Abstract
Acetaminophen (APAP) overdose is the most common cause of acute liver failure in the West. In mice, APAP hepatotoxicity can be rapidly induced with a single dose. Because it is both clinically relevant and experimentally convenient, APAP intoxication has become a popular model of liver injury. Early data demonstrated that rats are resistant to APAP toxicity. As a result, mice are the preferred species for mechanistic studies. Furthermore, recent work has shown that the mechanisms of APAP toxicity in humans are similar to mice. Nevertheless, some investigators still use rats. New mechanistic information from the last forty years invites a reevaluation of the differences between these species. Comparison may provide interesting insights and confirm or exclude the rat as an option for APAP studies. To this end, we treated rats and mice with APAP and measured parameters of liver injury, APAP metabolism, oxidative stress, and activation of the c-jun N-terminal kinase (JNK). Consistent with earlier data, we found that rats were highly resistant to APAP toxicity. Although overall APAP metabolism was similar in both species, mitochondrial protein adducts were significantly lower in rats. Accordingly, rats also had less oxidative stress. Finally, while mice showed extensive activation and mitochondrial translocation of JNK, this could not be detected in rat livers. These data support the hypothesis that mitochondrial dysfunction is critical for the development of necrosis after APAP treatment. Because mitochondrial damage also occurs in humans, rats are not a clinically relevant species for studies of APAP hepatotoxicity.
Keywords: Acetaminophen, hepatotoxicity, protein adducts, mitochondria, oxidant stress, c-jun-N-terminal kinase
INTRODUCTION
When used as directed, acetaminophen (APAP) is a safe and effective analgesic and fever reducer. However, large doses of APAP can cause serious liver injury. In fact, APAP overdose is the primary cause of acute liver failure in many countries throughout the West (Bernal, 2003; Gow et al., 2004; Larson et al., 2005), responsible for more than 70,000 hospitalizations each year in the U.S. alone (Budnitz et al., 2011). Research on the mechanism of APAP-induced liver injury began four decades ago, following the first published report of this toxicity in humans (Davidson and Eastham, 1966). Though many important questions have yet to be answered, the mechanism of APAP toxicity has been well investigated in rodents (Jaeschke et al., 2011; 2012) and progress is now being made in humans (Antoine et al., 2012; Atoniades et al., 2012; Davern et al., 2006; McGill et al., 2012) and with in vitro human models (McGill et al., 2011). This profusion of data likely makes APAP the best characterized hepatotoxicant.
Because APAP-induced liver injury is clinically relevant, well studied, and can be rapidly induced in vivo with a single dose, it has become a standard model in the pharmacology and toxicology literature. In particular, APAP overdose in rodents is frequently used to test the hepatoprotective potential of herbal therapeutics. While this can be a valid approach, a number of concerns have been raised (Jaeschke et al., 2010, 2011). For example, one of the most common issues in the complementary and alternative medicine literature is the use of rats to evaluate protection against APAP injury. It has been known since the early 1970s that rats are resistant to the liver-damaging effects of APAP (Mitchell et al., 1973). Doses which far exceed the LD50 for mice cause only minimal necrosis in rat liver. The reason for this difference in susceptibility is not well understood. In mice, APAP hepatotoxicity begins with metabolism of the parent compound to the reactive electrophile N-acetyl-p-benzoquinone imine (NAPQI). NAPQI depletes glutathione (GSH) and binds to proteins, primarily to the amino acid cysteine (Nelson, 1990; Cohen et al., 1997). Differences in APAP metabolism and protein binding could account for the difference between mice and rats. However, while protein binding appears to be a necessary first step toward injury, it is not sufficient to directly cause cell death (Jaeschke et al., 2012). 3’-hydroxyacetanilide (AMAP), a non-hepatotoxic isomer of APAP, also binds to proteins (Tirmenstein and Nelson, 1989). Moreover, toxicity develops only after the onset of oxidative stress and mitochondrial dysfunction, and preventing these phenomena protects against APAP (Kon et al., 2004; Cover et al., 2005; Ramachandran et al., 2011a,b). Moreover, activation and mitochondrial translocation of c-Jun N-terminal kinase (JNK) have repeatedly been shown to play a role in APAP toxicity in the liver (Gunawan et al., 2006; Latchoumycandane et al., 2007; Hanawa et al., 2008; Saito et al., 2010). Thus, mitochondrial dysfunction, oxidative stress, and/or JNK activation may also be different between the two species.
A better understanding of the differences between rats and mice will not only aid future researchers in selection of the best model for their experiments, it may provide important new mechanistic insights into APAP toxicity. Therefore, the objective of the present study was to investigate potential differences in the mechanism of APAP-induced liver injury between rats and mice with emphasis on protein adduct formation, oxidative stress, and JNK activation.
METHODS
Animals
C57Bl/6 mice (Jackson Laboratories, Bar Harbor, ME), Fischer 344 and Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) between 8–12 weeks of age were kept in a temperature controlled facility with a 12 hour light/dark cycle and free access to food and water. For all experiments, food was withdrawn 12–15 hours prior to treatment with APAP. The drug was administered i.p. or p.o. at the indicated doses in one of two metabolically inert vehicles: warm saline (mice) or 20% Tween-80 (rats). Saline and solutions of Tween are popular vehicles for the mouse and rat models, respectively, and have been shown to not interfere with APAP toxicity (Kelava et al., 2010). At various times, the animals were sacrificed by cervical dislocation (mice) or exsanguination (rats) under anesthesia. Blood was drawn from the caudal vena cava and centrifuged to obtain serum. After taking blood, livers were excised and portions were flash frozen for determination of glutathione (GSH), APAP-cysteine adducts on proteins (APAP-CYS), and western blotting, or fixed in 10% phosphate-buffered formalin for histology. For organelle isolation, fresh liver tissues were minced and gently homogenized on ice with 20–30 passes using a tight-fitting motor-driven Teflon pestle in a glass mortar. Subcellular fractions were collected by differential centrifugation. Briefly, cell debris was removed with 2,500 g spin for 10 min. The supernatant was then centrifuged at 20,000 g for 10 min to collect mitochondria and again at 110,000 g for 1 h to pellet the mixed microsomes and light membranes. The new supernatant was then collected as the cytosolic fraction.
Biochemical assays
Serum alanine aminotransferase (ALT) was measured using a kit (Pointe Scientific) and glutamate dehydrogenase (GDH) activity was determined as described (McGill et al., 2012). Liver GSH levels were measured using a modified Tietze assay as described (Jaeschke and Mitchell, 1990).
