Abstract
Previously, it was shown that microneedle-mediated transcutaneous immunization with plasmid DNA can potentially induce a stronger immune response than intramuscular injection of the same plasmid DNA. In the present study, we showed that the immune responses induced by transcutaneous immunization by applying plasmid DNA onto a skin area pretreated with solid microneedles were significantly enhanced by coating the plasmid DNA on the surface of cationic nanoparticles. In addition, the net surface charge of the DNA-coated nanoparticles significantly affected their in vitro skin permeation and their ability to induce immune responses in vivo. Transcutaneous immunization with plasmid DNA-coated net positively charged anoparticles elicited a stronger immune response than with plasmid DNA-coated net negatively charged nanoparticles or by intramuscular immunization with plasmid DNA alone. Transcutaneous immunization with plasmid DNA-coated net positively charged nanoparticles induced comparable immune responses as intramuscular injection of them, but transcutaneous immunization was able to induce specific mucosal immunity and a more balanced T helper type 1 and type 2 response. The ability of the net positively charged DNA-coated nanoparticles to induce a strong immune response through microneedle-mediated transcutaneous immunization may be attributed to their ability to increase the expression of the antigen gene encoded by the plasmid and to more effectively stimulate the maturation of antigen-presenting cells.
Keywords: Antibodies response, cytokines, splenocyte proliferation, skin permeation, antigen gene expression
1. INTRODUCTION
Microneedle-mediated transcutaneous immunization has evolved as a promising immunization modality to induce strong immune responses using a very low dose of antigen/vaccine [1]. Immunization with plasmid DNA is one of the most promising applications of gene therapy [2]. Normally, DNA immunization is carried out by intramuscular injection of naked plasmid DNA [3]. However, Mikszta et al. (2002) reported that microneedle-mediated transcutaneous immunization with naked plasmid DNA can potentially induce a stronger immune response than hypodermic needle-based intramuscular injection of the same plasmid DNA [4]. The present study was designed to test whether microneedle-mediated transcutaneous immunization with plasmid DNA coated on the surface of cationic nanoparticles will induce a stronger immune response than with plasmid DNA alone. Data from numerous previous studies have shown that coating of plasmid DNA onto cationic nanoparticles can significantly enhance the resultant immune responses, including when plasmid DNA-coated nanoparticles are applied topically onto the skin [5–7]. However, it remains unknown whether microneedle-mediated transcutaneous immunization with DNA carried by cationic nanoparticles is more effective than with the naked DNA alone. Microneedle-mediated transcutaneous DNA immunization has mainly been performed using plasmid DNA coated on solid microneedles [8–10], which allowed the physical delivery of the DNA directly into the skin. Another method of microneedle-mediated transcutaneous immunization is to apply vaccines or antigens onto a skin area before or after treatment with solid microneedles. This method of transcutaneous immunization allows the application of a large amount of antigens topically. Mass production of solid microneedles is commercially feasible, and solid microneedle rollers are currently being used in humans already. Moreover, data from several recent studies showed that nanoparticles can permeate through the micropores created by microneedles [11, 12], and transcutaneous immunization with protein antigens carried by nanoparticles onto a skin area pretreated with microneedles is feasible [13–15]. Therefore, in the present study, we carried out transcutaneous immunization by applying plasmid DNA coated on cationic nanoparticles onto a skin area pretreated with microneedles.
The plasmid DNA was carried by cationic nanoparticles prepared with poly (lactic-co-glycolic acid) (PLGA) and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), both are biocompatible. DNA is negatively charged under neutral pH. When mixed with cationic nanoparticles, it binds to the nanoparticles by electrostatic interaction. The net charge of the resultant plasmid DNA-cationic nanoparticle complexes is influenced by the ratio of the plasmid DNA to cationic nanoparticles in the mixture. We prepared net positively charged and net negatively charged plasmid DNA-nanoparticle complexes, which allowed us to evaluate the effect of the surface charge of the DNA-nanoparticle complexes on (i) their in vitro permeation through a skin area pretreated with microneedles and (ii) the immune responses induced by plasmid DNA coated on the nanoparticles in vivo, which has rarely been studied before.
2. MATERIALS AND METHODS
2.1. Materials
Dermaroller® microneedle roller was from Cynergy, LLC (Carson City, NV). There are 192 needles (1000 µm in length, 80 µm in base diameter) on the roller. The β-galactosidase gene-encoding pCMV-β was from American Type Culture Collection (ATCC, Manassas, VA) [16], and the anthrax protective antigen (PA63)-encoding pGPA plasmid was kindly provided by Dr. Dennis Klinman [17]. Large scale plasmid preparation was performed by GenScript (Piscataway, NJ). PLGA (Resomer RG 504H), acetone, pluronic F68, 3,3’,5,5’-tetramethylbenzidine (TMB) solution, sodium bicarbonate, sodium carbonate, Tween 20, phosphate-buffered saline (PBS) and fetal bovine serum (FBS) were from Sigma-Aldrich (St. Louis, MO). Horse serum, penicillin, streptomycin and picogreen were from Invitrogen (Carlsbad, CA). DOTAP and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-carboxyfluorescein (DOPE-fluorescein) were from Avanti Polar Lipids, Inc. (Alabaster, AL). Mouse IL-4 and IFN-γ ELISA sets were from BD Biosciences (San Diego, CA). Goat anti-mouse IgG, IgM, IgA, IgE, IgG1 and IgG2a were from Southern Biotechnology Associates, Inc. (Birmingham, AL).
2.2. Preparation of cationic PLGA nanoparticles
The cationic PLGA nanoparticles were prepared by the nanoprecipitation-solvent evaporation method [18]. PLGA polymer (3–20 mg) and DOTAP (0–40 mg) were accurately weighed and dissolved in acetone (2 ml). The organic phase was added drop wise into 10 ml of 1% pluronic F68 (as a hydrophilic stabilizer) with moderate stirring (800 rpm) for 3 h at room temperature until acetone is completely evaporated to form nanoparticles. Unentrapped DOTAP was removed by ultracentrifugation (23,000 rpm, 4°C, 45 min). The resultant nanoparticles were resuspended in 200 µl water. DOTAP-free nanoparticles were prepared similarly, except that DOTAP was not included in the acetone. To fluorescently label the nanoparticles, DOPE-fluorescein (5%, v/v) was included in the PLGA and DOTAP mixture during the nanoparticle preparation. The size and zeta potential of the nanoparticles were determined using a Malvern Zetasizer® Nano ZS (Westborough, MA). The morphology and size of the cationic nanoparticles were examined as previously described using an FEI Tecnai Transmission Electron Microscope (TEM) [19].
2.3. Determination of the final concentration of DOTAP in cationic nanoparticles
The DOTAP concentration was determined according to a previously described colorimetric method [20]. Briefly, 1% methyl orange (0.25 ml) was reacted with DOTAP in the presence of chloroform (6.5 ml) and a buffer solution (1.25 ml, 0.5 M citric acid and 0.2 M disodium hydrogen orthophosphate) to produce a yellow color complex. The intensity of the yellow color is proportional to the concentration of the methyl orange-DOTAP complexes measured spectrophotometrically at 415 nm.
2.4. Coating of plasmid DNA on the surface of the cationic nanoparticles
Plasmid DNA (pCMV-β or pGPA) was coated on the surface of the cationic nanoparticles by gently mixing equal volumes of cationic nanoparticles in a suspension with plasmid DNA in a solution to obtain a final DNA concentration of 200 µg/ml. To identify the appropriate ratios for the preparation of the net positively and net negatively charged DNA-coated PLGA nanoparticles, increasing amounts of nanoparticles (0.001–0.12 mg) were mixed with a fixed amount of DNA (1 µg), and the mixtures were incubated for at least 30 min at room temperature for the adsorption of the DNA onto the surface of the nanoparticles [5].
2.5. Stability of the plasmid DNA coated on the nanoparticles
To estimate the degree to which the plasmid DNA coated on the nanoparticles was protected from DNase I digestion, net positively and net negatively charged pCMV-β-nanoparticle complexes containing 10 µg of pCMV-β were treated with 1 unit of DNase I (Fermentas, Glenn Burnie, MD) at 37°C in 400 µl reaction buffer (10 mM Tris–HCl, pH 7.5, 2.5 mM MgCl2) for 30 min. The samples obtained were separated on a 1% agarose gel with ethidium bromide for 1 h at 100 V. Images were acquired using a high performance UV trans-illuminators (UVP LLC, Upland, CA). DNA alone, treated or untreated with DNase I, was used as a control.
