Abstract
Background
Delayed carotid endarterectomy (CEA) after a stroke or transient ischemic attack (TIA) is associated with risks of recurrent neurologic symptoms. In an effort to preserve cerebral function, urgent early CEA has been recommended in many circumstances. We analyzed outcomes of different time intervals in early CEA in comparison with delayed treatment.
Study Design
Retrospective chart review from a single university hospital tertiary care center between April 1999 and November 2010 revealed 312 patients who underwent CEA following stroke or TIA. Of these 312 patients, 69 received their CEA within 30 days of symptom onset and 243 received their CEA after 30 days from symptom onset. The early CEA cohort was further stratified according to the timing of surgery: Group A (27 patients), within 7 days; Group B (17), between 8 and 14 days; Group C (12), between 15 and 21 days; and Group D (12), between 22 and 30 days. Demographic data as well as 30-day (mortality, stroke, TIA, and myocardial infarction) and long-term (all-cause mortality and stroke) adverse outcome rates were analyzed for each group. These were also analyzed for the entire early CEA cohort and compared against the delayed CEA group.
Results
Demographics and co-morbid conditions were similar between groups. For 30-day outcomes, there were no deaths, 1 stroke (1.4%), 0 TIAs, and 0 myocardial infarctions in the early CEA cohort; in the delayed CEA cohort, there were 4 (1.6%), 4 (1.6%), 2 (0.8%), and 2 (0.8%) patients with these outcomes, respectively (p > 0.05 for all comparisons). Over the long-term, the early group had 1 ipsilateral stroke at 17 months and the delayed group had 2 ipsilateral strokes at 3 and 12 months. For long-term outcomes, there were 16 deaths in the early CEA cohort (21%) and 74 deaths in the delayed CEA cohort (30%, p > 0.05). Mean follow-up times were 4.5 years in the early CEA cohort and 5.8 years in the delayed CEA cohort.
Conclusion
There were no differences in 30-day and long-term adverse outcome rates between the early and delayed CEA cohorts. In symptomatic carotid stenosis patients without evidence of intracerebral hemorrhage, carotid occlusion, or permanent neurologic deficits early carotid endarterectomy can be safely performed and is preferred over delaying operative treatment.
INTRODUCTION
The role of carotid endarterectomy (CEA) in the management of symptomatic carotid stenosis has been firmly established based on several landmark prospective randomized trials from the 1990s.1–3 Early intervention within 2 weeks of symptom onset has been advocated and become standard of care for most patients with transient ischemic attacks (TIAs). However, for many years the approach in the setting of acute stroke has been to defer operative intervention for 4–6 weeks from the onset of symptoms due to the prospect of recurrent intraoperative stroke and ischemic to hemorrhagic stroke conversion. This is thought to occur as a result of compromise of the blood-brain barrier and loss of cerebrovascular autoregulation. The early literature from the second half of the 20th century reported several adverse outcomes related to early intervention for symptomatic carotid stenosis.4–7 However, many of these early reports were published before the era of computed tomography (CT) and magnetic resonance imaging (MRI) and before the ability to aggressively control blood pressure in an intensive care unit.8
While deferral of surgical intervention is intended to reduce the risks of periprocedural embolic or hemorrhagic events, there is an interval threat of ischemic recurrence. The most frequently cited reports have suggested rates of 1–2% of recurrent ischemic events within the first week and 2–4% in the first month.9–12 These rates, however, may be understated as other authors describe rates as high as 9 to 10% within 7 days.13,14 Results from the North American Symptomatic Carotid Endarterectomy Trial (NASCET) and European Carotid Surgery Trial (ECST) sub-group analyses suggest greatest benefit with early intervention and improved outcomes as shown by the National Institutes of Health Stroke Scale (NIHSS).15,16 Both trials demonstrated greatest risk reduction for recurrent stroke if endarterectomy was performed within 7 days of symptom onset. Restoration of cerebral blood flow can salvage neuronal tissue at risk in the penumbra, prevent recurrence of stroke, and avert progression to occlusion.17
We report a single-center 10-year experience of CEA performed for symptomatic carotid stenosis. We postulate that there is no difference in 30-day and long-term primary outcomes of stroke, myocardial infarction (MI), and death with early vs delayed treatment. Furthermore, outcomes of early CEA by time elapsed from symptom onset to treatment are evaluated in a subgroup analysis.