APAP-CYS
APAP-CYS protein adducts were measured using the high pressure liquid chromatography with electrochemical detection (HPLC-ED) method of Muldrew et al. (2002) with previously described modifications (Ni et al., 2012). For total liver adducts, tissues were homogenized with a blade-type homogenizer in 10 mM sodium acetate buffer (pH 6.5), filtered through Bio-Spin 6 columns (Bio-Rad) to remove low molecular weight compounds with the potential to interfere in the assay, and digested overnight with proteases to free the APAP-CYS. For measurement of protein adducts in the mitochondrial fraction, the pellets were resuspended in small volumes of sodium acetate buffer and subjected to 3 cycles of freeze-thaw to homogenize. The debris was then pelleted by centrifugation and supernatants were filtered and digested as above. Protein was measured using the bicinchoninic acid assay (BCA).
Histology and immunohistochemistry
Liver sections were stained with hematoxylin and eosin for assessment of tissue necrosis. Nitrotyrosine staining was performed as previously described (Knight et al., 2002) using a rabbit polyclonal anti-nitrotyrosine antibody (Life Technologies, Grand Island, NY) and the Dako LSAB peroxidase kit (Dako, Carpinteria, CA).
Statistical methods
One-way analysis of variance (ANOVA) was used to assess statistical significance between three or more groups. When a difference was detected, the Student Newman-Keul’s test was used for multiple comparisons. For non-normally distributed data, the Kruskal-Wallis test was used. p < 0.05 was considered significant.
RESULTS
APAP toxicity in rats
For our initial studies, two strains of rats were chosen based on previously published data reporting liver injury after APAP overdose: Fischer (F344) and Sprague-Dawley rats. The rats were treated orally with 1, 1.5, or 2 g APAP per kg body weight and sacrificed 24 h later. Doses were chosen based on the literature and on the limit of solubility of APAP (Mitchell et al., 1973). Though Fischer rats did have significantly elevated plasma ALT activity after the 1.5 g/kg dose, both strains showed considerable resistance (Table 1). Administering the drug i.p. at 1 g/kg in Fischer (F344) rats did not change these findings (Figure 1A). For direct comparison, time course studies with mice (300 mg/kg) and rats (1 g/kg) were executed in parallel. While mice had a dramatic increase in both plasma ALT and GDH over time, rat liver enzyme levels in plasma remained low despite receiving a much higher dose (Figure 1A,B). Moreover, we observed large areas of necrosis in mouse liver by histology, but little or no injury could be seen in rat samples (Figure 1C). These results are consistent with earlier reports (Mitchell et al., 1973) and show that rats are highly resistant to APAP-induced liver injury.
Table 1.
APAP Hepatotoxicity in Rats
| Strain | Dose | ALT |
|---|---|---|
| Fischer (F344) | Control | 35 ± 11 |
| Fischer (F344) | 1 g/kg | 39 ± 4 |
| Fischer (F344) | 1.5 g/kg | 101 ± 7* |
| Sprague-Dawley | Control | 30 ± 1 |
| Sprague-Dawley | 1 g/kg | 41 ± 11 |
| Sprague-Dawley | 2 g/kg | 31 ± 4 |
Two strains of rats were treated p.o. with the indicated doses of APAP for 24 h. The animals were then sacrificed and ALT activities were measured in serum. Data represent mean ± SE of n = 4 animals per group.
P < 0.05 (compared to control).
Figure 1.
