Abstract
We have designed and evaluated a dual anticancer delivery system to provide combined gene therapy and chemotherapy. Double-walled microspheres consisting of a poly(D,L-lactic-co-glycolic acid) (PLGA) core surrounded by a poly(lactic acid) (PLA) shell were fabricated via the precision particle fabrication (PPF) technique. We make use of the advantages of double-walled microspheres to deliver chitosan-DNA nanoparticles containing the gene encoding the p53 tumor suppressor protein (chi-p53) and/or doxorubicin (Dox), loaded in the shell and core phases, respectively. Different molecular weights of PLA were used to form the shell layer for each formulation. The microspheres were monodisperse with a mean diameter of 65 to 75 μm and uniform shell thickness of 8 to 17 μm. Blank and Dox-loaded microspheres typically exhibited a smooth surface with relatively few small pores, while chi-microspheres containing p53 nanoparticles, with and without Dox, presented rough and porous surfaces. The encapsulation efficiency of Dox was significantly higher when it was encapsulated alone compared to co-encapsulation with chi-p53 nanoparticles. The encapsulation efficiency of chi-p53 nanoparticles, on the other hand, was not affected by the presence of Dox. As desired, chi-p53 nanoparticles were released first, followed by simultaneous release of chi-p53 nanoparticles and Dox at a near zero-order rate. Thus, we have demonstrated that the PPF method is capable of producing double-walled microspheres and encapsulating dual agents for combined modality treatment, such as gene therapy and chemotherapy.
Keywords: Double-walled, microspheres, PLGA, PLA, chitosan, p53, doxorubicin, gene therapy, chemotherapy
INTRODUCTION
The tumor suppressor p53 is a transcriptional regulator that preserves genomic stability, and cells with p53 deficiencies accumulate DNA damage, while the abilities to activate cell cycle arrest and apoptosis are generally lost, favoring transformation into malignant cells [1, 2]. In addition, the loss of p53-mediated apoptosis significantly reduces the tumor response to many chemotherapeutics [2]. Gene therapy is a promising strategy for cancer treatment [3, 4]. In particular, correction of p53 deficiency by gene delivery has been shown to sensitize prostate, liver and breast tumors to doxorubicin (Dox), resulting in tumor growth inhibition and prolonged survival in murine models [5–7].
The focus of this work is to develop a system capable of simultaneous delivery of a gene delivery vector and chemotherapeutic small molecule providing controlled and sustained release. Double-walled microspheres [8–23] offer several advantages as compared to more conventional monolithic microspheres. Drug encapsulated in the core of double-walled microspheres may overcome the problem of high initial burst release commonly encountered in microspheres [8, 11, 16, 17]. Higher drug loads with improved drug stability may be achieved by using materials in the core phase that offer increased drug solubility [12]. Drug release rates may be controlled by controlling the shell material or thickness [13, 14]. Finally, drugs can be released either in a sequential or simultaneous manner by selectively loading them into the core or shell phase, thereby potentially enhancing drug efficacy [20–23].
We have reported the use of a precision particle fabrication (PPF) technique for fabrication of microspheres [24], double-walled microspheres [12–15], and microcapsules [25] with unprecedented control of the particle size and morphology. Here, we report the use of PPF to produce monodisperse double-walled microspheres loaded with Dox and gene delivery vectors comprising chitosan and a plasmid DNA encoding p53 (chi-p53 nanoparticles) in the poly(D,L-lactic-co-glycolic acid) (PLGA) and poly(lactic acid) (PLA) shell phases, respectively. The particles were characterized for drug encapsulation and intraparticle distribution, showing localization of chi-p53 and Dox to the appropriate regions of the particles. Finally, we have investigated the sequential in vitro release of these two drugs.
MATERIALS AND METHODS
Preparation of pCMV-p53 plasmid DNA
The pCMV-p53 plasmid DNA encoding wild-type p53 tumor suppressor protein driven by CMV promoter (Clontech Laboratories, Inc.; Mountain View, CA) was amplified in Escherichia coli DH5α cells and purified using the HiSpeed Plasmid Maxi Kit (Qiagen, Inc.; Valencia, CA). The DNA concentration was quantified by using the Quant-iT PicoGreen dsDNA Kit (Molecular Probes, Inc.; Eugene, OR).
Preparation of chi-p53 nanoparticles
Chi-p53 nanoparticles were formed according to a previously reported technique [26]. A chitosan (Mw = 190,000 – 310,000 Da, degree of deacetylation 75 – 85%; Sigma-Aldrich Corp.; St. Louis, MO) solution in 5 mM sodium acetate buffer, pH 5.5, and DNA solution (400 μg/ml in 5 mM sodium sulfate solution, pH 7.0) were preheated to 50 – 55°C separately. Equal volumes of both solutions were quickly mixed and vortexed for 30 s. The final volume of the mixture in each preparation was limited to < 500 μl in order to yield uniform nanoparticles. Chi-p53 nanoparticles were prepared at amine to phosphate ratio (N/P ratio) of seven.