METHODS
All CEA procedures performed for symptomatic carotid stenosis at Northwestern Memorial Hospital, Chicago, Illinois, between April 21, 1999, and November 30, 2010, were retrospectively reviewed per approved institutional review board protocols. Patients had one of the following within 3 months of their procedure in order to classify as a symptomatic neurologic event: hemispheric TIA or stroke resulting in facial, upper, or lower extremity weakness or dysarthria, aphasia, or syncope as evidenced by a formal neurology evaluation. Patients with amaurosis fugax or ocular lesions were excluded. Patients were referred by primary care practitioners, independent neurologists, or emergency room physicians. All patients underwent preoperative bilateral carotid artery duplex examination and were found to have stenosis ≥ 50% (NASCET criteria) on the symptomatic side. Patients received either a CT or MRI of the neck and brain to assess the carotid and cerebral circulation and the presence, location, and size of any infarct. Daily antiplatelet (aspirin 81 or 325 mg) therapy was implemented pre-operatively if not already active. Clopidogrel was utilized in certain patients dependant on the referring physician and anatomical characteristics of the carotid lesion.
Patients undergoing CEA within 30 days of symptom onset were classified in the “early CEA” group. In this cohort, patients were further categorized into one of four groups by time from symptom onset to treatment: Group A, 0–7 days; Group B, 8–14 days; Group C, 15–21 days; Group D, 22–30 days. Patients undergoing CEA after 30 days from symptom onset were classified into the “delayed CEA” group. Symptomatic patients who might otherwise have been offered CEA underwent carotid angioplasty and stenting (CAS) on the basis of anatomic criteria (surgically inaccessible lesion, neck irradiation, re-operative neck, or contralateral laryngeal nerve paralysis) or if part of an organized trial. These patients were excluded from the review. Also, patients with carotid occlusion, hemorrhagic stroke, or extensive permanent neurologic deficits were not offered operative management.
Operative Technique
All procedures were performed under general anesthesia by experienced, board-certified vascular surgeons using standard endarterectomy with patch closure or eversion endarterectomy. Selective shunting based on carotid stump pressure measurement was utilized at the discretion of the operating surgeon. Patients were neurologically monitored in the post-operative anesthesia care unit and then transferred to the standard surgical ward. High risk patients or those exhibiting hypertension or hypotension were observed in the intensive care unit.
Follow-up Protocol
Neurologic examinations were performed by the operating surgeon and neurologist in the immediate and long-term post-operative period. Stroke was defined as any new central neurological deficit that persisted beyond 24 hours regardless of evidence of an infarct on CT or MRI. TIA was defined as any new central neurological deficit that resolved within 24 hours after initial onset. MI was defined by at least two of the following criteria: typical chest pain lasting 20 minutes or more; serum levels of creatine kinase (CK), CK MB, or troponin at least twice the upper limit of the normal range; and new Q wave on at least two adjacent derivations or predominant R waves in V1 (R wave ≥1 mm >S wave in V1). Patients were clinically reevaluated at 1 and 12 months.
Outcomes
Thirty-day mortality, stroke, and MI rates for Groups A, B, C, and D, as well as for the “early CEA” group and the “delayed CEA” groups in total were analyzed. Long-term outcomes of stroke rate and all-cause mortality were also evaluated as far as 10 years from operative intervention. Data for patient characteristics by age, gender, hypertension, smoking status, hyperlipidemia, chronic renal disease, diabetes mellitus, history of MI, percutaneous coronary artery revascularization, clopidogrel use, side of operation, MCA territory stroke, contralateral carotid occlusion, intraoperative shunt use, and patch closure versus eversion technique were also collected for secondary analysis.
Statistical analysis
Baseline patient characteristics and post-operative outcomes between Groups A, B, C, and D, as well as between early and delayed CEA groups, were compared using the Chi-square or Fisher exact test (for small n or highly imbalanced table cells). Data were summarized using descriptive statistics (i.e., count and frequency for categorical variables; age as the lone continuous variable in the analysis was summarized using mean and standard deviation). Kaplan-Meier survival curves were fitted for the four early CEA groups as well the early and delayed CEA group in total and patient survival was compared using log-rank tests. Multivariate Cox proportional hazards models were constructed to identify independent risk factors of long-term post-operative mortality and stroke. The hazard ratio estimates were based on simultaneous analysis of all predicated variables. Assumption of proportionality was tested using log-minus-log plot and was met. Statistical significance was determined at a two-sided p-value < 0.05. All statistical analyses were conducted using SAS 9.2 (SAS Inc., Cary, NC).
