Abstract
The mechanisms that link bacterial infection to solid organ rejection remain unclear. Here we show that following the establishment of lung allograft acceptance in mice P. Aeruginosa (PA) airway infection induces a G-CSF-dependent neutrophilia that stimulates acute rejection. Graft-infiltrating neutrophils sharply upregulate the B7 molecules CD80 and CD86, but do not express CD40 or MHC Class II in response to PA infection. Neutrophil B7 promotes naïve CD4+ T cell activation and intragraft IL-2+, IFN-γ+ and IL-17+ T lymphocyte accumulation. Intravital 2-photon microscopy reveals direct interactions between neutrophils and CD4+ T cells within pulmonary allografts. Importantly, lung rejection in PA-infected recipients is triggered by CD80/86 on neutrophils and can be prevented by B7 blockade without affecting clearance of this pathogen. These data show that neutrophils enhance T cell activation through B7 trans-costimulation and suggest that inhibiting neutrophil-mediated alloimmunity can be accomplished without compromising bacterial immune surveillance.
In human lung recipients post-transplant airway colonization with PA is associated with graft rejection (1). Airway neutrophilia that often accompanies such infections has also been linked to both chronic and acute lung allograft rejection (2). In PA-infected lungs G-CSF is a critical mediator of neutrophil mobilization (3). Accordingly, we have reported that G-CSF-driven granulopoiesis leads to pulmonary tissue injury and prevents immunosuppression-mediated acceptance of mouse lung allografts (4, 5). Neutrophils have been proposed to regulate adaptive immune responses through a variety of mechanisms. Interestingly, neutrophils can express MHC Class II as well as costimulatory molecules and several studies have reported their capacity to act as APCs (6, 7). In addition to delivery of costimulatory signals by the cell that presents the antigen adaptive immune responses can be further enhanced by costimulatory signals expressed on bystander cells, a process referred to as trans-costimulation. Bystander APCs are thought to be the major mediators of B7 trans-costimulation and have been shown to a play a critical role in promoting solid organ rejection (8). In this report we provide evidence that G-CSF-mobilized neutrophils in response to PA infection upregulate and provide B7 trans-costimulatory signals to T cells and prevent established lung allograft tolerance.
Materials and Methods
Mice
C57BL/6J (B6), Balb/cJ (Balb/c) and B6 CD11b−/− mice are from Jackson Laboratories. B6 CD11c-EYFP was crossed with B6 LysM-GFP mice to generate double reporter mice (B6 CD11c-EYFP LysM-GFP). All experiments were approved by the Washington University Animal Studies Committee.
Lung transplantation, infection, Abs and neutrophil adoptive transfer
Lung transplantation was conducted as previously described (9) and all graft recipients were treated with CD154:CD40 blockade via CD154 Ab clone MR1 (250 μg, POD 0, Bio-X-Cell) and CD28:B7 blockade via CTLA4-Ig (200 μg, POD 2, Bio-X-Cell), which we have previously shown maintains acceptance for at least 100 days (10). 2.5 × 106 colony forming units (CFU) of P. aeruginosa strain P01, live (PA) or heat-killed dose equivalent (hkPA; 65°C for 1 hr) was resuspended in 50 μl normal saline for airway administration. 200 μg of G-CSF-Abs (Peprotech) or 250 μg clone 1A8 Ly6G (Bio-X-Cell) neutrophil-depleting Abs was administered i.v. 4 hrs prior to PA inoculation. Neutrophils were purified by negative selection as previously described (4). 107 neutrophils were injected i.v. into PA-infected G-CSF Ab-treated lung recipients once a day for up to 3 days.
Rejection Assessment
H & E sections of allograft tissue from uninfected and infected recipients were screened in a double blind fashion for the presentation of dense perivascular infiltrates and scored by the criteria set forth by the International Society of Heart and Lung Transplantation Working Lung Rejection Study Group of 2007 (11).
