Abstract
Heart rate is controlled by stimulatory sympathetic and inhibitory parasympathetic nerves innervating the sino-atrial node and cardiac conduction system. Sympathetic release of norepinephrine (NE) and parasympathetic release of acetylcholine (ACh) are controlled by the central nervous system, and by pre-synaptic inhibition of transmitter release within the atria. An increase in cardiac sympathetic transmission relative to parasympathetic transmission is pathological as it can lead to disturbances in heart rhythm, catecholaminergic toxicity and development of arrhythmias or fibrillation. Mice lacking the p75 neurotrophin receptor (p75−/−) have elevated atrial NE but a low heart rate suggesting autonomic dysregulation. Similarly, mice whose sympathetic neurons lack the gp130 cytokine receptor (gp130 KO) have a normal heart rate but enhanced bradycardia after vagal nerve stimulation. What is unclear is whether cardiac autonomic disturbances in these animals reflect systemic alterations in nerve activity or whether localized defects in neurotransmitter stores or release are involved. To examine local stimulus-evoked release of neurotransmitters, we have developed a novel method for simultaneous quantification of both NE and ACh after ex-vivo atrial field stimulation. Using HPLC with electrochemical detection for NE, and HPLC with mass spectrometry for ACh, we found that following field stimulation NE release was impaired in p75−/− atria while ACh content and release was elevated in gp130 KO atria. Thus, alterations in localized transmitter release from atrial explants are consistent with in vivo deficits in heart rate control, suggesting peripheral alterations in autonomic transmission in these mice.
Keywords: Heart, autonomic, HPLC-MS, HPLC-ED, sympathetic, parasympathetic
1. Introduction
Heart rate is controlled by sympathetic and parasympathetic innervation projecting to the atria (including the sino-atrial node) and cardiac conduction system. Norepinephrine (NE) released from sympathetic nerves stimulates heart rate and cardiac conduction, while acetylcholine (ACh) released from parasympathetic nerves slows heart rate and cardiac conduction. Activation of autonomic nerves by the central nervous system stimulates transmitter release, but NE and ACh release from cardiac nerves is additionally regulated in the atria by activation of inhibitory presynaptic receptors [1;2]. Neurotransmitter release from cardiac terminals can also be altered by a number of factors derived from cardiac and inflammatory cells [3–6]. An increase in cardiac sympathetic transmission relative to parasympathetic transmission is pathological [7;8], so it is important to understand local mechanisms that regulate NE and ACh release in the atria.
Cardiac autonomic disturbances are present in transgenic mice that lack the p75 neurotrophic receptor (p75−/−) and in mice whose noradrenergic neurons lack the gp130 cytokine receptor (gp130 KO). Nerve growth factor (NGF) stimulates sympathetic neuron survival, axon outgrowth, and NE production through activation of TrkA [9;10], and Trk signaling is enhanced in the absence of p75 [11]. Thus, NE content is elevated in p75−/− cardiac sympathetic nerves, but heart rate is low [12]. Although the p75 receptor is also present on cardiac parasympathetic neurons [13], parasympathetic innervation is normal in these animals [12], suggesting the low heart rate is due to impaired NE release. Inflammatory cytokines regulate neurochemistry and function of autonomic sympathetic neurons through activation of the gp130 receptor [9;14;15]. The absence of gp130 in neurons expressing the enzyme dopamine beta hydroxylase (gp130 KO) [16] results in mice that display cardiac autonomic disturbances including increased reperfusion arrhythmias and altered adrenergic and cholinergic transmission [16]. Therefore, autonomic dysregulation is present in both of these transgenic populations, but it is unclear whether local neurotransmitter stores and/or stimulus-evoked neurotransmitter release are compromised.
Since alterations in atrial neurotransmitter release will also impact co-projecting nerves, it is crucial to determine storage and release in both cardiac sympathetic and parasympathetic populations simultaneously. In order to verify the site of the autonomic defect in these animals, we have developed a novel method for simultaneous detection of autonomic neurotransmitters from the isolated right atrium following ex-vivo nerve stimulations. We found that the alterations in transmitter stores and release detected in atrial explants were consistent with in vivo generalized deficits in heart rate control.
