Abstract
Objective
To determine the rate of change of antimullerian hormone (AMH) in the late reproductive years and its associations with time to menopause (TTM). We hypothesized that the rate of change between 2 measures of AMH reflects follicular atresia and varies among women independent of age.
Design
14-year follow-up.
Setting
A randomly-identified, population-based cohort (Penn Ovarian Aging Study).
Subjects
293 women with 2 measures of AMH evaluated in survival analysis.
Intervention
None.
Main Outcome Measure
Time to menopause.
Results
The rate of AMH change was a strong independent predictor of TTM in multivariable analysis after adjusting for AMH baseline, age and smoking (hazard ratio for 1 SD change = 1.82, 95% CI: 1.56 – 2.14, P<0.0001). Among women with similar AMH levels, TTM differed by approximately 2 years when compared between a slow and fast rate of AMH change. A significant interaction of AMH rate of change and age (P<0.0001) indicated that a faster decrease in AMH was associated with an increased risk of menopause in women ages 35–39 years (hazard ratio 6.97, 95% CI: 3.81– 12.72, P<0.0001), with less dramatic but significant associations in women ages 40–44 and 45–48 years.
Conclusions
AMH rate of change was independently associated with TTM in late reproductive-age women and increased the precision of estimates of TTM when included with an AMH baseline level and age. The rate of AMH change may be a more direct surrogate than age and increases the precision of estimates of TTM during this clinically important time period.
Keywords: AMH, menopause, time to menopause, ovarian aging, follicular atresia, smoking
INTRODUCTION
Antimullerian hormone (AMH) is increasingly identified as an early predictor of decreased ovarian reserve (1–4). This hormone appears to modulate two regulatory steps of folliculogenesis: inhibiting recruitment of primordial follicles and decreasing the sensitivity of small antral follicles to follicle stimulating hormone (FSH) (5). Serum AMH levels are shown to decrease with age and are a stronger and more consistent correlate of age than the number of antral follicles observed on ultrasound, inhibin b or FSH levels (6, 7). Undetectable levels of AMH in women following ovariectomy as well as in natural menopause confirm its ovarian source, and transvaginal ultrasonography has demonstrated the correlation between AMH levels and the number of small antral follicles (8).
Several studies evaluated AMH as a predictor of age at menopause in naturally fertile or normoovulatory women (9, 10). We previously reported the value of one measure of AMH levels in late reproductive-age women for determining time to menopause (11). The study indicated that AMH was a strong predictor of time to menopause, and that inclusion of age with AMH significantly improved the estimates.
Models that have examined AMH in relation to menopause have assumed a steady rate of follicular atresia over time. We hypothesized that the rate of change between two measures of AMH at an interval of a year or more reflects the rate of follicular atresia; this rate of AMH change varies among women independent of age and may increase the precision of estimates of time to menopause during this clinically important time period. We further hypothesized that in addition to age, other risk factors of current smoking, body mass index (BMI) and race modify the associations between AMH and time to menopause.
MATERIALS AND METHODS
Study participants
The study evaluated 293 women in the Penn Ovarian Aging Study (POAS) who had a detectable baseline measure of AMH (>= 0.20 ng/mL) and at least one additional AMH measure one or more years later in order to define the rate of change (slope) of AMH in the late reproductive years. The cohort was randomly identified by telephone digit dialing to households in Philadelphia County, PA in 1996–1997, and sampling was stratified to obtain equal numbers of African American and white women (218 in each group), as described in previous reports (12). The Institutional Review Board of the University of Pennsylvania approved the study, and all participants provided written informed consent.
At cohort enrollment, all participants were premenopausal as defined by regular menstrual cycles in the reference range (22–35 days for the previous three menstrual cycles), ages 35–48 years, and an intact uterus with at least one ovary. Exclusion criteria included current use of psychotropic or hormonal medications, including hormonal contraception and hormone therapies, pregnancy or breast feeding, serious health problems known to compromise ovarian function (e.g., diabetes mellitus, liver disease, breast or endometrial cancer et al), uncontrolled hypertension, alcohol or drug abuse in the past year.