Preparation of double-walled PLA(PLGA) microspheres
Poly(D,L-lactic-co-glycolic acid) (PLGA) copolymer (50:50 lactic acid:glycolic acid; inherent viscosity (i.v.) = 0.61 dL/g in hexafluoroisopropanol), poly(D,L-lactic acid) (PDLLA) (i.v. = 0.37 and 0.70 dL/g in chloroform) and poly(L-lactic acid) (PLLA) (i.v. = 1.05 dL/g in chloroform) were purchased from Lactel Absorbable Polymers (Pelham, AL). Solutions of 30% (w/v) PLGA and 5% (w/v) PLA in DCM were passed through a coaxial nozzle to produce a jet of core PLGA surrounded by an annular stream containing PLA, protected by a 0.5% (w/v) poly(vinyl alcohol) (PVA; Mw = 25,000 Da, 88 mol% hydrolyzed; Polysciences, Inc.; Warrington, PA) carrier stream, and disrupted into uniform double-walled droplets by an ultrasonic transducer controlled by a frequency generator. The droplets were collected in 0.5% (w/v) PVA, stirred continuously for ~2 h, filtered, and rinsed with an equal volume of distilled water. Finally, the microspheres were freeze-dried and stored at −20 °C under desiccant.
For microspheres loaded with Dox, 1.2 ml of 50 mg/ml Dox (doxorubicin hydrochloride salt, >99% purity; LC Laboratories; Woburn, MA) in water was emulsified with 10 ml of PLGA/DCM solution using a Model 500 Sonic Dismembrator (Thermo Fisher Scientific, Inc.; Pittsburgh, PA) at 30% amplitude in an ice bath for 90 s to form a stable emulsion. For microspheres loaded with chi-p53, chi-p53 nanoparticles were prepared as described above and 1 ml containing 200 μg of DNA was emulsified with 10 ml of PLA/DCM solution. These emulsions were then used for fabrication of double-walled microspheres using PPF as described above. PLGA microspheres loaded with Dox only were also fabricated by using a single nozzle in the PPF technique.
Microscopy
The double-walled microspheres were examined using an Invertoskop inverted microscope (Carl Zeiss Microscopy LLC; Thornwood, NY). Images were captured using Digital Microscope Suite software. The surface morphology of the double-walled microspheres was examined using a JEOL JSM-6060LV scanning electron microscope (SEM) (JEOL Ltd.; Tokyo, Japan). The microspheres were placed directly onto a SEM sample holder coated with conductive carbon tape and sputter-coated with gold palladium prior to imaging at 10 kV. The intraparticle distributions of Dox and/or chi-DNA were examined using a TCS SP2 laser scanning confocal microscope (Leica Microsystems GmbH; Wetzlar, Germany). To visualize chi-DNA, chi-DNA nanoparticles were prepared with pCMV-p53 and fluorescently labeled pCy3 plasmid DNA (Mirus Bio LLC; Madison, WI) at 9:1 (w/w) ratio before preparing the microspheres. Samples were imaged using a 40x oil-immersion objective with 1.25 numerical aperture. Dox was excited with an argon laser at 488 nm, and the fluorescence emission was collected at 500 to 545 nm. The pCy3 plasmid DNA was excited with a helium-neon laser at 543 nm, and the fluorescence emission was collected at 545 to 600 nm. Optical cross-sections were taken at various depths for each sample in order to determine drug distribution at the centerline of the microspheres. Images were captured using Leica confocal software. The fluorescence intensity profiles were obtained using ImageJ software.
Drug loading
The drug loading was determined by dissolving ~50 mg of microspheres in 1 ml of hexafluoroisopropanol. The samples were allowed to stand until complete dissolution of the polymers, added to 5 ml of dilute acetic acid and incubated at 37 °C for 1 h. To measure Dox concentration, the aqueous supernatant was transferred in triplicate to a 96-well plate, and the absorbance was analyzed using a SpectraMax 340PC spectrophotometer (Molecular Devices LLC; Sunnyvale, CA) at a wavelength of 480 nm. To measure DNA concentration in the supernatant, chitosan was digested with chitosanase and lysozyme at 37°C for 4 h. The DNA concentration was determined by using the Quant-iT PicoGreen dsDNA Kit in triplicate, and the fluorescence was analyzed using a Cary Eclipse fluorescence spectrophotometer (Varian, Inc.; Palo Alto, CA) at excitation and emission wavelengths of 480 and 520 nm, respectively.