2. Materials and Methods
2.1. Animals
gp130DBH-Cre/lox mice lack gp130 in neurons expressing dopamine beta hydroxylase including peripheral sympathetic and a subpopulation of parasympathetic neurons. They were obtained from Dr. Hermann Rohrer and maintained as previously described [16]. p75 −/− (B6.129S4-Ngfrtm1Jae/J) mice, which lack functional p75 receptor [17] were obtained from Jackson Labs. Wild type C57BL/6J (WT) mice were obtained from Jackson Labs West (Sacramento, CA). All mice were kept on a 12h:12h light-dark cycle with ad libitum access to food and water. Male and female mice 12–18 weeks old were used for all experiments. All procedures were approved by the Institutional Animal Care and Use Committee and complied with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23, Revised 1996).
2.2. Atrial explants and stimulation
The right atrium was removed (n=4–6 per group) under isofluorane anesthesia and pinned to a thin layer of Sylgard (Dow Corning, Midland, MI) in a preheated (37°C), continuously oxygenated, water-jacketed organ bath containing 2 mLs of Ringer’s solution (120 mM NaCl, 1.2 mM KH2PO4, 4.7 mM KCl, 1.2 mM MgSO4, 2 mM CaCl2, 25 mM NaHCO3, 11 mM glucose, pH 7.4). The atrial tissue was placed between platinum stimulating electrodes, and after 60 min of equilibration, atria were stimulated using an S88X Stimulator (Grass Technologies, West Warwick, RI) in constant-voltage mode. Tissue was “primed” for 10 minutes with brief bouts of stimulation (10 sec stimulation, 20 sec rest; 15 V, 5 Hz, 0.1 msec pulse duration). The Ringer’s solution was replaced every 5 minutes. After the priming phase atria remained unstimulated during a 20 min rest phase. Fresh Ringer’s solution (1 mL) was then added, and replaced every 5 min for the remainder of the experiment. The initial 5 min period was used as a baseline for transmitter release. During the second 5 min period, the atrial nerves were stimulated (15 V, 5 Hz, 0.1 msec pulse duration) for 1 min to trigger neurotransmitter release. Two additional 5 min samples were collected without further stimulation. Each 1 mL sample was split into 0.5 mL samples for analysis of ACh and NE. Baseline levels of transmitter were subtracted from the amounts recovered during the stimulation and recovery periods. At the end of the experiment, atria were incubated in 1 mL 0.1 M perchloric acid (PCA) for 24 h to extract total remaining ACh and NE, which were quantified by HPLC coupled with Mass Spectrometry (HPLC-MS) or Electrochemical Detection (HPLC-ED) respectively.
2.3. HPLC-MS for ACh quantification
For each atria, 0.5 mL aliquots of the collected samples, and a 0.5 mL aliquot of the PCA extract of the atrial tissue, were spiked with deuterated ACh (d4 ACh; 0.1 pg/µL, CDN Isotopes, Quebec, Canada). Deuterated ACh was also added to standards of known concentrations of ACh (10 fg/µL-100 pg/µL, Sigma-Aldrich, St. Louis, MO). All samples and standards were dried by speedvac and re-constituted in 100 µL of acetonitrile + 6% formic acid. Quantification of d4ACh indicated that 49% of ACh was recovered following sample preparation. Samples were chromatographically separated on a hydrophilic interaction chromatography mode column (HILIC; PolyLC, Columbia, MD; mobile phase A: ammonium formate, B: acetonitrile) with a flow rate of 300 µL/min and detected and quantified by a linear ion trap mass spectrometer (Applied Biosystems MDS SCIEX 4000 QTrap mass spectrometer, Carlsbad, CA) [18]. Multiple reaction monitoring (MRM) was in positive ionization mode and electrospray ionization (ESI) source parameters were as follows: nebulising and curtain gas (N2) 50 psi, temperature 600 °C, ion spray voltage 3000 V. MRM was used to quantify d4 ACh (mass-to-charge ratio (m/z) 150→91.1, declustering potential 46 V, entrance potential 10 V, collision cell exit potential 16 V) and ACh (m/z 146→87, declustering potential 46 V, entrance potential 10 V, collision cell exit potential 16 V). Injections of 2.05 fmol (300 fg after 30µl injection) ACh consistently gave signal-to-noise ratios above 3 indicating the detection limit for ACh in our system (lower limit mean for 21 HPLC-MS runs =2.4 ± 0.04 fmol). There was excellent correlation for standards between individual HPLC-MS experimental runs (r2 = 0.999, 21 runs), and between the predicted concentration for standards within a run and actual experimental values (r2 mean= 0.999, 21 runs).