Study design
The cohort was followed for 14 years after enrollment. Follow-up assessments were at approximately 9-month intervals for the first five years and then annually, with a two-year gap between assessment 10 and 11. At each assessment period, the study data were collected at two in-home visits, which were timed to the early follicular phase of the menstrual cycle (days 2–6) in two consecutive menstrual cycles or approximately one month apart in non-cycling women.
The study was described to participants as a general women’s health study. Trained research interviewers obtained menstrual dates, structured interview data on overall health, blood samples for the hormone assays, and anthropometric measures (height, weight, waist and hip circumference); participants completed a set of validated self-report measures to assess health and other behavioral measures of the study.
Study variables
The primary outcome variable was time to menopause (TTM). This was measured in years from the first study assessment (when all participants were premenopausal) to the first follow-up assessment where the participant reported no menstrual bleeding for at least 12 months. The point one year before the 12-months of no menstrual bleeding was then defined as menopause.
AMH baseline level was the first available AMH value for each participant. AMH rate of change (slope) was calculated as the difference between the first of two consecutive undetectable log AMH levels (or the last AMH measurement if undetectable levels were not reached) minus the log AMH baseline level divided by time in years. Covariates that were selected as possible risk factors for TTM were obtained at the same visit as the AMH baseline measure and included age, race (African American or white), body mass index (kg/m2), and current smoker (yes, no).
AMH assays were conducted contemporaneously in 2011 in the Clinical and Translational Research Center of the University of Pennsylvania, using a second generation AMH enzyme-linked immunosorbent assay (ELISA) kits (Beckman Coulter Inc, Brea, CA). The blood samples were obtained at the scheduled study visits (days 2–6 of the menstrual cycle), centrifuged and frozen in aliquots at −80° C. The AMH assays were conducted for each participant at each assessment period that frozen samples were available until the participant’s AMH level was undetectable for two consecutive assessment periods (approximately 2 years) or the end of the 14-year follow-up, whichever occurred first. The intra- and interassay coefficients of variation were 4.6% and 6.8%, respectively. The lower limit of detection was 0.10 ng/mL.
Statistical analysis
A priori power calculations using NQuery Advisor 6.0 assumed type I alpha error of 5% and 80% power. Given that 146 of the 293 women in the study (50%) reached menopause in the follow-up period, the study has sufficient power to detect hazard ratios of 1.6 or larger for risk factors with 50% prevalence. The detectable hazard ratio for a risk factor with 30% prevalence of the women under study is 1.8 and for a 25% prevalence is 2.0.
Statistical analyses were performed using Kaplan-Meier estimations for TTM (13), log-rank tests, (14) and univariate and multivariable Cox proportional hazards models (15) to estimate the risk of menopause over the 14-year follow-up period. The hormone measures were transformed to natural log values to reduce the influence of their skewed distributions. To determine the nature of the decline in AMH, inspection of the distribution of log AMH to its undetectable level (0.10 ng/ml) indicated that the decline was principally linear. AMH baseline level, AMH rate of change (slope), age, current smoking, BMI and race were each evaluated for their association with TTM and then evaluated in multivariable models to identify their independent contributions after adjusting for the presence of the other variables.
In the Cox proportional hazards models (survival analysis), all participants contributed data to the analysis. In addition to TTM for women whose menopause was observed during the 14-year study, data from women who did not reach menopause were included and considered censored at last observed follow-up or study endpoint. Exogenous hormone use and hysterectomy were exclusions at enrollment in the cohort. Hormone use during follow-up was low and not associated with TTM. Observations of hormone use during the study were censored; observations of women who had a hysterectomy during the study were censored from the point of hysterectomy. A sensitivity analysis conducted for the multivariable Cox model indicated that the results remained consistent when the 16 women who reported a hysterectomy prior to menopause were omitted. At some time during the study, 17 women reported having one ovary (15 were prior to menopause). The rate of AMH decline did not differ between the women with one ovary and those with two ovaries (mean rate of change = 0.39 and 0.40, respectively, P=0.88). A sensitivity analysis omitted the women with one ovary and indicated that results for AMH rate of change remained consistent with the results in the full cohort (shown in Supplemental Table 1).
