Abstract
Objective
Because vein graft neointimal hyperplasia engenders vein graft failure, and because most vein graft neointimal cells derive from outside the vein graft, we sought to determine whether vein graft neointimal hyperplasia is affected by activity of the CXC chemokine receptor-4 (CXCR4), which is important for bone marrow-derived cell migration.
Methods
In congenic Cxcr4−/+ and wild-type (WT) recipient mice, we performed interposition grafting of the common carotid artery with the inferior vena cava of either Cxcr4−/+ or WT mice to create 4 surgically chimeric groups of mice (n ≥ 5 each), characterized by vein graft donor/recipient: WT/WT; Cxcr4−/+/WT; WT/Cxcr4−/+; and Cxcr4−/+/Cxcr4−/+; vein grafts were harvested 6 wk post-operatively.
Results
The agonist for CXCR4 is expressed by cells in the arterializing vein graft. Vein graft neointimal hyperplasia was reduced by reducing CXCR4 activity in vein graft-extrinsic cells, but not in vein graft-intrinsic cells: the rank order of neointimal hyperplasia was WT/WT ≈ Cxcr4 −/+/WT > WT/Cxcr4−/+ ≈ Cxcr4 −/+/Cxcr4−/+; CXCR4 deficiency in graft-extrinsic cells reduced neointimal hyperplasia by 39–47% (P < .05). Vein graft medial area was equivalent in all grafts except Cxcr4−/+/Cxcr4−/+, in which medial area was 60±20% greater (P < .05) Vein graft re-endothelialization was indistinguishable among all 3 vein graft groups. However, the prevalence of medial leukocytes was 40±10 % lower in Cxcr4−/+/Cxcr4−/+ thanin WT/WT vein grafts (P < .05), and the prevalence of SMC actin-positive cells was 45±20% higher (P < .05).
Conclusions
We conclude that CXCR4 contributes to vein graft neointimal hyperplasia through mechanisms that alter homing to the vein graft of graft-extrinsic cells, particularly leukocytes.
Clinical Relevance
The utility of autologous vein grafts is severely reduced by neointimal hyperplasia, which accelerates subsequent graft atherosclerosis. Our study demonstrates that vein graft neointimal hyperplasia is aggravated by activity of the cell-surface “CXC” chemokine receptor-4 (CXCR4), which is critical for recruitment of bone marrow-derived cells to sites of inflammation. Our model for CXCR4 deficiency used mice with heterozygous deficiency of Cxcr4. Consequently, our results suggest the possibility that a CXCR4 antagonist—like plerixafor, currently in clinical use—could be applied to vein grafts periadventitially, and perhaps achieve beneficial effects on vein graft neointimal hyperplasia.
INTRODUCTION
Although saphenous vein grafts remain the most commonly used conduit for arterial bypass surgery,1 their durability remains suboptimal: ~28% fail within one year of surgery,2 and ~75% are either occluded or atherosclerotic within 10 years of surgery.1 A major contributor to vein graft failure is neointimal hyperplasia, a process in which medial smooth muscle cells (SMCs) and vascular progenitor cells proliferate and migrate into the layer subjacent to the endothelium.3, 4 Even when it does not compromise vein graft patency, vein graft neointimal hyperplasia appears to accelerate vein graft atherosclerosis.5
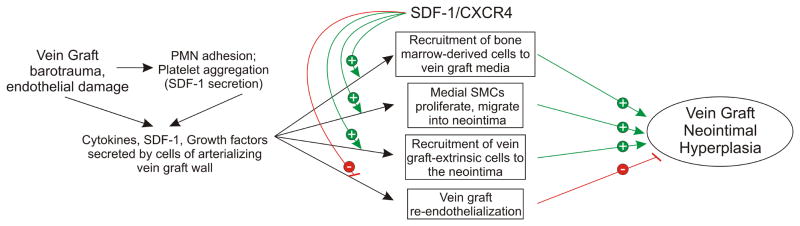
We have found that most vein graft neointimal cells derive from cells that reside outside of the vein graft at the time of its implantation, or from “graft-extrinsic” cells.4 Moreover, many of these graft-extrinsic cells derive from the bone marrow compartment.4 Because an arterializing vein graft constitutes an injured vessel,3 the pathogenesis of vein graft disease involves mechanisms pertinent to tissue repair—such as recruitment of bone marrow-derived cells. An important cell signaling system in this process is that comprising the chemokine known as stromal cell-derived factor-1α (SDF-1, or CXC chemokine ligand-12 [CXCL12]), and its primary chemokine receptor known as CXCR4.6, 7 The SDF-1/CXCR4 signaling system is critical for normal vascular development,8 and mediates cell-specific effects that engender distinct inflammatory phenotypes (Fig 1). For example, CXCR4 promotes migration of both bone marrow-derived cells9 and SMCs.10, 11 However, whereas CXCR4 signaling reduces proliferation of hematopoietic stem cells12 and induces endothelial cell apoptosis,13 it appears to promote proliferation of SMCs.11 In vivo, CXCR4 activation contributes to neointimal hyperplasia associated with wire-mediated arterial injury.14, 15 Nonetheless, CXCR4 activation appears to protect against atherosclerosis—by reducing atherosclerotic plaque infiltration by neutrophils.16 How the SDF-1/CXCR4 signaling system affects inflammation in the arterializing vein graft, and consequent neointimal hyperplasia, remains unknown.