2P Microscopy
Balb/c → B6. CD11c-EYFP LysM-GFP on POD 7 received PA and 5 × 106 CellTracker Red (CMTPX, Invitrogen) labeled B6 CD4+ T cells. On POD 8 time-lapse imaging was performed with a custom built 2P microscope running ImageWarp acquisition software (A&B Software) (5). For time-lapse imaging of neutrophil- CD4+ T cell interactions in the lung tissue, we averaged 15 video-rate frames (0.5 s per slice).
T cell analysis
Lung tissue digests was performed and T cell intracellular expression of IFN-γ, IL-17A and IL-2 was measured as previously described (4, 12). IL-2 culture production was measured by ELISA (ebioscience). Intragraft CD4+ T cells were isolated with anti-CD4 beads (Miltenyi) and cultured Balb/c bone marrow-derived DCs for 36 hrs. Splenic naïve CD4+ T cells were isolated by flow cytometric sort on a CD90.2+ CD25−CD62Lhi CD44lo CD4+ gate. Alloantigen-specific CD4+ T cell responses were generated with irradiated Balb/c T cell-depleted splenocytes for 36 hrs and IL-17 and IFN-γ was determined by FACS-cytokine secretion assay (Miltenyi).
Neutrophil Assessment
Neutrophils were identified as Ly6Ghi Gr1hi CD11b+CD115− cells by FACS and quantified by multiplying percent abundance by the total cell count in the bronchoalveolar lavage (BAL) as previously described (4). Neutrophils were stained with Abs (BD Pharmingen) to CD80 (16-10A1), CD86 (AF6-120.1), CD40 (3/23) and IAb (GL1).
Statistical Analysis
Data was analyzed using GraphPad Prism, version 5.0 and results were represented as mean ± s.e.m. An unpaired two-tailed Student’s t-test was used to evaluate pairs of means for significance. Values of p < 0.05 were considered significant and where *p < 0.05, ** p < 0.01.
Results and Discussion
P. Aeruginosa infection prevents established lung tolerance
Based on clinical reports that PA colonization shortens human pulmonary allograft survival (1) we asked if this infection abrogates established tolerance in a model of immunosuppression-mediated Balb/c→ B6 lung acceptance (4,10). On post-operative day (POD) 7 these lung recipients received saline or 2.5 × 106 CFU of PA intratracheally and allograft histology, T lymphocyte intragraft accumulation and neutrophilia was analyzed for up to three weeks post-infection (Fig. 1a–b). As compared to saline-treated control recipients there was progressive histological evidence of lymphocytic vascular rejection and the accumulation of graft-infiltrating IL-17+ CD4+, IFN-γ+CD4+ and IFN-γ+CD8+ T cells in PA-infected recipients. Additionally, lung rejection was associated with more alloantigen-specific Th1 and Th17 cells as compared to saline-treated lung recipients (Fig. 1c). Finally, allograft rejection could be induced by PA infection in recipients that had accepted their lungs for greater than 100 days suggesting the abrogation of regulatory mechanisms that also promote long term acceptance (Fig. S1a).
Figure 1.