2.6. In vitro permeation of DNA-coated nanoparticles through mouse skin pretreated with microneedles
In vitro permeation study was performed using jacketed Franz diffusion cells with a 0.64-cm2 diffusion area [11]. The lower dorsal skin of BALB/c mice was used for all permeation studies. Hair was trimmed 24 h before the collection of the skin. Skins were stored at −20°C for a maximum period of one month. The fat layer of skin was carefully removed. The skin was then placed onto the flat surface of a balance, and the microneedle roller was rolled in 4 perpendicular lines over the skin surface, 5 times each for a total of 20 times, with an applying pressure of 350–400 g [13, 21]. The pressure was constantly monitored using the balance. Treated skin area was then clamped in between the donor and the receiver compartments of the Franz diffusion cell (PermeGear, Inc., Hellertown, PA), dorsal side facing the donor compartment. The donor compartment was filled with pCMV-β alone or pCMV-β-coated net positively or net negatively charged nanoparticles in water (250 µl). The amount of pCMV-β plasmid placed into the donor compartment was 50 µg. The receiver compartment was filled with 5 ml of PBS (pH 7.4, 10 mM), which was constantly stirred with a magnetic stirrer, and the temperature was maintained at 37°C with the help of a Haake SC 100 Water Circulator from ThermoScientific (Wellington, NH). This maintained the skin surface temperature at 32°C [22]. At various time points, samples (150 µl) were withdrawn from the receiver compartment and immediately replenished with the same volume of fresh PBS. The amount of plasmid diffused into the receiver compartment was determined using picogreen dye and a BioTek SynergyTM HT Multi-Mode Microplate Reader (Winooski, VT). As a control, the permeation of DNA through intact skin was also evaluated.
2.7. In vitro release of plasmid from the nanoparticles
Net positively charged and net negatively charged DNA-coated nanoparticles with 10 µg of pCMV-β were incubated in 1 ml water at 37°C. At time points 2, 4, 10 and 24 h, the particles were centrifuged to collect the supernatant. One milliliter fresh water was added immediately back to the vials to resuspend the pellets. The amount of DNA in the supernatant was determined using picogreen.
2.8. In vitro transfection of DC2.4 cells
DC2.4 cells were from ATCC and maintained in RPMI1640 medium with 10% FBS, 10 U/ml of penicillin, and 100 µg/ml of streptomycin. On day 1, cells (20,000 per well) were plated in a 24-well plate and incubated overnight at 37°C, 5% CO2. On day 2, cells were incubated with the DNA formulations containing 0.3 µg/well of pCMV-β in a total volume of 400 µl culture medium. Four hours later, the medium was replaced with fresh medium, and the cells were incubated overnight. Cells were washed with PBS and harvested. Cell pellets were resuspended in a lysis buffer (20 mM Tris, 100 mM NaCl, 1 mM EDTA, 0.5% Triton X-100) and then freeze-and-thawed 3 times. Insoluble materials were removed by centrifugation (14,000 rpm, 4°C, 5 min), and supernatant was collected. β-Galactosidase activity was measured in the supernatant using a β-gal assay kit following the manufacturer’s instruction (Invitrogen). The total protein content in the supernatant was determined using Bradford reagent (Sigma-Aldrich) [7].
2.9. In vitro cellular uptake study
DC2.4 cells (2.5 × 104/well) were seeded in a 24-well plate and incubated at 37°C, 5% CO2 overnight. The pCMV-β plasmid, labeled using a Label IT® fluorescein nucleic acid labeling kit (Mirus, Madison, WI), was complexed with cationic nanoparticles to form net positively and net negatively charged nanoparticles. Cells were incubated with the nanoparticles for 4 h at 37°C, 5% CO2, washed 3 times with warm PBS, and re-suspended in 200 µl cell lysis buffer. The amount of pCMV-β added was 2 µg/well. The cell lysates were transferred to a clear bottom black side 96-well plate (Corning, NY), and the fluorescence intensities of the samples were measured at 485/528 nm using BioTek Synergy® Multi-Mode Microplate Reader. Data are reported as % cell uptake, which was calculated using a standard curve generated from known concentrations of fluorescein-labeled DNA [23].
2.10. Fluorescence microscopy
DC2.4 cells (1.5 × 105) were seeded on poly-D-lysine-coated glass cover slips and incubated in 6-well plates at 37°C, 5% CO2 for 24 h. After the cells were adhered to the cover slips, they were treated with fluorescein-labeled pCMV-β alone or fluorescein-labeled pCMV-β-coated nanoparticles and incubated for 4 h at 37°C, 5% CO2. For the net positively charged nanoparticles, cells were incubated for only 1 h. After the incubation, cells were washed three times with PBS and fixed with paraformaldehyde (3% in PBS) for 20 min at room temperature. The cover slips were again washed with warm PBS for 3 times and mounted onto clean glass slides using Vectashield H-1200 with 4´,6-diamidino-2-phenylindole (DAPI) (Vector laboratories, Burlingame, CA). Slides were observed under an Olympus BX60 microscope (Olympus America, Inc., Center Valley, PA).
2.11. Immunization studies
All animal studies were carried out following the National Institutes of Health guidelines for animal care and use. The animal protocol was approved by the Institutional Animal Care and Use Committee at The University of Texas at Austin. Female BALB/c (8–10 weeks), female hairless SKH1-Elite mice (14–16 weeks) or female C57BL/6 mice (8–10 weeks) were from Charles River (Wilmington, MA). Mice (n = 5/group) were immunized 3 times with either plasmid DNA or DNA-coated nanoparticles topically on an area treated with the microneedle roller. Briefly, 24 h prior to the initiation of the immunization, hair, if any, in the dorsal side of mice was carefully trimmed. Mice were anesthetized, the targeted skin area was wiped with an alcohol swab, and a 2-cm2 area was marked on the lower dorsal skin surface. Mice were placed onto the flat surface of a balance, and the microneedle roller was rolled in two perpendicular lines over the marked skin surface, 10 times each, for a total of 20 times [13, 24], with an applying pressure of 350–400 g, which was measured using the balance. Plasmids alone or plasmid-coated nanoparticles in water were carefully dripped onto the microneedle-treated area, which were then covered with a piece of self-adhesive Tegaderm® patch (3 M, St. Paul, MN) [25]. The Tegaderm patch was carefully removed 24 h later. Immunization was repeated 10 or 14 days apart for two more times. As a positive control, a group of mice were intramuscularly (gastrocnemius muscles) injected 3 times with plasmid alone or plasmid-coated net positively charged nanoparticles. Three weeks (or as where mentioned) after the last immunization, mice were bled to collect serum samples. Antibody response in blood serum was determined using enzyme-linked immunosorbent assay (ELISA) as previously described [26, 27].
2.12. In vitro cytokine release and splenocyte proliferation assays
Splenocyte preparation, cytokine release, and splenocyte proliferation assays were performed as previously described [7, 28]. Splenocytes (3 × 106 per well) were stimulated with 0 or 10 µg/ml of β-galactosidase for 48 h at 37°C, 5% CO2 before measuring cytokines (IL-4 and IFN-γ) in the supernatant using ELISA kits. Splenocytes (3 × 106 per well) were stimulated with 0 or 10 µg/ml of β-galactosidase for 120 h at 37°C, 5% CO2 before measuring cell numbers using an MTT assay [29].
2.13. Expression of MHC I/II and CD80/86 molecules on BMDCs
Bone marrow dendritic cells (BMDCs) were generated from bone marrow precursors obtained from femur bones of C57BL/6 mice [30]. Fully grown BMDCs were seeded into 6-well plate (10,000 cells/well) and incubated overnight at 37°C, 5% CO2. Cationic nanoparticles (200 µg per well), net positively charged DNA coated nanoparticles (5 µg pCMV-β/well), net negatively charged DNA coated nanoparticles (5 µg plasmid/well), or DNA alone (5 µg well) were added in the well, and the cells were incubated for 15 h at 37°C, 5% CO2. As controls, cells were also treated with PBS or lipopolysaccharide from E. coli (LPS, 200 ng/well, Sigma-Aldrich). Cells were washed with a staining buffer (1% FBS and 0.1% NaN3 in PBS, BD Pharmingen), stained with anti-CD80, anti-CD86, anti-I-A[b] MHC II, or anti-H-2Kb MHC I Ab for 20 min at 4°C, washed again with staining buffer, and analyzed with a Guava EasyCyte 8HT microcapillary flow cytometer (Millipore Corporation, Hayward, CA).
2.14. In vivo uptake and expression of pCMV-β plasmid, alone or coated on nanoparticles
Mice (n = 3/group) were treated with microneedles as mentioned previously, and pCMV-β alone or coated on nanoparticles (20 µg/mouse), was carefully applied onto the microneedle-treated area. Twenty-four hours later, the treated skin area was washed with water for 5 min and then carefully dissected. One half of the skin was used for the extraction of total RNA, while the other half was used to extract total DNA. Total cellular RNA was isolated from skin tissues using TRIzol reagent (Invitrogen). Isolated RNA was reverse transcribed with random hexamers using the SuperScript first-strand synthesis system (Invitrogen). Real-time PCR of β-galactosidase gene was carried out using the Power SYBR Green PCR Master Mix kit (Applied Biosystems, Foster City, CA) with the following primers: 5′-TTG ATC CGT TGT TCT TGT CA-3′ (forward) and 5′-GGC CAG GAA ATA CAA GAC AA-3′ (reverse). All samples were normalized to β-actin (5′-TTG ATC CGT TGT TCT TGT CA-3′ (forward) and 5′-GGC CAG GAA ATA CAA GAC AA-3′ (reverse)). Data were analyzed using the Applied Biosystems ViiATM 7 Software (Applied Biosystems). Genomic DNA was extracted from skin tissues using DNAzol reagent (Qiagen, Valencia, CA). Real-time PCR was carried out as mentioned above. Each 20-µl reaction contained 500 ng of genomic DNA, 100 nM of each primer, and 10 µl of 2× SYBR Premix (Applied Biosystems).
