each bar).
Discussion
We have shown that the homeostasis of antigen-specific CD4+ T cells, in the steady state as well as during a strong antigen-driven pathogenic response, is regulated by the same non-clonal neighboring T cells, that out-compete the targeted T cell for specific recognition of a sub-threshold peptide-MHC trophic ligand. These data help resolve several outstanding questions on the control of CD4+ T cell dynamics, discussed below, and suggest a conceptual framework for envisioning the functional architecture of the peripheral T cell repertoire (see Figure S7).
The first of these is regarding the role of public vs private factors in peripheral T cell homeostasis (Figure S7B). The extensive dataset in this report argues that competition between CD4+ T cells for generic trophic resources is unlikely to be the major regulator of helper T cell frequency in vivo. Clearly, a variety of such factors are critical for T cell survival in a global sense (Marrack and Kappler, 2004). In the absence of signals from the γc cytokines, for example, a profound lymphopenia is observed – although CD4+ T cells (of an activated phenotype) are less affected (DiSanto et al., 1996; Lantz et al., 2000). But, because T cells widely express receptors for these factors, it is not easy to explain how such a public competition can ensure the stable maintenance of individual specificities within a diverse repertoire. We resolved this question using a cellular strategy - packing mice with defined populations of T cells that can compete with the target T cell for some or all of the public factors. This strategy avoids the complexities of using genetic knockouts or blocking antibodies for individual factors and replaces it with cells that can compete physiologically for these resources. An argument can be made that it is still a low density sampling of the T cell universe, since the few TCR transgenics used in our packing experiments may not represent the full range of possibilities in a diverse repertoire. In this context, the negative results within our in vivo screen provide a more striking illustration. Here the bulk of the pools, effectively representing >99.9% of the peripheral T cell population from a polyclonal repertoire, fail to modulate the density of 5C.C7 T cells. While these data clearly eliminate public factors as the sole determinants of peripheral homeostasis, it is still possible that they play a critical but secondary role in the process.
Of the cognate factors (sensed via the TCR) that remain, the restricting MHC element itself (IEk for 5C.C7) was also not sufficient, because the similarly restricted A1(M) T cell did not affect the frequency of 5C.C7. A competition for the cognate antigen itself is certainly widely reported in models that use acute immunization (Smith et al., 2000). However, in the chronic antigen model, two T cells specific for the same antigen with TCRs that are known to have different affinities for IEk-PCC (AND & 5C.C7) did not affect each other’s numbers. Although the lack of an effect on AND numbers in particular could be attributed to the known cross-reactivities of AND to other ligands on IEk, 5C.C7 (not known for such cross-reactivities) also remains unaffected when competing with AND for PCC.
Finally, several elegant studies have shown that competition between large numbers of identical clones of T cells interfere with the survival and response of each other (Hataye et al., 2006; Moses et al., 2003; Troy and Shen, 2003). Indeed, clonal competition does work in our model as well, when measured by the steady state survival (Figure 5A) or acute activation of 5C.C7 T cells (Sojka et al., 2004). However, in the absence of a clear mechanism for this phenomenon, it was difficult to envision how it would operate within a polyclonal repertoire. In our data, the lifespan of 5C.C7 T cells was similar in hosts with a normal polyclonal population and in TCR transgenic mice with 10–15 million 5C.C7 T cells. This would imply that there are as many relevant clones in the polyclonal repertoire to mimic the clonal abundance of a monoclonal TCR transgenic animal. But most strikingly, the 5C.C7 T cells in a PCC+ but lymphopenic host routinely expand to 10–15 million. If clonal competition was a dominant determinant of the frequency of 5C.C7 T cells, these abundant clones should have efficiently interfered with one another - or for that matter a 2nd cohort of 5C.C7 T cells (Singh et al., 2006). The isolation of the Vα2+ deletor T cell finally allowed us to resolve these paradoxical observations and propose a unifying model. This deletor was not a clonal competitor of 5C.C7 but shares a sub-threshold ligand with it (Figure S7C). Most importantly, it did not share any cognate antigen specificity with its target that we could detect.