Serum enzymes in mice and rats treated with APAP. Mice and Fischer 344 rats were treated i.p. with 300 mg APAP/kg body weight or 1 g APAP/kg, respectively. At various times, the animals were sacrificed and serum was collected. (A) Time course of ALT activity in serum from mice and rats after APAP. (B) Time course of glutamate dehydrogenase (GDH) activity in serum from mice and rats. (C) Representative H&E stained liver sections from mice (top row) and rats (bottom row) treated with APAP. Data are expressed as mean ± SEM for n = 3–4 animals per group. *P < 0.05 (compared to t=0).
APAP metabolism in mice and rats
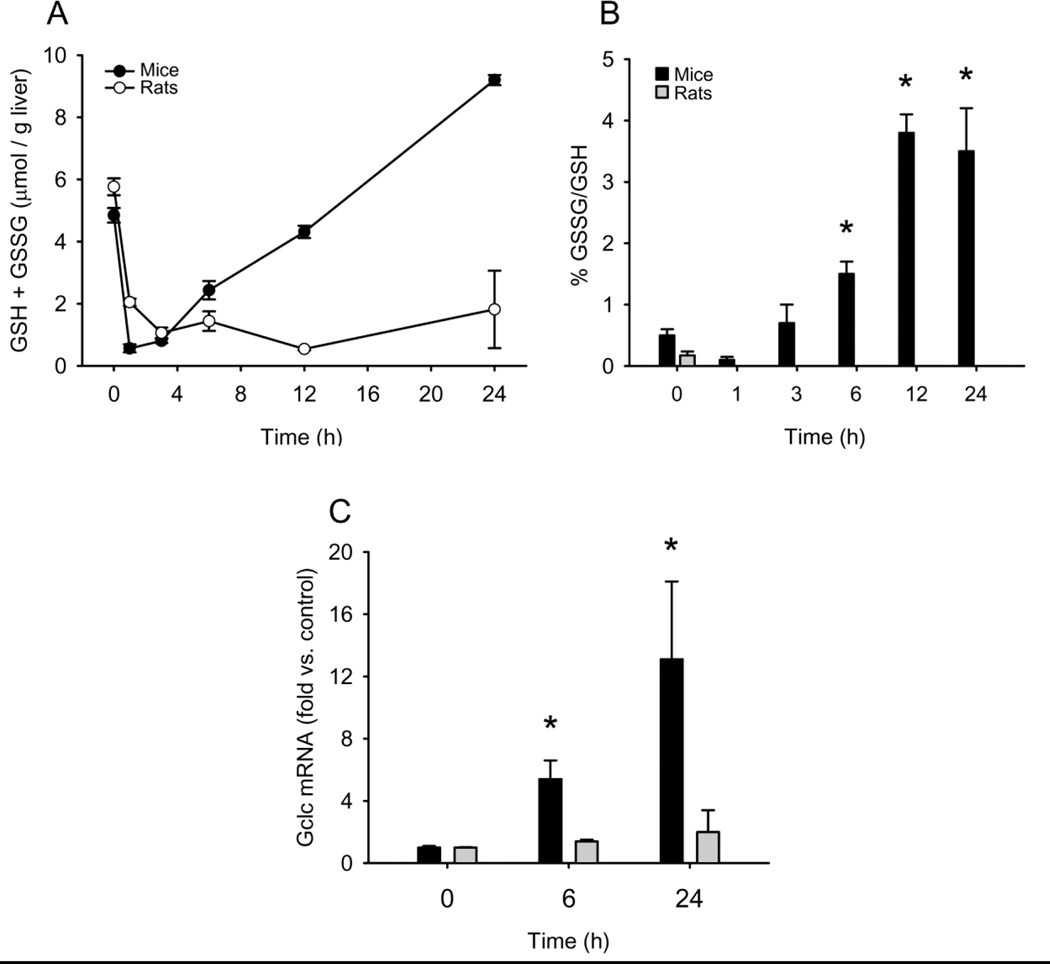
APAP toxicity depends upon the formation of the reactive metabolite NAPQI, which will deplete GSH and bind to proteins (Nelson, 1990). Protein binding is known to be the initiating event in the mechanism of injury. To determine whether or not there is a difference in metabolism in the liver between mice and rats, we measured liver GSH and total liver APAP-protein binding (Figure 2A, 3A). GSH concentrations were significantly reduced after 1 h in the livers of mice treated with the toxic dose of 300 mg/kg and remained low until 3 h and then started to recover (Figure 2A). In rats treated with 1 g/kg, there was a modest delay in GSH depletion so that the lowest concentrations were achieved at 3 h. Interestingly, there was no recovery of liver GSH in the rats even as late as 24 h after APAP treatment (Figure 2A). This is partially because the much higher dose took longer to clear, but it is likely also due to differences in GSH resynthesis. In mice, there was significant induction of the glutamyl-cysteine ligase catalytic subunit (Gclc) after APAP treatment, but this was not seen in rats (Figure 2C).
Figure 2.
Liver glutathione (GSH) and glutathione disulfide (GSSG) in mice and Fischer 344 rats after APAP treatment. Mice and rats were treated i.p. with 300 mg APAP/kg body weight or 1 g APAP/kg, respectively. At the indicated times, the animals were sacrificed and liver samples were flash frozen for later analysis of GSH and GSSG. (A) Total GSH levels. (B) GSSG-to-GSH ratio shown as a percentage. (C) mRNA levels of glutamate-cysteine ligase (gclc). Data are expressed as mean ± SEM for n = 3–4 animals per group. *P < 0.05 (compared to t=0).
Figure 3.
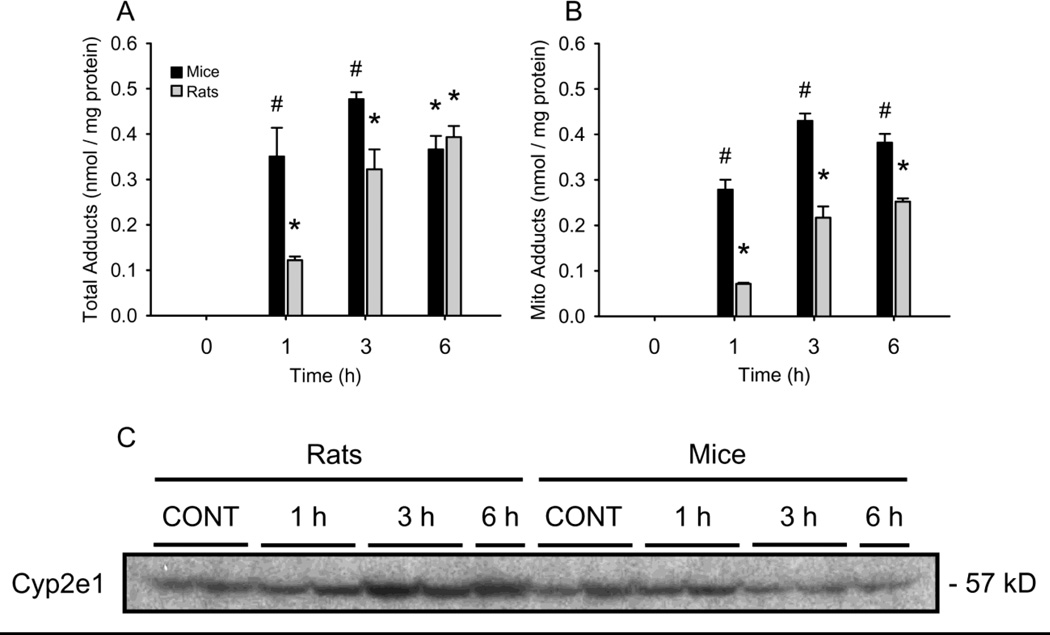
Total liver and mitochondrial APAP-protein adducts in mice and rats. Mice and rats were treated i.p.with 300 mg APAP/kg body weight or 1 g APAP/kg, respectively. At various times, the animals were sacrificed and livers were excised. One lobe from each was immediately homogenized for subcellular fractionation by differential centrifugation. The remaining tissue was flash frozen for later analysis of total liver adducts. (A) Total liver APAP-CYS time courses. (B) Liver mitochondria APAP-CYS time courses. Data are expressed as mean ± SEM for n = 3–4 animals per group. *P < 0.05 (compared to t=0) #P < 0.05 vs. rats.
Consistent with the delayed GSH depletion, the concentration of APAP-protein adducts increased more slowly in rat liver homogenates than in mouse samples, though similar levels were measured in both species by 6 h (Figure 3A). The fact that similar overall protein binding was achieved in rats given a dose of APAP more than threefold higher shows that formation of NAPQI occurs much less readily in these animals. Further, the fact that rats given this much higher dose were resistant to injury despite similar levels of protein binding when compared with mice suggests that there are factors other than total protein binding to consider.
