Abstract
Much is known concerning the cellular and molecular basis for CD8+ T memory immune responses. Nevertheless, conditions that selectively support memory generation have remained elusive. Here we show that an immunization regimen that delivers TCR signals through a defined antigenic peptide, inflammatory signals through LPS, and growth and differentiation signals through the IL-2R initially favors antigen-specific CD8+ T cells to rapidly and substantially develop into T effector-memory cells by TCR transgenic OVA-specific OT-I CD8+ T cells. Amplified CD8+ T memory development depends upon a critical frequency of antigen-specific T cells and direct responsiveness to IL-2. A homologous prime-boost immunization protocol with transiently enhanced IL-2R signaling in normal mice led to persistent polyclonal antigen-specific CD8+ T cells that supported protective immunity to Listeria monocytogenes. These results identify a general approach for amplified T memory development that may be useful to optimize vaccines aimed at generating robust cell-mediated immunity.
Introduction
There remains a pressing need for vaccines that develop robust CD8+ T cell-mediated immunity to counteract infectious agents that primarily reside within cells of an infected individual and evade the protective mechanisms of specific antibodies. CD8+ T cell responses are characterized by rapid expansion of rare antigen-specific T lymphocytes that usually eliminate infectious threats. These cells then rapidly contract to maintain homeostasis of the immune system (1). Those lymphocytes that persist after contraction represent immune memory (2) and the cells required for prophylactic immunity by vaccines. The decision to develop into memory cells appears to occur early during an immune response before contraction (3). An individual naïve T cell has the potential to develop into a T effector (Teff) or a T memory cell (4–6), suggesting that under the appropriate conditions T memory might be favored.
During priming, the high affinity IL-2R is transiently expressed (7, 8) and signaling through the IL-2R influences both the Teff and memory cell fates (9). IL-2 has been extensively used to boost immunity, including CD8+ T cell-dependent responses, with variable results. In conjunction with a viral infection or peptide immunization, administering IL-2 only minimally increased CD8+ T cell responses whereas experimental maneuvers that more persistently increased IL-2 levels or IL-2R signaling showed somewhat greater efficacy to promote CD8+ primary and memory responses (10–13). More recently, preformed agonist complexes of IL-2 bound to anti-IL-2 (IL2-IC) showed improved pharmacokinetics that greatly increased the half-life of IL-2 in vivo (14, 15). One such complex (IL-2/S4B6) primarily stimulates cells bearing IL-2Rβ and γc that generally boosts all memory CD8+ T cells and has been used to enhance immune responses to tumors and virus (11, 14, 16, 17). Another complex (IL-2/Jes-6.1A12) stimulates cells bearing the high affinity IL-2R (IL-2Rα, IL-2Rβ, and γc) and is thought to be primarily specific for CD4+ T regulatory (Treg) cells (14, 18, 19). However, antigen-responding T cells also express the high affinity IL-2R and respond to this IL2-IC (11).
The cellular and molecular basis for CD8+ T memory formation, persistence and function is increasingly defined. This understanding, however, has not readily yielded approaches that selectively support or amplify T memory responses. Most vaccines to induce cell-mediated immunity strive to prolong immune activation to induce memory. However, extensive antigen stimulation is not required, and may be detrimental, for optimal CD8+ T memory (20–24). Limiting antigen and inflammatory signal each individually favor memory development. However, memory cell numbers do not appreciably increase, but rather Teff development is opposed (25). During initial priming, extensive IL-2R signaling promotes short-lived Teff cells (8, 26, 27) while weaker IL-2R signaling programs CD8+ T memory development that contributes to TEM production and optimal secondary recall responses (7, 26, 28, 29). Based on these considerations, we tested whether T memory responses may be preferentially amplified when several of these conditions are met simultaneously. We report that optimal, but transient, TCR, inflammatory and IL-2R signaling represent conditions that favored CD8+ T memory development and readily support protective immunity by polyclonal T lymphocytes.
Materials and Methods
Mice
CD45.1-congenic C57BL/6, OT-I/Rag1−/−, and IL-15−/− mice were obtained from Taconic Farms and bred within our animal colony. IL-2Rβ−/− (CD122−/−) mice have been previously described (30) and were crossed to OT-I mice to yield OT-I CD122−/− mice. Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Miami.
Adoptive transfer and peptide immunizations
OT-I T cells were purified (typically ≥95%) by magnetic based positive selection using anti-CD8 beads (Miltenyi Biotec). For adoptive transfer, naïve OT-I T cells (CD45.2) were injected i.v. through the tail vein into naïve CD45.1 C57BL/6 recipient mice. Unless otherwise indicated, mice were immunized by i.v. injection in the tail vein with OVA257-264 (10 μg) (AnaSpec, San Jose, CA) and LPS (10 μg) (E.coli 055:B5, Sigma, St. Louis, Missouri). 24 hrs after immunization, the indicated mice received i.p. IL2-IC in 100 μl of HBSS. IL2-IC was prepared by the mixing 1.5 μg mouse IL-2 (eBiosciences) and 15 μg Jes-6.1A12 mAb to mouse IL-2 (eBiosciences) in 18 μl per injection for 15 min at room temperature followed by addition of HBSS.
FACS analysis
Flow cytometry for cell surface molecules, intracellular cytokines, and pStat5 and pS6 were performed as previously described (7, 31). DimerX was purchased from BD-Biosciences and loaded with OVA257-264 according to the manufacturer’s instructions such that 0.33 μg OVA257-264 to 0.5 μg of DimerX was added to ~1×106 cells. Typically 100,000–300,000 events were collected per sample. Intracellular cytokine production was performed after the culture of spleen cells (2.5 × 106 cells/well) from immunized mice in 24 well plates for 5 hrs with OVA257-264 (0.1 nM for OT-I; 100 nM for polyclonal T cells) in the presence of brefeldin A. For pStat5 and pS6, spleens cells were cultured (2.5 × 106 cells/well) in 24 well plates in medium for 30 min, stimulated with mouse IL-2 (10 ng/ml) and IL-15 (10 ng/ml) for 15 min, prior to p-STAT5 and pS6 staining. To examine signaling in vivo, immunized mice were euthanized, spleen suspensions were immediately prepared in RPMI 1640 medium containing 5% fetal calf serum and 1.5% formaldehyde. These cells were incubated at 37° C for 10 min and then permeabilized with ice-cold methanol and then stained for pSTAT5, pS6, and relevant surface markers.