This last property is crucial, since one of the consequences of chronic antigen stimulation in vivo is the induction of T cell tolerance – in this case, by a process involving the tuning of TCR proximal signaling molecules (Choi and Schwartz, 2007; Singh and Schwartz, 2003). As a result, a T cell that is constantly stimulated by agonistic self (or in some cases chronic-pathogen derived) antigens would get blunted in its ability to transduce signals via the TCR. This is likely to render that T cell a poor competitor for antigens. In essence, the tuning process eliminates the distraction afforded by clonal competition and allows one to hone in on the relevant activity within the truly polyclonal repertoire. Since the deletor also inhibits the proliferation of 5C.C7 T cells in PCC-negative lymphopenic hosts (in a manner similar to clonal competition), it is likely that the unifying feature underlying both phenomena is indeed the recognition of the same universe of self-ligands.
Other than the shared recognition of a sub-threshold ligand, the deletor T cell does not seem to possess a unique property that defines it as a separate lineage. But further studies are required to clearly establish if the deletor capable receptor does also trigger a new gene expression signature in the T cells. Furthermore, while the deletor activity did not enrich with regulatory T cells (Tregs), it must be pointed out that it was not depleted in the Foxp3+ve population either. In addition, although the early expansion of the 5C.C7 T cells was also blunted slightly by the Vα2+ deletor T cells, this effect was not as pronounced as the effect of total polyclonal T cells. It is likely that other mechanisms such as Tregs cells may therefore be involved during this phase (Vanasek et al., 2006).
Finally, the nature of the sub-threshold self-ligand itself raises interesting questions. At least one peptide that anchors the interaction between the deletor and 5C.C7 is one recently identified as a positively selecting ligand for the 5C.C7 TCR (Ebert et al., 2009). While it is tempting to speculate that a direct correlation might exist between thymic selection and peripheral regulation, previous studies have failed to validate a linear relationship (Bender et al., 1999; Clarke and Rudensky, 2000; Ebert et al., 2009; Ernst et al., 1999; Goldrath and Bevan, 1999; Kieper et al., 2004). In fact in our experiments, polyclonal T cells that are selected in a thymus that did not afford an IEk positively selecting signal to 5C.C7 T cells still possessed deletors capable of regulating them. Therefore, a more critical requirement might simply be for the ligand to be non-agonistic but consistently available on self MHC molecules in quantities that are limiting enough for a competitive process to operate. In many cases, positively selecting ligands are likely to satisfy these criteria and this might in fact be a strong teleological reason for retaining peptide-specific positive selection in the thymus. But certainly other sources of these ligands can arise in the periphery (from tissue-specific antigens, products of commensal organisms etc.) which might even qualitatively modulate the complexity of T cell repertoires in different tissue locations even before an antigen-specific response.
In this context, the ability of the deletor to regulate 5C.C7 numbers even in the presence of chronic PCC presentation is quite intriguing. This implies that interactions with the “non-stimulatory” weak ligands (that allow the deletor to dominate) are still relevant even though a stimulatory agonist is available. One potential explanation could be that the sub-threshold ligands and the agonists elicit qualitatively different signals downstream of the TCR. The former may in fact be more potent at triggering some pro-survival signals within the T cell than even the agonist. Alternately, the sub-threshold ligands may be segregating to specialized subsets of APCs which might allow the interacting T cells to obtain secondary survival signals. The segregation of the peripheral ligand on special APCs could also explain the ability of numerically fewer deletors to control more abundant target T cells after a clonal expansion. Since the relevant niches are limited, a few potent deletors could effectively block access to these, at least temporally. These mutually non-exclusive models will require further experimental validation, but have important consequences for our understanding of peripheral T cell responses.