Integrity of pCMV-p53 plasmid DNA
Agarose gel electrophoresis was performed to determine the integrity of pCMV-p53 plasmid DNA extracted from double-walled microspheres. Approximately 50 mg of microspheres were dissolved in 0.5 ml of DCM, added to 0.5 ml of PBS, incubated at 37°C for 1 h, and centrifuged at 10,000 rpm for 10 min. A portion of the aqueous supernatant was digested with chitosanase and lysozyme as described above. Samples were mixed with loading dye before loading in a 1% (w/v) agarose gel in Tris-acetate-EDTA (TAE) buffer. Naked pCMV-p53 plasmid DNA was loaded as control. Electrophoresis was carried out at 90 V for ~2 h in TAE buffer. The agarose gel was incubated in 0.5 μg/ml ethidium bromide for ~5 min before visualizing the DNA bands under an ultraviolet transilluminator. Images were acquired using a Gel Doc 2000 system (Bio-Rad Laboratories, Inc.; Hercules, CA).
In vitro drug release
Approximately 150 mg of microspheres was suspended in 5 ml PBS in centrifuge tubes. The tubes were maintained at 37°C with shaking at 240 rpm. At selected time points, the tubes were centrifuged at 10,000 rpm for 10 min before 1 ml of supernatant was collected and 1 ml of fresh PBS was replaced. To measure Dox concentration, supernatant fluorescence was analyzed at excitation and emission wavelengths of 480 and 590 nm, respectively. To measure DNA concentration, the supernatant was digested with chitosanase and lysozyme and analyzed by the Quant-iT PicoGreen dsDNA Kit.
RESULTS AND DISCUSSION
Fabrication and characterization of double-walled microspheres
An important advantage of the PPF technique is the capability of producing monodispersed double-walled microspheres with uniform shell thickness by varying the flow rates of core and shell polymer solutions. Moreover, microspheres with two agents loaded in the respective core and shell phases can be fabricated in a single step, and the agents can be released either in a sequential or parallel manner. In this study, the PPF technique was used to produce double-walled microspheres loaded with Dox and chi-p53 nanoparticles in the PLGA core and PLA shell phases, respectively.
For double-walled microsphere fabrication, a solution of PLGA (30% (w/v) in DCM) was arranged as the core stream while PLA (5% (w/v) in DCM) flowed as the annular stream. Three different molecular weights of PLA (i.v. = 0.37, 0.70 and 1.05 dL/g) were used to form the shell layer. The particle diameter ranged between 65 and 75 μm (Table 1). The shell thickness, calculated from the measured overall particle diameter and known mass flow rates of the polymer solutions assuming complete polymer phase separation, was ~8 to 17 μm. For blank double-walled PLA(PLGA) microspheres, the ratio of core diameter (Dcore) to overall particle diameter (Dparticle) is related to the volume fraction of PLGA in the microsphere as shown below.
Table 1.
Sizes and encapsulation efficiencies of double-walled PLA(PLGA) microspheres.
| Sample | Drug | PLA i.v. = 0.37 dL/g | PLA i.v. = 0.70 dL/g | PLA i.v. = 1.05 dL/g | Avg size (μm) | Core diameter (μm) | Shell thickness (μm) | |||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Size (μm) | e.e. (%) | Size (μm) | e.e. (%) | Size (μm) | e.e. (%) | |||||
| A | Blank | 64.5 ± 4.3 | - | 65.2 ± 4.0 | - | 62.3 ± 3.7 | - | 64.0 | 46.2 | 8.9 |
|
| ||||||||||
| B | Dox | 63.6 ± 4.5 | 79.3 ± 1.5 | 64.5 ± 4.2 | 80.0 ± 2.6 | 65.2 ± 2.9 | 82.8 ± 1.5 | 64.4 | 49.4 | 7.5 |
|
| ||||||||||
| C | Chi-p53 | 71.9 ± 3.6 | 24.7 ± 2.1 | 73.5 ± 3.7 | 32.5 ± 1.7 | 72.2 ± 4.1 | 36.8 ± 1.1 | 72.5 | 38.7 | 16.9 |
|
| ||||||||||
| D | Dox | 74.1 ± 3.6 | 32.3 ± 0.6 | 72.1 ± 3.6 | 40.5 ± 2.1 | 74.5 ± 3.7 | 46.8 ± 0.7 | 73.6 | 42.8 | 15.4 |
| Chi-p53 | 27.0 ± 2.2 | 32.6 ± 1.6 | 36.8 ± 1.4 | |||||||
| (1) |
where vPLGA and vPLA are the flow rates of PLGA and PLA respectively (cm3 of polymer/h), xPLGA and xPLA are the volume fractions of PLGA and PLA in the core and shell solutions respectively, Fcore and Fshell are the flow rates of core and shell solutions respectively (cm3 of polymer solution/h), CPLGA and CPLA are the concentrations of PLGA and PLA in DCM respectively (g of polymer/cm3 of DCM), and ρPLGA and ρPLA are the densities of PLGA and PLA respectively (g/cm3).