RESULTS
There were 69 patients who underwent CEA within 30 days of symptom onset and 243 after 30 days. Within the early CEA group, 27 patients received their CEA within the first week, 17 the second week, and 12 each in the third and fourth weeks. One patient was not categorized in the subgroup analysis as the patient underwent CEA within 30 days, but it was unclear exactly when the onset of symptoms occurred. Demographic data are presented in Table 1. Comparison of the four early CEA groups did not reveal any differences with regard to mean age, percentage of octogenarians, males, patients with hypertension, diabetes, history of tobacco use, hypercholesterolemia, dialysis, or preoperative clopidogrel use. There was a higher proportion of smoking and history of MI in the early CEA group (27/69 vs. 58/243, p = 0.012).
Table 1.
Demographic Characteristics
| Early CEA (within 30 days) | Late CEA (after 30 days) | P by groups | P early v. late | |||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Early CEA | ||||
| n | 27 | 17 | 12 | 12 | 69* | 243 | ||
| Mean Age, yr (SD) | 68.6 (9.8) | 68.8 (14.7) | 67.5 (14.3) | 70.7 (9.3) | 68.9 (11.7) | 70.4 (9.7) | 0.93 | 0.27 |
| Age ≥ 80 yr | 4 | 6 | 2 | 2 | 14 (20%) | 46 (19%) | 0.42 | 0.80 |
| Male | 16 | 9 | 8 | 8 | 42 (61%) | 159 (65%) | 0.92 | 0.48 |
| Hypertension | 24 | 14 | 10 | 12 | 61 (88%) | 205 (84%) | 0.51 | 0.40 |
| Diabetes | 7 | 3 | 3 | 3 | 16 (23%) | 61 (25%) | 0.71 | 0.74 |
| Smoker | 11 | 7 | 6 | 3 | 27 (39%) | 58 (24%) | 0.68 | 0.012 |
| PTCA | 2 | 0 | 1 | 0 | 3 (4%) | 28 (11%) | 0.66 | 0.07 |
| Hyperlipidemia | 16 | 12 | 9 | 9 | 47 (68%) | 160 (66%) | 0.42 | 0.72 |
| h/o MI | 7 | 3 | 2 | 3 | 15 (22%) | 26 (11%) | 0.90 | 0.016 |
| Dialysis | 0 | 1 | 0 | 0 | 1 (1%) | 2 (1%) | 0.60 | 0.63 |
| Clopidogrel | 8 | 5 | 2 | 3 | 18 (26%) | 52 (21%) | 0.48 | 0.79 |
| Indication: stroke | 20 | 14 | 9 | 11 | 55 (80%) | 151 (62%) | 0.66 | 0.006 |
| Indication: TIA | 7 | 3 | 3 | 1 | 14 (20%) | 92(38%) | 0.66 | 0.006 |
| Shunt | 15 | 11 | 3 | 6 | 36 (52%) | 68 (28%) | 0.20 | 0.0002 |
| Patch | 25 | 12 | 9 | 10 | 57 (83%) | 189 (78%) | 0.23 | 0.38 |
One patient underwent early CEA, but exact symptom onset was unclear
With regard to surgical indications, 80% of patients underwent early CEA for stroke vs 62% in the delayed group (55/69 vs 151/243, respectively). In terms of anatomical considerations, there was very little difference by subgroup or by early versus delayed CEA in terms of patch vs eversion endarterectomy. There was a statistically significant difference in the shunt use between groups (p < 0.05). Fifty two percent (36/69) of patients had a shunt utilized in the early endarterectomy group vs 28% (68/243) in the delayed group. It is unclear why this trend occurred. Perhaps this is due to increased collateral development in the delayed group, therefore not requiring a shunt. There was no correlation between stroke or death and eversion vs patch or shunt use.