These data are the strongest experimental evidence yet, in support of an emerging model envisioning the peripheral T cell repertoire as subdivided into small homeostatic units or colonies (Figure S7D) (Hao et al., 2006; Hataye et al., 2006; Leitao et al., 2009; Min et al., 2004; Takada and Jameson, 2009). Each colony can be defined as a group of TCRs that share the recognition of a specific set of endogenous sub-threshold ligands - although their cognate agonists differ greatly. In such a model, the size of each colony would likely depend on the amounts of the sub-threshold ligand presented in vivo and be strictly regulated by intra-colony competition for these ligands. Within each colony, individual clones would be able to respond to their disparate cognate antigens during an infection or injury (Figure S7E). But most importantly, after the clearance of antigen (or the induction of tolerance), the number of antigen-specific T cells would be controlled primarily on the basis of competition between members of its own colony (Figure S7F). Since this process avoids rampant bystander losses that might be a side effect of competition for public resources, the overall diversity of the repertoire would be minimally affected. The teleological advantage of such a mechanism would be to maintain the broad diversity of the naive and memory T cell repertoire, especially in the context of multiple recurrent infections from diverse pathogens. Clearly such a model would have profound implications on our attempts to manipulate the immune response in various clinical contexts. Learning to identify and manipulate the dynamics of individual micro-colonies could be key to developing vaccines capable of generating long lasting antigen-specific T cells or conversely, ameliorating autoimmunity by reducing the life-span of auto-antigen-specific effectors.
Experimental Procedures
Please refer to Supplementary Online Text for more detailed experimental procedures.
Mice and cells
Strains used were bred to a B10.A (H-2a) background. All TCR transgenics were additionally on a Rag2−/− background, unless specifically stated. The PCC transgenic was originally generated by Oehen et. al. using a plasma membrane targeting sequence linked to the synthetic PCC exon. T cells for transfer experiments were isolated from pooled lymph nodes (LN). All animal experiments were approved by the DIR/NIAID Animal Care and Use committee.
FACS analysis and sorting
5C.C7 T cells were detected by double staining for CD4 and Vβ3 in CD3e−/− mice and additionally with Ly5.1 in intact mice or Ly5.1 or Vα11 in the presence of other retrogenic T cells. For flow cytometry sorting, LN cells were enriched by negative selection using a lineage cocktail bound to Dynabeads indirectly followed by staining for appropriate. Sorts were typically >99% purity.
In vitro culture
T cell proliferation was assayed using 10,000 cells, 25 fold excess of B10.A, CD3e−/− splenocytes (irradiated at 3000R) and indicated doses of peptide for 60 hours before pulsing with 1μCi of 3H-Thymidine. After 20–24 hours harvested cells were analyzed for incorporated radioactivity. The peptides used for stimulating T cells in this study are as follows: MCC (88-103), PCC (81-104), Dby (197-211) and GP (557-611).
Screening for “deletor” activity
T cells from B10.A, Vβ3 tg, TCRα+/− mice that express the beta chain from the 5C.C7 TCR and an assortment of endogenous alpha chains were seeded at a frequency of 100 cells per well by dilution, in a 24 well plate. These cells were stimulated with 1ug/ml Ionomycin, 200ng/ml PMA, 10U/ml IL-2 and diluted anti-CD28 ascites solution or with 10μg/ml Staphylococcus enterotoxin A, a superantigen that activates this particular TCR beta chain. All wells received 1 million irradiated B10.A, CD3e−/− splenocytes as a source of feeder cells/APCs and the culture medium added to bring up the volume to 1ml. Three days later, the media was replaced with one supplemented with 20U/ml of IL-2 and IL-7. Three days later, the cells were harvested and transferred to 6 well plates. The medium was replaced every 2 days as before and after 8 days, the cell number in each well was enumerated. Wells with at least 2 million T cells were used for transfers to fresh B10.A, PCC+, CD3e−/− mice. 7 days later, 5C.C7 T cells were transferred in and their frequency enumerated 40–60 days later.