2.4. HPLC-ED for NE quantification
A 0.5 mL aliquot of each sample, and a 0.5 mL aliquot of the PCA atrial extract were spiked with an internal standard dihydroxybenzylamine (DHBA, 9 nM; Sigma-Aldrich, St. Louis, MO). A similar amount of DHBA was also added to standards of known amounts of NE (4–40 nM) and catecholamines were precipitated from samples and standards with alumina (15 mg, 30 min). The alumina was twice washed with ddH2O, and the catecholamines, NE and DHBA, were desorbed with 0.1 M PCA [19]. Samples were chromatographically separated by reverse-phase HPLC (C18 column; 15 × 0.46 cm, 5 µm particle size; Varian, Lake Forest, CA) using a mobile phase containing 75 mM NaH2PO4 (pH 3.0), 1.7 mM sodium octane sulfonate, and 4% (v/v) acetonitrile. A coulometric detector (ESA, Bedford, MA) was used to detect and quantify NE (electrode potential 180 mV, 50 nA) with area under curve normalized to DHBA area. Detection limits were ~50 fmol with recoveries >60% [20].
2.6. Data analysis
The amount of ACh was calculated by comparing the ratio of ACh to d4 ACh in samples to those ratios from known standards for ACh and d4 ACh run in parallel. Similarly, NE concentration was determined by comparison of the ratios of NE to DHBA in the samples to those ratios from known standards for NE and DHBA run in parallel with the experimental samples. Data were acquired using Analyst software (AB SCIEX, Framingham, MA) for HPLC-MS runs and with LCsolution software (Shimadzu, Columbia, MD) for HPLC-ED runs. Multiple groups were compared to each other by or one-way ANOVA with a Newman-Keuls post-hoc test or Kruskal-Wallis ANOVA using Prism software (v5.0, GraphPad Software, San Diego, CA). Stimulation-evoked release was the amount of NE or ACh in the bath after stimulation less the NE or ACh present prior to stimulation.
3. Results
Mouse atria were stimulated with platinum electrodes in an ex-vivo organ dish system. Atrial weight was similar between the three genotypes (wildtype 16.5±2.4 mg, p75 −/− 12.4±3.5 mg, gp130 KO 16.2± 2.2 mg). Samples of ACh and the internal standard, d4 ACh, showed appropriate fragmentation pattern under tandem MS-MS conditions (data not shown). The total pool of ACh remaining in atria after stimulation (Fig. 1A–C) was normalized to tissue weight (Fig. 1D). The amount of ACh released into media during the 5 minute baseline phase, normalized to tissue weight, was approximately 1/10th of the ACh released during stimulation and did not differ significantly between genotypes (wildtype 11.4±3.2 fmol/mg, p75−/− 7.2±1.5 fmol/mg, gp130 KO 17.9±11.2 fmol/mg). Stimulus-evoked release of ACh, also normalized to tissue weight, constituted only a small fraction of the total atrial pool of ACh (3–4 %) in all groups of animals (Fig. 1E). The gp130 KO animals showed interesting differences in parasympathetic function compared to WT or p75 −/− animals. Atrial ACh content was significantly higher in gp130 KO animals (Fig. 1D, p<0.01), and stimulation-evoked release of ACh from parasympathetic nerves was significantly greater compared to WT and p75 −/− atria (Fig. 1E, p<0.05). This increased release was due to the elevated ACh content in gp130 KO atria, since ACh secretion normalized to atrial ACh content was similar in all genotypes.
Figure 1. HPLC-MS quantification of atrial ACh content and release.
Panels A–C show representative ACh (top) and d4 ACh (bottom) chromatograms from wildtype (A), p75 −/− (B), and gp130 KO (C) mice. (D) Total atrial ACh content was greater in gp130 KO mice (5.4±0.4 pmol/mg, n=4) than in WT (2.5±0.4 pmol/mg, n=10) and p75 −/− (2.9±0.4 pmol/mg, n=6) mice. (E) Stimulus-evoked release of ACh was also higher in the gp130 KO mice (200±30 fmol/mg) when compared to WT (100±10 fmol/mg) or p75 −/− (90±20 fmol/mg) mice. Data are mean±SEM, *p<0.05, **p<0.01.