Rates of 30-day mortality, stroke, and MI, as well as long-term stroke and death rates are shown in Table 2. Of patients receiving early CEA, the only adverse outcome was a postoperative hemorrhagic stroke in a patient who underwent CEA 9 days after the onset of symptoms resulting in hemiplegia. This was postulated to have occurred as a result of hyperperfusion syndrome and poor blood pressure management. No 30-day deaths or MIs were observed in this cohort. The rates of 30-day death, stroke, and MI were similarly low (4/243, 4/243, 2/243, respectively) in patients undergoing delayed CEA; there were no statically significant differences in any of these outcomes by subgroup or by early versus delayed CEA.
Table 2.
30-day and Long-Term Mortality, Stroke, and MI Rates
| Early CEA (< 30 days) | Late CEA (> 30 days) | P by groups | P early v. late | |||||
|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group D | Early CEA | ||||
| n | 27 | 17 | 12 | 12 | 69* | 243 | ||
| 30-day outcomes | ||||||||
| Mortality | 0 | 0 | 0 | 0 | 0 | (1.6%) 4 | 1.00 | 0.58 |
| Stroke | 0 | (6%) 1 | 0 | 0 | (1.4%) 1 | (1.6%) 4 | 0.60 | 1.00 |
| MI | 0 | 0 | 0 | 0 | 0 | (0.8%) 2 | 1.00 | 1.00 |
| Long-term outcomes (10 years) | ||||||||
| Mortality | (26%) 7 | (29%) 5 | (16%) 2 | (16%) 2 | (23%) 16 | (30%) 74 | 0.86 | 0.24 |
| Stroke | (11%) 3 | (6%) 1 | (8%) 1 | (16%) 2 | (10%) 7 | (7%) 16 | 0.82 | 0.30 |
| MI | (7%) 2 | 0 | 0 | 0 | (3%) 2 | (5%) 12 | 0.79 | 0.74 |
One patient underwent early CEA, but exact symptom onset was unclear
Anatomical characteristics, including location and side of the intracranial lesions comparing early and delayed groups are shown in Table 3. Also, contralateral carotid occlusion is reported. There was no statistically significant difference in the two groups, however there was a larger percentage of early endarterectomy patients with middle cerebral artery (MCA) territory strokes and contralateral carotid occlusions. MCA territory strokes were seen in nearly half of all symptomatic patients (46% early, and 39% delayed groups).
Table 3.
Anatomical characteristics
| n | Early CEA | Delayed CEA | P value |
|---|---|---|---|
| MCA territory stroke | 32 (46%) | 94 (39%) | 0.29 |
| Left side | 40 (58%) | 128 (53%) | 0.43 |
| Contralateral Carotid Occlusion | 10 (14%) | 22 (9%) | 0.14 |
Of the patients undergoing early CEA, all cause mortality was seen in 16 patients during the 10-year follow-up period; 7 in Group A, 5 in Group B, and 2 each in Groups C and D. Seventy-four patients in the delayed CEA group expired during long-term follow-up. There were 7 strokes including contralateral or other embolic sources during follow-up in the early CEA cohort. There was no difference in the rate of either mortality or stroke by group or by early versus delayed CEA.
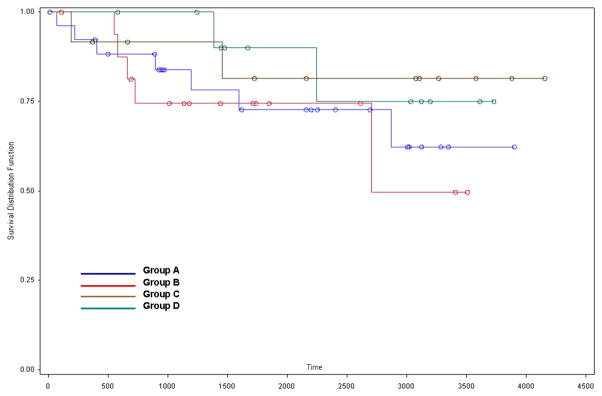
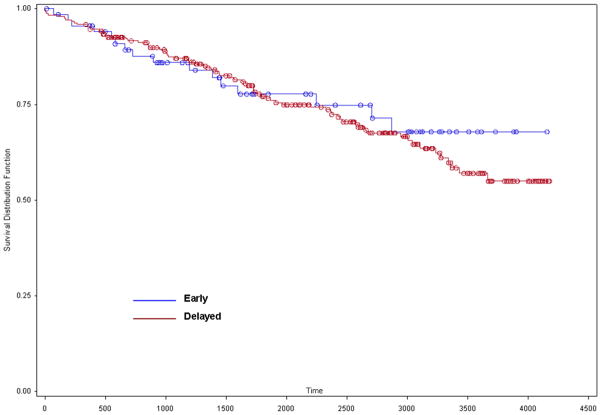
Figures 1 and 2 show Kaplan-Meier survival curves for each of the four subgroups and for the early and delayed CEA groups, respectively. Log-rank analyses revealed no difference in survival in either comparison. Mean survival of patients in the early CEA group was 6.6 years and 7.9 years for patients in the delayed CEA group.