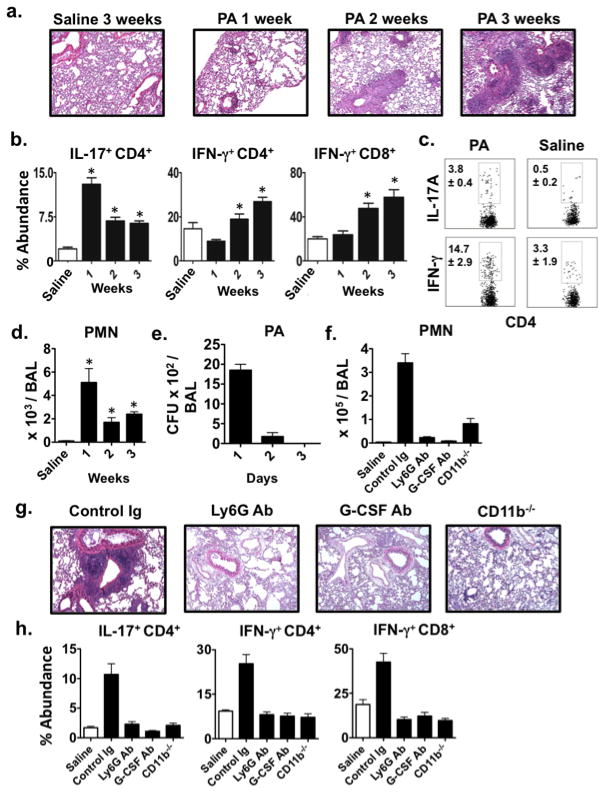
PA-induced neutrophilia prevents established lung allograft tolerance. Balb/c → B6 lung allografts analyzed by (a) H & E graft histology (representative of N ≥ 4) or for (b) the accumulation of indicated intragraft T lymphocytes (N ≥ 4) up to 3-weeks after either saline or PA airway instillation. (c) Intragraft CD4+ T cells isolated 3-weeks after either saline or PA airway instillation into Balb/c → B6 recipients were stimulated with Balb/c splenocytes and analyzed for IL-17 or IFN-γ expression (N ≥ 3). (d) Balb/c → B6 lung allograft BAL neutrophil numbers after either saline or PA airway instillation (N ≥ 4) or (e) CFU after PA infection (N=3). (f) BAL neutrophils quantified 1-day after hkPA or saline airway instillation into Balb/c → B6 recipients (N=4) treated with indicated Abs or 1-day after hkPA airway instillation into Balb/c → CD11b−/− recipients (N=3). (g) Allograft tissue isolated 3-weeks after PA infection from lung recipients treated as in (f) and analyzed by H & E histology (N ≥ 3) or for (h) indicated intragraft T lymphocyte accumulation (N ≥ 3).
PA infection also stimulated high levels of lung neutrophilia (Fig. 1d), which resolved only partially despite the rapid clearance of this pathogen from lung airways (Fig. 1e). To assess the role of neutrophilia on lung survival we utilized several approaches to prevent neutrophil graft accumulation in heat killed PA (hkPA)-treated lung recipients (Fig. 1f) as live strain administration is lethal to neutrophil-depleted lung recipients due to the inability to clear this infection (13). Importantly, hkPA induced patterns of allograft rejection as well as intragraft Th1 and Th17 cell accumulation comparable to the live strain (Fig. S1b). Inhibiting neutrophil accumulation with either G-CSF Ab blockade or Ly6G Ab-mediated neutrophil depletion led to similar reductions in intragraft Th1 and Th17 cells as well as protection against lung allograft rejection (Fig. 1g–h). Moreover, allograft survival was also maintained in CD11b−/− recipients, a critical mediator of neutrophil trafficking into the lung (14). Therefore, these data collectively show that P. Aeruginosa initiates a pulmonary neutrophilia that prevents established allograft tolerance.
Neutrophil B7 trans costimulation promotes CD4+ T cell activation
Reports of neutrophils acquiring the characteristics of APCs in both humans (7) and mice (6) led us to investigate if these cells could directly promote naïve CD4+ T cell activation. Although neutrophils did not express MHC Class II or CD40 in PA-infected lung recipients we detected high levels of CD80 and CD86 on these cells (Fig. 2a) suggesting CD4+ T cell activation potential through B7 trans-costimulation. To investigate this possibility we co-cultured neutrophils from PA-infected mice with naïve CFSE-labeled CD4+ T cells in the presence or absence of latex beads coupled to CD3 Abs and analyzed IL-2 expression and proliferation (Fig. 2b–c). In the absence of CD3 beads neutrophils from PA-infected mice were unable to stimulate CD4+ T cell IL-2 expression or proliferation consistent with a lack of MHC Class II expression on these cells. However, in the presence of CD3 beads CD4+ T cell IL-2 expression and proliferation was significantly augmented by neutrophils from PA-infected mice when compared to neutrophils from either uninfected or PA-infected CD80−/−86−/− mice. Consistent with these observations B7 blockade mediated by CTLA4Ig markedly reduced the ability of neutrophils from PA-infected mice to enhance CD4+ T cell IL-2 expression and proliferation to levels that were comparable to co-cultures with neutrophils from PA-infected CD80−/−86−/− mice. Collectively, these data show that neutrophil B7 trans-costimulation enhances naïve CD4+ T cell activation.