Mitochondrial protein adducts, oxidative stress and JNK activation in mice and rats
Mitochondrial dysfunction is known to play a role in APAP hepatotoxicity in both mice (Meyers et al., 1988; Jaeschke, 1990) and humans (McGill et al., 2012). It is well-established that NAPQI binds to mitochondrial proteins (Tirmenstein and Nelson, 1989) and it is generally accepted that this is an important early event in the mitochondrial dysfunction and associated oxidative stress seen after APAP overdose. For this reason, although there was little difference in total liver protein binding, mitochondrial APAP-protein adducts were evaluated (Figure 3B). The increase in mitochondrial adduct levels from rats paralleled the increase seen in mice. Importantly, however, at all time points the levels were significantly lower in the samples from rats. This was observed even at 6 h when the total adduct levels between the two species were similar (Figure 3A). It is possible that the lower mitochondrial protein binding is responsible for the resistance to injury. Cyp2e1 is the major P450 enzyme responsible for APAP metabolism in rodents (Lee et al., 1996) and there is evidence that mitochondrial Cyp2e1 can contribute to, and may itself be sufficient for, the formation of mitochondrial APAP-protein adducts and oxidative stress (Knockaert et al., 2011). To determine whether or not this species difference in mitochondrial protein binding could be due to differences in mitochondrial P450 protein expression we immunoblotted for Cyp2e1 in mitochondria from livers of both species before and after APAP treatment (Figure 3C). There was no difference in basal mitochondrial Cyp2e1 between mice and rats. In fact, we observed an increase in rats at later time points after APAP treatment. Thus, the lower mitochondrial APAP-protein adduct levels are likely not due to differences in mitochondrial Cyp2e1.
Mitochondrial protein binding after APAP treatment is thought to lead to downstream mitochondrial oxidative stress. The major reactive oxygen species in the mechanism of APAP toxicity in mice is superoxide (O2−), which dismutates to molecular oxygen and hydrogen peroxide or reacts with nitric oxide (NO) to form peroxynitrite (ONOO−), a potent oxidant and nitrating species. To determine whether or not oxidative stress developed in either species, tissue levels of GSSG were measured and the GSSG-to-GSH ratio calculated. Hepatic GSSG levels in mice were 0.5% of the total in controls and increased significantly after 3 h, reflecting an oxidant stress in mouse livers (Figure 2B). In contrast, GSSG levels in rats were 0.2% in controls and remained <0.1% at all time points after APAP administration. These data indicate a substantial oxidant stress after APAP in mice but not in rats. These data were confirmed by immunostaining for nitrotyrosine protein adducts (Figure 4), which are a footprint of peroxynitrite formation. Extensive centrilobular nitrotyrosine staining was evident in mouse livers but not in rats, suggesting that peroxynitrite formation occurred only in mice (Figure 4).
Figure 4.
Nitrotyrosine staining in mice (top row) and rats (bottom row) after APAP treatment. Mice and rats were treated i.p. with 300 mg APAP /kg body weight or 1 g APAP/kg, respectively. At the indicated times, the animals were sacrificed and livers were fixed in phosphate-buffered formalin. Sections were stained using an anti-3-nitrotyrosine antibody.
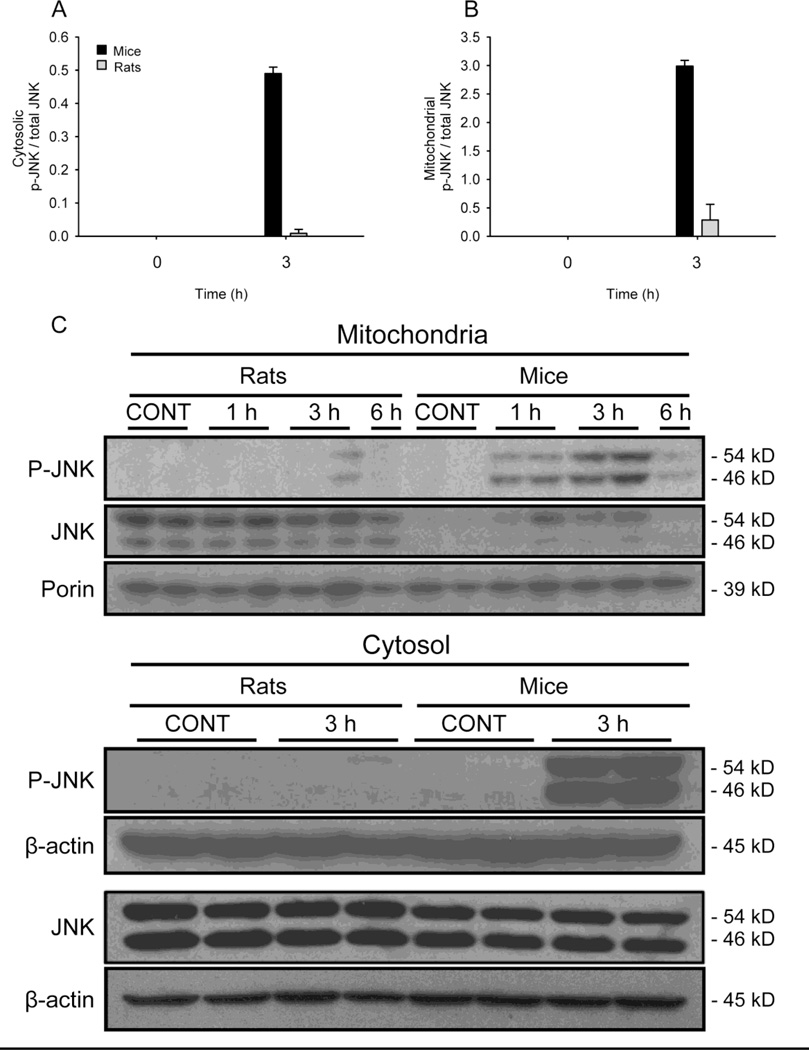
JNK activation has been shown to be critical in the mechanism of APAP-induced liver injury (Gunawan et al., 2006; Latchoumycandane et al., 2007; Hanawa et al., 2008; Saito et al., 2010). Because JNK activation may be the result of oxidative stress after APAP treatment in mice (Nakagawa et al., 2008; Saito et al., 2010), we next tested whether or not we could detect JNK phosphorylation (p-JNK) and mitochondrial translocation in liver samples from these two species (Figure 5A,C). Very little phosphorylated JNK could be detected in mitochondrial fractions from rats after APAP, though there was a clear increase in mouse samples at 1 and 3 h post- APAP. The difference in the mitochondrial p-JNK results could have been due to lack of activation or to lack of translocation. To test the latter possibility, we also blotted for p-JNK in the cytosol fractions (Figure 5B,C), as well as for total (non-phosphorylated + phosphorylated) JNK in both fractions (Figure 5C). Interestingly, non-phosphorylated JNK appears to be constitutively present in mitochondria from rat liver but not from mice. The reason for this is not yet known. In any case, very little p-JNK could be detected in rat samples when compared with samples from mice. Together, these data show that there is no relevant oxidative stress or JNK activation in rats after APAP overdose.