Figure 1.
Subgroup KM curve.
Figure 2.
Early vs. delayed KM curve.
DISCUSSION
The timing of carotid endarterectomy after recent neurological event has been debated for several decades and is still controversial. Many factors come into play when assessing timing of symptom onset. Patient delay to endarterectomy may be a result of delay in presentation, delay in referral, or at the discretion of the operating surgeon. The initial literature4–7 presented clinical data supporting delay of surgical intervention for more than 6 weeks due to concern for ischemic to hemorrhagic conversion. Giordano et al18 reported an 18.5% perioperative stroke rate in 27 patients undergoing CEA within 5 weeks of acute stroke compared with 0% in the delayed CEA group. Rockman et al19 reviewed their 10-year CEA experience and reported a significantly higher rate (5.1%) of perioperative stroke in their early CEA group (n = 604) than in their delayed CEA group (1.6%, n = 442, p < 0.01). The evidence was significant, but most was reported in the era before improved imaging modalities (CT, MRI) could better characterize infarcts and close pre- and post-operative blood pressure management could be carried out in an intensive care setting.
The vast majority of contemporary literature supports early intervention. The foundation of early endarterectomy in symptomatic patients began with the improved results of pooled analysis from the ECST and NASCET trials in the 1990s.1–3 Rantner et al demonstrated no difference in major complication rates in 29 CEAs performed before or 62 CEAs performed after 28 days from symptoms.20 More importantly, they observed secondary ischemic stroke in 11.8% of their delayed CEA cohort during the 4-week waiting period. Ballotta et al21 showed in a prospective study of 45 patients randomized to early CEA and 41 randomized to delayed CEA that there was no difference in perioperative stroke rates, survival, or stroke-free survival at 3 years. Capoccia et al17 reported that 93.5% (58 of 62) patients undergoing CEA within 2 weeks of symptom onset experienced National Institutes of Health Stroke Scale (NIHSS) score improvement upon treatment. Finally, as in this study, Paty et al22 reported no difference in the rates of perioperative stroke resulting in permanent neurologic deficit by time elapsed after symptom onset (Week 1: 2/72, 2.8%; Week 2: 2/59, 3.4%; Week 3: 1/29, 3.4%; Week 4: 2/78, 2.6%).
Retrospective analysis from this study indicates that the majority of the delay to operative treatment was a result of patient presentation and referral patterns. Many patients did not recognize the severity of initial symptom onset and presented upon recurrence, delaying care. Furthermore, referral for endarterectomy in many instances did not occur until complete workup and identification of carotid disease. Referring physicians became more aggressive about early consultation for surgery towards the latter part of the decade. Practice patterns within the vascular surgery group also shifted towards earlier endarterectomy.
Anatomical considerations showed interesting findings. Nearly half of all patients exhibited MCA territory strokes. Greater than 10% of the entire cohort had contralateral carotid occlusion. Patients with occlusion or >80% stenosis of the contralateral carotid had a shunt utilized. At our institution, selective shunting is implemented by checking stump pressures. Approximately 50% of the symptomatic patients had a shunt used, but this included patients with TIAs or old infarcts that had resolution of symptoms. We advocate mandatory shunting of all patients with a documented clinical or radiological acute ipsilateral cerebral infarction. In further analyzing the data, there was a trend toward greater shunt usage for acute strokes in the latter part of the decade. There was an increase in shunt use in the early endarterectomy group suggesting increased symptom severity and greater carotid disease, but this is unclear.