Figure 2.
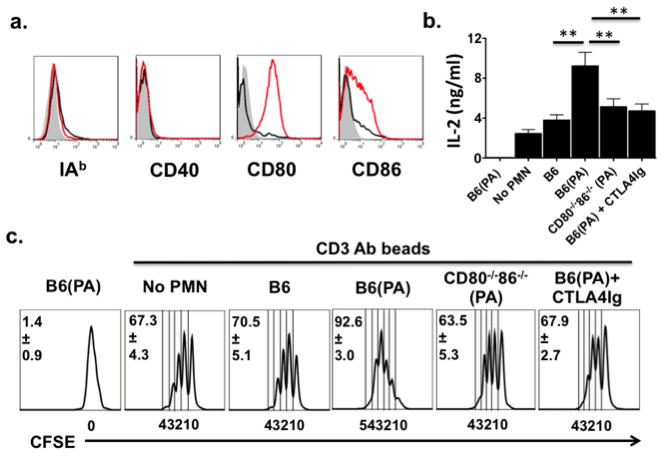
Neutrophil B7 directly enhances naïve CD4+ T cell activation. (a) BAL neutrophils from uninfected (black line) or PA-infected (red line) Balb/c → B6 recipients were stained with indicated Abs or isotype (shaded histogram) Abs (N=4). (b) IL-2 levels from naïve CD4+ T cells cultured with neutrophils from PA-infected mice (B6(PA)) or stimulated by CD3 beads in the absence (no PMN) or presence of neutrophils from uninfected mice (B6), B6(PA), PA-infected CD80−/−86−/− mice (CD80−/− 86−/− (PA)) or B6(PA) and 15 μg/ml CTLA4Ig. (c) CFSE-labeled naïve CD4+ T cells cultured as in (b) for 72 hrs and analyzed for responder frequency where mitotic divisions are shown below the x ordinate (N=4).
Neutrophil B7 prevents established lung allograft tolerance
We next asked if neutrophil B7 stimulated CD4+ T cell activation in vivo. To this end we sought to normalize for APC costimulatory molecule expression by adoptively-transferring neutrophils isolated from either B6 or B6 CD80−/−86−/− mice into PA-infected Balb/c → B6 lung recipients that were also treated with G-CSF Abs to ablate endogenous granulocyte mobilization (Fig. 3a). Strikingly, thirty-six hrs after infection intragraft IL-2+ and IFN-γ+ CD4+ T cell percent abundance increased to levels nearly equivalent to Control Ig-treated PA-infected lung recipients. Also, neutrophil B7 significantly augmented intragraft IL-17+ CD4+ and IFN-γ+ CD8+ T cell accumulation. We then analyzed the effect of neutrophil B7 on allograft tolerance by continuing adoptive transfers of B6 or CD80−/−86−/− neutrophils for two additional days into G-CSF Ab-treated PA-infected lung recipients (Fig. 3b). CD80−/−CD86−/− neutrophils had little impact on allograft inflammation three weeks after infection while B6 neutrophil recipients rejected their lungs in a manner similar to control Ig-treated PA-infected recipients. As B7 dependent responses suggested direct association of graft-resident T cells with neutrophils we employed intravital 2-photon microscopy to analyze cell-cell interactions within the allograft tissue (Fig. 3c, Movie 1). One day after infection we detected graft-infiltrating neutrophils making contact with CD4+ T cells that were simultaneously associated with pulmonary CD11c+ DC lending support for the B7 trans-costimulation three-cell contact model in vivo (15). To further assess if these interactions can enhance graft-resident T lymphocyte activation we purified CD4+ T cells from Balb/c allografts and analyzed IL-2 expression following co-culture with Balb/c bone marrow-derived DC and neutrophils from PA-infected mice (Fig. 3d). B6 neutrophils were significantly better at simulating IL-2 production than CD80−/−86−/− neutrophils. However, when B6 neutrophils were separated by transwells from DC-stimulated CD4+ T cells IL-2 expression fell sharply underscoring the requirement for neutrophil direct contact with intragraft T cells to promote alloimmunity.