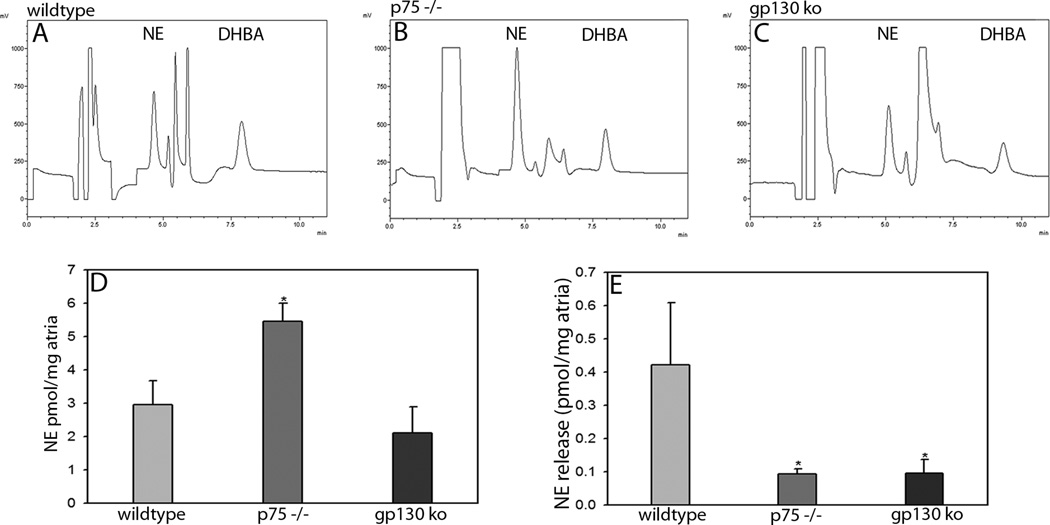
NE and the internal standard, DHBA, were well separated by the chromatographic conditions used (Fig. 2 A–C). The total pool of NE remaining in atria after stimulation was quantified by HPLC-ED and normalized to tissue weight. Total atrial NE was elevated in p75−/− mice compared to other genotypes (Fig. 2D). NE release during the 5 minute baseline phase, normalized to tissue weight, did not differ significantly between genotypes (wildtype 14.0±5.7 fmol/mg, p75−/− 14.2±7.3 fmol/mg, gp130 KO 22.4±10.5 fmol/mg). Stimulus-evoked release of NE, also normalized to tissue weight, constituted only a small fraction of the total atrial pool of NE (2–14 %) in all groups of animals. Stimulus-evoked release of NE was significantly lower in atria from p75 −/− and gp130 KO mice compared to WT (Fig. 2E), even though atrial NE content was greater than (p75−/−) or the same as (gp130 KO) WT mice (Fig. 2D). NE secretion normalized to atrial NE content was significantly lower in gp130 and p75−/− compared to WT, but not significantly different between the two transgenic strains.
Figure 2. HPLC-ED quantification of atrial NE content and release.
Panels A–C show representative chromatograms of total atrial NE, and the internal control DHBA, from wildtype (A), p75 −/− (B) and gp130 KO (C) mice. (D) Total atrial NE was greater in p75 −/− mice (5.4±0.5 pmol/mg, n=4) than in WT (3.0±0.7 pmol/mg, n=7) and gp130 KO (2.1±0.7 pmol/mg, n=6) mice. (E) In contrast, atrial NE release was higher in WT mice (420±180 fmol/mg) compared to either p75 −/− (90±10 fmol/mg) or gp130 KO (90±40 fmol/mg) mice. Data are mean±SEM, *p<0.05.