2.15. Statistical analysis
Statistical analyses were performed using analysis of variance followed by Fisher’s protected least significant difference procedure. A P value of ≤ 0.05 (two-tailed) was considered statistically significant.
3. RESULTS AND DISCUSSION
3.1. Preparation and characterization of cationic PLGA nanoparticles
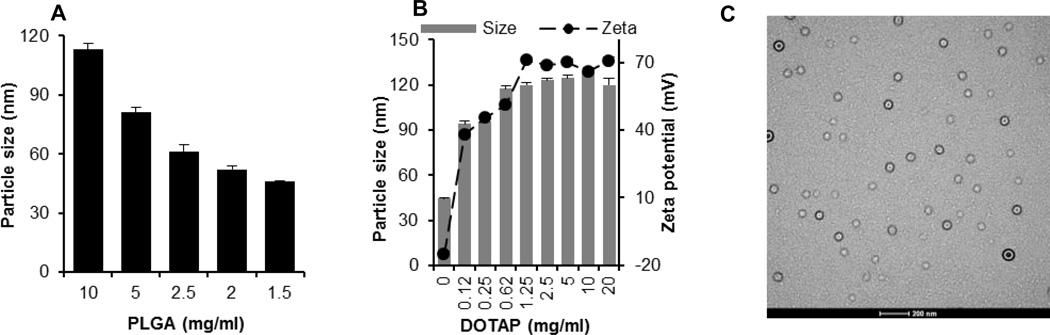
Plasmid DNA-coated nanoparticles were prepared by coating pre-prepared cationic PLGA nanoparticles with plasmid DNA. PLGA nanoparticles were prepared by the nanoprecipitation-solvent evaporation method. As shown in Fig. 1A, the size of PLGA nanoparticles was dependent on the concentration of PLGA used. Decreasing the concentration of PLGA resulted in smaller nanoparticles, in agreement with findings by Chorny et al. [31] and Nafee et al. [32]. With 1.5 mg/ml of PLGA, nanoparticles of 46 ± 1 nm were obtained (Fig. 1A). Nanoparticles prepared with less than 1.5 mg/ml PLGA were not reproducible. Therefore, 1.5 mg/ml of PLGA was used to prepare cationic nanoparticles by including various amount of the cationic DOTAP lipid. As shown in Fig. 1B, the concentration of DOTAP significantly affected the size and zeta potential of the resultant nanoparticles; increasing the concentration of DOTAP increased the size and zeta potential of the resultant nanoparticles. The size and zeta potential of the nanoparticles stopped increasing when about 1 mg/ml of DOTAP was used (i.e., between 0.62 mg/ml and 1.25 mg/ml) (Fig. 1B). In the end, the PLGA nanoparticles prepared with 20 mg/ml of DOTAP were selected to coat plasmid DNA, because in our pilot studies, nanoparticles prepared using less than 20 mg/ml DOTAP were not able to bind a sufficient amount of plasmid DNA onto their surface, which is required for in vivo studies [33]. The final amount of DOTAP remaining in the PLGA nanoparticles was determined to be 1.5 %. Thus, the theoretical PLGA/DOTAP ratio in the nanoparticles was 5:1 (w/w). Fig. 1C is a typical TEM picture of the cationic PLGA nanoparticles, which were spherical and uniform in size, with a diameter of less than 100 nm. The size of the cationic nanoparticles did not significantly change after one month of storage at room temperature or at 4°C (data not shown). The polydispersity index of nanoparticles did not change as well (data not shown).
Fig. 1.
(A) The effect of the concentration of PLGA on the size of PLGA nanoparticles. (B) The effect of the concentration of DOTAP on the size and zeta potential of the cationic nanoparticles. (C) A typical TEM of cationic nanoparticles (bar = 200 nm). Data reported in A + B are mean ± S.E.M. (n = 3).
3.2. Preparation and characterization of plasmid DNA-coated PLGA nanoparticles
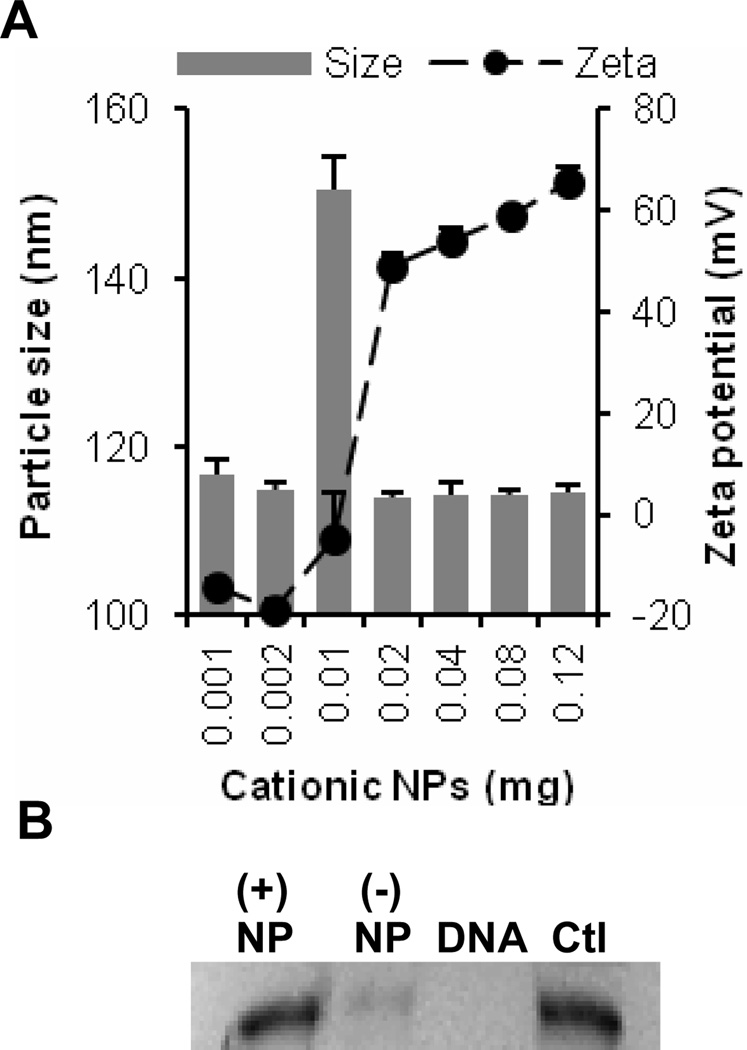
A study was performed to identify the effect of the ratio of the cationic nanoparticles to plasmid DNA on the size and zeta potential of the resultant DNA-nanoparticle complexes. To accomplish this, increasing amounts of cationic nanoparticles (0.001–0.12 mg) were mixed with a fixed amount of pCMV-β (1 µg), and the size and zeta potential of the resultant complexes were determined. The sizes of the resultant DNA-nanoparticle complexes were around 115 nm for all ratios tested, except that for the 10:1 ratio (i.e., 0.01 mg NPs), a relatively larger size was observed (Fig. 2A). In fact, at the 10:1 ratio, the zeta potential of the DNA-nanoparticle complexes was close to zero, suggesting that the larger particle size was likely a result of particle aggregation [5]. The DNA-nanoparticle complexes prepared at 2:1 and 40:1 ratios were used to prepare net negatively charged ((−) NP) and net positively charged ((+) NP) plasmid DNA-coated nanoparticles, respectively, for further studies. At room temperature, both net positively and net negatively charged DNA-coated nanoparticles were stable in an aqueous suspension (data not shown). In order to evaluate whether the DNA coated on the surface of the nanoparticles was protected from enzymatic degradation, the DNA-coated nanoparticles were incubated with DNase I for 30 min. As shown in Fig. 2B, free DNA was completely digested after incubation with DNase I, while the DNA that was coated on the surface of the nanoparticles was protected, to a certain extent, from DNase digestion. The level of protection was higher for the net positively charged pCMV-β-coated nanoparticles (lane 1) than for the net negatively charged nanoparticles (lane 2).
Fig. 2.
(A) The size and zeta potential of pCMV-β-cationic nanoparticle complexes at various nanoparticles to plasmid ratios. Increasing amounts of cationic nanoparticles (0.001–0.12 mg) were mixed with a fixed amount of DNA (1 µg) in equal volumes and allowed to incubate at room temperature for at least 30 min before measuring size and zeta potential. All data reported are mean ± S.E.M. (n = 3). (B) Agarose gel electrophoresis assessing the stability of pCMV-β coated on the cationic nanoparticles. (+) NP, pCMV-β-coated net positively charged nanoparticles; (−) NP, pCMV-β-coated net negatively charged nanoparticles; DNA, pCMV-β alone; Ctl, pCMV-β not treated with DNase I.