Fig 1.
Possible roles of the SDF-1/CXCR4 signaling system in vein graft neointimal hyperplasia. Green arrows (with “+”) indicate augmentation; red lines (with “−”) indicate inhibition, or reduction. For explanation and references, please see the text of the Introduction.
This study tests the hypothesis that CXCR4 activity, particularly in vein graft-extrinsic cells, contributes to vein graft neointimal hyperplasia. To do so, we used a mouse vein graft model that mimics human vein graft disease,17 in that non-occlusive, SMC-rich neointimal hyperplasia develops over 4–6 weeks. Moreover, in this model, vein graft neointimal hyperplasia is sensitive to effects of single-gene deletions in either the vein graft donor or the congenic vein graft recipient.18, 19
MATERIALS AND METHODS
Studies of immature vein grafts
To investigate the role of CXCR4 in the recruitment of bone marrow-derived progenitor cells to vein graft lesions, we first evaluated whether its ligand SDF-1 is expressed in cells constituting the arterializing vein graft. For this purpose, we harvested carotid interposition vein grafts from WT mice 2 wk post-operatively, when the vein graft wall remains incompletely arterialized (wall thickness and neointimal hyperplasia reach steady state by 4 wk post-operatively).17 We compared immature (2-wk-old) vein grafts with ungrafted IVCs from congenic mice (see below, under “Histology”). In addition, we compared vein grafts (n = 4/group) implanted into WT and Cxcr4−/+ recipients (see below).
Study design for CXCR4 experiments
To study the effects of CXCR4 physiology on vein graft disease, we used CXCR4-deficient and congenic wild-type (WT), C57BL/6 mice. However, because CXCR4 deficiency is lethal in the embryonic or perinatal period,8 we used Cxcr4−/+ mice as our model for CXCR4 loss of function. We implanted interposition vein grafts from WT and congenic Cxcr4−/+ mice into the common carotid arteries of WT or Cxcr4−/+ mice, and thereby created four groups of vein graft donor/recipient mice: WT/WT, Cxcr4−/+/WT, WT/Cxcr4−/+, and Cxcr4−/+/Cxcr4−/+. With these four groups, we could test whether neointimal hyperplasia is reduced by CXCR4 loss of function (heterozygosity) in vein graft-intrinsic cells, vein graft-extrinsic cells, or both cell types. Vein grafts were harvested 6 weeks post-operatively, after neointimal hyperplasia in WT grafts reaches steady state.17
Mice
Wild-type (WT) and congenic Cxcr4−/+ mice on the C57BL/6 genetic background were purchased from Jackson Labs, and maintained as we reported.17 All animal experiments were performed according to protocols approved by the Duke University Institutional Animal Care and Use Committee, and complied with the Guide for the Care and Use of Laboratory Animals (National Research Council).
Vein graft surgery
Interposition vein graft surgery was performed as described previously.17–19 Inferior vena cavae from WT or congenic Cxcr4−/+ donor mice were anastomosed end-to-side to the right common carotid artery of WT or congenic Cxcr4−/+ recipient mice, to create 4 distinct donor/recipient combinations: WT/WT; Cxcr4−/+/WT; WT/Cxcr4−/+; Cxcr4−/+/Cxcr4−/+. After both vein graft anastomoses were secured, the intervening common carotid artery was ligated and cut. All vein graft donors and recipients were matched for gender and age (10–15 wks old), and there were at least 5 independent vein graft donors/recipients for each genotypic group. All 4 surgical groups underwent surgery contemporaneously, and the surgeon was blinded to the genotype of the mice. At 2 or 6 wk post-op, mice were euthanized and vein grafts were harvested as described,17, 18 with PBS perfusion to achieve exsanguination followed by perfusion fixation with PBS/formalin (paraffin-embedded specimens) or incubation in 30% sucrose/PBS overnight followed by embedding in OCT compound (frozen sections).
Histology
All specimens were sectioned at 5 μm, from the distal or middle third of the vein graft specimens; all four vein graft groups were matched for section location, so as to minimize variation among grafts of identical donor/recipient genotype groups.17 WT vein grafts implanted into WT recipient mice (“WT/WT”) developed neointimal hyperplasia characteristic of the pre-atherosclerotic stages of human vein graft disease17: multiple layers of smooth muscle cells (SMCs) that do not cause significant luminal stenosis.
Morphometry was performed as described,17 on perfusion-fixed specimens stained with a modified Masson’s trichrome and Verhoeff’s elastic tissue stain that facilitates the simultaneous identification of collagen (green), elastin (black), cytoplasm (red), and nuclei (black).17 The neointimal/medial boundary was defined as the transition from the cytoplasm-rich, disorganized neointima to the collagen-rich media.17 The medial/adventitial boundary was defined as the transition from the more densely organized medial collagen to the less densely organized, vasa vasora-containing collagenous network of the adventitia.17 Neointimal area was measured as the cross-sectional area subtended by the luminal perimeter and the neointimal/medial boundary. Medial area was measured as the cross-sectional area subtended by the neointimal/medial and medial/adventitial boundaries. All measurements were made on two sections per vein graft, by observers blinded to specimen identity.