TCR alpha chains and retrogenic mice
RNA isolated from expanded centi-pools was reverse transcribed and the cDNA amplified using previously published 5′ primers for the alpha chain (Alli et al., 2008) (see Table S1) and modified 3′ TCR Cα primer (5′-CTCCCTGCAGGGACTGGACCACAGCCTCAGCGT-3′). Amplified cDNA from pools of TCRs was cloned and individual clones sequenced. Verified sequences from the PB5 centi-pool were subcloned into a modified pMSCV-puro vector. Ecotropic retroviral particles generated from these constructs were used to infect bone marrow cells from B10.A, Vβ3+, Rag2−/− mice (PCC+ or PCC-), which were seeded into irradiated recipient mice (B10.A, CD3e−/− or Rag2−/−). In experiments requiring peripheral PCC expression, the transfected bone marrow was also mixed (1:1) with B10.A, PCC+, CD3e−/− BM cells.
Microarray & statistical analysis
Microarray data (GEO accession # GSE35220) was analyzed using BRB-ArrayTools developed by Dr. Richard Simon and BRB-ArrayTools Development Team using the Quantitative Trait Analysis module. All statistical tests were performed using the modules in Graphpad prism v5 (Graphpad Software Inc, CA)
Supplementary Material
Highlights.
Competition for public trophic factors is insufficient to limit CD4+ T cell numbers
Different subsets in the repertoire specifically regulate individual T cell clones
Regulators outcompete the target TCR for recognition of a sub-threshold ligand
Regulators can eliminate autoimmune pathology even in lymphopenic hosts.
Acknowledgments
We thank Chuan Chen & Eleanore Chuang for assistance with experiments; Carol Henry & Cal Eigsti for FACS sorting; Qin Su & Tim Myers for microarray hybridization; Pascal Chappert for hearty discussions; Ron Germain and Yasmine Belkaid for comments on improving the manuscript.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alli R, Nguyen P, Geiger TL. Retrogenic modeling of experimental allergic encephalomyelitis associates T cell frequency but not TCR functional affinity with pathogenicity. J Immunol. 2008;181:136–145. doi: 10.4049/jimmunol.181.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender J, Mitchell T, Kappler J, Marrack P. CD4+ T cell division in irradiated mice requires peptides distinct from those responsible for thymic selection. J Exp Med. 1999;190:367–374. doi: 10.1084/jem.190.3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown IE, Blank C, Kline J, Kacha AK, Gajewski TF. Homeostatic proliferation as an isolated variable reverses CD8+ T cell anergy and promotes tumor rejection. J Immunol. 2006;177:4521–4529. doi: 10.4049/jimmunol.177.7.4521. [DOI] [PubMed] [Google Scholar]
- Cho BK, Rao VP, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Schwartz RH. Molecular mechanisms for adaptive tolerance and other T cell anergy models. Semin Immunol. 2007;19:140–152. doi: 10.1016/j.smim.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke SR, Rudensky AY. Survival and homeostatic proliferation of naive peripheral CD4+ T cells in the absence of self peptide:MHC complexes. J Immunol. 2000;165:2458–2464. doi: 10.4049/jimmunol.165.5.2458. [DOI] [PubMed] [Google Scholar]
- DiSanto JP, Guy-Grand D, Fisher A, Tarakhovsky A. Critical role for the common cytokine receptor gamma chain in intrathymic and peripheral T cell selection. J Exp Med. 1996;183:1111–1118. doi: 10.1084/jem.183.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorfman JR, Stefanova I, Yasutomo K, Germain RN. CD4+ T cell survival is not directly linked to self-MHC-induced TCR signaling. Nat Immunol. 2000;1:329–335. doi: 10.1038/79783. [DOI] [PubMed] [Google Scholar]
- Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009;10:1162–1169. doi: 10.1038/ni.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst B, Lee DS, Chang JM, Sprent J, Surh CD. The peptide ligands mediating positive selection in the thymus control T cell survival and homeostatic proliferation in the periphery. Immunity. 1999;11:173–181. doi: 10.1016/s1074-7613(00)80092-8. [DOI] [PubMed] [Google Scholar]
- Freitas AA, Rocha B. Population biology of lymphocytes: the flight for survival. Annu Rev Immunol. 2000;18:83–111. doi: 10.1146/annurev.immunol.18.1.83. [DOI] [PubMed] [Google Scholar]
- Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, Johannes KP, Su MA, Chang HY, Krummel MF, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–847. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldrath AW, Bevan MJ. Low-affinity ligands for the TCR drive proliferation of mature CD8+ T cells in lymphopenic hosts. Immunity. 1999;11:183–190. doi: 10.1016/s1074-7613(00)80093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandjean I, Duban L, Bonney EA, Corcuff E, Di Santo JP, Matzinger P, Lantz O. Are major histocompatibility complex molecules involved in the survival of naive CD4+ T cells? J Exp Med. 2003;198:1089–1102. doi: 10.1084/jem.20030963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y, Legrand N, Freitas AA. The clone size of peripheral CD8 T cells is regulated by TCR promiscuity. J Exp Med. 2006;203:1643–1649. doi: 10.1084/jem.20052174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hataye J, Moon JJ, Khoruts A, Reilly C, Jenkins MK. Naive and memory CD4+ T cell survival controlled by clonal abundance. Science. 2006;312:114–116. doi: 10.1126/science.1124228. [DOI] [PubMed] [Google Scholar]
- Kieper WC, Burghardt JT, Surh CD. A role for TCR affinity in regulating naive T cell homeostasis. J Immunol. 2004;172:40–44. doi: 10.4049/jimmunol.172.1.40. [DOI] [PubMed] [Google Scholar]
- Kirberg J, Berns A, Von Boehmer H. Peripheral T cell survival requires continual ligation of the T cell receptor to major histocompatibility complex-encoded molecules. J Exp Med. 1997;186:1269–1275. doi: 10.1084/jem.186.8.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantz O, Grandjean I, Matzinger P, Di Santo JP. Gamma chain required for naive CD4+ T cell survival but not for antigen proliferation. Nat Immunol. 2000;1:54–58. doi: 10.1038/76917. [DOI] [PubMed] [Google Scholar]
- Leitao C, Freitas AA, Garcia S. The role of TCR specificity and clonal competition during reconstruction of the peripheral T cell pool. J Immunol. 2009;182:5232–5239. doi: 10.4049/jimmunol.0804071. [DOI] [PubMed] [Google Scholar]
- Marrack P, Kappler J. Control of T cell viability. Annu Rev Immunol. 2004;22:765–787. doi: 10.1146/annurev.immunol.22.012703.104554. [DOI] [PubMed] [Google Scholar]
- Min B, Foucras G, Meier-Schellersheim M, Paul WE. Spontaneous proliferation, a response of naive CD4 T cells determined by the diversity of the memory cell repertoire. Proc Natl Acad Sci USA. 2004;101:3874–3879. doi: 10.1073/pnas.0400606101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses CT, Thorstenson KM, Jameson SC, Khoruts A. Competition for self ligands restrains homeostatic proliferation of naive CD4 T cells. Proc Natl Acad Sci USA. 2003;100:1185–1190. doi: 10.1073/pnas.0334572100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obar JJ, Khanna KM, Lefrancois L. Endogenous naive CD8+ T cell precursor frequency regulates primary and memory responses to infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oehen S, Brduscha-Riem K. Naive cytotoxic T lymphocytes spontaneously acquire effector function in lymphocytopenic recipients: A pitfall for T cell memory studies? Eur J Immunol. 