3.3. Cationic PLGA nanoparticles facilitated the cellular uptake of plasmid DNA coated on their surface
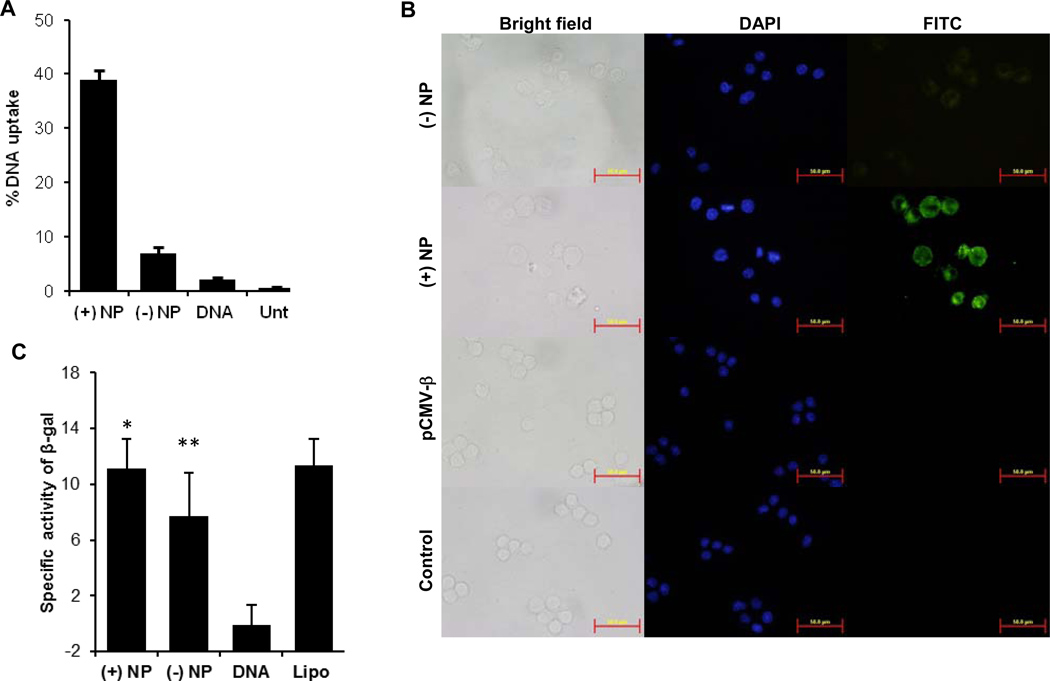
To evaluate the extent to which the cationic PLGA nanoparticles can deliver plasmid DNA coated on their surface into antigen-presenting cells (APC), the uptake of fluorescein-labeled pCMV-β, alone or coated on the cationic PLGA nanoparticles, by dendritic cells (DC2.4) was measured. As expected, the uptake of free pCMV-β by DC2.4 cells was minimal (Fig. 3A). However, coating of pCMV-β on the nanoparticles significantly increased its uptake by DC2.4 cells, especially for the net positively charged pCMV-β-coated nanoparticles (Fig. 3A).
Fig. 3.
In vitro uptake of plasmid DNA coated on nanoparticles by DC2.4 cells. (A) DC2.4 cells were incubated with fluorescein-labeled pCMV-β-coated nanoparticles or fluorescein-labeled pCMV-β alone for 4 h, and the extent of nanoparticle uptake was determined by measuring the fluorescence intensity. (B) Fluorescent microscopic images of DC2.4 cells after up to 4 h of incubation with pCMV-β-coated nanoparticles or pCMV-β alone. For the net positively charged pCMV-β-coated nanoparticles, cells were incubated for only 1 h. Cell nucleus was stained with DAPI (blue). pCMV-β was labeled with FITC (green). (C) In vitro transfection of DC2.4 cells with pCMV-β-coated nanoparticles, pCMV-β alone, or pCMV-β complexed with Lipofectamine. (*, p = 0.94, (+) NP vs. Lipo; **, p = 0.34, (−) NP vs. Lipo). Data shown are mean ± S.E.M. (n = 4 in A, 8 in C). Lipo, pCMV-β complexed with lipofectamine; Unt, Untreated.
Fluorescence microscopic data also confirmed the enhancement of the uptake of the pCMV-β by coating it on the cationic PLGA nanoparticles. As shown in Fig. 3B, the green fluorescence signal was significantly stronger in DC2.4 cells that were incubated with the net positively charged pCMV-β-coated PLGA nanoparticles for only 1 h than in C2.4 cells that were incubated with the net negatively charged pCMV-β-coated PLGA nanoparticles for even 4 h. For a comparison, a green fluorescence signal was not detected in DC2.4 cells incubated with the free fluorescein-labeled pCMV-β alone (Fig. 3B).
Finally, the ability of the cationic PLGA nanoparticles to deliver plasmid DNA coated on their surface into cells was further evaluated by measuring the expression of β-galactosidase gene in DC2.4 cells. As expected, β-galactosidase activity was not detected in cells incubated with pCMV-β alone, but was significantly higher in cells that were incubated with pCMV-β coated on PLGA nanoparticles (Fig. 3C). The β-galactosidase activity in cells transfected with the net negatively charged pCMV-β-coated PLGA nanoparticles was comparable to that in cells transfected with the net positively charged pCMV-β-coated PLGA nanoparticles (Fig. 3C), despite that the uptake of the DNA in the net positively charged pCMV-β-coated PLGA nanoparticles was significantly higher than that in the net negatively charged pCMV-β-coated PLGA nanoparticles (Fig. 3A). This is likely because the incubation time with the nanoparticles in cell uptake experiment was only up to 4 h, whereas the transfection study was performed for a longer time period (24 h). In addition, in the transfection study, we measured the β-galactosidase activity, not the amount of β-galactosidase. It is possible that above a certain level, the amount of β-galactosidase protein in a sample and its activity measured are not directly proportional.
Taken together, it is clear that the cationic PLGA nanoparticles increased the cellular uptake of plasmid DNA coated on their surface in vitro, and the net positively charged DNA-coated PLGA nanoparticles were more effective than the net negatively charged DNA-coated nanoparticles in increasing the cellular uptake of the plasmid DNA, likely because of the strong electrostatic interactions between the net positively charged nanoparticles and the negatively charged cell surface [34].
3.4. Pretreatment of mouse skin with microneedles allowed the permeation of plasmid DNA coated on the surface of cationic PLGA nanoparticles through the skin in vitro
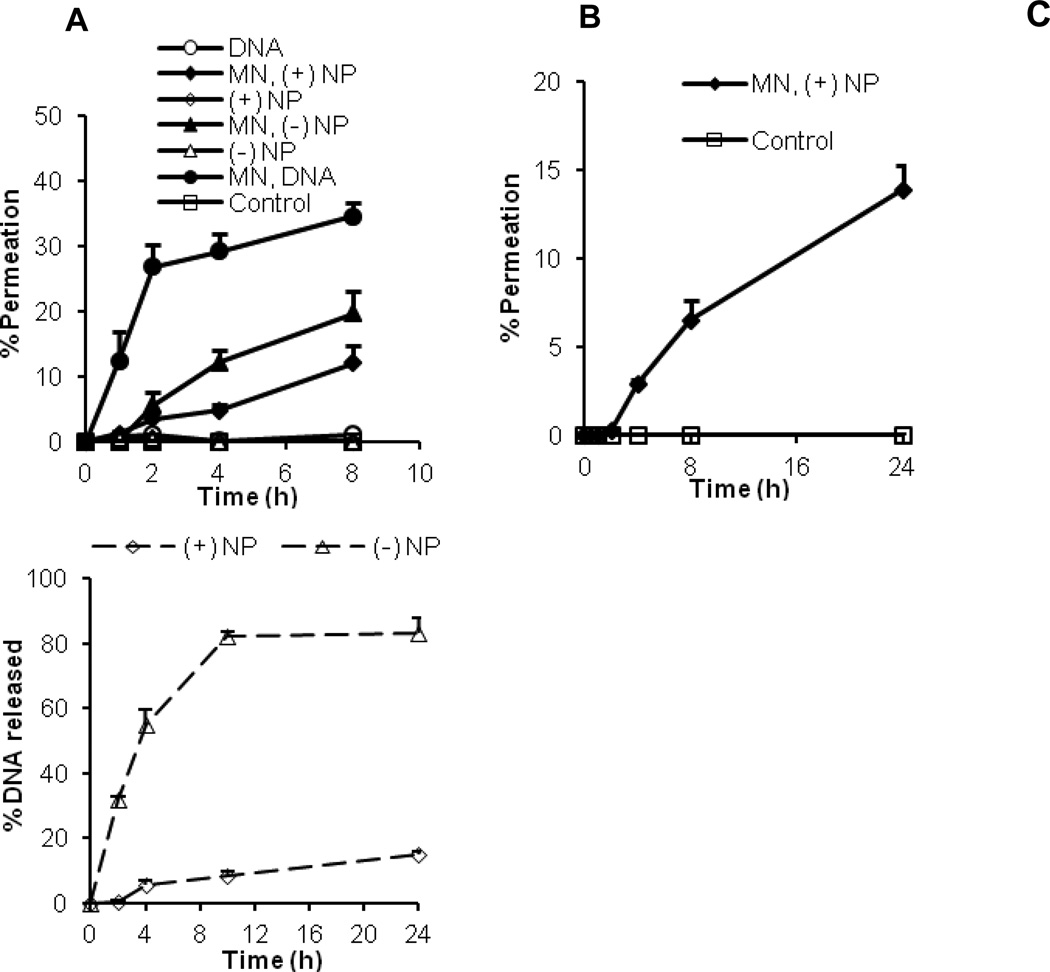
To evaluate the extent to which plasmid DNA coated on the surface of the cationic PLGA nanoparticles can permeate into mouse skin pretreated with microneedles, the diffusion of pCMV-β, alone or coated on the cationic PLGA nanoparticles, through a mouse skin area pretreated with microneedles was measured. As shown in Fig. 4A, pCMV-β, alone or coated on the nanoparticles, was not able to permeate through intact skin (i.e., skin that was not pretreated with microneedles), but it can permeate through the skin that was pretreated with microneedles. The permeation of the naked pCMV-β plasmid was more extensive than the pCMV-β coated on the cationic PLGA nanoparticles (Fig. 4A). Moreover, the permeation of pCMV-β on the net negatively charged pCMV-β-coated PLGA nanoparticles through the skin pretreated with microneedles was more extensive than the permeation of pCMV-β on the net positively charged pCMV-β-coated PLGA nanoparticles (Fig. 4A). For example, 19.6 ± 3.5% of pCMV-β that was on the net negatively charged pCMV-β-coated PLGA nanoparticles permeated through the microneedle-treated mouse skin within 8 h, as compared to only 12.1 ± 2.8% for the pCMV-β on the net positively charged pCMV-β-coated PLGA nanoparticles during the same period (Fig. 4A).