For drug loaded double-walled PLA(PLGA) microspheres with the creation of water-in-oil emulsion in core and/or shell phase(s), the above equation is modified to include the pore volume originally occupied by the water phase that contributed to the total volume of the microsphere as shown below.
| (2) |
where vwater,core and vwater,shell are the flow rates of water in the core and shell phases respectively (cm3 of water/h), xwater,core and xwater,shell are the volume fractions of water in the core and shell solutions respectively, and Vwater,core and Vwater,shell are the volume ratios of water to DCM used to create the emulsion in the core and shell solutions respectively.
Then, the shell thickness (tshell) can be calculated as follows.
| (3) |
Particles containing chi-p53 nanoparticles in the shell were slightly larger than those without chi-p53, which may be attributed to the emulsion that formed the shell phase (Table 1). While the core diameter of various microsphere formulations was comparable to one another, the shell thickness of particles containing chi-p53 nanoparticles was larger than those without chi-p53. In this case, the water/organic volume ratio of the chi-p53 emulsion is 1/10. However, the organic phase is 5% (w/v) polymer. Thus, the volume of pores resulting from the aqueous phase is on the order of the volume of polymer after solvent removal. The presence of the emulsion then roughly doubles the volume of the polymer shell, which explains the increase in shell thickness observed.
Optical micrographs suggest that fully formed double-walled microspheres were produced in all the formulations, with no sign of partial or incomplete encapsulation (Figure 1). However, FTIR spectroscopy suggested that both PLGA and PLA were present in the shells of the microspheres (Supplementary Information).
Figure 1.

Transmitted light and laser scanning confocal (overlay) micrographs depicting blank and drug loaded double-walled PLA(PLGA) microspheres. The distribution of doxorubicin in formulations B and D microspheres is indicated in green. The distribution of chi-p53 nanoparticles in formulations C and D microspheres is indicated in red and yellow (colocalization of red and green), respectively. Scale bar = 50 μm.
Blank and Dox-loaded microspheres (formulations A and B) typically exhibited smooth morphology with relatively few small pores on the surface (Figure 2). On the other hand, microspheres encapsulating chi-p53 nanoparticles or combined Dox and chi-p53 nanoparticles (formulations C and D) presented a rough and porous surface structure, likely due to the presence of the chi-p53 emulsion in the shell layer. In fact, the emulsion in the shell phase appeared to have a significant effect on the drug distribution, initial burst release of drug and subsequent drug release rates (see below).
Figure 2.
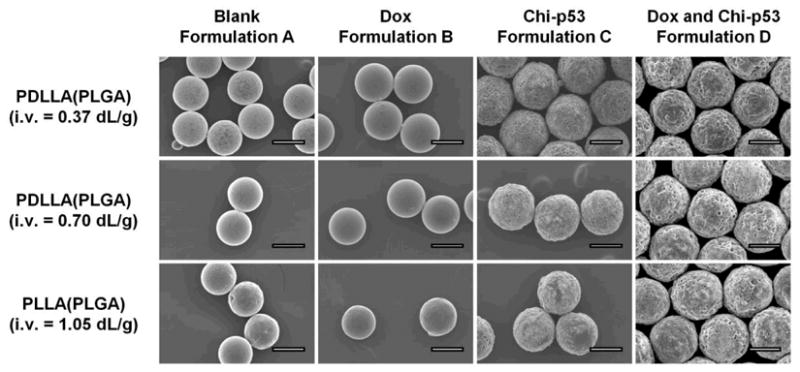
Scanning electron micrographs depicting the surface morphology of blank and drug loaded double-walled PLA(PLGA) microspheres. Scale bar = 50 μm.
Distinct core-shell particles, encapsulating Dox in the core were produced with high molecular weight of the PLA shell layer (i.v. = 0.70 and 1.05 dL/g) (Figure 1f, j and 3d, g). On the other hand, microspheres containing low molecular weight PLA shell (i.v. = 0.37 dL/g) exhibited irregular of Dox distributions (Figure 3a). For formulation C microspheres, chi-p53 fluorescence was present mainly at the particle periphery, corresponding the PLA shell (Figure 3b, e, h). For formulation D microspheres, Dox was homogeneously distributed throughout the microsphere (Figure 3c, f, i). However, chi-p53 remained near the particle edge, similar to that for formulation C microspheres.