Gene expression analysis
Total RNA was isolated using TRizol and further purified with RNAeasy Minikit (QIAGEN). RNA quantity and quality was evaluated using an Agilent 2100 BioAnalyzer. A single round of linear RNA probe amplification and labeling was performed using NuGEN Ovation Pico WTA system, WT-Ovation Exon module and Encore Biotin Module (NuGEN, San Carlos, CA, USA). Gene expression was assessed using Affymetrix Mouse Gene ST 1.0 arrays at the Microarray and Gene Expression Core within the John P. Hussman Institute for Human Genomics at the University of Miami. Image analysis was performed using the Affymetrix Command Console Software (AGCC). Resulting data were normalized with the RMA method and GEA was performed using software at GeneSifter (Seattle, WA). The expression data are available through the Gene Expression Omnibus data base (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE39110.
Bacterial infections and determination of CFU
Recombinant Listeria monocytogenes that express OVA134-387 (LM-OVA) (32), kindly provided by H. Chen (University of Pennsylvania), was grown using brain heart infusion broth (BHI). Log phase growing (OD560 of 0.2) LM-OVA was diluted in PBS to 5 x103 CFU for primary responses or to 5 × 105 CFU for challenging previously immunized mice and injected i.v. in the tail vein. The actual number of bacteria injected was confirmed by growth on BHI agar plates. 3 days post infection, the spleen and liver were disrupted using a 0.2 μm screen in 0.05% Trition X and CFUs were determined by serial dilutions after incubation for 18 hr at 37° C on BHI agar plates.
Statistical Analysis
Data were analyzed using Prism 5.0 software. All data were analyzed by unpaired Student’s t-test except for protection from Listeria infections where the Mann-Whitney test was used. The p values are shown for statistically significant differences.
Results
IL-2R signaling in amplified CD8+ T memory development
Mice were immunized to induce optimal, but transient, TCR, inflammatory and IL-2R signaling. Initially, we examined CD8+ T memory development by TCR transgenic OVA-specific class I MHC-restricted CD8+ OT-I T cells. OT-I T cells (CD45.2) were adoptively transferred into wild-type (WT) congenic CD45.1-recipient mice, which facilitated identification of donor OT-I T cells. One day after the OT-I cell transfer, recipient mice were immunized with OVA257-264 to induce TCR signaling and LPS to provide an inflammatory signal. IL-2 in the form of a single application of an agonist IL-2/anti-IL-2 complex (IL2-IC) that targets the high affinity IL-2R was administered 20–24 hr after antigen to coincide with expression of the antigen-induced high affinity IL-2R and to take advantage of the improved pharmacokinetics of IL2-IC (14, 15).
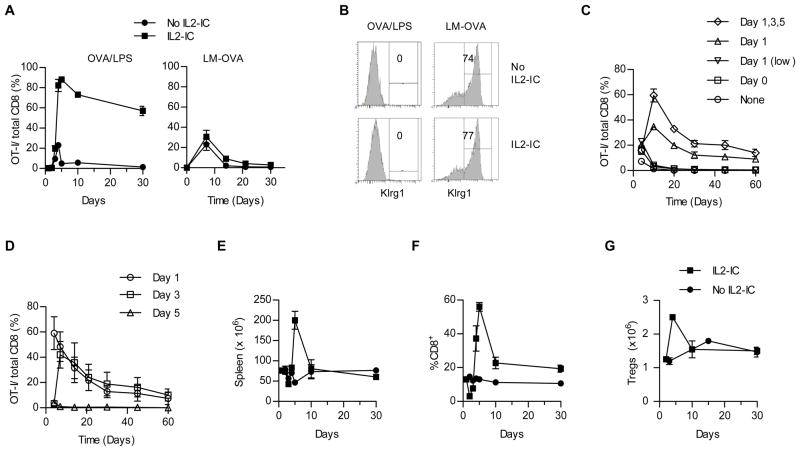
Immunization with MHC class I binding peptides is a direct approach to drive an exogenous antigen into the class I presentation pathway. Immunization with peptides provides a convenient means for transient antigen, but the short half-life of peptides in vivo have generally resulted in disappointing immune responses. However, peptide immunization with OVA257-264 and transient inflammation induced by LPS favored a rapid large production of persistent CD8+ T cells when IL-2R signaling was enhanced by IL2-IC (Fig. 1A left). In the absence of IL2-IC, the magnitude of the primary response to OVA-containing Listeria monocytogenes (LM-OVA) was comparable to that elicited by OVA/LPS (Fig. 1A). Importantly, IL2-IC did not substantially amplify the memory response to LM-OVA (Fig. 1A, right), suggesting that IL-2-amplified CD8+ T memory is specific for immunization with OVA peptide/LPS. Immunization with OVA/LPS did not support production of CD8+ Klrg1+ short-lived Teff cells whereas these cells dominated the OT-I response to LM-OVA (Fig. 1B). This trend was not influenced by IL2-IC, indicating that the production of CD8+ Klrg1+ OT-I cells was primarily due to the nature of the antigen and inflammatory signals and represented conditions that do not support an amplified T memory response.
FIGURE 1.