We stained immunofluorescently as described,4, 18, 20 with the following IgGs (or corresponding isotype negative-control IgGs): rabbit anti-SDF-1 (Santa Cruz Biotechnology), mouse anti-collagen I (Sigma-Aldrich), and anti-SMC-α-actin (Cy5-conjugated 1A4, Sigma-Aldrich) and rabbit anti-CD45 (H-230, Santa Cruz Biotechnology), for the purpose of recognizing fibrocytes21 as well as leukocytes. Immunofluorescence microscopy with narrow band-pass filters was performed as described.4, 18, 19, 22 Protein immunofluorescence was normalized to DNA fluorescence in the same microscopic field, and quantitated as described.4, 18–20, 22, 23 Morphometric and immunofluorescence data were quantitated by observers blinded to specimen identity.
Vein graft endothelialization
Work from our group19 and others24 has shown that during the early phase of arterialization, there is significant damage to the vein graft endothelium. To quantitate re-endothelialization, we stained vein graft cross sections for vWF (as described19); an observer blinded to specimen identity assessed the percentage of the luminal surface that stained positive for vWF.19
Statistical analysis
One-way ANOVA with Tukey’s post-hoc test for multiple comparisons was used to analyze morphometric and protein expression data. Data are presented as means ± SD in the text and ±SE in the figures.
RESULTS
Immature vein grafts express SDF-1
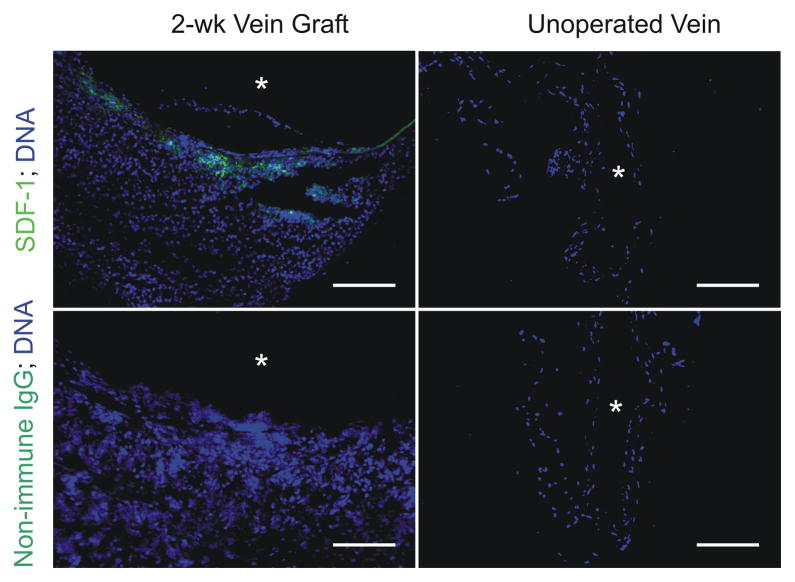
In our mouse model of vein grafting, immature,17 arterializing vein grafts express SDF-1 protein, discerned by immunofluorescence microscopy of 2-wk-old vein grafts (Fig 2). However, no SDF-1 could be detected in cognate IVCs that had not been subjected to vein grafting (Fig 2). Thus, like atherosclerotic25 and mechanically injured arteries,26 vein grafts may use SDF-1 to recruit cells.
Fig 2.
SDF-1 is expressed in arterializing vein grafts. Carotid interposition vein grafts in WT mice were created with syngeneic inferior venae cavae and harvested 2 wk post-op, before the completion of arterialization. IVCs from WT mice of equivalent age were also harvested, for comparison. Serial frozen sections were stained with SDF-1-specific or non-immune rabbit IgG, anti-rabbit IgG/Alexa-488 (green), and Hoechst 33342 to visualize DNA (blue). Shown are sections from single specimens, representative of 4 independent vein graft and IVC specimens. Scale bar = 100 μm (original mag nification ×220). Equivalent results were obtained with WT/WT, WT/Cxcr4−/+, and Cxcr4−/+/Cxcr4−/+ vein grafts (see Methods).