In the proportional hazards analyses, hazard ratios with 95% confidence intervals indicate the estimated ratio for risk of reaching menopause between the exposure variable and its reference group and the risk of reaching menopause at a time point during the observation period. Hazard ratios >1.0 indicate greater risk (shorter TTM), and <1.0 less risk (longer TTM) in the exposure group compared to the reference group. Proportionality of hazards was evaluated by plots of transformed hazard estimates and smoothed residuals (16, 17). No violations of modeling assumptions were observed.
The AMH level and slope were evaluated as continuous variables (natural log transformed) and also as group variables in order to provide more information and larger cell sizes for evaluating additional variables in multivariable models. When used as a group variable, AMH rate of change was divided into two groups at the median of the log values. The slope values above the median had the higher rate of change; values below the median had a slower rate of change. AMH baseline was divided into two approximately equal size groups with the cut point at greater or equal to 1.0 ng/mL.
All analyses were conducted using the SAS 9.3 statistical package (SAS, Inc, Cary, NC). Statistical tests were two-sided with P<0.05 considered significant.
RESULTS
At baseline, the mean (SD) age of the 293 participants was 40.93 (3.29) years (range 35–48 years); all participants had regular menstrual cycles in normal range (22–35 days) and were premenopausal. During the follow-up interval, 50% (146/293) of the participants were observed to reach menopause, with a median time to menopause of 10.05 years (interquartile range 6.07 to 13.05 years). The study variables were compared at baseline between the participants and the remainder of the cohort (who were excluded because their baseline AMH was undetectable or they lacked two AMH measures to calculate a slope); race, BMI and smoking did not significantly differ. However, the omitted participants were older, had higher FSH levels, lower inhibin b levels and lower estradiol levels, values that are consistent with undetectable AMH levels (Table 1).
Table 1.
Baseline Characteristics
| Variable | Included in study, N=2931 | Excluded in study, N=1432 | P Value |
|---|---|---|---|
| Age, yrs (mean, 95% CI) | 40.93 (40.6 – 41.3) | 43.35 (42.8 – 43.9) | <0.001 |
| Race, N (%) | 0.143 | ||
| African American | 140 (47.8) | 79 (55.2) | |
| White | 153 (52.2) | 64 (44.8) | |
| BMI, kg/m2 (N, %) | 0.295 | ||
| ≥30 | 108 (37.0) | 61 (42.7) | |
| <30 | 184 (63.0) | 82 (57.3) | |
| Smoker (N, %) | 110 (37.5) | 56 (39.2) | 0.754 |
| AMH (ng/mL)3 | 0.93 (0.84 – 1.03) | 0.18 (0.15 – 0.22) | <0.001 |
| FSH (mIU/mL)3 | 6.49 (6.23 – 6.76) | 10.53 (9.45 – 11.74) | <0.001 |
| Inhibin b (pg/mL)3 | 76.60 (71.9 – 81.5) | 39.22 (34.6 – 44.5) | <0.001 |
| Estradiol (pg/mL)3 | 36.1 (33.9 – 38.35) | 30.9 (27.1 – 35.2) | 0.048 |
Study participants had a detectable AMH level (≥0.20 ng/mL) at their first measurement and at least one follow-up measure of AMH in order to define a slope (rate of change).
The excluded women were enrolled in the randomly-identified cohort but did not have an AMH slope due to an undetectable AMH at baseline or not having at least 1 follow-up measure of AMH to calculate the slope.
Hormone values are the geometric mean with 95% confidence interval at baseline.
The median AMH level at baseline was 0.98 ng/mL; range 0.20 to 7.51 ng/mL; inter- quartile range 0.49 to 1.82 ng/mL. The median time interval between the two AMH measures (i.e., baseline and the first of two consecutive undetectable AMH values or study endpoint, whichever occurred first) was 4.93 years (interquartile range 2.54 to 7.27 years).
Table 2 shows the associations of the study variables with time to menopause. AMH rate of change, AMH baseline and age were each significantly associated with TTM in unadjusted analysis (P<0.0001). In multivariable analysis, the rate of AMH change was a strong independent predictor of TTM after adjusting for AMH baseline, age and smoking (hazard ratio for 1 SD change = 1.82, 95% CI: 1.56 – 2.14, P<0.0001). AMH baseline level and age also remained independent predictors of TTM in the adjusted model (P<0.0001), as shown in Table 2. We further investigated whether the small number of women who reported having only one ovary at some point in the study, impacted these findings. The mean rate of AMH decline between women with one ovary (N=17) and the remaining sample was notably similar (0.39, 95% CI 0.29–0.49 and 0.40, 95% CI 0.36–0.44, respectively, P=0.88). When the women with one ovary were omitted in multivariable analysis, the hazard ratio for AMH rate of change did not change from the hazard ratio for the full cohort shown in Table 2 (the sensitivity analysis is shown in supplemental Table 1).