Fig. 4.
Permeation of plasmid DNA alone or coated on nanoparticles (A), and permeation of net positively charged DNA-coated nanoparticles (B) through a mouse skin area treated or untreated with microneedles. (C) Release of DNA from net positively charged and net negatively charged DNA coated nanoparticles after incubation in vitro at 37°C. Plasmid used was pCMV-β. MN = microneedle. All data reported are mean ± S.E.M. (n = 3).
To confirm that it was the DNA-coated nanoparticles that permeated through micropores, not the DNA alone released from the DNA coated nanoparticles, the permeation of the fluorescein-labeled net positively charged nanoparticles (i.e., nanoparticle were labeled with fluorescein) was also monitored. As shown in Fig. 4B, almost 6.5 ± 1.1% of net positively charged nanoparticles permeated through the microneedle-treated mouse skin within 8 h, which indicates that it was the DNA-coated nanoparticles that permeated through the skin, not just the DNA alone released from the nanoparticles. The permeation of net positively charged nanoparticles determined by this method was found less, as compared to the permeation of net positively charged nanoparticles determined by measuring plasmid DNA using picogreen (Fig. 4A), likely because some of the DNA may have been released from the nanoparticles in the donor compartment and permeated to the receiver compartment themselves through the micropores.
Fig. 4C shows the cumulative release of pCMV-β from the pCMV-β-coated net positively and net negatively charged nanoparticles. It is clear that the release of the pCMV-β from net negatively charged nanoparticles was faster than from the net positively charged nanoparticles (Fig. 4C). Therefore, it is likely that the relatively more extensive permeation of pCMV-β in the net negatively charged nanoparticles through the skin that was pretreated with microneedles was partially due to the direct diffusion of pCMV-β that was released from the PLGA nanoparticles in the donor compartment of the Franz diffusion apparatus.
3.5. Microneedle-mediated transcutaneous immunization using the plasmid DNA-coated cationic PLGA nanoparticles induced strong immune responses
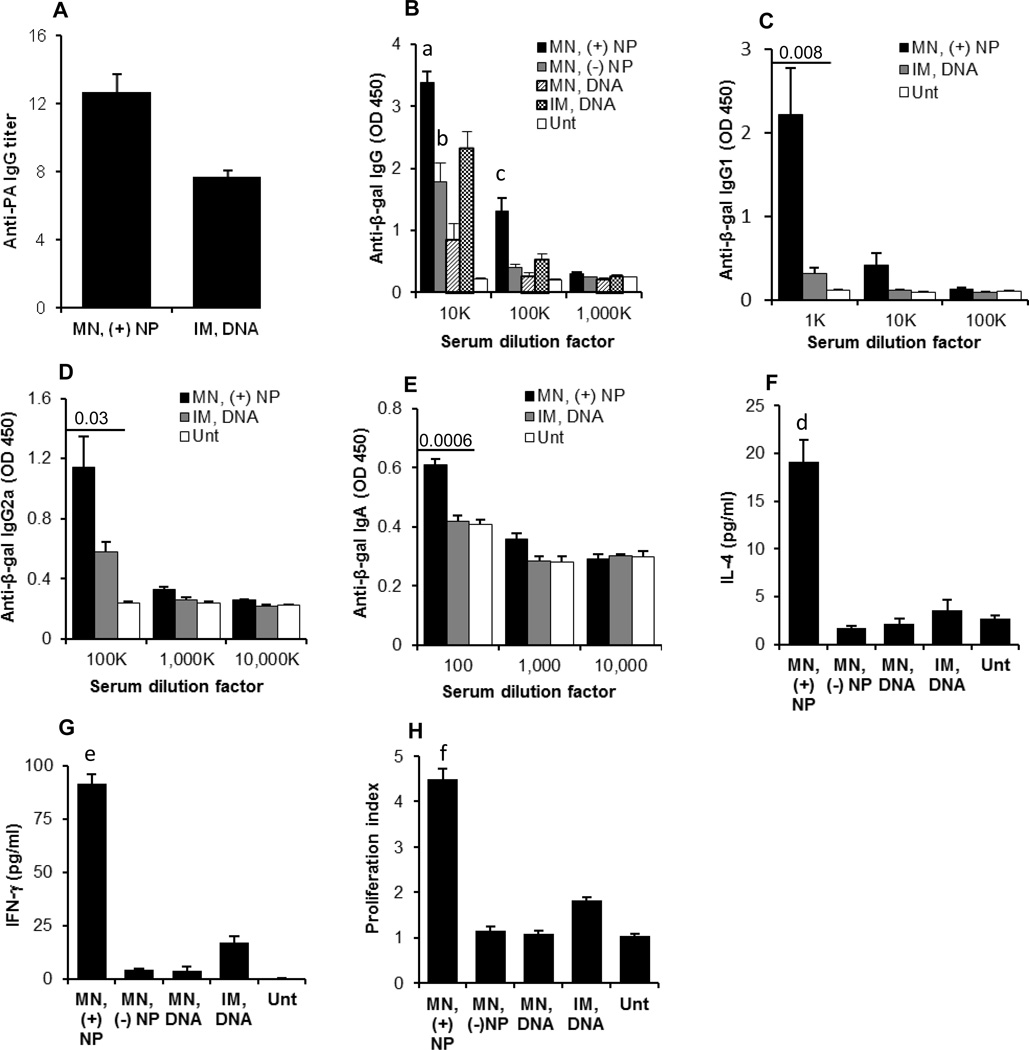
In a pilot study, female BALB/c mice were transcutaneously immunized with an anthrax protective antigen (PA63) gene-encoding plasmid, pGPA (10 µg/mouse/dose), three times, 10 days apart. Six weeks after the last immunization, anti-PA IgG response was detected only in mice that were pretreated with microneedles before the transcutaneous immunization (data not shown), and the pGPA-coated net positively charged PLGA nanoparticles induced the strongest anti-PA IgG response (see Fig. 5A for anti-PA IgG titer). Therefore, further transcutaneous immunization studies were carried out only on a mouse skin area that was pretreated with microneedles. In the second immunization study, the β-galactosidase-encoding pCMV-β plasmid, alone or coated on the cationic PLGA nanoparticles, was used to immunize the immunocompetent hairless SKH-1 Elite mice [35]. The SKH-1 mice were used because the hair (and hair follicle) density in them is significantly lower than that in the hairy BALB/c or C57BL/6 mice and close to that of humans [36]. Mice were dosed every two weeks for three times with 20 µg of pCMV-β per mouse per dose. Shown in Fig. 5B are the anti-β-gal IgG levels in mouse serum samples 21 days after the last immunization. Transcutaneous immunization with pCMV-β alone induced an anti-β-gal IgG response, but it was significantly weaker than when the pCMV-β was coated on the PLGA nanoparticles (Fig. 5B), supporting our hypothesis that microneedle-mediated transcutaneous immunization with plasmid DNA coated on cationic nanoparticles can induce a stronger immune response than with plasmid DNA alone. Moreover, the net positively charged pCMV-β-coated PLGA nanoparticles induced a stronger anti-β-gal IgG response than the net negatively charged pCMV-β-coated PLGA nanoparticles (Fig. 5B). Importantly, the anti-β-gal IgG level in mice that were transcutaneously immunized with the net positively charged pCMV-β-coated PLGA nanoparticles was higher than in mice that were intramuscularly injected with the same amount of pCMV-β alone (p = 0.009). Therefore, we further characterized only the immune responses induced by transcutaneous immunization with the net positively charged pCMV-β-coated PLGA nanoparticles.