Due to the increasing evidence, there has been an evolution in the guidelines regarding carotid endarterectomy after acute stroke and TIA. Previous recommendations23 entailed delay of carotid endarterectomy after acute stroke for 4–6 weeks. The most recent 2011 SVS (Society of Vascular Surgery) guidelines have taken the aggregate data of the NASCET and ECST trials as well as recent reports into account and encourage CEA to be performed within 2 weeks of the neurological event.24,25 The ECVS (European Society for Vascular Surgery) guidelines also recommend CEA within 2 weeks of symptom onset.26
There are some limitations to this study. It was relatively small, especially for the subgroup comparison within the early CEA groups. Also, the data was retrospectively analyzed from a single institution and patients were not randomized. The patient groups, despite the results in Table 1, may not be directly comparable, particularly as selection criteria for early CEA may be different from the criteria for delayed CEA. The size of the preoperative infarct on imaging was not described within the confines of this review. Nonetheless, the results from this study appear to be consistent with most of the recent retrospective analyses regarding the timing of carotid endarterectomy after acute stroke.
CONCLUSION
Consistent with recent evidence, our single-institution experience does not show any statistically significant difference in rates of 30-day or long-term mortality or stroke between early and delayed carotid endarterectomy. Additionally, we have demonstrated no difference in adverse event rates by time elapsed from symptom onset to surgical intervention. There was a small, though not statistically significant, increase in perioperative stroke and death rate in the delayed endarterectomy group, perhaps due to a statistical error. Early endarterectomy may prevent recurrent embolic events and interval strokes without increased perioperative stroke and death risk. Carotid endarterectomy may be performed with success as early as two weeks in neurologically symptomatic patients. Appropriate stroke severity and risk stratification should be taken into account when performing early CEA.
Footnotes
NOTE: Presented at the 36th Annual Meeting of the Southern Association for Vascular Surgery, Scottsdale, AZ, January 18–21, 2012.
Disclosures
Stipend for Dr. Park was partially supported by National Institutes of Health Grant #5T32HL094293.
Dr. Morasch receives honoraria for serving as training course director for W. L. Gore & Associates, Inc. and as consultant for King Pharmaceuticals.
Dr. Rodriguez has received honoraria from W. L. Gore & Associates, Medtronic, and Abbott as a speaker and from Abbott and Medtronic as a consultant.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.NASCET Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325:445–53. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 2.ECST Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–87. [PubMed] [Google Scholar]
- 3.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. N Engl J Med. 339:1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 4.Wylie EJ, Hein MF, Adams JE. Intracranial hemorrhage following surgical revascularization for treatment of acute strokes. J Neurosurg. 1964;21:212–5. doi: 10.3171/jns.1964.21.3.0212. [DOI] [PubMed] [Google Scholar]
- 5.Rob CG. Operation for acute completed stroke due to thrombosis of the internal carotid artery. Surgery. 1969;65:862–5. [PubMed] [Google Scholar]
- 6.Bruetman ME, Fields WS, Crawford ES, DeBakey ME. Cerebral hemorrhage in carotid artery surgery. Arch Neurol. 1963;9:458–67. doi: 10.1001/archneur.1963.00460110026002. [DOI] [PubMed] [Google Scholar]
- 7.Hunter JA, Julian OC, Dye WS, Javid H. Emergency operation for acute cerebral ischemia due to carotid artery obstruction. Ann Surg. 1965;162:901–4. doi: 10.1097/00000658-196511000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston SC, Gress DR, Browner WS, Sidney S. Short term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–6. doi: 10.1001/jama.284.22.2901. [DOI] [PubMed] [Google Scholar]