The effect of IL2-IC on the magnitude and persistence of antigen-activated OT-I T cells. OT -I T cells (2.5–5 × 105 for OVA/LPS or 1 × 104 for LM-OVA) were transferred into CD45.1-congenic B6 mice and 24 hr later were immunized with OVA257-264 (10 μg) and LPS (10 μg) or challenged with LM-OVA (5–25 × 103 CFUs) with and without IL2-IC. Unless otherwise stated, IL2-IC was always administered 24 hr after antigenic challenge using 1.5 μg IL-2/15 μg Jes-6.1A12 mAb. (A) Time course of the OT-I frequency in spleen after challenge with OVA/LPS or in PBL after infection with LM-OVA. Data (mean ± SE) are from 3–9 mice/time point derived from at least 2 experiments. (B) Expression of Klrg1 by OT-I T cells at the peak of the response (day 4 for OVA/LPS; day 7 for LM-OVA). Data are representative of at least 3 mice/group. (C, D) Frequency of OT-I T cells after challenge with OVA/LPS after varying the application of IL2-IC, where low represents the use of 0.5 μg IL-2/μg Jes-6.1A12 mAb. Data (mean ± SE) is representative of 3 (C) and 6 mice/group (D), the latter from 2 experiments. Spleen cells cellularity (E), the frequency of splenic CD8+ T cells (F) and the numbers of splenic Treg cells (G) were assessed for mice immunized with OVA/LPS with and without IL2-IC. Data from (E–G) (mean ± SE) are representative of 3 to 9 mice/time point from 2–3 experiments.
When compared to administering IL-2-IC 1 day after OVA257-264 and LPS priming, the application of a lower amount of IL-2-IC at this interval or the simultaneous immunization with OVA/LPS and IL2-IC did not support increased persistent OT-I T cells (Fig. 1C). Multiple applications of IL2-IC substantially increased the primary response, but only modestly increased persistent OT-I cells when compare to a single application of IL2-IC 24 hr after immunization (Fig. 1C). In addition, a similar level of OT-I memory cells was obtained when IL2-IC was applied 1 and 3, but not 5, days after priming (Fig. 1D). Thus, early after a primary immunization, the antigen-activated T cells showed a window of responsiveness to IL2-IC to promote increased persistent OT-I memory T cells, where antigen-dependent activation must precede that of IL-2. Based on these results, in the remaining experiments in this report, we always applied IL2-IC 24 hr after immunization with OVA/LPS.
IL2-IC using Jes-6.1, which targets the high affinity IL-2R, also caused a transient increased in spleen cell cellularity (Fig. 1E). This effect in part represents a substantial transient increased in CD8+ T cells due to IL2-IC-driven expansion of OVA/LPS activated OT-I T cells (Fig. 1F) and an expected transient increase in CD4+ Foxp3+ Treg cells (Fig. 1G). Collectively, these experiments show that targeting the high affinity IL-2R for a brief period of time is sufficient to selectively support substantial expansion of not only Treg cells but antigen-reactive CD8+ T cells, the latter which persist for an extended period of time.
IL2-IC promotes rapid development of functional TEM cells
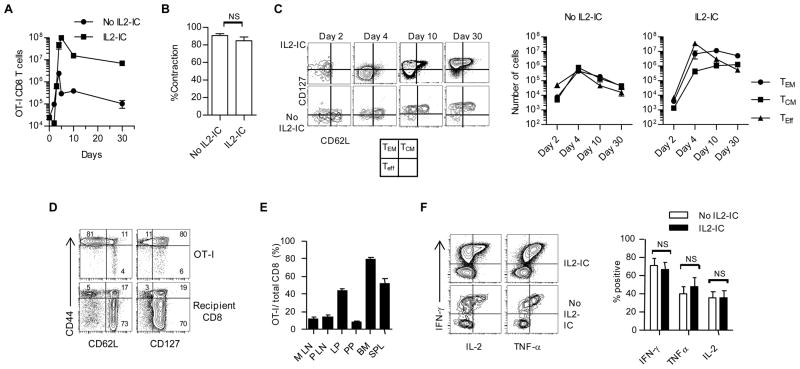
IL2-IC caused the CD8+ T compartment to be dominated by OT-I cells 3–30 days post-immunization (Fig. 2A). This effect is primarily due to enhanced expansion (Fig. 2A, day 4–5) as the level of contraction was similar with and without IL2-IC (Fig. 2B). The overall effect of IL2-IC resulted in a 40- to 70-fold increased in the absolute number of OT-I memory cells by 10 and 30 days post-priming, respectively. At 4 days post priming, IL2-IC favored production of OT-I Teff cells (CD62Llo, CD127lo), but these cells substantially decreased by 10 and 30 days post-immunization (Fig. 2C). In contrast, mainly T effector-memory (TEM) OT-I cells (CD62Llo, CD127hi) were seen 10 and 30 days post-immunization with a minor population of T central memory (TCM) cells (CD62Lhi, CD127hi) (Fig. 2C). Importantly, as assessed 15 days after immunization for mice receiving IL2-IC, only OT-I T cells developed into CD44hi CD62Llo CD127hi TEM cells (84.0 ± 1.0%; mean ± SE) as the remaining recipient-derived CD8+ T cells were mostly naïve CD44lo/int, CD62Lhi and CD127hi cells (69.0 ± 2.4%; mean ± SE) (Fig. 2D). High expression of Ly-6C, which also marks CD8+ memory cells (33), was noted by day 10 post priming, consistent with a rapid development of CD8+ T memory (data not shown). At 30 days post-priming, these memory cells were widely distributed in other lymphoid tissues, including the mesenteric lymph node (MLN), peripheral LN, and Peyer’s patch (PP), and non-lymphoid tissues, including the bone marrow (BM) and the lamina propria (LP) of the small intestine (Fig. 2E). Functional analyses of splenic OT-I cells indicated that at 30 days after priming IL2-IC led to equivalent high production of IFNγ, TNFα, and IL-2 (Fig. 2F). Thus, IL2-IC rapidly amplified the development of highly functional TEM cells.
FIGURE 2.