Fig. 5.
(A) Anti-PA IgG titers in the sera of mice transcutaneously immunized with net positively charged pGPA-coated nanoparticles or intramuscularly injected with pGPA alone. BALB/c mice were dosed on days 0, 10, and 20 with 10 µg pGPA plasmid. Data reported are 42 days after last immunization. (B) Serum anti-β-gal IgG induced by pCMV-β, alone or coated on nanoparticles, applied onto a skin area pre-treated with microneedles (a, p = 0.009, MN, (+) NP vs. IM, DNA, 10K dilution; b, p = 0.196, MN, (−) NP vs. IM, DNA, 10K dilution; c, p = 0.008, MN, (+) NP vs. IM, DNA, 100K dilution). (C.E) Anti-β-gal IgG1 (C), anti-β-gal IgG2a (D), and anti-β-gal IgA (E) induced by pCMV-β-coated net positively charged nanoparticles applied onto a skin area pre-treated with microneedles. (F.G) In vitro release of IL-4 (F) and IFN-γ (G) from splenocytes after in vitro restimulation with β-galactosidase for 48 h. (d, p = 0.004, MN, (+) NP vs. IM, DNA; e, p = 0.0001, MN, (+) NP vs. IM, DNA). (H) In vitro proliferation of splenocytes after restimulation with β-galactosidase for 120 h (f, p = 0.0007, MN, (+) NP vs. IM, DNA). In B-H, SKH-1 Elite mice were dosed on days 0, 14, and 28 with 20 µg pCMV-β plasmid per mouse. Data in B are 21 days after the last immunization. Data in C.H are 42 days after the last immunization. All data are reported as mean ± S.E.M (n = 5). (MN, microneedle; IM, intramuscular; Unt, untreated).
Shown in Figs. 5C–E are the anti-β-gal IgG1, IgG2a, and IgA responses in the serum samples of SKH-1 Elite mice 42 days after the last immunization with the net positively charged pCMV-β-coated PLGA nanoparticles. Both strong IgG1 and IgG2a were induced. As expected, the antibody response induced by intramuscularly injected pCMV-β alone was slightly IgG2a biased [37]. Anti-IgA was detected in the transcutaneously immunized mice, but not in mice intramuscularly injected with the pCMV-β (Fig. 5E), indicating that transcutaneous immunization with the net positively charged pCMV-β-coated PLGA nanoparticles also induced a specific mucosal response. The skin is an integrated part of the mucosal immune system. It was previously reported that transcutaneous immunization can induce mucosal immunity [38, 39]. Anti-β-gal IgE was not detected in any of the immunized mice (data not shown), indicating the lack of allergic responses.
Data in Figs. 5F–G showed that the splenocytes isolated from mice that were transcutaneously immunized with the net positively charged pCMV-β-coated PLGA nanoparticles, after re-stimulation with β-galactosidase, secreted high levels of both IL-4 and IFN-γ which in combination with the IgG1/IgG2a subtypes in Figs. 5C–D, show that the immune responses induced by transcutaneous immunization with the net positively charged pCMV-β-coated PLGA nanoparticles were CD4+ T helper (Th) type Th1 and Th2 balanced. Furthermore, data in Fig. 5H showed that the splenocytes isolated from mice that were transcutaneously immunized with the net positively charged pCMV-β-coated PLGA nanoparticles also proliferated significantly after in vitro re-stimulation with β-galactosidase.
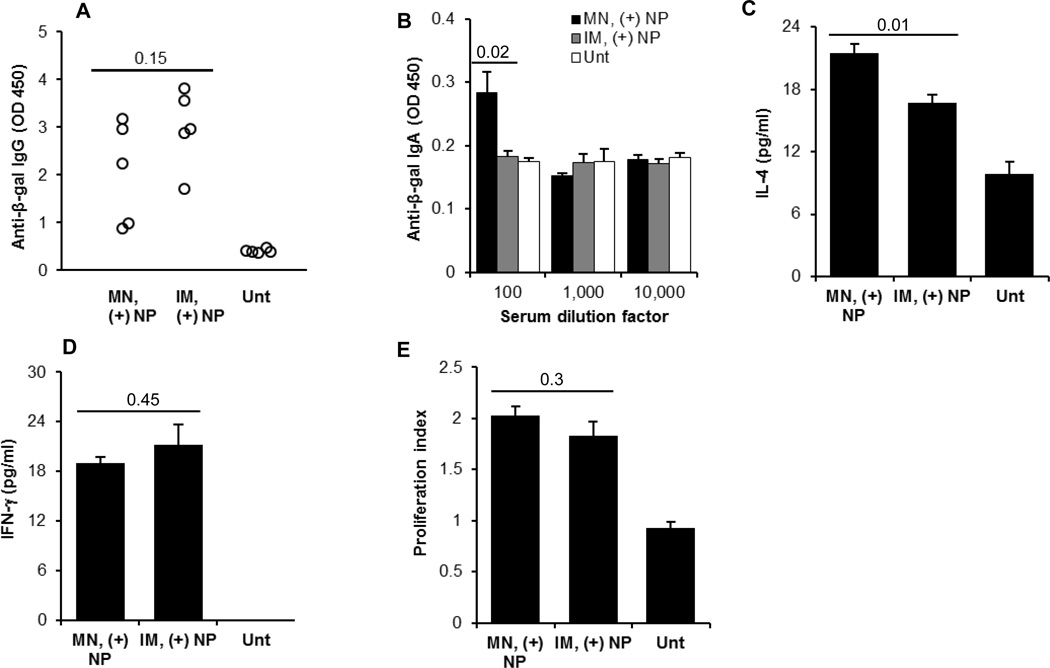
Data in Fig. 5 showed that transcutaneous immunization with the net positively charged pCMV-β-coated PLGA nanoparticles induced a stronger immune response than intramuscular immunization with the same dose of the pCMV-β plasmid alone. In order to understand whether the pCMV-β-coated on the PLGA nanoparticles are as immunogenic when dosed by transcutaneous immunization as when dosed by intramuscular injection, a third immunization study was carried out by dosing C57BL/6 mice with the net positively charged pCMV-β-coated PLGA nanoparticles by the transcutaneous route (after pretreatment with microneedles) or by intramuscular injection (every two weeks for 3 times, 20 µg of pCMV-β per mouse per dose). When measured 21 days after the last immunization, the serum total anti-β-galactosidase IgG levels induced by both routes were not significantly different (Fig. 6A), although there were a couple of weak responders in the transcutaneously immunized mice (Fig. 6A). This is significant considering that only a small fraction of the pCMV-β coated on the PLGA nanoparticles had likely entered the skin after transcutaneous immunization, whereas all the pCMV-β coated on the PLGA nanoparticles were injected into mice by the intramuscular route. We expect that transcutaneous immunization using a lower dose of pCMV-β coated cationic nanoparticles (e.g., 1 or 5 µg/mouse/dose) will induce a stronger immune response than intramuscular injection of the same DNA coated cationic nanoparticles due to dose sparing effect [1]. In addition, data shown below are supportive of the immunological advantages of transcutaneous immunization using the DNA-coated nanoparticles over intramuscular injection.
Fig. 6.
(A–B) Serum anti-β-gal IgG (from individual mice after 10,000-fold dilution) (A) and anti-β-gal IgA (B) induced by pCMV-β-coated net positively charged nanoparticles applied onto a skin area pre-treated with microneedles or injected intramuscularly. (C–D) In vitro release of IL-4 and IFN-γ from splenocytes restimulated with β-galactosidase for 48 h. (E) In vitro proliferation of splenocytes restimulated with β-galactosidase for 120 h. C57BL/6 mice were dosed on day 0, 14, and 28 with 20 µg pCM-β plasmid per mouse. Data in A are 21 days after the last immunization, while data in B–E are 49 days after the last immunization. All data reported are mean ± S.E.M. from 5 mice per group. (MN, microneedle; IM, intramuscular; Unt, untreated).
Shown in Fig. 6B is the anti-β-gal IgA response in the serum samples 49 days after the last immunization with the net positively charged pCMV-β-coated PLGA nanoparticles. Anti-β-gal IgA was detected only in the transcutaneously immunized mice, but not in the intramuscularly injected mice (Fig. 6B), again indicating the ability of transcutaneous immunization to induce specific mucosal responses. Figs. 6C–D showed that high levels of IL-4 and IFN-γ were secreted by the splenocytes isolated from the mice immunized with net positively charged pCMV-β-coated PLGA nanoparticles transcutaneously or intramuscularly. However, IL-4 secretion in transcutaneously immunized mice was significantly higher than in the intramuscularly immunized mice (Fig. 6C), which is also advantageous considering that there is a need to increase the humoral immune responses induced by intramuscularly injected plasmid DNA, especially in large animals and humans [40]. Finally, the splenocytes isolated from transcutaneously immunized mice and intramuscularly immunized mice both proliferated at comparable levels, after in vitro re-stimulation with β-galactosidase (Fig. 6E). Taken together, transcutaneous and intramuscular immunizations with net positively charged pCMV-β-coated nanoparticles induced comparable levels of total IgG and proliferative responses, but transcutaneous immunization induced mucosal response as well, which is significant, considering mucosal immunity is needed to prevent infection through the mucosa.