CXCR4 promotes vein graft neointimal hyperplasia
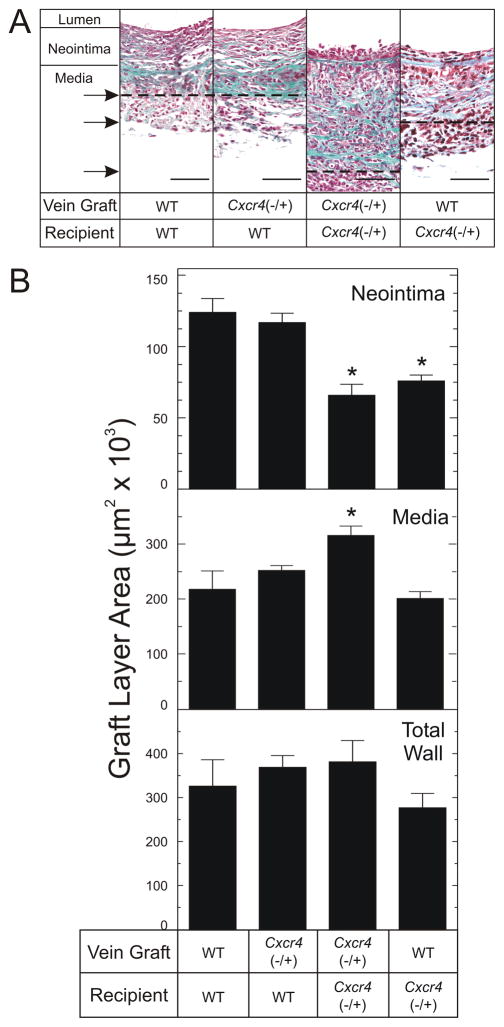
Compared with the WT/WT control vein grafts, the Cxcr4−/+/WT vein grafts demonstrated equivalent neointimal hyperplasia, as assessed by neointimal area (120±20×103 μm2 versus 120±10×103 μm2, Fig 3). Thus, CXCR4 activity in vein graft-intrinsic cells did not appear to affect vein graft neointimal hyperplasia. In contrast to these vein graft findings in WT recipient mice, however, vein grafts implanted into Cxcr4−/+ recipient mice showed substantially less neointimal hyperplasia—whether the vein graft donor was WT or Cxcr4−/+ (Fig 3). Compared with neointimal area in WT/WT or Cxcr4−/+/WT vein grafts, neointimal area was ~39–47% less in vein grafts that were WT/Cxcr4−/+ (76±9×103 μm2) or Cxcr4−/+/Cxcr4−/+ (70±20×103μm2) (P < .05, Fig 3B). Intriguingly, medial area was 60±20% greater (P < .05) in Cxcr4−/+/Cxcr4−/+ vein grafts (320±40×103μm2) than in WT/WT control vein grafts (200±40×103μm2, Fig 3). Consequently, total vein graft wall area (media plus neointima) did not differ among the 4 vein graft groups (Fig 3), and neither did luminal area (data not shown). Thus, unlike CXCR4 activity in graft-intrinsic cells, CXCR4 activity in vein graft-extrinsic cells promotes neointimal hyperplasia.
Fig 3.
CXCR4 activity in vein graft-extrinsic cells contributes to vein graft neointimal hyperplasia. Carotid interposition vein grafts from the indicated mouse donors were placed into the indicated congenic recipient mice and harvested 6 wks later, after perfusion fixation. Vein graft sections were stained with a modified connective tissue stain. A, Photomicrographs of single specimens, representative of ≥ 5 obtained of each type. Specimens are aligned at the neointimal/medial border; the medial/adventitial borders are indicated by the arrows and corresponding dashed lines. Scale bar = 50 μm (original magnification ×440). B, Neointimal and medial areas were measured for each specimen cross section by an observer blinded to specimen identity, using computerized planimetry. “Total Wall” refers to the sum of neointimal plus medial areas. Shown are means ± S.E. from ≥ 5 independent vein graft specimens from each group. Compared with WT/WT control vein grafts: *, P < .05.
CXCR4 does not affect vein graft re-endothelialization
Because the mature vein graft endothelium comprises both vein graft-intrinsic and –extrinsic endothelial cells4 that can be affected by CXCR4 activity,13 we sought to determine whether CXCR4 in graft-intrinsic or –extrinsic cells affected vein graft re-endothelialization. In mature vein grafts, the degree of endothelialization was indistinguishable among our 4 vein graft groups: WT/WT (93±6%), Cxcr4−/+/WT (92±7%), WT/Cxcr4−/+ (94±2%) and Cxcr4−/+/Cxcr4−/+ (91±4%) (Fig 4). Thus, as with arterial injury,15 in vein graft arterialization there appears to be no effect of CXCR4 on re-endothelialization.
Fig 4.
CXCR4 does not affect vein graft re-endothelialization. Vein grafts of the indicated donor/recipient combinations were harvested 6 weeks post-operatively and fixed in formalin. A, Specimen cross sections were fluorescently stained simultaneously for von Willebrand factor to identify endothelial cells (green), and for DNA (blue). Shown are photomicrographs from single samples, representative of ≥ 5 vein grafts in each group. Scale bar = 100 μm (original magnification ×440, lumen oriented upward). B, The length of luminal border staining positively for von Willebrand factor was divided by the total lumen perimeter, to obtain the “% re-endothelialization,” which is plotted as mean ± SE of ≥ 5 independent vein graft specimens from each group.