Figure 3.
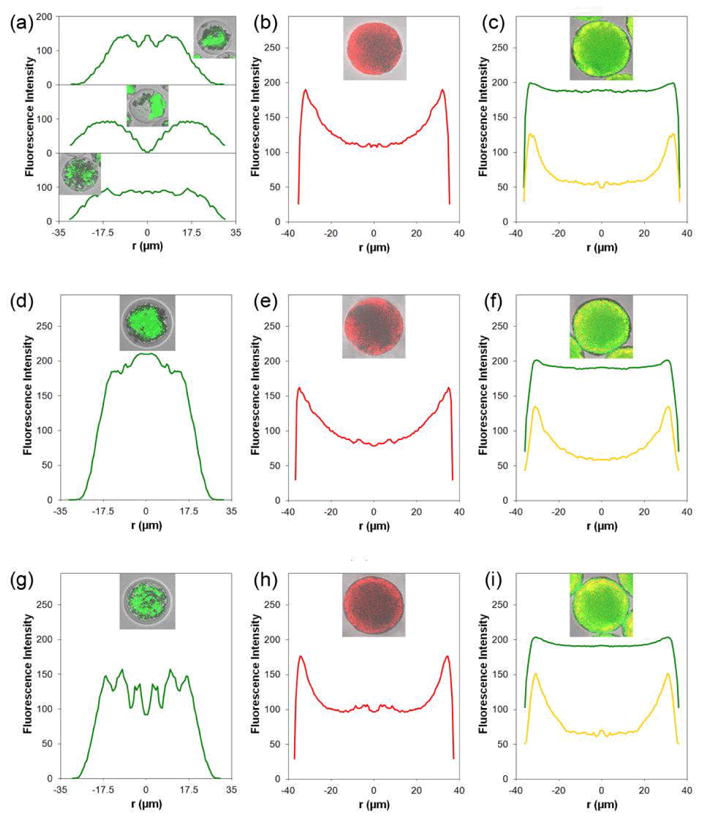
Radially averaged fluorescence intensity profiles of doxorubicin and/or chi-p53 nanoparticles in representative double-walled PLA(PLGA) microspheres. The inserts are the confocal images captured at the centerline of the microspheres. (a), (d) and (g) are the profiles of doxorubicin (green) for formulation B microspheres with increasing molecular weights of PLA shell layer, i.v. = 0.37, 0.70 and 1.05 dL/g, respectively. (b), (e) and (h) are the profiles of chi-p53 nanoparticles (red) for formulation C microspheres with increasing molecular weights of PLA shell layer, i.v. = 0.37, 0.70 and 1.05 dL/g, respectively. (c), (f) and (i) are the profiles of doxorubicin (green) and chi-p53 nanoparticles (yellow) for formulation D microspheres with increasing molecular weights of PLA shell layer, i.v. = 0.37, 0.70 and 1.05 dL/g, respectively.
In order to load Dox and chi-p53 in PLGA core and PLA shell phases, respectively, a water-in-oil emulsion of drug in polymer/DCM solution was created in each phase. During the DCM extraction process, the water phases created in both core and shell phases of the PLA(PLGA) droplets may spread and coalesce, resulting in the diffusion of Dox and chi-p53 into the neighboring phase. Since Dox is a hydrophilic drug, the presence of a water-in-oil emulsion in the shell phase may facilitate Dox diffusion from the PLGA core into the PLA shell layer. Thus, Dox was homogeneously distributed in formulation D microspheres, resulting in co-localization of Dox and chi-p53 in the shell layer.
Encapsulation efficiency of doxorubicin and chi-p53 nanoparticles
To investigate the effect of the PLA shell, PLGA microspheres loaded with Dox (formulation E) were fabricated with particle diameter of 50 μm corresponding to the approximate diameter of the PLGA core encapsulated within the double-walled microspheres. The encapsulation efficiency of Dox in double-walled PLA(PLGA) microspheres (formulation B, ~80%; Table 1) was higher than that in single-polymer PLGA microspheres (formulation E, 61%). Thus, it appears that the presence of the PLA shell layer inhibits the premature release of Dox into the PVA solution during DCM extraction. However, the encapsulation efficiency of Dox decreased (~32 to 47%) when the drug was loaded together with chi-p53 nanoparticles. The emulsion created in the PLA shell phase likely facilitated the diffusion of Dox out of the microspheres during DCM extraction. The encapsulation efficiency of chi-p53 nanoparticles for formulations C and D microspheres was similar (~25 to 37%). The rather low encapsulation efficiency of chi-p53 nanoparticles could be due to the exposure of the PLA shell phase emulsion to the PVA solution during DCM extraction. Overall, the encapsulation efficiency of Dox and chi-p53 nanoparticles was observed to improve with increasing molecular weight of the PLA shell layer.