Table 2.
Associations of Study Variables with Time to Menopause
| Variable | Unadjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| Hazard Ratio1 | 95% CI | P | Hazard Ratio1 | 95% CI | P | |
| AMH rate of change2, 3 | 1.31 | 1.23 – 1.40 | <0.0001 | 1.82 | 1.56 – 2.14 | <0.0001 |
| AMH level <1.0 ng/mL | 2.84 | 2.00 – 4.05 | <0.0001 | 2.30 | 1.58 – 3.33 | <0.0001 |
| Age | <0.0001 | <0.0001 | ||||
| 35 – 39 | 1.00 | Reference | --- | 1.00 | Reference | --- |
| 40 – 44 | 3.53 | 2.37 – 5.25 | <0.0001 | 3.03 | 2.01 – 4.56 | <0.0001 |
| 45 – 48 | 9.16 | 5.41– 15.53 | <0.0001 | 3.82 | 2.09 – 7.00 | <0.0001 |
| Smoking, current | 1.36 | 0.97 – 1.90 | 0.070 | 1.41 | 1.01 – 1.98 | 0.045 |
| BMI ≥ 30 | 0.91 | 0.65 – 1.27 | 0.566 | |||
| Race | ||||||
| African American | 1.07 | 0.77 – 1.48 | 0.687 | |||
| White | 1.00 | Reference | --- | |||
N=293
Hazard ratio (HR) > 1.0 indicates greater risk (shorter time to menopause; HR < 1.0 indicates less risk (longer time to menopause).
Rate of change is calculated as the difference between the first undetectable level or the last detectable value of log AMH and log AMH baseline per year.
Hazard ratio is for 1 SD of AMH rate of change, SD=0.33.
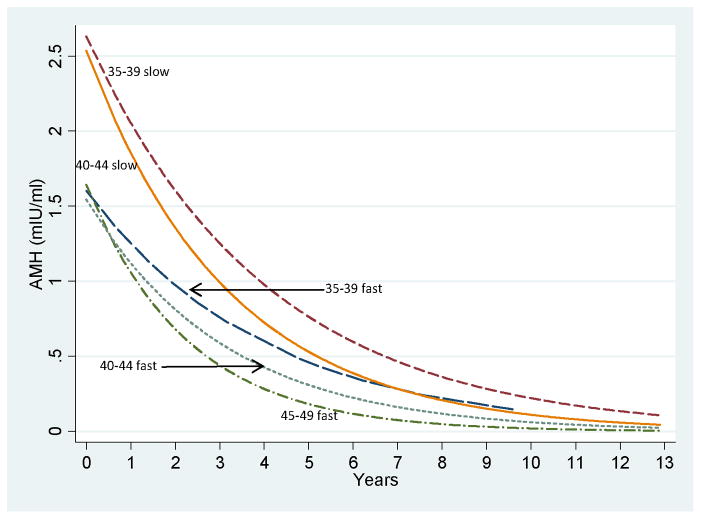
We further evaluated the rate of AMH change (fast or slow) in combination with AMH baseline level (low or high) and age group in estimates of TTM. Figure 1 depicts the curves for AMH rate of decline during the study by age groups in the subset of women who had a baseline AMH >=1.0 ng/mL. (The subgroups with a baseline AMH <1.0 ng/ml are not shown because further AMH decline was minimal and the curves of these subgroups were nearly identical.
Figure 1.
AMH rate of decline over the study interval for women with baseline AMH >=1.0 ng/mL in 5 subgroups. Subgroups are the following (starting at lower left): ages 45–49 years, fast rate of decline; 40–44 years, fast rate of decline; 35–39 years, fast rate of decline; 40–44 years, slow rate of decline; 35–39 years, slow rate of decline. The subgroups with AMH <1.0 ng/mL at baseline had little additional AMH decline and the curves of these subgroups were nearly identical (not shown).