4. Discussion
In this study, we have adapted methods to quantify ACh and NE released from cardiac parasympathetic and sympathetic nerves following atrial field stimulation. Our technique overcomes important limitations of classical methods for monitoring transmitter release, which track a single neurotransmitter and only provide information on the fraction of the total pool of that neurotransmitter released but not on the actual amount of transmitter released. This method could readily be adapted to monitor NE together with metabolites such as 3-methoxy-4-hydroxyphenylethyleneglycol (MHPG), providing additional information about neurotransmission in vivo. Given the pathophysiological importance of relative changes in ACh and NE release in the atria [7;8], we believe this new method will provide important benefits for studying regulation of endogenous transmitter release in pathological situations. For example, neuropeptides released after cardiac damage can lower ACh release from parasympathetic nerves [21] in addition to altering sympathetic transmission [22], and release of these peptides is increased in cardiac pathology. Simultaneous analysis of ACh and NE release from cardiac nerves in mice lacking neuropeptides or their receptors will allow for dissection of the factors that cause autonomic imbalance during cardiovascular pathology.
We examined the release of NE and ACh in atria from animals that exhibited altered heart rate control in vivo. Mice lacking p75 have altered sympathetic control of heart rate [12]; atrial NE content is high in these mice but they exhibit a blunted tachycardia response to stress and to tyramine-induced release of endogenous NE [12] suggesting impaired NE release. Indeed, consistent with in vivo observations, we demonstrated a decrease in NE release from p75−/− atria. In addition, we observed that atrial content of NE was higher than in wildtype animals, a finding similar to previous observations in the atria [12], and ventricles [23] from these animals. The increased NE content is entirely consistent with enhanced Trk signaling in these mice which will promote NE synthesis [11;24]. How lack of p75 signaling directly attenuates NE release from atrial terminals is unclear, but p75 modulates sympathetic firing patterns and activation of voltage-gated channels [25], in addition to altering synapse formation in sympathetic ganglia [26]. In contrast to sympathetic innervation, atrial parasympathetic nerve density, and parasympathetic tone, are normal in p75−/− atria [12], and our results showing similar levels of ACh content and release in p75−/− and wildtype mice are consistent with innervation and functional data.
Mice whose autonomic neurons lack gp130, exhibit exacerbated bradycardia in response to vagal nerve stimulation [16] but a blunted tachycardia response to tyramine [16] suggesting enhanced ACh release and impaired NE release. Our data from stimulation-evoked release of ACh and NE are consistent with, and support, the physiological findings we reported previously [16]. Total NE content was normal in gp130 KO atria compared to WT, but NE release was impaired consistent with their blunted tyramine responses. ACh content and release were elevated in the gp130 KO atria consistent with the large vagally-induced bradycardia observed in vivo.
In summary, we present a novel method for simultaneous detection of ACh and NE from the same sample after field stimulation of mouse atria. This method offers excellent sensitivity for ACh (~2.1 fmol) and NE detection (50 fmol) allowing detection of absolute neurotransmitter levels released from atrial nerve terminals. With this novel methodology we demonstrate that autonomic disturbances in p75−/− and gp130 KO transgenic mice have a strong peripheral component, including attenuated atrial NE release in both populations and elevated ACh atrial content and release in gp130 KO mice. We are now in a position to dissect out the role of specific neurotrophins and inflammatory cytokines in regulating autonomic nerve cross-talk at the level of the peripheral effector organ.
Highlights.
Simultaneous quantification acetylcholine and norepinephrine released from atrial nerve fibers.
Norepinephrine release is impaired in p75 −/− atria.
Atrial acetylcholine content and release are augmented in neuronal gp130 KO mice.
Acknowledgements
This work was supported by HL093056 and used instrumentation in the OHSU Bioanalytical Shared Resource and Pharmacokinetics core. We thank Core Director Dr. Dennis Koop for assistance setting up the ACh method, and Jenny Luo for expert technical assistance.