Figure 3.
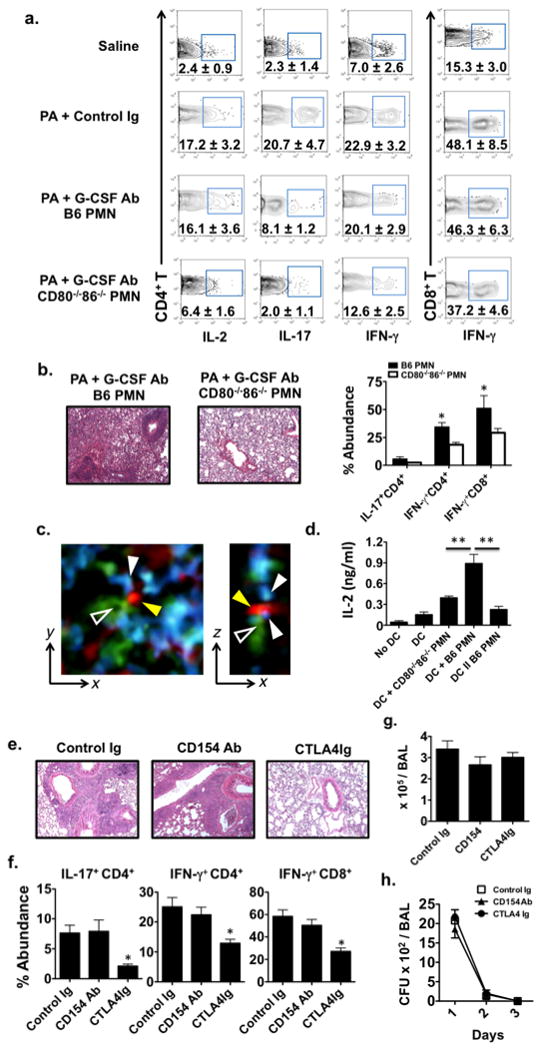
Lung allograft rejection in PA-infected recipients is neutrophil B7 dependent. (a) Percent abundance of indicated intragraft CD4+ and CD8+ T cells one day after saline or PA inoculation into Balb/c → B6 lung recipients that either received control Ig or G-CSF Abs with B6 or CD80−/−86−/− neutrophils (N ≥ 3). (b) H & E graft histology (N=4) and indicated intragraft T lymphocyte accumulation (N=4) of Balb/c → B6 lung recipients 3-weeks after that receiving PA, G-CSF Abs and either B6 or CD80−/−86−/− neutrophils. (c) Intravital 2-photon imaging within allografts of Balb/c → B6 CD11c-EYFP LysM-GFP lung recipients that received CMTPX-labeled CD4+ T cells one day after PA-infection. A zoomed view of a typical interaction between a CD4+ T cell (red-yellow arrows), DC (green-hollow arrow) and neutrophil (blue-white arrows) in the y-x (left) and z-x (right) plane (N=2). (d) IL-2 produced by intragraft CD4+ T cells from uninfected Balb/c → B6 lungs cultured alone (No DC) or co-cultured with allogeneic Balb/c bone-marrow derived DC (DC) or co-cultured with DC in combination with B6 neutrophils from PA-infected B6 mice (DC + B6 PMN) or DC in combination with PMN from PA-infected CD80−/−86−/− mice (DC + CD80−/−86−/− PMN) or co-cultured with DC separated from B6 neutrophils by transwells (DC II B6 PMN) (N ≥ 3) Lung recipients on POD 7 received PA along with 200μg of Control Ig, CD154 or CTLA4Ig and were either analyzed 3-weeks later for (e) H & E graft histology (N ≥ 3) and (f) intragraft T lymphocyte accumulation (N ≥ 3) or assessed for (g) BAL PMN numbers (N ≥ 3) one day after infection or (f) BAL CFU (N ≥ 3) for up to 3-days after infection.