Integrity of plasmid DNA encapsulated within microspheres
Agarose gel electrophoresis was performed to determine the integrity of pCMV-p53 plasmid DNA complexed with chitosan within the double-walled PLA(PLGA) microspheres (formulation C; Figure 4). No bands were observed for chi-p53 nanoparticles extracted from PLA(PLGA) particles, indicating that chitosan effectively bound plasmid DNA. After enzymatic digestion of chitosan to release the plasmid DNA, the observed bands matched those of the unencapsulated pCMV-p53. This indicates that the plasmid DNA retained its structure through particle fabrication, solvent extraction and freeze-drying processes.
Figure 4.
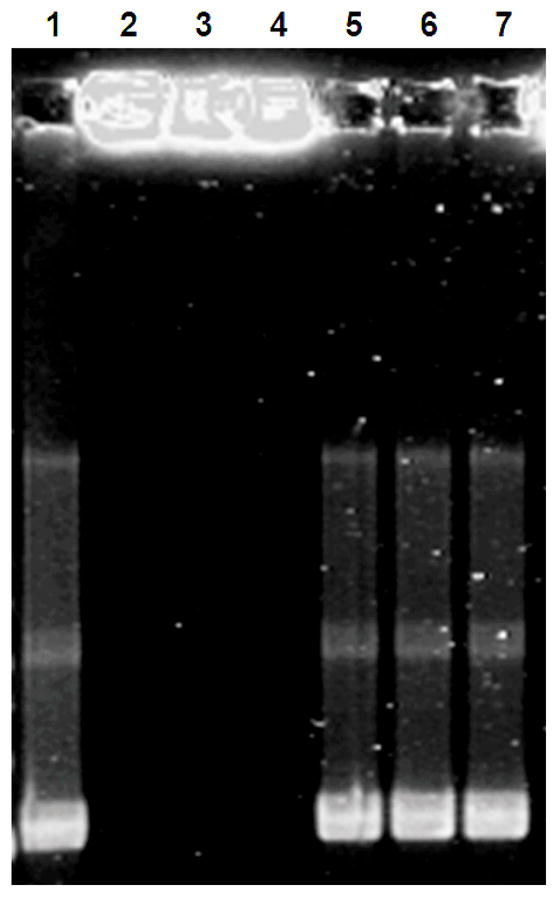
Agarose gel electrophoresis of chi-p53 nanoparticles extracted from double-walled PLA(PLGA) microspheres (formulation C). Lane 1: Naked pCMV-p53 plasmid DNA. Lanes 2 to 4: Chi-p53 nanoparticles, N/P = 7, from microspheres with increasing molecular weights of PLA shell layer, i.v. = 0.37, 0.70 and 1.05 dL/g, respectively, before chitosanase and lysozyme digestion. Lanes 5 to 7: Chi-p53 nanoparticles, N/P = 7, from microspheres with increasing molecular weights of PLA shell layer, i.v. = 0.37, 0.70 and 1.05 dL/g, respectively, after chitosanase and lysozyme digestion.
In vitro release of doxorubicin and chi-p53 nanoparticles
For formulation B microspheres, release of Dox showed a small initial burst (~2 to 10%) before entering a lag phase of about 25 days (Figure 5a). The drug was subsequently released slowly over a period of ~100 days. On the other hand, when chi-p53 was present in the shell (formulation D), release of Dox exhibited a larger initial burst (~18 to 29%) before entering a lag phase of about 25 days (Figure 5c) followed by slow release at a relatively constant rate. The release profiles of chi-p53 nanoparticles were similar regardless of Dox content (Figure 5b, d). The nanoparticles were released quickly over the first week and then at a decreased rate over a period of 120 days. The initial burst of Dox and chi-p53 varied inversely with PLA molecular weight. However, the molecular weight of the PLA shell layer had a minimal effect on the subsequent release rates of Dox and chi-p53.
Figure 5.