- 9.Lovett JK, Dennis MS, Sandercock PA, Bamford J, Warlow CP, Rothwell PM. Very early risk of stroke after a first transient ischemic attack. Stroke. 2003;34:e138–40. doi: 10.1161/01.STR.0000080935.01264.91. [DOI] [PubMed] [Google Scholar]
- 10.Coull AJ, Lovett JK, Rothwell PM on behalf of the Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326–8. doi: 10.1136/bmj.37991.635266.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovett JK, Coull AJ, Rothwell PM. Early risk of recurrence by subtype of ischemic stroke in population- based incidence studies. Neurology. 2004;62:569–73. doi: 10.1212/01.wnl.0000110311.09970.83. [DOI] [PubMed] [Google Scholar]
- 12.Rantner B, Eckstein HH, Ringleb P, Woelfle KD, Bruijnen H, Schmidauer C, et al. American Society of Anesthesiology and Rankin as predictive parameters for the outcome of carotid endarterectomy within 28 days after an ischemic stroke. J Stroke Cerebrovasc Dis. 2006;15:114–20. doi: 10.1016/j.jstrokecerebrovasdis.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Rothwell PM, Warlow CP. Timing of TIAs preceding stroke: time window for prevention is very short. Neurology. 2005;64:817–20. doi: 10.1212/01.WNL.0000152985.32732.EE. [DOI] [PubMed] [Google Scholar]
- 14.Keldahl ML, Eskandari MK. Timing of carotid surgery after acute stroke. Expert Rev Cardiovasc Ther. 2010;8:1399–403. doi: 10.1586/erc.10.122. [DOI] [PubMed] [Google Scholar]
- 15.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 16.Gasecki AP, Ferguson GG, Eliasziw M, Clagett GP, Fox AJ, Hachinski V, et al. Early endarterectomy for severe carotid artery stenosis after a nondisabling stroke: results from the North American Symptomatic Carotid Endarterectomy Trial. J Vasc Surg. 1994;20:288–95. doi: 10.1016/0741-5214(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 17.Capoccia L, Sbarigia E, Speziale F, Toni D, Fiorani P. Urgent carotid endarterectomy to prevent recurrence and improve neurologic outcome in mild-to-moderate acute neurologic events. J Vasc Surg. 2011;53:622–8. doi: 10.1016/j.jvs.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 18.Giordano JM. The timing of carotid endarterectomy after acute stroke. Semin Vasc Surg. 1998;11:19–23. [PubMed] [Google Scholar]
- 19.Rockman CB, Maldonado TS, Jacobowitz GR, Cayne NS, Gagne PJ, Riles TS. Early carotid endarterectomy in symptomatic patients is associated with poorer perioperative outcomes. J Vasc Surg. 2006;44:480–7. doi: 10.1016/j.jvs.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 20.Rantner B, Pavelka M, Posch L, Schmidauer C, Fraedrich G. Carotid endarterectomy after ischemic stroke – is there a justification for delayed surgery? Eur J Vasc Endovasc Surg. 2005;30:36–40. doi: 10.1016/j.ejvs.2005.02.045. [DOI] [PubMed] [Google Scholar]
- 21.Ballotta E, Da Giau G, Baracchini C, Abbruzzese E, Saladini M, Meneghetti G. Early versus delayed carotid endarterectomy after a nondisabling ischemic stroke: a prospective randomized study. Surgery. 2002;131:287–93. doi: 10.1067/msy.2002.119987. [DOI] [PubMed] [Google Scholar]
- 22.Paty PS, Darling RC, 3rd, Feustel PJ, Bernardini GL, Mehta M, Ozsvath KJ, et al. Early carotid endarterectomy after acute stroke. J Vasc Surg. 2004;39:148–54. doi: 10.1016/j.jvs.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Blaisdell WF, Clauss RH, Galbraith JG, Imparato AM, Wylie EJ. Joint study of extracranial arterial occlusion IV: A review of surgical considerations. JAMA. 1969;209:1889–95. [PubMed] [Google Scholar]
- 24.National Institute for Health and Clinical Excellence. Diagnosis and initial management of acute stroke and transient ischemic attack (TIA) London, UK: National Institute for Health and Clinical Excellence; 2008. http://www.nice.org.uk/nicemedia/pdf/CG68NICEGuideline.pdf. [Google Scholar]
- 25.Ricotta JJ, Aburahma A, Ascher E, Eskandari M, Faries P, Lal BK, et al. Updated Society for Vascular Surgery guidelines for management of extracranial carotid disease. J Vasc Surg. 2011;54:e1–31. doi: 10.1016/j.jvs.2011.07.031. [DOI] [PubMed] [Google Scholar]
- 26.Liapis C, Mackey WC, Perler B, Cao P. Comparison of SVS and ESVS carotid disease management guidelines. Eur J Vasc Endovasc Surg. 2009;38:243–5. doi: 10.1016/j.ejvs.2009.05.017. [DOI] [PubMed] [Google Scholar]