In murine transplantation models (16) and human kidney recipients (17) B7-CD28 blockade strategies have been employed to promote allograft acceptance, but its use to maintain established tolerance in infected recipients has not been reported. We therefore asked if either CD154 Ab or CTLA4Ig administration at the time of PA infection can promote established lung tolerance in B6 lung allograft recipients (Fig 3e). CD154 Ab treatment did not prevent acute rejection consistent with the absence of CD40 expression on neutrophils in PA-infected lung recipients. In contrast, CTLA4Ig-treated recipients maintained lung survival and had patterns of intragraft Th1 and Th17 cell abundance comparable to uninfected lung recipients (Fig. 3f vs. 1b). Also, CTLA4Ig treatment did not affect airway neutrophil infiltration or PA infection clearance indicating that targeting B7 function does not impair pulmonary bacterial immune surveillance (Fig. 3g–h).
In summary, we have shown that neutrophil B7 expression induced by PA infection plays a critical role in preventing established lung tolerance through promoting T cell trans-costimulation. In light of previous work that has shown the importance of toll-like receptors in regulating organ tolerance (18, 19) our data provide additional insight into innate immune responses to infected allografts. As pulmonary allografts are especially vulnerable to infection given the organ’s direct exposure to the external environment a better understanding of how neutrophils regulate alloimmunity will be important for the development of more effective immunotherapeutic strategies for lung recipients.
Supplementary Material
References
- 1.Botha P, Archer L, Anderson RL, Lordan J, Dark JH, Corris PA, Gould K, Fisher AJ. Pseudomonas aeruginosa colonization of the allograft after lung transplantation and the risk of bronchiolitis obliterans syndrome. Transplantation. 2008;85:771–774. doi: 10.1097/TP.0b013e31816651de. [DOI] [PubMed] [Google Scholar]
- 2.Vos R, Vanaudenaerde BM, Verleden SE, De Vleeschauwer SI, Willems-Widyastuti A, Van Raemdonck DE, Dupont LJ, Nawrot TS, Verbeken EK, Verleden GM. Bronchoalveolar lavage neutrophilia in acute lung allograft rejection and lymphocytic bronchiolitis. J Heart Lung Transplant. 2010;29:1259–1269. doi: 10.1016/j.healun.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Gregory AD, Hogue LA, Ferkol TW, Link DC. Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood. 2007;109:3235–3243. doi: 10.1182/blood-2005-01-015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kreisel D, Sugimoto S, Zhu J, Nava R, Li W, Okazaki M, Yamamoto S, Ibrahim M, Huang HJ, Toth KA, Ritter JH, Krupnick AS, Miller MJ, Gelman AE. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood. 2011;118:6172–6182. doi: 10.1182/blood-2011-04-347823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kreisel D, Sugimoto S, Tietjens J, Zhu J, Yamamoto S, Krupnick AS, Carmody RJ, Gelman AE. Bcl3 prevents acute inflammatory lung injury in mice by restraining emergency granulopoiesis. J Clin Invest. 2011;121:265–276. doi: 10.1172/JCI42596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abi Abdallah DS, Egan CE, Butcher BA, Denkers EY. Mouse neutrophils are professional antigen-presenting cells programmed to instruct Th1 and Th17 T-cell differentiation. Int Immunol. 2011;23:317–326. doi: 10.1093/intimm/dxr007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iking-Konert C, Vogt S, Radsak M, Wagner C, Hansch GM, Andrassy K. Polymorphonuclear neutrophils in Wegener’s granulomatosis acquire characteristics of antigen presenting cells. Kidney Int. 2001;60:2247–2262. doi: 10.1046/j.1523-1755.2001.00068.x. [DOI] [PubMed] [Google Scholar]