IL2-IC rapidly amplifies the development of highly functional TEM cells. Mice containing OT-I T cells were then immunized with OVA/LPS (see Fig. 1) in the presence or absence of IL2-IC. (A) Number of OT-I cells in the spleen. (B) Percent contraction of OT-I T cells was calculated from the data in Fig. 2A data where the peak of the response was considered day 4 without IL2-IC and day 5 with IL2-IC and contraction was assessed on day 5 and 10, respectively. (C) OT-I T cells in the spleen were examined for the distribution of Teff, TCM, and TEM based on expression of CD62L and CD127. Data in (A,C) (mean ± SE) are representative 3 to 9 mice/time point from at least 2 experiments. (D) OT-I and recipient-derived CD8+ T cells in the PBL from mice that received IL2-IC were examined at 15 days post-immunization for naïve and TEM cells based on expression of CD44, CD62L and CD127. Data are representative of 4 mice. Numbers within contour plots represent %+ cells. (E) Persistent OT-I memory cells from mice treated with IL2-IC in the MLN, peripheral LN (PLN), LP, PP, BM, and spleen (SPL). Data (mean ± SE) are from 1 experiment containing 4 mice/group. (F) Functional activity of OT-I T cells in the spleen 30 days after immunization by stimulation with OVA257-264 ex vivo. Data were derived from 3 experiments each containing 3–4 mice/group.
Amplified TEM development leads to TCM cells
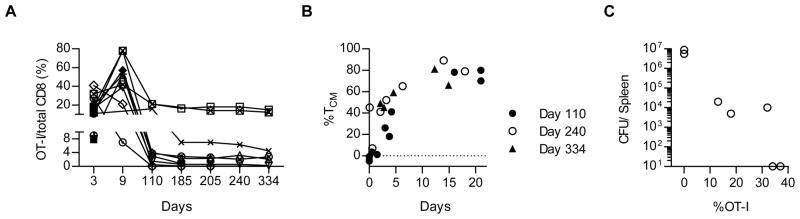
When induced in response to infection, CD8+ TEM cells persist for a relatively short period of time, but appear to give rise to long-lasting TCM cells (3). Similarly, the high level of OT-I TEM cells found on day 30 post-immunization with IL2-IC decreased when mice were examined for up to 334 days (Fig. 3A). For 10 mice followed for ≥110 days, the majority (n=7) contained a substantial fraction of OT-I memory cells (at least 1% of the total CD8+ T cells or ~2 × 105 memory cells, assuming 20 × 106 CD8+ T cells/mouse) and these cells were typically >40% TCM cells (Fig. 3B). For 7 mice followed for 334 days, OT-I memory cells were found in 5 mice and they persisted at levels similar to that found on day 110 post-immunization (Fig. 3A). When these 7 mice were challenged on day 334 with LM-OVA, the 5 mice with detectable OT-I memory cells each generated a recall response, as the proportion of OT-I cells increased 3- to 5-fold in the spleen (Fig. 3C) when compared to the pre-challenged levels in the PBL (Fig. 3A). An anti-LM-OVA protective response was also found based on a substantial reduction in the number of colony forming units (CFU) in the spleen that was proportional to the fraction of recalled OT-I T cells (Fig. 3C). Collectively, these data indicate that a peptide based immunization scheme with IL2-IC initially favors high production of TEM cells but eventually generates long-term persistent TCM cells that induce a potent functional recall response.
FIGURE 3.
Persistence of IL2-IC driven CD8+ T memory cells. OT-I T cells (5 × 105) were transferred into CD45.1 B6 mice and then immunized with OVA257-264, LPS, and IL2-IC. At the indicated days, (A) the proportion of total and (B) the relative representation of TCM by OT-I T cells in the PBL were determined. (C) On day 334 mice were infected with 5 × 105 CFUs of LM-OVA and 3 days later the CFUs and donor OT-I T cells in the spleen were determined. Each point represents an individual mouse.
IL-2R and IL-15R signaling for TEM development
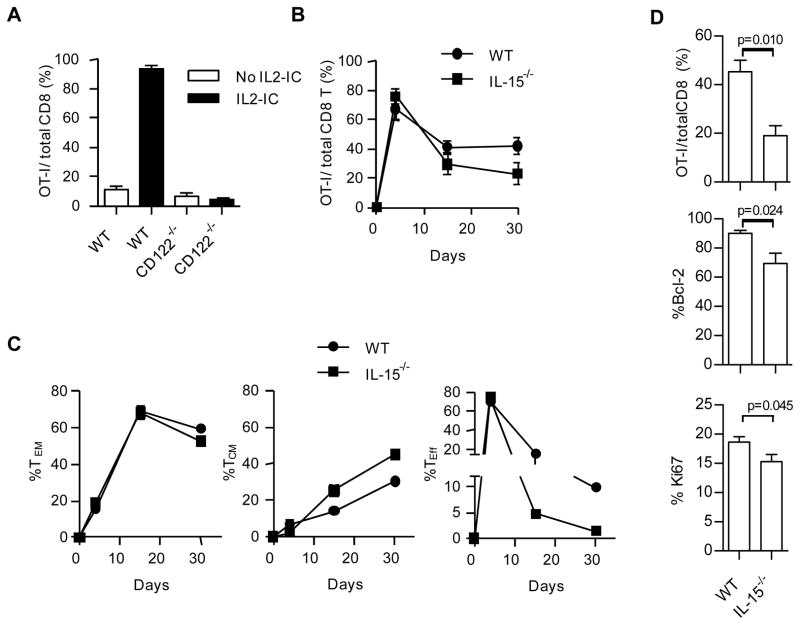
Given the striking effect of IL2-IC on TEM production, we assessed the capacity of IL2-IC to alter IL-2R-dependent signaling by the antigen-responsive OT-I T cells. Our past work showed that in response to OVA257-264 and LPS by OT-I T cells without IL2-IC, IL-2Rα is very transiently expressed and easily detected 24–48 hours post-immunization whereas IL-2Rβ, γc, and IL-15R remained expressed for at least 30 days (7). In this study, IL-2Rα was similar expressed by OT-I after immunization without IL2-IC (Fig. 4A, left). However, for OT-I T cells immunized in the presence of IL2-IC, IL-2Rα was detected for an extended period of time, albeit at a somewhat lower level after day 10 (Fig. 4A, right). This result is consistent with the known role of IL-2 to upregulate expression of IL-2Rα(34) and indicates that the large increased in IL-2Rα expression by IL2-IC is transient.