Figure 5.
JNK phosphorylation and mitochondrial translocation in livers from mice and rats after APAP treatment. P-JNK was measured by western blotting in mitochondrial and cytosolic fractions from mice and rats after treatment with 300 mg APAP /kg body weight or 1 g APAP/kg, respectively for the indicated times (C). Densitometric analysis of P-JNK and total JNK in the cytosol (A) and the mitochondria (B).
DISCUSSION
The objective of this study was to evaluate potential mechanistic differences of APAP hepatotoxicity between rats and mice. Although it is known that rats are resistant to APAP hepatotoxicity (Mitchell et al., 1973), many investigators continue to choose this species for their studies of potentially hepatoprotective compounds. This is especially true in the area of herbal therapeutics and natural products (Jaeschke et al., 2011) but it is not limited to this field (Laskin et al., 1995; Miyamoto et al., 2008; Ahmed et al., 2011). Initially, species differences in APAP toxicity were thought to result from different rates of APAP metabolism (Davis et al., 1974). Consistent with this, our data revealed that APAP protein binding in rats was similar to a standard mouse model of toxicity when a threefold higher dose was administered. Moreover, we observed a delay in hepatic GSH depletion and APAP-protein adduct formation in rats compared with mice. In this study, the rats and mice were fasted for approximately the same amount of time (12–15 h) before APAP treatment. It is possible that this was simply insufficient for the rat model due to a difference in GSH turnover between species. Indeed, the starting GSH levels in livers from fasted rats were on average 1 µmol/g liver higher than in livers from control mice. However, optimization of this value would be difficult due to the fact that the rate of GSH synthesis in rat liver is actually increased during fasting (Lauterburg and Mitchell, 1981). In any case, induction of cytochrome P450 enzymes with phenobarbital or a similar compound is one strategy that has been used to compensate for the apparent difference in metabolism (Mitchell et al., 1973). However, this usually requires several days of pretreatment. More importantly, many of these compounds are nuclear receptor activators and the effect of these treatment regimens on the mechanism of toxicity has not been well investigated. The mouse model is more convenient and better characterized. Interestingly, despite the delayed metabolism in rats, at later time points APAP protein binding was similar in both species at the doses used. Thus, it is likely that there are other downstream factors responsible for the difference in susceptibility. After several decades of research on the mechanisms of APAP toxicity, it is now possible to compare some of these downstream events between mice, rats, and even humans, in greater detail.
Mitochondrial dysfunction and oxidative stress in mice and rats
Mitochondrial dysfunction is known to occur after APAP overdose in mice (Jaeschke and Bajt, 2006). Protein adducts in mitochondria are higher after APAP treatment compared with the non-hepatotoxic isomer 3’-hydroxyacetanilide (Tirmenstein and Nelson, 1989). It is generally accepted that this increased mitochondrial protein binding leads to mitochondrial dysfunction and oxidative stress (Jaeschke and Bajt, 2006). APAP overdose inhibits mitochondrial respiration (Meyers et al., 1988) and causes a decrease in hepatic ATP levels in the liver (Jaeschke, 1990). Using electron microscopy, swelling and lysis of mitochondria were also observed (Placke et al., 1987). Evidence for superoxide and peroxynitrite formation selectively in mitochondria has also been found (Jaeschke, 1990; Cover et al., 2005) and it was later discovered that mitochondrial depolarization occurs in primary mouse hepatocytes treated with high concentrations of APAP (Kon et al., 2004; Reid et al., 2005). Importantly, well-characterized inhibitors of the mitochondrial permeability transition (MPT) were protective in this model (Kon et al., 2004), and mice deficient for the MPT pore regulator cyclophilin D had reduced liver injury in vivo (Ramachandran et al., 2011a). However, the MPT is only regulated by cyclophilin D after low but not high overdoses of APAP (LoGuidice and Boelsterli, 2011). Similar to the results with AMAP mentioned above, we saw reduced mitochondrial APAP-protein adducts in rats. Together with the absence of GSSG, nitrotyrosine protein adducts, p-JNK formation or p-JNK translocation to the mitochondria in this species, these data strongly suggest that no mitochondrial dysfunction or oxidative stress occurs in rats after APAP overdose. Moreover, there was no elevation of serum GDH activity, which has been used as a marker of mitochondrial damage (McGill et al., 2012), though this could be due to the lack of necrosis and enzyme release.
Protein binding, especially mitochondrial protein binding, is necessary for initiation of APAP toxicity (Tirmenstein and Nelson, 1989). A large number of compounds (extracts from natural products) have been claimed to protect against APAP through antioxidant effects or through prevention of mitochondrial damage. However, the metabolic activation of APAP is rarely evaluated. Any reduction in APAP-protein adducts by inhibition of metabolism or scavenging of NAPQI will be protective against APAP-induced liver injury. Without protein binding, downstream events in the mechanism of toxicity (e.g. mitochondrial dysfunction, oxidative stress, JNK activation) will not occur and one could mistakenly conclude that the compound of interest protects by blocking one or more of these events. For this reason, measurement of GSH or APAP-CYS should be the first experiment performed in any test of potentially hepatoprotective compounds relying on the APAP model. In both cases, an early time point (0.5 – 1 h post-APAP) should be used. Observations later than 1 h may miss early differences in protein adduct formation, and in mice GSH levels begin to recover by 4 – 6 h (Jaeschke et al., 2011).
JNK activation in mice and rats
JNK is phosphorylated and translocates to mitochondria early in APAP hepatotoxicity in mice (Gunawan et al., 2006; Hanawa et al., 2008; Ramachandran et al., 2011b) and this is thought to occur at least partly as a result of an initial oxidative stress (Nakagawa et al., 2008; Saito et al., 2010; Ramachandran et al., 2011a). Our results confirmed these findings (Figure 5). Importantly, inhibition of JNK activation in mice reduces ALT activity in plasma as well as the appearance of necrosis in liver sections, reduces nuclear DNA fragmentation, and prevents the further development of mitochondrial oxidative stress after APAP. In contrast, we could not detect JNK phosphorylation in mitochondria from rats. This supports the conclusion that these animals did not develop mitochondrial dysfunction or oxidative stress. The exact relationship between JNK activity and oxidative stress after APAP intoxication is not fully understood. However, there is evidence that the early oxidant stress is involved in JNK activation, which appears to amplify the mitochondrial oxidant stress (Saito et al., 2010). Interestingly, non-phosphorylated JNK was present in mitochondria from control rat liver but not from control mice (Figure 5A). This may suggest that liver injury involving JNK requires both mitochondrial localization and phosphorylation. Localization or translocation alone is insufficient.