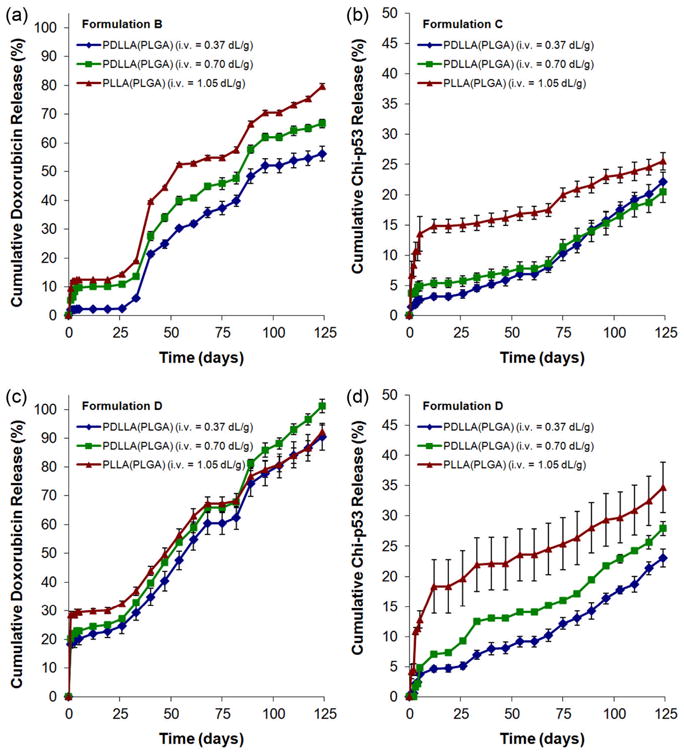
In vitro Dox and chi-p53 release from double-walled PLA(PLGA) microspheres: (a) doxorubicin from formulation B microspheres, (b) chi-p53 nanoparticles from formulation C microspheres, (c) doxorubicin from formulation D microspheres, and (d) chi-p53 nanoparticles from formulation D microspheres.
CONCLUSIONS
In this study, several formulations of double-walled PLA(PLGA) microspheres loaded with Dox and/or chi-p53 nanoparticles have been fabricated and characterized as potential agents for combined gene therapy and chemotherapy. The drug loaded microspheres exhibited an early release of chi-p53 nanoparticles from the PLA shell layer, followed by a sustained release of Dox from the PLGA core. By performing a washing step to eliminate the initial burst release of Dox, particles similar to formulation D may provide a promising synergistic cancer treatment.
Supplementary Material
Acknowledgments
The authors acknowledge the funding support from National Institutes of Health (NIH, USA) and National Medical Research Council (NMRC, Singapore) under the grant numbers 1R01EB005181 and NMRC EDG11may084, respectively. Qingxing Xu acknowledges the scholarship support from Agency for Science, Technology and Research (A*STAR, Singapore) for NUS-UIUC Joint Ph.D. Program.
References
- 1.Whibley C, Pharoah PD, Hollstein M. p53 polymorphisms: Cancer implications. Nat Rev Cancer. 2009;9:95–107. doi: 10.1038/nrc2584. [DOI] [PubMed] [Google Scholar]
- 2.Millau JF, Bastien N, Drouin R. p53 transcription activities: A general overview and some thoughts. Mutation Res. 2009;681:118–133. doi: 10.1016/j.mrrev.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 3.El-Aneed A. An overview of current delivery systems in cancer gene therapy. J Controlled Release. 2004;94:1–14. doi: 10.1016/j.jconrel.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 4.Samaranayake H, Maatta AM, Pikkarainen J, Yla-Herttuala S. Future prospects and challenges of antiangiogenic cancer gene therapy. Hum Gene Ther. 2010;21:381–396. doi: 10.1089/hum.2010.017. [DOI] [PubMed] [Google Scholar]
- 5.Xu L, Pirollo KF, Chang EH. Tumor-targeted p53-gene therapy enhances the efficacy of conventional chemo-radiotherapy. J Controlled Rel. 2001;74:115–128. doi: 10.1016/s0168-3659(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 6.Wiradharma N, Tong YW, Yang YY. Self-assembled oligopeptide nanostructures for co-delivery of drug and gene with synergistic therapeutic effect. Biomatls. 2009;30:3100–3109. doi: 10.1016/j.biomaterials.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Lu X, Wang QQ, Xu FJ, Tang GP, Yang WT. A cationic prodrug/therapeutic gene nanocomplex for the synergistic treatment of tumors. Biomatls. 2011;32:4849–4856. doi: 10.1016/j.biomaterials.2011.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Lee TH, Wang J, Wang C. Double-walled microspheres for the sustained release of a highly water soluble drug: Characterization and irradiation studies. J Controlled Release. 2002;83:437–452. doi: 10.1016/s0168-3659(02)00235-3. [DOI] [PubMed] [Google Scholar]
- 9.Yang Y, Shi M, Goh S, Moochhala SM, Ng S, Heller J. POE/PLGA composite microspheres: Formation and in vitro behavior of double walled microspheres. J Controlled Release. 2003;88:201–213. doi: 10.1016/s0168-3659(02)00491-1. [DOI] [PubMed] [Google Scholar]
- 10.Shi M, Yang Y, Chaw C, Goh S, Moochhala SM, Ng S, Heller J. Double walled POE/PLGA microspheres: Encapsulation of water-soluble and water-insoluble proteins and their release properties. J Controlled Release. 2003;89:167–177. doi: 10.1016/s0168-3659(02)00493-5. [DOI] [PubMed] [Google Scholar]