Abbreviations
- HPLC-MS
High performance liquid chromatography-mass spectrometry
- HPLC-ED
High performance liquid chromatography-electrochemical detection
- ACh
Acetylcholine
- NE
Norepinephrine
- P75−/−
p75 neurotrophic receptor knockout mice
- GP130 KO
mice lacking gp130 in noradrenergic neurons
- DHBA
Dihydroxybenzylamine
- PCA
Perchloric acid
- NET
Norepinephrine transporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Levy MN, Blattberg B. Effect of vagal stimulation on the overflow of norepinephrine into the coronary sinus during cardiac sympathetic nerve stimulation in the dog. Circ Res. 1976;38:81–84. doi: 10.1161/01.res.38.2.81. [DOI] [PubMed] [Google Scholar]
- 2.Wetzel GT, Brown JH. Presynaptic modulation of acetylcholine release from cardiac parasympathetic neurons. Am J Physiol Heart Circ Physiol. 1985;248:H33–H39. doi: 10.1152/ajpheart.1985.248.1.H33. [DOI] [PubMed] [Google Scholar]
- 3.Mackins CJ, Kano S, Seyedi N, Schafer U, Reid AC, Machida T, Silver RB, Levi R. Cardiac mast cell-derived renin promotes local angiotensin formation, norepinephrine release, and arrhythmias in ischemia/reperfusion. J Clin Invest. 2006;116:1063–1070. doi: 10.1172/JCI25713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schutte F, Burgdorf C, Richardt G, Kurz T. Adenosine A1 receptor-mediated inhibition of myocardial norepinephrine release involves neither phospholipase C nor protein kinase C but does involve adenylyl cyclase, Can. J. Physiol Pharmacol. 2006;84:573–577. doi: 10.1139/y06-007. [DOI] [PubMed] [Google Scholar]
- 5.Isaka M, Kudo A, Imamura M, Kawakami H, Yasuda K. Endothelin receptors, localized in sympathetic nerve terminals of the heart, modulate norepinephrine release and reperfusion arrhythmias. Basic Research in Cardiology. 2007;102:154–162. doi: 10.1007/s00395-006-0623-2. [DOI] [PubMed] [Google Scholar]
- 6.Sasaoka T, Egi Y, Tawa M, Yamamoto A, Ohkita M, Takaoka M, Maruyama T, Akira T, Matsumura Y. Angiotensin II type 2 receptor-mediated inhibition of norepinephrine release in isolated rat hearts. J. Cardiovasc Pharmacol. 2008;52:176–183. doi: 10.1097/FJC.0b013e31818127f8. [DOI] [PubMed] [Google Scholar]
- 7.La Rovere MT, Pinna GD, Hohnloser SH, Marcus FI, Mortara A, Nohara R, Bigger JT, Jr, Camm AJ, Schwartz PJ. Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation. 2001;103:2072–2077. doi: 10.1161/01.cir.103.16.2072. [DOI] [PubMed] [Google Scholar]
- 8.Schwartz PJ, La Rovere MT. ATRAMI: a mark in the quest for the prognostic value of autonomic markers. Autonomic Tone and Reflexes After Myocardial Infarction. Eur Heart J. 1998;19:1593–1595. doi: 10.1053/euhj.1998.1292. [DOI] [PubMed] [Google Scholar]
- 9.Glebova NO, Ginty DD. Growth and survival signals controlling sympathetic nervous system development. Annu. Rev Neurosci. 2005;28:191–222. doi: 10.1146/annurev.neuro.28.061604.135659. [DOI] [PubMed] [Google Scholar]
- 10.Kuruvilla R, Zweifel LS, Glebova NO, Lonze BE, Valdez G, Ye H, Ginty DD. A neurotrophin signaling cascade coordinates sympathetic neuron development through differential control of TrkA trafficking and retrograde signaling. Cell. 2004;118:243–255. doi: 10.1016/j.cell.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 11.Hannila SS, Lawrance GM, Ross GM, Kawaja MD. TrkA and mitogen-activated protein kinase phosphorylation are enhanced in sympathetic neurons lacking functional p75 neurotrophin receptor expression. Eur J Neurosci. 2004;19:2903–2908. doi: 10.1111/j.0953-816X.2004.03381.x. [DOI] [PubMed] [Google Scholar]