Mechanisms of APAP toxicity in humans
Progress is now being made in the study of APAP toxicity in humans (McGill et al., 2012; Antoine et al., 2012; Antoniades et al., 2012). Data from the human cell line HepaRG and from human samples have provided evidence that mitochondrial damage also occurs in humans (McGill et al., 2011, 2012). APAP selectively causes necrosis of hepatocyte-like cells in HepaRG cultures, and this is preceded by loss of mitochondrial membrane potential and the development of mitochondrial oxidative stress (McGill et al., 2011). In humans, glutamate dehydrogenase (GDH) and mitochondrial DNA (mtDNA) are detectable in plasma during APAP-induced liver injury but are low or nondetectable in samples from overdose patients without serious liver injury or from healthy controls (McGill et al., 2012). These biomarkers were also elevated in mice after treatment with high doses of APAP but not after treatment with furosemide, a diuretic which can cause similar centrilobular necrosis but without the antecedent mitochondrial dysfunction. These data suggest that GDH and mtDNA in plasma are specific biomarkers for mitochondrial injury and that humans develop this injury after APAP overdose. Because there was lower mitochondrial protein binding in rats and they were resistant to mitochondrial damage in our study, this species is probably not a clinically relevant model for APAP-induced liver injury and the mouse is preferred.
Potential issues with the mouse model
A caveat to our interpretation of these data is that APAP toxicity in some strains of mice has not been as thoroughly studied as in others and there is wide variation in sensitivity to APAP (Harrill et al., 2009). It is tempting to speculate that, in addition to differences in expression of cell death genes (Harrill et al., 2009), this may be due in part to variation in mitochondrial protein binding and/or mitochondrial dysfunction and oxidative stress. For our experiments, we chose C57Bl/6 mice. This strain is most commonly used for studies of APAP hepatotoxicity and the mechanism of toxicity in these animals is well understood. Nevertheless, other susceptible strains such as ICR mice (Jaeschke, 1990), C3Heb/FeJ mice (Knight et al., 2001; Cover et al., 2005) and B6C3F1 mice (Agarwal et al., 2011) consistently show evidence of mitochondrial oxidant stress and peroxynitrite formation after APAP overdose.
While the mouse, in general, appears to be more clinically relevant than the rat model, there may still be important differences between mice and humans. For example, although Cyp2e1-deficient mice are protected against APAP-induced liver injury (Lee et al., 1996), another study found that recombinant human CYP3A4 was much more active than human 2E1 in converting APAP to APAP-GSH (Laine et al., 2009). Further, CYP2E1 activity is low in the metabolically competent human liver cell line HepaRG (Anthérieu et al., 2010), but these cells metabolize APAP and develop toxicity (McGill et al., 2011). Thus, the enzymes responsible for APAP metabolism may be different in mice and humans.
Conclusions
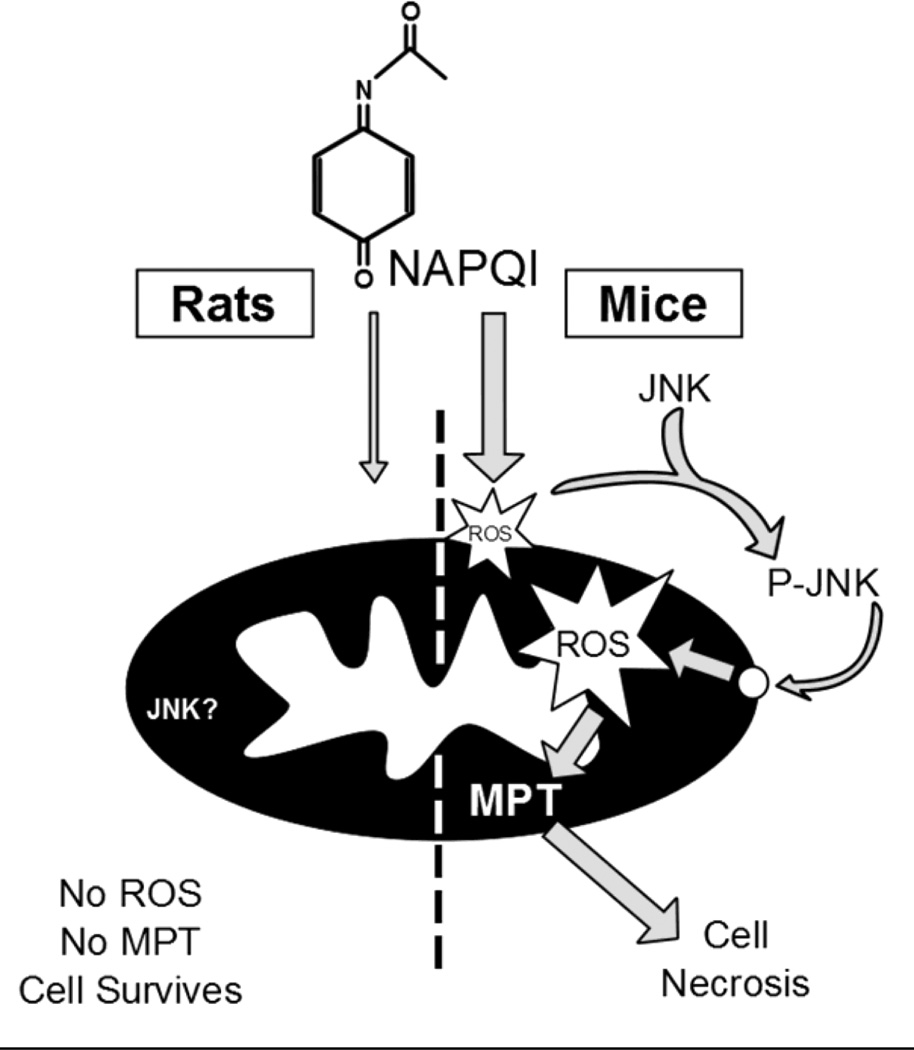
Rats are much more resistant to APAP hepatotoxicity than mice. This is likely the result of reduced mitochondrial protein binding, which limits mitochondrial dysfunction and prevents the oxidative stress and peroxynitrite formation in rats (Figure 6). These data support the already well-established role of mitochondria in the mechanism of APAP toxicity. Furthermore, because mitochondrial dysfunction occurs in humans and probably leads to the necrosis observed after APAP overdose, rats are not a human-relevant species for studies using the APAP liver injury model.