Additional analysis to explore the AMH decline within age groups showed a significant interaction between AMH rate of change and age in TTM (P<0.0001). The rate of change had the strongest effect in the 35–39 year age group, where a faster decrease in AMH was associated with an increased risk of menopause (hazard ratio 6.97, 95% CI 3.81 – 12.72, P<0.0001). This association was less dramatic but remained significant for ages 40–44 years (hazard ratio 1.52, 95% CI: 1.21 – 1.91, P=0.0003 and for 45–48 years (hazard ratio 2.39, 95% CI: 1.84 – 3.11, P=<0.0001) (See supplemental Table 2).
Table 3 shows that the three variables of age, AMH rate of change and AMH baseline provided further refinement in estimates of TTM. For example, with a low AMH level and fast rate of change, the median TTM was 7.99 years (95% CI 6.26 – 8.69) in the 45–48 year age group. However, in the 35–39 year age group with the same low AMH level and fast rate of AMH change, the TTM increased to 10.12 years (95% CI 5.93 - NA).
Table 3.
Median Time to Menopause by AMH Level, AMH Rate of Change and Age
| AMH BASELINE | AMH CHANGE1 | Ages 35–39 | Ages 40–44 | Ages 45–48 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Median time2 | 95% CI | N | Median time2 | 95% CI | N | Median time2 | 95% CI | ||
| Low <1.0 ng/mL | Fast | 18 | 10.12 | 5.93 – NA3 | 37 | 8.83 | 8.01 – 9.81 | 29 | 7.99 | 6.26 – 8.69 |
| Slow | 30 | 12.67 | 10.58 – 12.84 | 37 | 9.47 | 8.61 – 10.26 | 4 | 8.60 | 5.03 – 10.27 | |
| High ≥1.0 ng/mL | Fast | 20 | 12.26 | 8.85 – 13.01 | 39 | 10.29 | 9.12 – 11.13 | 3 | 8.00 | 6.23 – NA3 |
| Slow | 56 | NA4 | ------ | 20 | 12.76 | 9.40 – NA3 | 0 | NA4 | ------ | |
N=293
Rate of change is calculated as the difference between the first undetectable level or the last detectable value of log AMH and log AMH baseline level per year. The rate of AMH change is divided at the sample median; below the median (slow change) is the reference group.
Median time to menopause (yrs) from Kaplan-Meier estimates.
No upper confidence limit due to continuing follow-up.
Estimate uninterpretable due to insufficient number reaching menopause.
Smokers had a shorter time to menopause compared to non-smokers, with a median time of 10.12 years (95% CI: 9.27 – 11.08) versus 11.35 years (95% CI: 10.04 – 12.58) for non-smokers. The association of smoking with TTM was marginally significant when adjusted for age, AMH rate of change, AMH level (P=0.045) (Table 2). Further examination of smoking in the multivariable model indicated that its association with TTM was significant when adjusted for age but decreased with the addition of each AMH term. This decrease in the association of smoking with the addition of each AMH term suggested that AMH may be in the pathway between smoking and TTM.
Although we hypothesized that BMI was associated with TTM, the association was not significant in adjusted or unadjusted analysis (Table 2). Race (African American versus white) was not associated with time to menopause in either unadjusted (P=0.69) or multivariable analysis (P=0.74).
DISCUSSION
Previous studies suggested that a single measure of the AMH level together with a woman’s age contributed to estimations of time to menopause (9–11, 18). However, the models did not account for variable rates of follicular atresia over time. We hypothesized that the rate of change in AMH, a sensitive measure of ovarian reserve, reflects the rate of follicular atresia independent of age and may contribute to more precise estimates of time to menopause in late reproductive-age women.
As hypothesized, the rate of AMH change was strongly associated with TTM and provided greater precision in estimates of time to menopause when included with a baseline measure of AMH than either a single AMH measure or age alone. When age was included with the AMH terms, the precision in estimates of TTM increased further. For instance, low AMH levels (<1.0 ng/mL) were observed for nearly all women in the 45–48-year age group but were also observed for 39% of the younger women in our sample (ages 35–39 years). With the same low AMH levels and a slow rate of AMH change, the older women had a median TTM of approximately 8.6 years while the younger women had a median time of 12.7 or more years. Although the clinical meaning of low AMH levels in younger menstruating women (ages 35–39 years) remains unclear, information about the rate of AMH change in addition to the AMH level improves the estimates of TTM for these women.