- 12.Habecker BA, Bilimoria P, Linick C, Gritman K, Lorentz CU, Woodward W, Birren SJ. Regulation of cardiac innervation and function via the p75 neurotrophin receptor. Auton. Neurosci. 2008;140:40–48. doi: 10.1016/j.autneu.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoard JL, Hoover DB, Mabe AM, Blakely RD, Feng N, Paolocci N. Cholinergic neurons of mouse intrinsic cardiac ganglia contain noradrenergic enzymes, norepinephrine transporters, and the neurotrophin receptors tropomyosin-related kinase A and p75. Neuroscience. 2008;156:129–142. doi: 10.1016/j.neuroscience.2008.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stanke M, Duong CV, Pape M, Geissen M, Burbach G, Deller T, Gascan H, Otto C, Parlato R, Schutz G, Rohrer H. Target-dependent specification of the neurotransmitter phenotype: cholinergic differentiation of sympathetic neurons is mediated in vivo by gp 130 signaling. Development. 2006;133:141–150. doi: 10.1242/dev.02189. [DOI] [PubMed] [Google Scholar]
- 15.Habecker BA, Sachs HH, Rohrer H, Zigmond RE. The dependence on gp130 cytokines of axotomy induced neuropeptide expression in adult sympathetic neurons. Dev. Neurobiol. 2009;69:392–400. doi: 10.1002/dneu.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parrish DC, Alston EN, Rohrer H, Hermes SM, Aicher SA, Nkadi P, Woodward WR, Stubbusch J, Gardner RT, Habecker BA. The absence of gp130 in dopamine {beta} hydroxylase-expressing neurons leads to autonomic imbalance and increased reperfusion arrhythmias. Am J Physiol Heart Circ Physiol. 2009;297:H960–H967. doi: 10.1152/ajpheart.00409.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee KF, Li E, Huber LJ, Landis SC, Sharpe AH, Chao MV, Jaenisch R. Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell. 1992;69:737–749. doi: 10.1016/0092-8674(92)90286-l. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Rauch A, Lee H, Xiao H, Rainer G, Logothetis NK. Capillary hydrophilic interaction chromatography/mass spectrometry for simultaneous determination of multiple neurotransmitters in primate cerebral cortex, Rapid Commun. Mass Spectrom. 2007;21:3621–3628. doi: 10.1002/rcm.3251. [DOI] [PubMed] [Google Scholar]
- 19.Woodward WR, Seil FJ, Hammerstad JP. Cerebellum plus locus coeruleus in tissue culture. II: Development and metabolism of catecholamines. J. Neurosci. Res. 1987;17:184–188. doi: 10.1002/jnr.490170214. [DOI] [PubMed] [Google Scholar]
- 20.Lorentz CU, Woodward WR, Tharp K, Habecker BA. Altered norepinephrine content and ventricular function in p75NTR−/− mice after myocardial infarction. Auton. Neurosci. 2011;164:13–19. doi: 10.1016/j.autneu.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herring N, Lokale MN, Danson EJ, Heaton DA, Paterson DJ. Neuropeptide Y reduces acetylcholine release and vagal bradycardia via a Y2 receptor-mediated, protein kinase C-dependent pathway. J. Mol. Cell Cardiol. 2008;44:477–485. doi: 10.1016/j.yjmcc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Potter EK, Tripovic D. Modulation of sympathetic neurotransmission by neuropeptide Y Y(2) receptors in rats and guinea pigs. Exp. Brain Res. 2006 doi: 10.1007/s00221-006-0550-3. [DOI] [PubMed] [Google Scholar]
- 23.Lorentz CU, Alston EN, Belcik JT, Lindner JR, Giraud GD, Habecker BA. Heterogeneous ventricular sympathetic innervation, altered {beta} adrenergic receptor expression, and rhythm instability in mice lacking p75 neurotrophin receptor. Am J Physiol Heart Circ Physiol. 2010;298:H1652–H1660. doi: 10.1152/ajpheart.01128.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohn J, Aloyz RS, Toma JG, Haak-Frendscho M, Miller FD. Functionally antagonistic interactions between the TrkA and p75 neurotrophin receptors regulate sympathetic neuron growth and target innervation. J. Neurosci. 1999;19:5393–5408. doi: 10.1523/JNEUROSCI.19-13-05393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luther JA, Birren SJ. p75 and TrkA signaling regulates sympathetic neuronal firing patterns via differential modulation of voltage-gated currents. J Neurosci. 2009;29:5411–5424. doi: 10.1523/JNEUROSCI.3503-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma N, Deppmann CD, Harrington AW, St HC, Chen ZY, Lee FS, Ginty DD. Long-Distance Control of Synapse Assembly by Target-Derived NGF. Neuron. 2010;67:422–434. doi: 10.1016/j.neuron.2010.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]