It was noted that the values of IgG, IgA, IFN-γ and proliferation index are all relatively lower in Fig. 6 than in Fig. 5; this is likely because in Fig. 6, the mice used were C57BL/6, whereas in Fig. 5, the immuno-competent hairless SKH-1 mice were used. As expected, the anti-β-gal IgA levels in mouse serum samples were low (Fig. 5E and 6B). This is likely because IgA is mainly in the mucous secretion, and only small amount is in the blood. In fact, in an ongoing experiment, we immunized C57BL/6 mice with net positively charged plasmid DNA-coated nanoparticles transcutaneously on a skin area pretreated with microneedles (every two weeks for 3 times, 4 µg of an OVA-encoding plasmid, pCI-neo-sOVA, per mouse), anti-OVA IgA with a titer of 80–160 was detectable in the mouse fecal sample extracts, but not in the serum samples (data not shown).
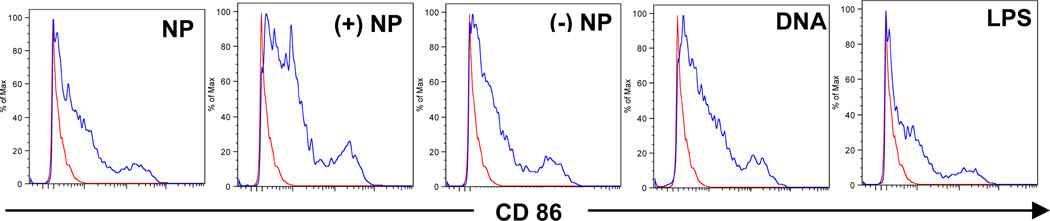
3.6. Net positively charged DNA-coated nanoparticles more effectively up-regulated the expression of CD86 on BMDCs
To evaluate the effect of nanoparticles on the expression of major histocompatibility complex (MHC) and co-stimulatory molecules on dendritic cells, the expression of MHC I, MHC II, CD80 and CD86 on mouse BMDCs was measured after in vitro stimulation with cationic nanoparticles, net positively charged DNA-coated nanoparticles and net negatively charged DNA-coated nanoparticles. Plasmid DNA alone and LPS were used as controls. As shown in Fig. 7, all formulations effectively up-regulated the expression of CD86 molecules. However, it appears that the net positively charged pCMV-β-coated nanoparticles were more effective than the others, suggesting that net positively charged DNA-coated nanoparticles can more efficiently stimulate the maturation of DCs. There was no difference in the expression of CD80, MHC I and MHC II among all the treatments (data not shown).
Fig. 7.
Flow cytometric graphs showing the expression of CD86 on mouse BMDCs after 15 h incubation with cationic PLGA nanoparticles (NP), pCMV-β-coated net positively charged nanoparticles ((+) NP), pCMV-β-coated net negatively charged nanoparticles ((−) NP), pCMV-β alone (DNA), or LPS. Experiment was repeated three times with similar results. Peaks in the left are cells incubated with fresh medium.
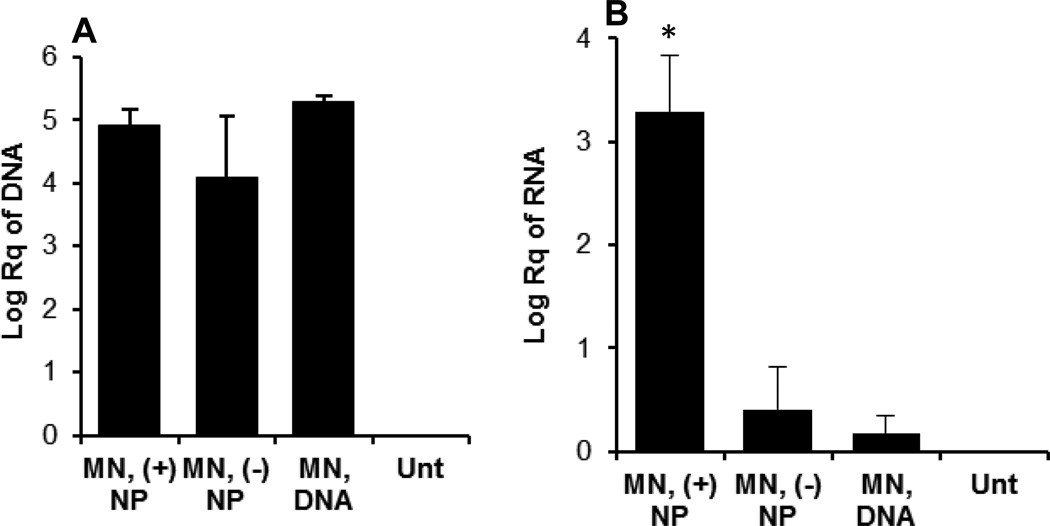
3.7. Net positively charged DNA-coated nanoparticles significantly enhanced the expression of the antigen encoded by the plasmid applied topically onto a skin area pretreated with microneedles
As shown in Fig. 8A, a significant amount of pCMV-β plasmid was recovered in the skin area pretreated with microneedles after the administration of net positively charged pCMV-β-coated nanoparticles, net negatively charged pCMV-β-coated nanoparticles or pCMV-β alone, but there was no difference among them. However, the net positively charged pCMV-β-coated nanoparticles led to a significantly higher level of β-galactosidase mRNA expression in the skin samples, as compared to the net negatively charged pCMV-β-coated nanoparticle or pCMV-β alone (Fig. 8B).
Fig. 8.
In vivo uptake of pCMV-β plasmid (A) and expression of β-galactosidase gene (mRNA level) (B) 24 h after pCMV-β, alone or coated on nanoparticles, was applied on a mouse skin area pretreated with microneedles. A 100 µl (20 µg pCMV-β) of each formulation was applied on C57BL/6 mice (8–10 weeks) at 0 h. pCMV-β recovered from the skin samples and the β-galactosidase mRNA level in the skin samples were determined 24 h later using qRT-PCR. MN, microneedles. Data reported are mean ± S.E.M. (n = 3). (*, p = 0.005, MN, (+) NP vs. MN, (−) NP; MN, (+) NP vs. MN, DNA).
Taken the data in Fig. 7 and Fig. 8 together, it seems that the ability of the net positively charged DNA-coated nanoparticles to induce a strong immune response after transcutaneous immunization is related to their ability to increase the expression of the antigen gene encoded by the plasmid coated on them and to more effectively stimulate dendritic cell maturation.
4. CONCLUSION
A biodegradable PLGA-DOTAP nanoparticle-based plasmid DNA delivery system for microneedle-mediated transcutaneous immunization was successfully developed. To our best knowledge, this represents the first report showing that microneedle-mediated transcutaneous immunization with plasmid DNA carried by the nanoparticles induced a stronger immune response than with the plasmid DNA alone. Moreover, we also found that the net surface charge of the DNA-coated nanoparticles affected their in vitro skin permeation and ability to induce immune responses in vivo. Transcutaneous immunization with plasmid DNA-coated net positively charged nanoparticles induced a stronger immune response than with plasmid DNA-coated net negatively charged nanoparticles. Furthermore, transcutaneous immunization was able to induce specific mucosal immunity as well. The ability of the net positively charged plasmid DNA-coated nanoparticles to induce a strong immune response after transcutaneous immunization is likely related to their ability to increase the expression of the antigen gene encoded by the plasmid and to stimulate dendritic cell maturation.