FIGURE 4.
Contribution of IL-2 and IL-15 signaling in preferential development of CD8+ T memory cells. OT-I T cells were transferred into the indicated mice and immunized as described in Fig. 1. (A) OT-I cells from the spleen were assessed for expression of CD25 for the indicated days after immunization. The mean florescence intensity (MFI) for CD25 expression is displayed for each histogram. Data are representative of at least 2–4 mice/time point. (B,C) Phospho-flow analysis for pSTAT5 and pS6 activation by OT-I T cells from the indicated immunized mice after stimulation with IL-2 and IL-15 for 15 min ex vivo (B) or directly analyzed in vivo (C), the latter evaluated by immediately fixing spleen cells from euthanized mice. For the ex vivo OT-I T cells, the values for pSTAT5 and pS6 in the absence of IL-2 and IL-15 were subtracted from those observed at 15 min with IL-2 and IL-15. Data (B,C) (mean ± SE) are representative of 4 (days 2–10) and 2 (day 30) mice/group.
IL-2- and IL-15-dependent signaling activates the STAT5 and PI3K/Akt pathways through the shared use of IL-2Rβ and γc by their receptors (35, 36). Activation of these pathways by antigen-activated OT-I T cells in the presence or absence of IL2-IC in vivo or ex vivo after re-stimulation with IL-2 or IL-15 was assessed by measuring pSTAT5 and pS6 (downstream of the PI3K/Akt) by phospho-flow analysis. pSTAT5, but not pS6, increased when OT-I cells were cultured with IL-2 ex vivo for 15 min (Fig. 4B, left) and 60 min (not shown).This effect was long-lasting only for the mice that were immunized with IL2-IC, consistent with expression of IL-2Rα. This finding suggests that IL-2-dependent activation of STAT5 is primarily responsible for amplified CD8+ T memory development. Essentially identical responsiveness to IL-15 was noted for OT-I T cells immunized with and without IL2-IC, with only pSTAT5 readily detected (Fig. 4B, right). Thus, immunization with IL2-IC increased the potential of OT-I T cells to respond to IL-2, which may facilitate T memory development and survival, while not altering responsiveness to IL-15. In contrast, pSTAT5 and pS6 activation by OT-I T cells in vivo was observed, but only between days 2–4 post-immunization with IL-2-IC (Fig. 4C). The effect on pS6 likely reflects TCR and/or co-stimulatory signaling because culture of these OT-I cells with IL-2 did not lead to increased pS6 levels (Fig. 4B). Collectively, these experiments are consistent with the main contribution of IL2-IC in amplified TEM development by inducing strong transient IL-2R STAT5-dependent signaling by antigen-responsive T cells early after priming. We cannot, however, rule a out that the increased potential of OT-I cells to activated STAT5 after immunization with IL2-IC may result in weak IL-2R signaling that is difficult to detect in vivo but still contributes to maintain these TEM cells.
The expansion and development of TEM cells depended upon a direct effect of IL2-IC on the OT-I cells because the response by IL-2Rβ−/− (CD122−/−) OT-I cells with IL2-IC resembled that of WT OT-I cells immunized without IL2-IC (Fig. 5A). We also tested the possible contribution of IL-15 in amplified development of TEM cells in the presence of IL2-IC because IL-15R signaling overlaps with IL-2R due to sharing of IL-2Rβ and γc, IL-2 and IL-15 induce similar levels of STAT5 in IL2-IC treated OVA/LPS-primed OT-I T cells (Fig. 4B), and because lL-15 is important for the persistence of CD8+ T memory cells (37). For these experiments, WT CD45.1 OT-I T cells were transferred into CD45.2 IL-15−/− recipients. In the absence of IL-15, time course experiments revealed that OT-I T cells in PBL initially expanded to high numbers but were found at significantly lower levels 30 days post immunization (Fig. 5B). The relative development of TEM cells, however, was not affected by the absence of IL-15 whereas substantially lower Teff cells were detected (Fig. 5C). Analysis of the spleen at 30 days post-immunization confirmed this lower proportion of OT-I T cells in IL-15−/− recipients (Fig. 5D). This lower level is likely due to a role for IL-15 in the homeostasis of the persistent OT-I T cells because in the absence of IL-15 expression of Bcl-2 and the proliferative marker Ki67 was significantly lower (Fig. 5D). Thus, transient and relatively high IL-2R signaling by the responding antigen-specific T cells is required for the development of amplified TEM development while IL-15 supports their survival.
FIGURE 5.
IL-2R signaling is required for the development of TEM development while IL-15 supports their survival. WT or CD122−/− OT-I T cells were transferred into WT CD45.1 B6 (A) or the indicated mice (B–D) and immunized as described in Fig. 1. Requirement for CD122-dependent signaling by examining the fraction of OT-I T cells in the PBL 4 days post immunization in the absence or presence of IL2-IC. Data (mean ± SE) are from 2 experiments each containing 3 mice/group. (B–D) Requirement for IL-15 signaling by enumerating the fraction of OT-I T cells in the PBL (B,C) or in the spleen (D), the latter 30 days post-immunization, where all mice were immunized in the presence of IL2-IC. As indicated, OT-I cells were examined for the distributed of Teff, TCM, and TEM based on expression of CD62L and CD127. (D) OT-I cells were examined for expression of Bcl-2 and Ki67 on day 30 post-immunization. (B–D) Data (mean ± SE) are from a single experiment containing 5 mice/group.