CXCR4 alters vein graft composition
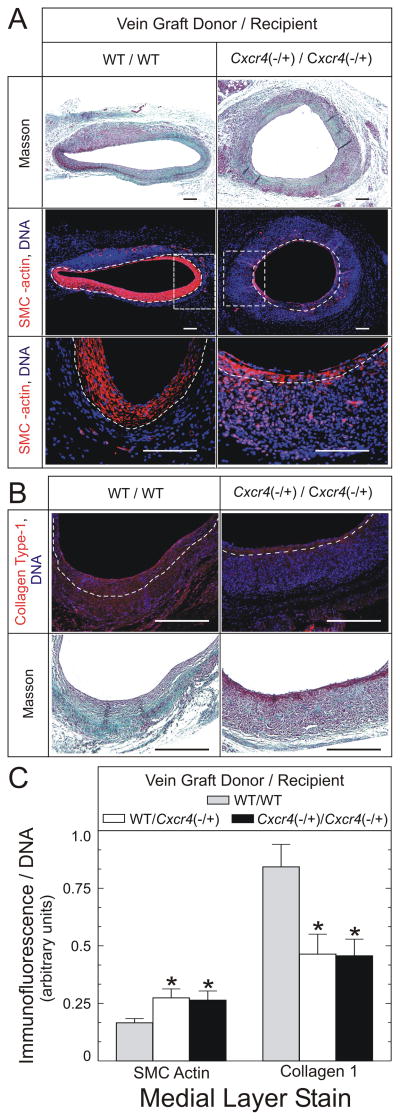
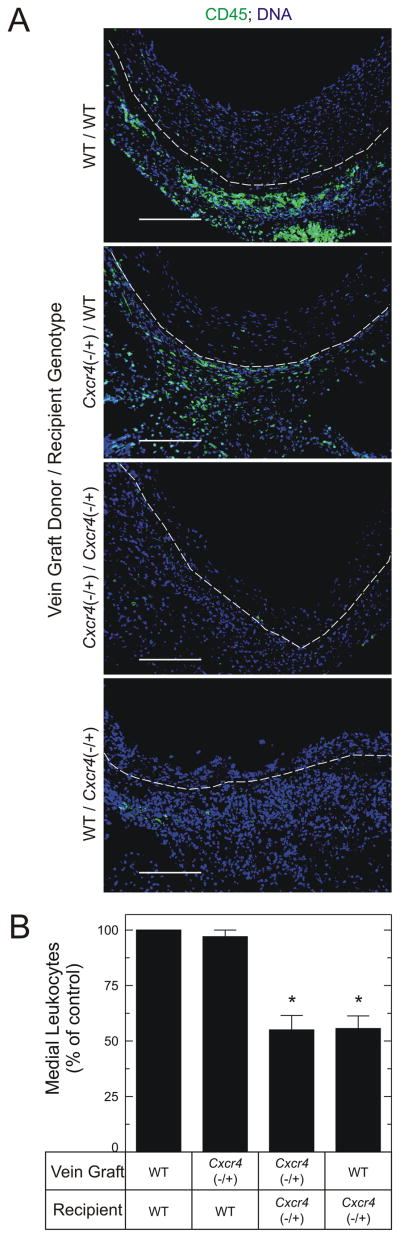
To determine whether CXCR4 in vein graft-intrinsic or –extrinsic cells altered the cellular composition of the vein graft, we first determined the vein graft prevalence of α-SMC actin-positive cells. Whereas SMCs were more abundant in the larger neointimas of WT/WT vein grafts (Figs 3 and 5), they were 1.6±0.2-fold more prevalent in the media of vein grafts from Cxcr4−/+ recipients (P < .05, Fig 5). Despite the higher prevalence of medial SMCs, however, vein grafts from Cxcr4−/+ recipients had only 55±8% as much medial collagen I as WT/WT vein grafts (P < .05, Fig 5). Nevertheless, congruently with a higher prevalence of SMCs, the media of vein grafts from Cxcr4−/+ recipients had a lower prevalence of leukocytes, as demonstrated by staining for the pan-leukocyte marker CD45: as compared with WT/WT or Cxcr4−/+/WT vein grafts, WT/Cxcr4−/+ and Cxcr4−/+/Cxcr4−/+ vein grafts had 56±5% and 55±6% as many medial leukocytes, respectively (P < .05, Fig 6). Thus, the media of vein grafts from Cxcr4−/+ recipients appears to be enriched in SMCs and poor in leukocytes, compared with vein grafts from WT recipients.
Fig 5.
CXCR4 activity affects vein graft composition. Vein grafts of the indicated donor/recipient groups were harvested 6 weeks postoperatively and stained with a modified connective tissue stain (“Masson”), from which neointimal/medial boundaries were identified. Serial sections were fluorescently stained simultaneously for α-SMC actin (A, middle and lower panels, red) and DNA (blue), or for collagen type I (B, upper panel, red) and DNA (blue). Dotted white lines indicate the neointimal/medial boundaries. A, SMC-actin-stained sections. In the middle panels, the dotted rectangles delimit the areas further enlarged for depiction in the lower panels. Scale bars = 100 μm (original magnification ×110, middle panels; ×440, lower panels). B, Collagen I-stained sections; scale bar = 100 μm (original magnification ×440). C, SMC actin and collagen type I fluorescence in the medial layer of each vein graft were normalized to cognate DNA fluorescence intensity within each microscopic field. The resulting ratios were normalized, within each staining group, to those of WT/WT samples to yield “% of control.” Data are plotted as the means ± S.E. of ≥ 4 independent vein graft specimens from each group. Compared with WT/WT control vein grafts: *, P < .05.
Fig 6.
CXCR4 activity in vein graft-extrinsic cells promotes leukocyte recruitment to vein grafts. Vein grafts with donors and recipients of the indicated genotypes were harvested 6 weeks postoperatively, and were fluorescently stained simultaneously for CD45 (green) and DNA (blue). A, Dotted white lines indicate the neointimal/medial boundaries. Shown are photomicrographs from single samples, representative of ≥ 4 in each group. Scale bar = 100μm (original magnification ×220, lumen oriented upward). B, CD45 fluorescence in the medial layer of each vein graft was normalized to cognate DNA fluorescence intensity; the resulting ratios were normalized within each staining group to those of WT/WT samples to yield “% of control,” plotted as the means ± S.E. of ≥ 4 independent vein graft specimens from each group. Compared with WT/WT control vein grafts: *, P < .05.