ACKNOWLEDGEMENTS
This work was supported in part by a National Institute of Allergy and Infectious Diseases grant (AI078304) to ZC. The authors acknowledge the assistance of Dr. Gang Xiao in splenocyte preparation and B. Leticia Rodriguez in the DNase I protection assay. The authors also acknowledge Cynergy, LLC (Carson City, NV) for providing Dermaroller®microneedle rollers.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Matriano JA, Cormier M, Johnson J, Young WA, Buttery M, Nyam K, Daddona PE. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm Res. 2002;19:63–70. doi: 10.1023/a:1013607400040. [DOI] [PubMed] [Google Scholar]
- 2.Kutzler MA, Weiner DB. DNA vaccines: ready for prime time? Nat Rev Genet. 2008;9:776–788. doi: 10.1038/nrg2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ulmer JB, Donnelly JJ, Parker SE, Rhodes GH, Felgner PL, Dwarki VJ, Gromkowski SH, Deck RR, DeWitt CM, Friedman A, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 4.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 5.Cui Z, Mumper RJ. Chitosan-based nanoparticles for topical genetic immunization. J Control Release. 2001;75:409–419. doi: 10.1016/s0168-3659(01)00407-2. [DOI] [PubMed] [Google Scholar]
- 6.Cui Z, Baizer L, Mumper RJ. Intradermal immunization with novel plasmid DNAcoated nanoparticles via a needle-free injection device. J Biotechnol. 2003;102:105–115. doi: 10.1016/s0168-1656(03)00029-4. [DOI] [PubMed] [Google Scholar]
- 7.Cui Z, Mumper RJ. Topical immunization using nanoengineered genetic vaccines. J Control Release. 2002;81:173–184. doi: 10.1016/s0168-3659(02)00051-2. [DOI] [PubMed] [Google Scholar]
- 8.Gill HS, Soderholm J, Prausnitz MR, Sallberg M. Cutaneous vaccination using microneedles coated with hepatitis C DNA vaccine. Gene Ther. 2010;17:811–814. doi: 10.1038/gt.2010.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hooper JW, Golden JW, Ferro AM, King AD. Smallpox DNA vaccine delivered by novel skin electroporation device protects mice against intranasal poxvirus challenge. Vaccine. 2007;25:1814–1823. doi: 10.1016/j.vaccine.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen X, Kask AS, Crichton ML, McNeilly C, Yukiko S, Dong L, Marshak JO, Jarrahian C, Fernando GJ, Chen D, Koelle DM, Kendall MA. Improved DNA vaccination by skin-targeted delivery using dry-coated densely-packed microprojection arrays. J Control Release. 2010;148:327–333. doi: 10.1016/j.jconrel.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 11.McAllister DV, Wang PM, Davis SP, Park JH, Canatella PJ, Allen MG, Prausnitz MR. Microfabricated needles for transdermal delivery of macromolecules and nanoparticles: fabrication methods and transport studies. Proc Natl Acad Sci U S A. 2003;100:13755–13760. doi: 10.1073/pnas.2331316100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, Birchall JC. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366:190–200. doi: 10.1016/j.ijpharm.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Li X, Sandoval MA, Rodriguez BL, Sloat BR, Cui Z. Permeation of antigen protein-conjugated nanoparticles and live bacteria through microneedle-treated mouse skin. Int J Nanomedicine. 2011;6:1253–1264. doi: 10.2147/IJN.S20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bal SM, Ding Z, Kersten GF, Jiskoot W, Bouwstra JA. Microneedle-based transcutaneous immunisation in mice with N-trimethyl chitosan adjuvanted diphtheria toxoid formulations. Pharm Res. 2010;27:1837–1847. doi: 10.1007/s11095-010-0182-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bal SM, Slutter B, Jiskoot W, Bouwstra JA. Small is beautiful: N-trimethyl chitosan-ovalbumin conjugates for microneedle-based transcutaneous immunisation. Vaccine. 2011;29:4025–4032. doi: 10.1016/j.vaccine.2011.03.039. [DOI] [PubMed] [Google Scholar]
- 16.MacGregor GR, Caskey CT. Construction of plasmids that express E. coli beta-galactosidase in mammalian cells. Nucleic Acids Res. 1989;17:2365. doi: 10.1093/nar/17.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu ML, Leppla SH, Klinman DM. Protection against anthrax toxin by vaccination with a DNA plasmid encoding anthrax protective antigen. Vaccine. 1999;17:340–344. doi: 10.1016/s0264-410x(98)00210-2. [DOI] [PubMed] [Google Scholar]
- 18.Barichello JM, Morishita M, Takayama K, Nagai T. Encapsulation of hydrophilic and lipophilic drugs in PLGA nanoparticles by the nanoprecipitation method. Drug Dev Ind Pharm. 1999;25:471–476. doi: 10.1081/ddc-100102197. [DOI] [PubMed] [Google Scholar]
- 19.Sloat BR, Sandoval MA, Li D, Chung WG, Lansakara PD, Proteau PJ, Kiguchi K, DiGiovanni J, Cui Z. In vitro and in vivo anti-tumor activities of a gemcitabine derivative carried by nanoparticles. Int J Pharm. 2011;409:278–288. doi: 10.1016/j.ijpharm.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang LW, Langley DF. Identification and determination of ionic surface active agents. Arch Environ Contam Toxicol. 1977;5:447–456. doi: 10.1007/BF02220924. [DOI] [PubMed] [Google Scholar]
- 21.Li G, Badkar A, Kalluri H, Banga AK. Microchannels created by sugar and metal microneedles: characterization by microscopy, macromolecular flux and other techniques. J Pharm Sci. 2010;99:1931–1941. doi: 10.1002/jps.21981. [DOI] [PubMed] [Google Scholar]
- 22.Kolli CS, Banga AK. Characterization of solid maltose microneedles and their use for transdermal delivery. Pharm Res. 2008;25:104–113. doi: 10.1007/s11095-007-9350-0. [DOI] [PubMed] [Google Scholar]
- 23.Sandoval MA, Sloat BR, Lansakara PD, Kumar A, Rodriguez BL, Kiguchi K, Digiovanni J, Cui Z. EGFR-targeted stearoyl gemcitabine nanoparticles show enhanced anti-tumor activity. J Control Release. 2012;157:287–296. doi: 10.1016/j.jconrel.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou CP, Liu YL, Wang HL, Zhang PX, Zhang JL. Transdermal delivery of insulin using microneedle rollers in vivo. Int J Pharm. 2010;392:127–133. doi: 10.1016/j.ijpharm.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 25.Sloat BR, Kiguchi K, Xiao G, DiGiovanni J, Maury W, Cui Z. Transcutaneous DNA immunization following waxing-based hair depilation. J Control Release. 2012;157:94–102. doi: 10.1016/j.jconrel.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sloat BR, Sandoval MA, Hau AM, He Y, Cui Z. Strong antibody responses induced by protein antigens conjugated onto the surface of lecithin-based nanoparticles. J Control Release. 2010;141:93–100. doi: 10.1016/j.jconrel.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sloat BR, Sandoval MA, Cui Z. Towards preserving the immunogenicity of protein antigens carried by nanoparticles while avoiding the cold chain. Int J Pharm. 2010;393:197–202. doi: 10.1016/j.ijpharm.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui Z, Mumper RJ. Genetic immunization using nanoparticles engineered from microemulsion precursors. Pharm Res. 2002;19:939–946. doi: 10.1023/a:1016402019380. [DOI] [PubMed] [Google Scholar]
- 29.Sloat BR, Cui Z. Nasal immunization with anthrax protective antigen protein adjuvanted with polyriboinosinic-polyribocytidylic acid induced strong mucosal and systemic immunities. Pharm Res. 2006;23:1217–1226. doi: 10.1007/s11095-006-0206-9. [DOI] [PubMed] [Google Scholar]
- 30.Lutz MB, Kukutsch N, Ogilvie AL, Rossner S, Koch F, Romani N, Schuler G. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. J Immunol Methods. 1999;223:77–92. doi: 10.1016/s0022-1759(98)00204-x. [DOI] [PubMed] [Google Scholar]
- 31.Chorny M, Fishbein I, Danenberg HD, Golomb G. Lipophilic drug loaded nanospheres prepared by nanoprecipitation: effect of formulation variables on size, drug recovery and release kinetics. J Control Release. 2002;83:389–400. doi: 10.1016/s0168-3659(02)00211-0. [DOI] [PubMed] [Google Scholar]
- 32.Nafee N, Taetz S, Schneider M, Schaefer UF, Lehr CM. Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of the formulation parameters on complexation and transfection of antisense oligonucleotides. Nanomedicine. 2007;3:173–183. doi: 10.1016/j.nano.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Pang S-W, Park H-Y, Jang Y-S, Kim W-S, Kim J-H. Effects of charge density and particle size of poly(styrene/(dimethylamino)ethyl methacrylate) nanoparticle for gene delivery in 293 cells. Colloids and Surfaces B: Biointerfaces. 2002;26:213–222. [Google Scholar]
- 34.Mansouri S, Cuie Y, Winnik F, Shi Q, Lavigne P, Benderdour M, Beaumont E, Fernandes JC. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27:2060–2065. doi: 10.1016/j.biomaterials.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 35.Benavides F, Oberyszyn TM, VanBuskirk AM, Reeve VE, Kusewitt DF. The hairless mouse in skin research. J Dermatol Sci. 2009;53:10–18. doi: 10.1016/j.jdermsci.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bramson J, Dayball K, Evelegh C, Wan YH, Page D, Smith A. Enabling topical immunization via microporation: a novel method for pain-free and needle-free delivery of adenovirus-based vaccines. Gene Ther. 2003;10:251–260. doi: 10.1038/sj.gt.3301886. [DOI] [PubMed] [Google Scholar]
- 37.Oran AE, Robinson HL. DNA vaccines, combining form of antigen and method of delivery to raise a spectrum of IFN-gamma and IL-4-producing CD4+ and CD8+ T cells. J Immunol. 2003;171:1999–2005. doi: 10.4049/jimmunol.171.4.1999. [DOI] [PubMed] [Google Scholar]
- 38.Glenn GM, Scharton-Kersten T, Vassell R, Mallett CP, Hale TL, Alving CR. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J Immunol. 1998;161:3211–3214. [PubMed] [Google Scholar]
- 39.Heckert RA, Elankumaran S, Oshop GL, Vakharia VN. A novel transcutaneous plasmid-dimethylsulfoxide delivery technique for avian nucleic acid immunization. Vet Immunol Immunopathol. 2002;89:67–81. doi: 10.1016/s0165-2427(02)00186-1. [DOI] [PubMed] [Google Scholar]
- 40.Cui Z. DNA vaccine. Adv Genet. 2005;54:257–289. doi: 10.1016/S0065-2660(05)54011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]