IL-2-dependent gene expression profile and TEM development
To begin to explore the molecular mechanisms that contributes to amplified TEM production, genome-wide transcriptional profiling was performed for OT-I cells immunized in the presence or absence of IL2-IC near the peak of pSTAT5 activation and OT-I expansion and after contraction by OT-I T cells without IL2-IC, i.e. day 3 and 5 post-immunization. Comparison across these groups revealed 1990 Affymetrix targets that varied by at least 2-fold between these samples. Euclidian clustering of these differentially expressed genes revealed 3 clades, with transcripts from day 3 OT-I cells with IL2-IC in a distinct clade, indicating that IL-2 exerted its most distinctive influence on gene expression at this time (Fig. 6A). Gene enrichment analyses (GEA) annotated 1089 of 1990 Affymetrix targets (54.7%) into 5 Gene Ontology functional classifications with z scores from 4.0–17.2. GEA after pairwise analysis revealed that immunization with IL-2-IC increased transcripts in metabolism and cell cycle but down-regulated transcripts for cell death and immune system processes (Fig. 6B). Genes listed within metabolism were also highly enriched in transcripts related to the mitochondrion and 80.4% of these 97 unique targets were over-expressed selectively on day 3 with IL2-IC, suggesting that mitochondria-dependent functions were enhanced by IL-2R signaling. Transcripts for histone modification were selectively enriched on day 3 after priming, suggesting that IL-2 may regulate chromatin remodeling to favors TEM development. Even though individual transcripts between groups varied, similar trends in GEA (down-regulated: metabolism, cell cycle, histone modification; upregulated: cell death, immune system processes) were noted when comparing gene expression day 3 vs. day 5 post-immunization irrespective of administering IL2-IC. This pattern corresponds to gene expression as antigen-activated T cells contract, but T memory cells persist.
FIGURE 6.
Gene expression by OT-I T cells immunized in the presence and absence of IL2-IC. Differential expressed genes from triplicate biological independent replicates were selected based on a 2-fold cut-off using one way ANOVA (p<0.05) after applying the Benjamini and Hochberg correction for false positives.(A) Euclidean clustering of sample relatedness based on differentially expressed genes. (B) Z-score analysis of GEA groups of differentially expressed genes based on the indicated pairwise analysis. Z score >2 was considered to represent significant gene enrichment within a given category. (C) Selected individual differentially expressed genes. All genes shown differed by at least 2-fold except those underlined which are <2-fold.
Considering expression of selected transcripts (Fig. 6C), OT-I cells expressed a gene profile of TEM cells by day 5 after immunization with IL2-IC [Selllow (CD62L), Ccr7lo, Cd44hi, IL7rhi (CD127), Cd27hi, Ly6chi, Gzmbhi, Prf1hi, IFNghi]. This result is consistent with FACS analysis of these cells, further demonstrating the rapidity of TEM development. The development of IL2-IC-amplified TEM cells is likely due in part to low expression of inhibitory receptors (Ctla4 and Lag3) and pro-apoptotic molecules [Fas and Bcl2l11(bim)] selectively on day 3 post-immunization. The accumulation of IL2-IC-driven TEM cells is also reflected by increased expression of transcripts related to cell cycle progression (Cdc34, Nudc, Cdkn3, Birc5, Mki67) as noted day 5 post-priming. IL2-IC caused several transcriptional regulators associated with Teff [Prdm1 (Blimp-1) and Irf4] and TEM (Eomes and Id2) cells to increased, but other factors (Id3, Tcf7) supporting long-lived TCM cells to decrease. Other transcriptional regulators related to Teff (Tcf3, Tbx21, Gata3) and T memory (Bcl6) cells were not substantially altered. As suggested by other studies (29, 38–41), the relative levels of Blimp-1, Eomes, and Id3 and perhaps IRF4 may contribute to preferred TEM development by this immunization scheme.
A prime-boost regimen with IL2-IC supports TEM development by a low number of OT-I
The number of antigen-specific T cells to an individual epitope has been estimated between 50 and 1000 cells within a mouse (42). We have determined that after naïve OT-I T cells are transferred into normal recipient mice, approximately 5% of the cells initially engraft and are available to respond to an antigenic challenge (see Fig. 2A, cell number on Day 0). Thus, preferred TEM development might reflect a response to antigen and IL2-IC by a high number (~2.5 × 104 cells) of naïve OT-I cells. Indeed, when lower numbers of OT-I T cells (0.1–10 × 103) were transferred into CD45.1 B6 mice and immunized in the presence of IL2-IC, a low primary response was detected on day 8 post-immunization, and the cells readily contracted to low levels (Fig. 7A). Nevertheless, successful priming was confirmed based on the proportion and number of OT-I T cells detected in the spleen after these mice were re-challenged with OVA254-264 and LPS (Fig. 7B). This finding indicates that amplified CD8+ TEM development in the primary response (Fig. 1, 2) depends on a relatively high frequency of naïve OT-I T cells.
FIGURE 7.
Memory development by low numbers of antigen-specific naïve T cells. CD45.1-B6 mice received the indicated number of naïve OT-I T cells and were immunized with OVA257-264, LPS, and IL2-IC. (A) OT-I T cells were enumerated in the PBL at the indicated time. (B) At 30 days post immunization, mice were re-challenged with OVA257-264 and LPS and OT-I cells were enumerated in the spleen 3 days later. Data (mean ± SE) are derived from 1 experiment containing 3 mice/group. (C) CD45.1-B6 mice received 2,000 OT-I T cells and were primed and boosted with OVA257-264 (10 μg) and LPS (10 μg) with and without IL2-IC as indicated. (D) 30 days after the initial immunization, the distributed of Teff, TCM, and TEM based on expression of CD62L and CD127 were enumerated for the persistent OT-I T cells in the spleen. Data (C, D) (mean ± SE) are derived from 3 experiments each containing 3 mice/group.