Figure 6.
Diagram of APAP metabolism and downstream events in mice and rats. The reactive metabolite binds to mitochondrial proteins more in the mouse than in the rat. This leads to an initial mitochondrial oxidative stress with JNK activation and the amplification of the mitochondrial oxidant stress and the membrane permeability transition (MPT) pore opening in the mouse, which do not occur in the rat.
Highlights.
Acetaminophen overdose causes severe liver injury only in mice but not in rats
APAP causes hepatic GSH depletion and protein adduct formation in rats and mice
Less protein adducts were measured in rat liver mitochondria compared to mouse
No oxidant stress, peroxynitrite formation or JNK activation was present in rats
The limited mitochondrial adducts in rats are insufficient to trigger cell necrosis
ACKNOWLEDGMENT
This investigation was supported in part by National Institutes of Health Grants AA12916 and DK070195 and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. M.R. McGill and C.D. Williams were supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26A2) from the National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
The authors do not have any conflict of interest to disclose.
REFERENCES
- Agarwal R, MacMillan-Crow LA, Rafferty TM, Saba H, Roberts DW, Fifer EK, James LP, Hinson JA. Acetaminophen-induced hepatotoxicity in mice occurs with inhibition of activity and nitration of mitochondrial manganese superoxide dismutase. J. Pharmacol. Exp. Ther. 2011;337:110–116. doi: 10.1124/jpet.110.176321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed MM, Wang T, Luo Y, Ye S, Wu Q, Guo Z, Roebuck BD, Sutter TR, Yang JY. Aldo-keto reductase-7A protects liver cells and tissues from acetaminophen induced oxidative stress and hepatotoxicity. Hepatology. 2011;54:1322–1332. doi: 10.1002/hep.24493. [DOI] [PubMed] [Google Scholar]
- Anthérieu S, Chesné C, Li R, Camus S, Lahoz A, Picazo L, Turpeinen M, Tolonen A, Uusitalo J, Guguen-Guillouzo C, Guillouzo A. Stable expression, activity, and inducibility of cytochromes P450 in differentiated HepaRG cells. Drug Metab. Dispos. 2010;38:516–525. doi: 10.1124/dmd.109.030197. [DOI] [PubMed] [Google Scholar]
- Antoine DJ, Jenkins RE, Dear JW, Williams DP, McGill MR, Sharpe MR, Craig DG, Simpson KJ, Jaeschke H, Park BK. Molecular forms of HMGB1 and keratin-18 as mechanistic biomarkers for mode of cell death and prognosis during clinical acetaminophen hepatotoxicity. J. Hepatol. 2012;56:1070–1079. doi: 10.1016/j.jhep.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Antoniades CG, Quaglia A, Taams LS, Mitry RR, Hussain M, Abeles R, Possamai LA, Bruce M, McPhail M, Starling C, Wagner B, Barnardo A, Pomplun S, Auzinger G, Bernal W, Heaton N, Vergani D, Thursz MR, Wendon J. Source and characterisation of hepatic macrophages in acetaminophen-induced acute liver failure in humans. Hepatology. 2012 Feb 15; doi: 10.1002/hep.25657. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bernal W. Changing patterns of causation and the use of transplantation in the United Kingdom. Semin. Liver Dis. 2003;23:227–237. doi: 10.1055/s-2003-42640. [DOI] [PubMed] [Google Scholar]
- Budnitz DS, Lovegrove MC, Crosby AE. Emergency department visits for overdoses of acetaminophen-containing products. Am. J. Prev. Med. 2011;40:585–592. doi: 10.1016/j.amepre.2011.02.026. [DOI] [PubMed] [Google Scholar]
- Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, Hinson JA. Selective protein covalent binding and target organ toxicity. Toxicol. Appl. Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, Jaeschke H. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J. Pharmacol. Exp. Ther. 2005;315:879–887. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- Davern TJ, 2nd, James LP, Hinson JA, Polson J, Larson AM, Fontana RJ, Lalani E, Munoz S, Shakil AO, Lee WM Acute Liver Failure Study Group. Measurement of serum acetaminophen-protein adducts in patients with acute liver failure. Gastroenterology. 2006;130:687–694. doi: 10.1053/j.gastro.2006.01.033. [DOI] [PubMed] [Google Scholar]
- Davidson DG, Eastham WN. Acute liver necrosis following overdose of paracetamol. Br. Med. J. 1966;2:497–499. doi: 10.1136/bmj.2.5512.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DC, Potter WZ, Jollow DJ, Mitchell JR. Species differences in hepatic glutathione depletion, covalent binding and hepatic necrosis after acetaminophen. Life Sci. 1974;14:2099–2109. doi: 10.1016/0024-3205(74)90092-7. [DOI] [PubMed] [Google Scholar]
- Gow PJ, Jones RM, Dobson JL, Angus PW. Etiology and outcome of fulminant hepatic failure managed at an Australian liver transplant unit. J. Gastroenterol. Hepatol. 2004;19:154–159. doi: 10.1111/j.1440-1746.2004.03273.x. [DOI] [PubMed] [Google Scholar]
- Gunawan BK, Liu ZX, Han D, Hanawa N, Gaarde WA, Kaplowitz N. c-Jun N-terminal kinase plays a major role in murine acetaminophen hepatotoxicity. Gastroenterology. 2006;131:165–178. doi: 10.1053/j.gastro.2006.03.045. [DOI] [PubMed] [Google Scholar]
- Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J. Biol. Chem. 2008;283:13565–13577. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrill AH, Ross PK, Gatti DM, Threadgill DW, Rusyn I. Population-based discovery of toxicogenomics biomarkers for hepatotoxicity using a laboratory strain diversity panel. Toxicol. Sci. 2009;110:235–243. doi: 10.1093/toxsci/kfp096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminopheninduced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J. Pharmacol. Exp. Ther. 1990;255:935–941. [PubMed] [Google Scholar]