1999;29:608–614. doi: 10.1002/(SICI)1521-4141(199902)29:02<608::AID-IMMU608>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Oehen S, Feng L, Xia Y, Surh CD, Hedrick SM. Antigen compartmentation and T helper cell tolerance induction. J Exp Med. 1996;183:2617–2626. doi: 10.1084/jem.183.6.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polic B, Kunkel D, Scheffold A, Rajewsky K. How alpha beta T cells deal with induced TCR alpha ablation. Proc Natl Acad Sci USA. 2001;98:8744–8749. doi: 10.1073/pnas.141218898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiel J, Caucheteux S, Laurence A, Singh NJ, Bocharov G, Ben-Sasson SZ, Grossman Z, Paul WE. Antigen-stimulated CD4 T-cell expansion is inversely and log-linearly related to precursor number. Proc Natl Acad Sci USA. 2011;108:3312–3317. doi: 10.1073/pnas.1018525108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schietinger A, Delrow JJ, Basom RS, Blattman JN, Greenberg PD. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science. 2012;335:723–727. doi: 10.1126/science.1214277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluns KS, Lefrancois L. Cytokine control of memory T-cell development and survival. Nat Rev Immunol. 2003;3:269–279. doi: 10.1038/nri1052. [DOI] [PubMed] [Google Scholar]
- Singh NJ, Chen C, Schwartz RH. The impact of T cell intrinsic antigen adaptation on peripheral immune tolerance. PLoS Biol. 2006;4:e340. doi: 10.1371/journal.pbio.0040340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NJ, Schwartz RH. The strength of persistent antigenic stimulation modulates adaptive tolerance in peripheral CD4+ T cells. J Exp Med. 2003;198:1107–1117. doi: 10.1084/jem.20030913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AL, Wikstrom ME, Fazekas De St GB. Visualizing T cell competition for peptide/MHC complexes: a specific mechanism to minimize the effect of precursor frequency. Immunity. 2000;13:783–794. doi: 10.1016/s1074-7613(00)00076-5. [DOI] [PubMed] [Google Scholar]
- Sojka DK, Bruniquel D, Schwartz RH, Singh NJ. IL-2 secretion by CD4+ T cells in vivo is rapid, transient, and influenced by TCR-specific competition. J Immunol. 2004;172:6136–6143. doi: 10.4049/jimmunol.172.10.6136. [DOI] [PubMed] [Google Scholar]
- Surh CD, Sprent J. Regulation of mature T cell homeostasis. Semin Immunol. 2005;17:183–191. doi: 10.1016/j.smim.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Takada K, Jameson SC. Naive T cell homeostasis: from awareness of space to a sense of place. Nature reviews Immunology. 2009;9:823–832. doi: 10.1038/nri2657. [DOI] [PubMed] [Google Scholar]
- Takeda S, Rodewald HR, Arakawa H, Bluethmann H, Shimizu T. MHC class II molecules are not required for survival of newly generated CD4+ T cells, but affect their long-term life span. Immunity. 1996;5:217–228. doi: 10.1016/s1074-7613(00)80317-9. [DOI] [PubMed] [Google Scholar]
- Tanchot C, Lemonnier FA, Perarnau B, Freitas AA, Rocha B. Differential requirements for survival and proliferation of CD8 naive or memory T cells. Science. 1997;276:2057–2062. doi: 10.1126/science.276.5321.2057. [DOI] [PubMed] [Google Scholar]
- Troy AE, Shen H. Cutting edge: homeostatic proliferation of peripheral T lymphocytes is regulated by clonal competition. J Immunol. 2003;170:672–676. doi: 10.4049/jimmunol.170.2.672. [DOI] [PubMed] [Google Scholar]
- Vanasek TL, Nandiwada SL, Jenkins MK, Mueller DL. CD25+Foxp3+ regulatory T cells facilitate CD4+ T cell clonal anergy induction during the recovery from lymphopenia. J Immunol. 2006;176:5880–5889. doi: 10.4049/jimmunol.176.10.5880. [DOI] [PubMed] [Google Scholar]
- Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.