Since low number of naïve OT-I T cells increased after immunization with IL2-IC, we tested whether a homologous primed-boost immunization strategy would suffice for the required high OT-I frequency for the accumulation of TEM cells. WT recipient mice were adoptively transferred with 1,000–2,000 naïve OT-I T cells (estimated 50–100 engrafted naïve OT-I T cells) and then were immunized and boosted 1 and 14 days later with OVA257-264 and LPS with or without IL2-IC. Marked expansion and minimal contraction of OT-I T cells were noted only after the boost with the IL2-IC (Fig. 7C) and these cells were mostly TEM cells (Fig. 7D). Thus, a low number of antigen-specific CD8+ T cells are driven into memory cells by a prime-boost after immunization with IL2-IC.
A prime-boost regimen with IL2-IC supports immunity to LM-OVA in normal mice
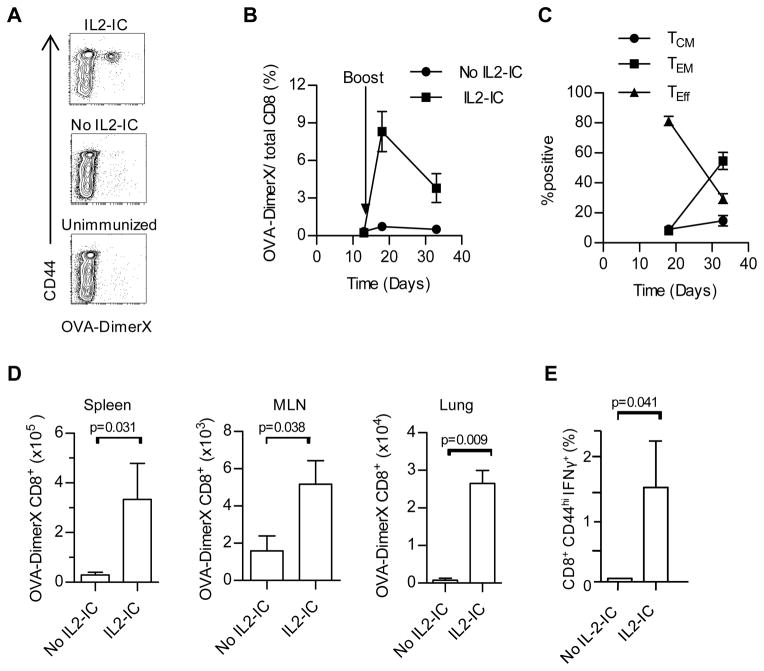
To test the utility of this approach for immune protection by polyclonal T cells, normal mice without OT-I T cells were vaccinated with a prime-boost regimen with OVA257-264 and LPS in the presence or absence of IL2-IC. The frequency of OVA-specific T cells was enumerated using OVA257-264 bound to H-2Kb:Ig fusion protein (OVA-DimerX) (Fig. 8A). When examined 5 and 19 days after the boost (i.e. day 18 and 33 after the primary immunization), 8.3 and 3.8% of the CD8+ T cells in the PBL were OVA257-264 specific (Fig. 8B). When considering the more numerous OVA-specific CD8+ T cells in the PBL from mice receiving IL2-IC, initially most were Teff cells just after the boost, but the dominant populations was TEM cells 19 days after the boost (Fig. 8C). Analysis of various tissues revealed a significance preference for OVA-specific CD8+ T cells in the spleen, lung, and MLN after the prime and boost with IL2-IC, with greatest numbers of such cells in the spleen and lung (Fig. 8D). Moreover, a similar frequency and preference for OVA-specific CD8+ T cells were found in the spleen when enumerated as IFNγ+ cells after stimulation with OVA257-264 in vitro (Fig. 8E). Thus, these data indicate that IL2-IC readily supports production of a substantial number of OVA-specific polyclonal CD8+ T cells that preferentially persist as TEM cells
FIGURE 8.
IL2-IC supports development of OVA-specific polyclonal CD8+ memory T cells. Normal B6 mice were primed (100 μg OVA254-264/10 μg LPS) and 14 days later boosted (50 μg OVA254-264/10 μg LPS) in the presence or absence of IL2-IC. (A) Contour plots of OVA-specific CD8+ T cells in the PBL 19 days after the boost from the indicated mice after gating CD8+ T cells. OVA-specific T cells were detected using OVA257-264 bound to a H-2Kb:Ig fusion protein (OVA-DimerX). (B) The frequency of OVA-specific CD8+ T cells in the PBL was enumerated 5 and 19 days after the boost. (C) OVA-specific CD8+ T cells in the PBL were examined for the distribution of Teff, TCM, and TEM based on expression of CD62L and CD127. (D) OVA-specific CD8+ T cells in the spleen, MLN and lung. Data (mean ± SE) in (A-D) are derived from 2 independent experiments containing a total of 6 mice/group with the exception of the lung tissue data which contains 3 mice/group from 1 experiment. (E) 19–27 days after the boost, OVA-specific CD8+ T cells were measured by enumerating the CD44hi IFN-γ+ cells after stimulation with 100 nM OVA257-264 ex vivo. Data (mean ± SE) represent 5 mice/group.