- Jaeschke H, Bajt ML. Intracellular signaling mechanisms of acetaminophen-induced liver cell death. Toxicol. Sci. 2006;89:31–41. doi: 10.1093/toxsci/kfi336. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab. Rev. 2012;44:88–106. doi: 10.3109/03602532.2011.602688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, McGill MR, Williams CD, Ramachandran A. Current issues with acetaminophen hepatotoxicity – a clinically relevant model to test the efficacy of natural products. Life Sci. 2011;88:737–745. doi: 10.1016/j.lfs.2011.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–759. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, McGill MR, Farhood A. Herbal extracts as hepatoprotectants against acetaminophen hepatotoxicity. World J. Gastroenterol. 2010;16:2448–2450. doi: 10.3748/wjg.v16.i19.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelava T, Cavar I, Culo F. Influence of small doses of various drug vehicles on acetaminophen-induced liver injury. Can. J. Physiol. Pharmacol. 2010;88:960–967. doi: 10.1139/y10-065. [DOI] [PubMed] [Google Scholar]
- Knight TR, Ho YS, Farhood A, Jaeschke H. Peroxynitrite is a critical mediator of acetaminophen hepatotoxicity in murine livers: protection by glutathione. J. Pharmacol. Exp. Ther. 2002;303:468–475. doi: 10.1124/jpet.102.038968. [DOI] [PubMed] [Google Scholar]
- Knight TR, Kurtz A, Bajt ML, Hinson JA, Jaeschke H. Vascular and hepatocellular peroxynitrite formation during acetaminophen toxicity: role of mitochondrial oxidant stress. Toxicol. Sci. 2001;62:212–220. doi: 10.1093/toxsci/62.2.212. [DOI] [PubMed] [Google Scholar]
- Knockaert L, Descatoire V, Vadrot N, Fromenty B, Robin MA. Mitochondrial CYP2E1 is sufficient to mediate oxidative stress and cytotoxicity induced by ethanol and acetaminophen. Toxicol. In Vitro. 2011;25:475–484. doi: 10.1016/j.tiv.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animals microsomes. Xenobiotica. 2009;39:11–21. doi: 10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, Reisch JS, SchiØdt FV, Ostapowicz G, Shakil AO, Lee WM Acute Liver Failure Study Group. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–1372. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- Laskin DL, Gardner CR, Price VF, Jollow DJ. Modulation of macrophage functioning abrogates the acute hepatotoxicity of acetaminophen. Hepatology. 1995;21:1045–1050. [PubMed] [Google Scholar]
- Latchoumycandane C, Goh CW, Ong MM, Boelsterli UA. Mitochondrial protection by the JNK inhibitor leflunomide rescues mice from acetaminophen-induced liver injury. Hepatology. 2007;45:412–421. doi: 10.1002/hep.21475. [DOI] [PubMed] [Google Scholar]
- Lauterburg BH, Mitchell JR. Regulation of hepatic glutathione turnover in rats in vivo and evidence for kinetic homogeneity of the hepatic glutathione pool. J. Clin. Invest. 1981;67:1415–1424. doi: 10.1172/JCI110170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Buters JT, Pineau T, Fernandez-Salquero P, Gonzalez FJ. Role of CYP2E1 in the hepatotoxicity of acetaminophen. J. Biol. Chem. 1996;271:12063–12067. doi: 10.1074/jbc.271.20.12063. [DOI] [PubMed] [Google Scholar]
- LoGuidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin D-regulated permeability transition. Hepatology. 2011;54:969–978. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J. Clin. Invest. 2012;122:1574–1583. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–982. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol. Appl. Pharmacol. 1988;36:1193–1196. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- Mitchell JR, Jollow DJ, Potter WZ, Davis DC, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. I. Role of drug metabolism. J. Pharmacol. Exp. Ther. 1973;187:185–194. [PubMed] [Google Scholar]
- Miyamoto M, Yanai M, Ookubo S, Awasaki N, Takami K, Imai R. Detection of cell-free, liver-specific mRNAs in peripheral blood from rats with hepatotoxicity: a potential toxicological biomarker for safety evaluation. Toxicol. Sci. 2008;106:538–545. doi: 10.1093/toxsci/kfn188. [DOI] [PubMed] [Google Scholar]
- Muldrew KL, James LP, Coop L, McCullough SS, Hendrickson HP, Hinson JA, Mayeux PR. Determination of acetaminophen-protein adducts in mouse liver and serum and human serum after hepatotoxic doses of acetaminophen using high-performance liquid chromatography with electrochemical detection. Drug Metab. Dispos. 2002;30:446–451. doi: 10.1124/dmd.30.4.446. [DOI] [PubMed] [Google Scholar]
- Nakagawa H, Maeda S, Hikiba Y, Ohmae T, Shibata W, Yanai A, Sakamoto K, Ogura K, Noguchi T, Karin M, Ichijo H, Omata M. Deletion of apoptosis signal-regulating kinase 1 attenuates acetaminophen-induced liver injury by inhibiting c-Jun N-terminal kinase activation. Gastroenterology. 2008;135:1311–1321. doi: 10.1053/j.gastro.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Nelson SD. Molecular mechanisms of the hepatotoxicity caused by acetaminophen. Semin. Liver Dis. 1990;10:267–278. doi: 10.1055/s-2008-1040482. [DOI] [PubMed] [Google Scholar]
- Ni HM, Boggess N, McGill MR, Lebofsky M, Borude P, Apte U, Jaeschke H, Ding WX. Liver specific loss of Atg5 causes persistent activation of Nrf2 and protects against acetaminophen-induced liver injury. Toxicol. Sci. 2012;127:438–450. doi: 10.1093/toxsci/kfs133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Placke ME, Ginsberg GL, Wyand DS, Cohen SD. Ultrastructural changes during acute acetaminophen-induced hepatotoxicity in the mouse: a time and dose study. Toxicol. Pathol. 1987;15:481–488. doi: 10.1177/019262338701500407. [DOI] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic. Res. 2011a;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran A, Lebofsky M, Weinman SA, Jaeschke H. The impact of partial manganese superoxide dismutase (SOD2)-deficiency on mitochondrial oxidant stress, DNA fragmentation and liver injury during acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2011b;251:226–233. doi: 10.1016/j.taap.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid AB, Kurten RC, McCullough SS, Brock RW, Hinson JA. Mechanisms of acetaminophen-induced hepatotoxicity: role of oxidative stress and mitochondrial permeability transition in freshly isolated hepatocytes. J. Pharmacol. Exp. Ther. 2005;312:509–516. doi: 10.1124/jpet.104.075945. [DOI] [PubMed] [Google Scholar]
- Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol. Appl. Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3’-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 1989;264:9814–9819. [PubMed] [Google Scholar]