Several other covariates including smoking, obesity and race were explored as risk factors for TTM. We found that smoking had a marginally significant association with TTM in multivariable analysis adjusted for age and the AMH terms. The toxic effects of smoking on oocyte and/or granulosa cells may lead to more rapid follicular atresia and ultimately a shorter time to menopause (19–24). We found no significant association between BMI or race and TTM.
A limitation to consider is that AMH rate of change (the slope) was based on two time points (the first detectable value and the first of two consecutive undetectable levels or the last observed value in the follow-up interval). This definition minimized the overall variability in the slope estimates while maximizing the time between the two AMH measures and may not fully describe the “true” trend of the decrease in AMH as women approach menopause. Other limitations include the possibility of misclassification of AMH slopes due to differences in measurement of the time interval or other measurement error that may underestimate the association of AMH slope with TTM. Although the rate of AMH decline for women who reported having one ovary during the study was similar to women with two ovaries, the exact timing of removal of one ovary could not be determined and the small number of women may not fully describe AMH decline in this subgroup. Data were obtained from a population-based cohort of urban women in general good health with baseline ages 35–48 years and may not predict the risk of menopause in younger women or in women with menstrual cycle irregularities, infertility or other health problems.
A strength of the study is the new information about the rate of decline in AMH in a healthy population of mid-life women. The data were from women had regular menstrual cycles in normal range at cohort enrollment, the participants were followed annually for 14 years to provide prospective identification of menopause, and stratification of cohort enrollment on race provided equal numbers of African American and white women for analysis. AMH and all other study measures were obtained at the annual follow-up assessments, and statistical power was adequate for the aims of the study.
This information on the rate of AMH change and its strong, independent association with TTM is potentially important for clinical practice when counseling about fertility or infertility issues or considering adverse health outcomes that are known to be associated with menopause, such as increased bone loss and risk of coronary heart disease. However, at this time, the clinical utility of AMH is limited by several factors. Most importantly, defining a shorter time interval to reliably define the rate of AMH change is needed. We found that while a median time interval of 3.5 years between two AMH measures might reliably predict time to menopause, intervals less than approximately three years were unreliable due to large variability in AMH levels within a woman over time. It also appeared that at least three annual measures of AMH were needed in order to identify when an undetectable level of AMH occurred. Second, improved precision of commercial assays for AMH is needed. In this study, the AMH samples were measured concurrently in the same laboratory, which likely provided greater precision than can be obtained in clinical practice. Third, the lower limit of the AMH assay is reached by most women several years before menopause, and increased sensitivity of the AMH assay is needed for its use as a marker of TTM. Finally, these statistical models predict a median time to menopause and do not provide precise individual estimates that are important for clinical practice.
This study indicates that the rate of change in AMH reflects follicular atresia and is a strong and independent surrogate for ovarian reserve in estimating time to menopause. Further studies to validate these findings and improve assessments of ovarian reserve will contribute to greater precision in estimating TTM for individual women.
Acknowledgments
Supported by the National Institutes of Public Health, Grants RO1 AG12745 (to EWF, Principal Investigator) and RR024134 (to the Translational and Clinical Research Center, School of Medicine).