- 8.Mandelbrot DA, Kishimoto K, Auchincloss H, Jr, Sharpe AH, Sayegh MH. Rejection of mouse cardiac allografts by costimulation in trans. J Immunol. 2001;167:1174–1178. doi: 10.4049/jimmunol.167.3.1174. [DOI] [PubMed] [Google Scholar]
- 9.Okazaki M, Krupnick AS, Kornfeld CG, Lai JM, Ritter JH, Richardson SB, Huang HJ, Das NA, Patterson GA, Gelman AE, Kreisel D. A mouse model of orthotopic vascularized aerated lung transplantation. Am J Transplant. 2007;7:1672–1679. doi: 10.1111/j.1600-6143.2007.01819.x. [DOI] [PubMed] [Google Scholar]
- 10.Okazaki M, Gelman AE, Tietjens JR, Ibricevic A, Kornfeld CG, Huang HJ, Richardson SB, Lai J, Garbow JR, Patterson GA, Krupnick AS, Brody SL, Kreisel D. Maintenance of airway epithelium in acutely rejected orthotopic vascularized mouse lung transplants. Am J Respir Cell Mol Biol. 2007;37:625–630. doi: 10.1165/rcmb.2007-0257RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trulock EP, Christie JD, Edwards LB, Boucek MM, Aurora P, Taylor DO, Dobbels F, Rahmel AO, Keck BM, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult lung and heart-lung transplantation report-2007. J Heart Lung Transplant. 2007;26:782–795. doi: 10.1016/j.healun.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 12.Gelman AE, Okazaki M, Sugimoto S, Li W, Kornfeld CG, Lai J, Richardson SB, Kreisel FH, Huang HJ, Tietjens JR, Zinselmeyer BH, Patterson GA, Miller MJ, Krupnick AS, Kreisel D. CCR2 regulates monocyte recruitment as well as CD4 T1 allorecognition after lung transplantation. Am J Transplant. 2010;10:1189–1199. doi: 10.1111/j.1600-6143.2010.03101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koh AY, Priebe GP, Ray C, Van Rooijen N, Pier GB. Inescapable need for neutrophils as mediators of cellular innate immunity to acute Pseudomonas aeruginosa pneumonia. Infect Immun. 2009;77:5300–5310. doi: 10.1128/IAI.00501-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seekamp A, Mulligan MS, Till GO, Smith CW, Miyasaka M, Tamatani T, Todd RF, 3rd, Ward PA. Role of beta 2 integrins and ICAM-1 in lung injury following ischemia-reperfusion of rat hind limbs. Am J Pathol. 1993;143:464–472. [PMC free article] [PubMed] [Google Scholar]
- 15.Rudd CE, Raab M. Independent CD28 signaling via VAV and SLP-76: a model for in trans costimulation. Immunol Rev. 2003;192:32–41. doi: 10.1034/j.1600-065x.2003.00005.x. [DOI] [PubMed] [Google Scholar]
- 16.Schaub M, Stadlbauer TH, Chandraker A, Vella JP, Turka LA, Sayegh MH. Comparative strategies to induce long-term graft acceptance in fully allogeneic renal versus cardiac allograft models by CD28-B7 T cell costimulatory blockade: role of thymus and spleen. J Am Soc Nephrol. 1998;9:891–898. doi: 10.1681/ASN.V95891. [DOI] [PubMed] [Google Scholar]
- 17.Vincenti F, Larsen CP, Alberu J, Bresnahan B, Garcia VD, Kothari J, Lang P, Urrea EM, Massari P, Mondragon-Ramirez G, Reyes-Acevedo R, Rice K, Rostaing L, Steinberg S, Xing J, Agarwal M, Harler MB, Charpentier B. Three-year outcomes from BENEFIT, a randomized, active-controlled, parallel-group study in adult kidney transplant recipients. Am J Transplant. 2012;12:210–217. doi: 10.1111/j.1600-6143.2011.03785.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen L, Ahmed E, Wang T, Wang Y, Ochando J, Chong AS, Alegre ML. TLR signals promote IL-6/IL-17-dependent transplant rejection. J Immunol. 2009;182:6217–6225. doi: 10.4049/jimmunol.0803842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Palmer SM, Burch LH, Davis RD, Herczyk WF, Howell DN, Reinsmoen NL, Schwartz DA. The role of innate immunity in acute allograft rejection after lung transplantation. Am J Respir Crit Care Med. 2003;168:628–632. doi: 10.1164/rccm.200303-447OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