DISCUSSION
Using a genetic approach to reduce CXCR4 activity, our work demonstrates that vein graft neointimal hyperplasia is exacerbated by CXCR4 activity in vein graft-extrinsic cells. Our work also suggests that SDF-1/CXCR4 signaling system promotes inflammation associated with vein graft arterialization, by demonstrating that Cxcr4−/+ vein graft recipients have fewer leukocytes in the vein graft media. In vein grafts, the beneficial effects attributable to CXCR4 deficiency were observed when CXCR4 expression was reduced by only ~50%,27 in Cxcr4−/+ graft recipient mice. Consequently, these studies highlight the possibility that inhibiting CXCR4—at least in the local vein graft environment—could achieve clinically beneficial effects.
The importance of the SDF-1/CXCR4 signaling system in vein graft arterialization accords with current concepts regarding the pathophysiology of vein graft disease. Consequent to the barotrauma associated with implantation in the arterial circuit,3, 24, 28 defects in the vein graft endothelium trigger platelet adhesion to the vein graft’s luminal surface; platelet-secreted SDF-129 then functions to recruit vein graft-extrinsic cells to the vein graft. During vein graft arterialization, cells of the burgeoning vein graft media and neointima also secrete SDF-1—as Fig 2 demonstrates. Reducing CXCR4 activity in both vein graft-extrinsic and graft-intrinsic cells attenuates neointimal hyperplasia substantially. However, inhibiting CXCR4 activity globally does not appear to engender thinning of the vein graft wall overall, because of increased medial hypertrophy (Fig 3). Thus, because they may not prevent medial or total vein graft wall thickening, vein graft therapies that inhibit CXCR4 may decrease neointimal hyperplasia without increasing vein graft wall stress (by the Law of LaPlace).3, 5, 30
That the Cxcr4−/+/Cxcr4−/+ vein grafts demonstrated larger medial areas than other vein graft types was surprising, in light of our previous finding that a large proportion of vein graft medial cells derive from the bone marrow.4 The higher prevalence of SMC-actin-positive cells in the media of Cxcr4−/+/Cxcr4−/+ vein grafts (Fig 5) suggested the possibility that Cxcr4−/+/Cxcr4−/+ vein grafts, compared with WT/WT vein grafts, comprised more fibrocytes—bone marrow-derived cells that differentiate from monocyte-like cells into SMC actin-expressing myofibroblasts.21, 31 However, the SMC actin-expressing cells of the Cxcr4−/+/Cxcr4−/+ vein grafts appeared relatively deficient in two other key markers of fibrocytes21: collagen I and CD45 (Figs 5 and 6). Consequently, we infer that the media of Cxcr4−/+/Cxcr4−/+ vein grafts is populated principally by SMCs, and not bone marrow-derived fibrocytes.
Reduction in CXCR4 activity reduced the prevalence of leukocytes in arterialized vein grafts (Fig 6), just as CXCR4 antagonism reduced macrophage density in injured mouse femoral arteries.15 These observations accord with models of CXCR4-dependent recruitment of inflammatory cells,6 and may explain the reduction of vein graft neointimal hyperplasia we observed in Cxcr4−/+ vein graft recipients. By decreasing leukocyte density in arterializing vein grafts, reduction of vein graft-extrinsic cells’ CXCR4 activity diminishes the number of cytokine-secreting cells in the vein graft.18 A reduction in vein graft cytokine levels, in turn, would be expected to reduce medial SMC activation, manifest as proliferation and migration into the neointimal layer of the vein graft.3 Perhaps for these reasons, our data suggest that vein graft neointimal hyperplasia depends upon CXCR4 activity in vein graft-extrinsic cells, rather than vein graft-intrinsic cells—even though CXCR4 activation can drive SMC proliferation and migration.32
CXCR4 deficiency had no effect on vein graft re-endothelialization, just as CXCR4 antagonism failed to affect re-endothelialization of wire-injured arteries.14, 15 This result may be attributable to one or more of several factors. First, graft-extrinsic endothelial cells constitute only ~10% of total vein graft endothelial cells in our model,4 and consequently our approach may not have been sensitive enough to discern CXCR4-dependent differences in such a small fraction of the vein graft endothelial cells. Second, CXCR4-mediated endothelial progenitor cell recruitment27 may be offset by CXCR4-promoted endothelial cell apoptosis and cytokine secretion,13 which by recruiting inflammatory cells to the vein graft may further promote endothelial cell apoptosis.19
Study Limitations
These mouse studies were performed with interposition vein grafts, and thus cannot model all of the hemodynamic parameters that obtain in vein grafts used to bypass partially patent, atherosclerotic vessels. In addition, our genetic approach to reducing CXCR4 activity—heterozygous gene deletion—cannot be applied to humans. However, the CXCR4 antagonist plerixafor (AMD3100) is safely used in humans,33, 34 and could conceivably be used to treat vein grafts focally, at the time of implantation. Because our experiments did not use plerixafor, our work cannot determine whether vein graft-localized plerixafor treatment, perhaps in the form of a peri-adventitial gel,35, 36 could reduce vein graft neointimal hyperplasia—by inhibiting CXCR4 not only on vein graft-intrinsic cells but also on graft-extrinsic cells, as they endeavor to migrate into the graft.35, 36 Thus, the clinical translatability of our CXCR4 findings remains to be determined.