Footnotes
Disclosure Summary: Grant support (outside the submitted work) to the University of Pennsylvania from Forest Research, Inc. and Bionovo (EWF); honoraria and consultancies (MDS); nothing to declare (DWB, CRG).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77:357–62. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 2.Feyereisen E, Méndez Lozano DH, Taieb J, Hesters L, Frydman R, Fanchin R. Anti-Müllerian hormone: clinical insights into a promising biomarker of ovarian follicular status. Reprod Biomed Online. 2006;12:695–03. doi: 10.1016/s1472-6483(10)61081-4. [DOI] [PubMed] [Google Scholar]
- 3.Scheffer GJ, Broekmans FJ, Looman CW, Blankenstein M, Fauser BC, teJong FH, et al. The number of antral follicles in normal women with proven fertility is the best reflection of reproductive age. Hum Reprod. 2003;18:700–6. doi: 10.1093/humrep/deg135. [DOI] [PubMed] [Google Scholar]
- 4.van Rooij IA, Broekmans FJ, te Velde ER, Fauser BC, Bancsi LF, de Jong FH, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17:3065–71. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 5.Gruijters MJ, Visser JA, Durlinger AL, Themmen AP. Anti-Müllerian hormone and its role in ovarian function. Mol Cell Endocrinol. 2003;211:85–90. doi: 10.1016/j.mce.2003.09.024. [DOI] [PubMed] [Google Scholar]
- 6.Yang YS, Hur MH, Kim SY, Young K. Correlation between sonographic and endocrine markers of ovarian aging as predictors for late menopausal transition. Menopause. 2011;18:138–45. [PubMed] [Google Scholar]
- 7.Barad DH, Weghofer A, Gleicher N. Comparing anti-Müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91:1553–5. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 8.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95:170–5. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause. 2011;18:766–70. doi: 10.1097/gme.0b013e318205e2ac. [DOI] [PubMed] [Google Scholar]
- 10.Broer SL, Eijkemans MJ, Scheffer GJ, van Rooij IA, de Vet A, Themmen AP, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96:2532–9. doi: 10.1210/jc.2010-2776. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-Mullerian Hormone as a Predictor of Time to Menopause in Late Reproductive Age Women. J Clin Endocrinol Metab. 2012;97(5):1673–80. doi: 10.1210/jc.2011-3032. Epub 2012 Feb 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freeman EW, Sammel MD, Gracia CR, Kapoor S, Lin H, Liu L, et al. Follicular phase hormone levels and menstrual bleeding status in the approach to menopause. Fertil Steril. 2005;83:383–92. doi: 10.1016/j.fertnstert.2004.06.066. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 14.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–70. [PubMed] [Google Scholar]
- 15.Cox DR. Regression models and life-tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 16.Kleinman DG, Klein M. Survival analysis: a self-learning text. 2. Vol. 140 New York: Springer-Verlag; 2005. [Google Scholar]
- 17.Hess KR. Graphical methods for assessing violations of the proportional hazards assumption in Cox regression. Stat Med. 1995;14:1707–23. doi: 10.1002/sim.4780141510. [DOI] [PubMed] [Google Scholar]
- 18.Sowers MR, Eyvazzadeh AD, McConnell D, Yosef M, Jannausch ML, Zhang D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93:3478–83. doi: 10.1210/jc.2008-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Westoff C, Murphy P, Heller D. Predictors of ovarian follicle number. Fertil Steril. 2000;74 (4):624–8. doi: 10.1016/s0015-0282(00)01527-2. [DOI] [PubMed] [Google Scholar]
- 20.Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, et al. Maternal exposure to polycyclic aromatic hydorcarbons diminished murine ovarian reserave via induction of hara-kiri. J Clin Investigation. 2007;117 (12):3971–8. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L, Tan L, Yang F, Luo Y, Li X, Deng HW, et al. Meta-analysis suggests that smoking is associated with an increased risk of early natural menopause. Menopause. 2012;19:126–32. doi: 10.1097/gme.0b013e318224f9ac. [DOI] [PubMed] [Google Scholar]
- 22.Plante BJ, Cooper GS, Baird DD, Steiner AZ. The impact of smoking on antimüllerian hormone levels in women aged 38 to 50 years. Menopause. 2010;17:571–6. doi: 10.1097/gme.0b013e3181c7deba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Asselt KM, Kok HS, van Der Schouw YT, Grobbee DE, te Velde ER, Pearson PL, et al. Current smoking at menopause rather than duration determines the onset of natural menopause. Epidemiology. 2004;15:634–9. doi: 10.1097/01.ede.0000134868.53468.b7. [DOI] [PubMed] [Google Scholar]
- 24.Fleming LE, Levis S, LeBlanc WG, Dietz NA, Arheart KL, Wilkinson JD, et al. Earlier age at menopause, work, and tobacco smoke exposure. Menopause. 2008;15:1103–8. doi: 10.1097/gme.0b013e3181706292. [DOI] [PMC free article] [PubMed] [Google Scholar]