- 11.Rahman NA, Mathiowitz E. Localization of bovine serum albumin in double-walled microspheres. J Controlled Release. 2004;94:163–175. doi: 10.1016/j.jconrel.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 12.Berkland C, Pollauf E, Pack DW, Kim K. Uniform double-walled polymer microspheres of controllable shell thickness. J Controlled Release. 2004;96:101–111. doi: 10.1016/j.jconrel.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 13.Berkland C, Cox A, Kim K, Pack DW. Three-month, zero-order piroxicam release from monodispersed double-walled microspheres of controlled shell thickness. J Biomed Mater Res. 2004;70A:576–584. doi: 10.1002/jbm.a.30114. [DOI] [PubMed] [Google Scholar]
- 14.Pollauf E, Kim KK, Pack DW. Small-molecule release from poly(d,l-lactide)/poly(d,l-lactide-co-glycolide) composite microparticles. J Pharm Sci. 2005 doi: 10.1002/jps.20408. in press. [DOI] [PubMed] [Google Scholar]
- 15.Pollauf EJ, Berkland C, Kim KK, Pack DW. In vitro degradation of polyanhydride/polyester core-shell double-wall microspheres. Int J Pharm. 2005 doi: 10.1016/j.ijpharm.2005.06.004. in press. [DOI] [PubMed] [Google Scholar]
- 16.Tan EC, Lin R, Wang CH. Fabrication of double-walled microspheres for the sustained release of doxorubicin. J Controlled Rel. 2005;291:135–143. doi: 10.1016/j.jcis.2005.04.089. [DOI] [PubMed] [Google Scholar]
- 17.Zheng W. A water-in-oil-in-oil-in-water (W/O/O/W) method for producing drug-release, double-walled microspheres. Int J Pharm. 374;90:95. doi: 10.1016/j.ijpharm.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 18.Kokai LE, Tan H, Jhunjhunwala S, Little SR, Frank JW, Marra KG. Protein bioactivity and polymer orientation is affected by stabilizer incorporation for double-walled microspheres. J Controlled Rel. 2010;141:168–176. doi: 10.1016/j.jconrel.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Kokai LE, Ghaznavi AM, Marra KG. Incorporation of double-walled microspheres into polymer nerve guides for the sustained delivery of glial cell line-derived neurotrophic factor. Biomatls. 2010;31:2313–2322. doi: 10.1016/j.biomaterials.2009.11.075. [DOI] [PubMed] [Google Scholar]
- 20.Choi DH, Park CH, Kim IH, Chun HJ, Park K, Han DK. Fabrication of core-shell microcapsules using PLGA and alginate for dual growth factor delivery system. J Controlled Rel. 2010;147:193–201. doi: 10.1016/j.jconrel.2010.07.103. [DOI] [PubMed] [Google Scholar]
- 21.Nie H, Dong Z, Arifin DY, Hu Y, Wang CH. Core/shell microspheres via coaxial electrohydrodynamic atomization for sequential and parallel release of drugs. J Biomed Mater Res A. 2010;95:709–716. doi: 10.1002/jbm.a.32867. [DOI] [PubMed] [Google Scholar]
- 22.Nie H, Fu Y, Wang CH. Paclitaxel and suramin-loaded core/shell microspheres in the treatment of brain tumors. Biomatls. 2010;31:8732–8740. doi: 10.1016/j.biomaterials.2010.07.080. [DOI] [PubMed] [Google Scholar]
- 23.Lee WL, Loei C, Widjaja E, Loo SC. Altering the drug release profiles of double-layered ternary-phase microparticles. J Controlled Rel. 2011;151:229–238. doi: 10.1016/j.jconrel.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Berkland C, Kim K, Pack DW. Fabrication of PLG microspheres with precisely controlled and monodisperse size distributions. J Control Release. 2001;73:59–74. doi: 10.1016/s0168-3659(01)00289-9. [DOI] [PubMed] [Google Scholar]
- 25.Berkland C, Pollauf EJ, Varde NK, Pack DW, Kim KK. Monodisperse liquid-filled biodegradable microcapsules. Pharm Res. 2007;24:1007–1013. doi: 10.1007/s11095-006-9197-9. [DOI] [PubMed] [Google Scholar]
- 26.Mao HQ, Roy K, Troung-Le VL, Janes KA, Lin KY, Wang Y, August JT, Leong KW. Chitosan-DNA nanoparticles as gene carriers: Synthesis, characterization and transfection efficiency. J Controlled Rel. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