CONCLUSIONS
The present study demonstrates that the SDF-1/CXCR4 signaling system in vein graft-extrinsic cells contributes to vein graft neointimal hyperplasia. Mechanisms underlying this phenomenon include CXCR4-mediated recruitment of inflammatory cells to the vein graft.
Acknowledgments
This work was supported, in part, by NIH grants HL77185 and HL73042 (to NJF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sabik JF., 3rd Understanding saphenous vein graft patency. Circulation. 2011;124:273–5. doi: 10.1161/CIRCULATIONAHA.111.039842. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JH, Hafley G, Harrington RA, Peterson ED, Ferguson TB, Jr, Lorenz TJ, et al. Efficacy and safety of edifoligide, an E2F transcription factor decoy, for prevention of vein graft failure following coronary artery bypass graft surgery: PREVENT IV: a randomized controlled trial. JAMA. 2005;294:2446–54. doi: 10.1001/jama.294.19.2446. [DOI] [PubMed] [Google Scholar]
- 3.Cai X, Freedman NJ. New therapeutic possibilities for vein graft disease in the post-edifoligide era. Future Cardiol. 2006;2:493–501. doi: 10.2217/14796678.2.4.493. [DOI] [PubMed] [Google Scholar]
- 4.Zhang L, Freedman NJ, Brian L, Peppel K. Graft-extrinsic cells predominate in vein graft arterialization. Arterioscler Thromb Vasc Biol. 2004;24:470–6. doi: 10.1161/01.ATV.0000116865.98067.31. [DOI] [PubMed] [Google Scholar]
- 5.Mann MJ, Gibbons GH, Kernoff RS, Diet FP, Tsao PS, Cooke JP, et al. Genetic engineering of vein grafts resistant to atherosclerosis. Proc Natl Acad Sci U S A. 1995;92:4502–6. doi: 10.1073/pnas.92.10.4502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: an update. Arterioscler Thromb Vasc Biol. 2008;28:1897–908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 7.Warren DT, Shanahan CM. Defective DNA-damage repair induced by nuclear lamina dysfunction is a key mediator of smooth muscle cell aging. Biochem Soc Trans. 2011;39:1780–5. doi: 10.1042/BST20110703. [DOI] [PubMed] [Google Scholar]
- 8.Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–4. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 9.Jujo K, Hamada H, Iwakura A, Thorne T, Sekiguchi H, Clarke T, et al. CXCR4 blockade augments bone marrow progenitor cell recruitment to the neovasculature and reduces mortality after myocardial infarction. Proc Natl Acad Sci U S A. 2010;107:11008–13. doi: 10.1073/pnas.0914248107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kodali R, Hajjou M, Berman AB, Bansal MB, Zhang S, Pan JJ, et al. Chemokines induce matrix metalloproteinase-2 through activation of epidermal growth factor receptor in arterial smooth muscle cells. Cardiovasc Res. 2006;69:706–15. doi: 10.1016/j.cardiores.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Huynh J, Nishimura N, Rana K, Peloquin JM, Califano JP, Montague CR, et al. Age-related intimal stiffening enhances endothelial permeability and leukocyte transmigration. Sci Transl Med. 2011;3:112ra22. doi: 10.1126/scitranslmed.3002761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nie Y, Han YC, Zou YR. CXCR4 is required for the quiescence of primitive hematopoietic cells. J Exp Med. 2008;205:777–83. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Melchionna R, Porcelli D, Mangoni A, Carlini D, Liuzzo G, Spinetti G, et al. Laminar shear stress inhibits CXCR4 expression on endothelial cells: functional consequences for atherogenesis. FASEB J. 2005;19:629–31. doi: 10.1096/fj.04-2219fje. [DOI] [PubMed] [Google Scholar]
- 14.Zernecke A, Schober A, Bot I, von Hundelshausen P, Liehn EA, Mopps B, et al. SDF-1alpha/CXCR4 axis is instrumental in neointimal hyperplasia and recruitment of smooth muscle progenitor cells. Circ Res. 2005;96:784–91. doi: 10.1161/01.RES.0000162100.52009.38. [DOI] [PubMed] [Google Scholar]
- 15.Shiba Y, Takahashi M, Yoshioka T, Yajima N, Morimoto H, Izawa A, et al. M-CSF Accelerates Neointimal Formation in the Early Phase After Vascular Injury in Mice. The Critical Role of the SDF-1-CXCR4 System. Arterioscler Thromb Vasc Biol. 2007;27:283–9. doi: 10.1161/01.ATV.0000250606.70669.14. [DOI] [PubMed] [Google Scholar]
- 16.Zernecke A, Bot I, Djalali-Talab Y, Shagdarsuren E, Bidzhekov K, Meiler S, et al. Protective role of CXC receptor 4/CXC ligand 12 unveils the importance of neutrophils in atherosclerosis. Circ Res. 2008;102:209–17. doi: 10.1161/CIRCRESAHA.107.160697. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Hagen PO, Kisslo J, Peppel K, Freedman NJ. Neointimal hyperplasia rapidly reaches steady state in a novel murine vein graft model. J Vasc Surg. 2002;36:824–32. [PubMed] [Google Scholar]
- 18.Zhang L, Peppel K, Brian L, Chien L, Freedman NJ. Vein graft neointimal hyperplasia is exacerbated by tumor necrosis factor receptor-1 signaling in graft-intrinsic cells. Arterioscler Thromb Vasc Biol. 2004;24:2277–83. doi: 10.1161/01.ATV.0000147766.68987.0d. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L, Sivashanmugam P, Wu JH, Brian L, Exum ST, Freedman NJ, et al. Tumor necrosis factor receptor-2 signaling attenuates vein graft neointima formation by promoting endothelial recovery. Arterioscler Thromb Vasc Biol. 2008;28:284–9. doi: 10.1161/ATVBAHA.107.151613. [DOI] [PubMed] [Google Scholar]
- 20.Wu JH, Zhang L, Fanaroff AC, Cai X, Sharma KC, Brian L, et al. G Protein-coupled Receptor Kinase-5 Attenuates Atherosclerosis by Regulating Receptor Tyrosine Kinases and 7-transmembrane Receptors. Arterioscler Thromb Vasc Biol. 2012;32:308–16. doi: 10.1161/ATVBAHA.111.239608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–46. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L, Peppel K, Sivashanmugam P, Orman ES, Brian L, Exum ST, et al. Expression of tumor necrosis factor receptor-1 in arterial wall cells promotes atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1087–94. doi: 10.1161/ATVBAHA.0000261548.49790.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang L, Connelly JJ, Peppel K, Brian L, Shah SH, Nelson S, et al. Aging-related atherosclerosis is exacerbated by arterial expression of tumor necrosis factor receptor-1: evidence from mouse models and human association studies. Hum Mol Genet. 2010;19:2754–66. doi: 10.1093/hmg/ddq172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davies MG, Klyachkin ML, Dalen H, Massey MF, Svendsen E, Hagen PO. The integrity of experimental vein graft endothelium--implications on the etiology of early graft failure. Eur J Vasc Surg. 1993;7:156–65. doi: 10.1016/s0950-821x(05)80756-x. [DOI] [PubMed] [Google Scholar]
- 25.Abi-Younes S, Sauty A, Mach F, Sukhova GK, Libby P, Luster AD. The stromal cell-derived factor-1 chemokine is a potent platelet agonist highly expressed in atherosclerotic plaques. Circ Res. 2000;86:131–8. doi: 10.1161/01.res.86.2.131. [DOI] [PubMed] [Google Scholar]
- 26.Schober A, Knarren S, Lietz M, Lin EA, Weber C. Crucial role of stromal cell-derived factor-1alpha in neointima formation after vascular injury in apolipoprotein E-deficient mice. Circulation. 2003;108:2491–7. doi: 10.1161/01.CIR.0000099508.76665.9A. [DOI] [PubMed] [Google Scholar]
- 27.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, et al. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–51. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 28.Brown MA, Zhang L, Levering VW, Wu JH, Satterwhite LL, Brian L, et al. Human umbilical cord blood-derived endothelial cells reendothelialize vein grafts and prevent thrombosis. Arterioscler Thromb Vasc Biol. 2010;30:2150–5. doi: 10.1161/ATVBAHA.110.207076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Massberg S, Konrad I, Schurzinger K, Lorenz M, Schneider S, Zohlnhoefer D, et al. Platelets secrete stromal cell-derived factor 1alpha and recruit bone marrow-derived progenitor cells to arterial thrombi in vivo. J Exp Med. 2006;203:1221–33. doi: 10.1084/jem.20051772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ehsan A, Mann MJ, Dell’Acqua G, Dzau VJ. Long-term stabilization of vein graft wall architecture and prolonged resistance to experimental atherosclerosis after E2F decoy oligonucleotide gene therapy. J Thorac Cardiovasc Surg. 2001;121:714–22. doi: 10.1067/mtc.2001.111204. [DOI] [PubMed] [Google Scholar]
- 31.Maharjan AS, Pilling D, Gomer RH. High and low molecular weight hyaluronic acid differentially regulate human fibrocyte differentiation. PLoS ONE. 2011;6:e26078. doi: 10.1371/journal.pone.0026078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jie W, Wang X, Zhang Y, Guo J, Kuang D, Zhu P, et al. SDF-1alpha/CXCR4 axis is involved in glucose-potentiated proliferation and chemotaxis in rat vascular smooth muscle cells. Int J Exp Pathol. 2010;91:436–44. doi: 10.1111/j.1365-2613.2010.00720.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:4767–73. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- 34.DiPersio JF, Stadtmauer EA, Nademanee A, Micallef IN, Stiff PJ, Kaufman JL, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–6. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- 35.Giangrande PH, Zhang J, Tanner A, Eckhart AD, Rempel RE, Andrechek ER, et al. Distinct roles of E2F proteins in vascular smooth muscle cell proliferation and intimal hyperplasia. Proc Natl Acad Sci U S A. 2007;104:12988–93. doi: 10.1073/pnas.0704754104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah SH, Freedman NJ, Zhang L, Crosslin DR, Stone DH, Haynes C, et al. Neuropeptide Y gene polymorphisms confer risk of early-onset atherosclerosis. PLoS Genet. 2009;5:e1000318, 1–10. doi: 10.1371/journal.pgen.1000318. [DOI] [PMC free article] [PubMed] [Google Scholar]