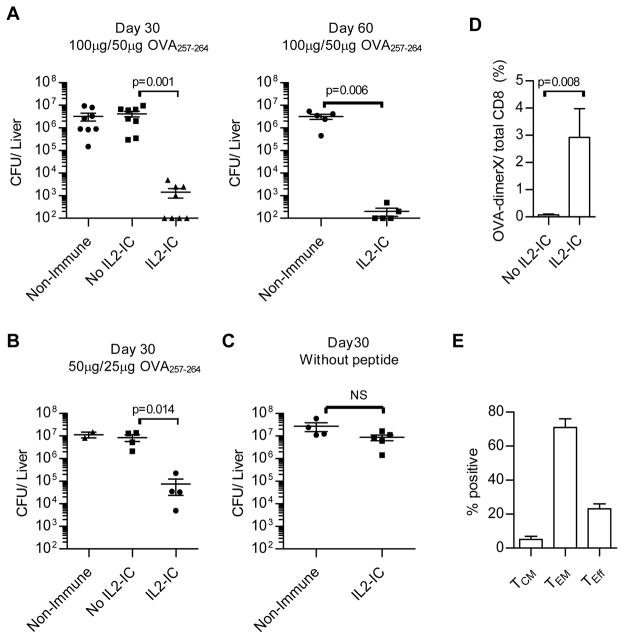
To test the functional activity of these OVA-specific CD8+ T cells, at 30 and 60 days after the primary immunization (15 and 45 days after the boost), other mice were infected with a lethal dose of LM-OVA, which requires a CD8+ T cell response for protection (43). When examined 3 days after infection, LM-OVA CFUs were reduced by 103–104-fold in the liver (Fig. 9A) and spleen (not shown) for only those mice that received the homologous prime-boost vaccination with OVA257-264 and IL2-IC. A similar protective effect was found for mice that received a lower dose of OVA257-264 and IL2-IC for the primary and booster injections (Fig. 9B). This protective response was antigen-dependent because no protection was noted for mice that received only LPS and IL2-IC on days 1 and 14 (Fig. 9C). The frequency of OVA-specific CD8+ T cells in the spleen after challenge with LM-OVA (Fig. 9D) was similar to that detected in the PBL just prior to the LM-OVA challenge (Fig. 8B). For the IL2-IC treated mice, the large majority of these were TEM cells (Fig. 9E). Thus, this immunization protocol that supports amplified TEM development provides potent antigen-dependent protective immunity when applied to normal mice.
FIGURE 9.
Protective immunity to LM-OVA by CD8+ memory T cells. B6 mice without OT-I T cells were primed and boosted 14 days later with the indicated amount of OVA257-264 and LPS (10 μg) (A and B) or (C) identically treated with only LPS and IL-2-IC. At the indicated day after the first immunization, mice were infected with LM-OVA (5 x105 CFUs) and 3 days later CFUs were enumerated for the liver. Control is unimmunized B6 mice. Each symbol represents an individual mouse. Data (mean ± SE) in (A,B) are derived from 3 independent experiments and in (C) from a single experiment. (D) The frequency of OVA-specific CD8+ T cell in the spleen after LM-OVA challenge by FACS using OVA/DimerX. (E) OVA-specific CD8+ T cell cells in the spleen were examined for the distribution of Teff, TCM, and TEM based on expression of CD62L and CD127. Data (mean ± SE) in (D, E) are derived from one experiments containing 3 mice/group.
Discussion
This study demonstrates that an immune response that is initially dominated Teff cells yields an IL-2-dependent amplified T memory response by a strategy that limits TCR, inflammatory, and IL-2R signaling after priming and booster injections. This finding is consistent with the notion that development of antigen-activated CD8+ T cells into memory cells can be optimized when receiving proper instructive signals. These conditions were met by immunization with a class I binding peptide, LPS, and transient application of IL2-IC. By targeting the high affinity IL-2R for a relatively short time after priming, three beneficial activities occurred for antigen-reactive CD8+ T cells, enhanced clonal expansion, largely uniform and rapid differentiation to TEM cells, and persistence of highly functional memory cells. For OT-I T cells, memory cells persisted at least for approximately 1 year that led to an increased representation of TCM cells.
In an infection, antigen load and the associated inflammation increase until immune clearance. The more persistent antigenic and inflammatory signals and endogenous IL-2, likely through CD4+ T cells, is known to favored production of terminally differentiated short-lived CD8+ Klrg1+ Teff cells (8, 44). These conditions appear to subvert amplified TEM development, as CD8+ TEM development was not strongly favored for mice infected with LM-OVA and treated with IL2-IC. Indeed, the lack of CD8+ Klrg1+ short-lived Teff cells after immunization with OVA257-264, LPS, and IL2-IC provide further support that antigenic and inflammatory signals are limited by this immunization scheme.
Our data indicate that the sequence of administration of IL2-IC is critical to promote amplified CD8+ T memory development. Even though the IL2-IC complex we used has a half-life of approximately 72 hr (15), the simultaneous immunization with OVA257-264, LPS and IL2-IC did not support amplified CD8+ T memory. Presumably high early IL-2R signaling by IL2-IC aborts the process by which IL-2R signaling supports large numbers of TEM cells, most likely by altering the sequence of expression of a critical set of genes. However, a single application of a relatively high amount of IL2-IC 24 hr after antigen immunization was sufficient to support substantial amplified TEM production that was only marginally improved by increased application of IL2-IC. Our data indicate that there is a window of time, approximately 1–3 days after immunization, where antigen-activated CD8+ T cells are receptive to IL2-IC to amplify TEM cells.
Past work by others have also showed that IL2-IC using Jes-6.1 also increased the primary response by OVA254-264 stimulated OT-I T cells, but a large expansion of antigen-specific memory cells did not occur (11). Our study found the same result when we used a similar low amount of IL2-IC. Thus, the level of IL-2 is another key variable for amplified T memory responses. In addition, enhanced IL-2R signaling primarily through stimulation of the intermediate affinity IL-2R, comprised of IL-2Rβ and γc, using a distinct IL2-IC, (IL-2/S4B6 mAb), increased the numbers of all memory phenotypic CD8+ T cells because IL-2Rβ and γc are expressed by most CD8+ T memory cells (11, 16, 17). Correspondingly, the augmented antigen-specific responses observed were not selective and were secondary to this generalized increase in memory cells. In marked contrast, an important advantage of targeting the high affinity IL-2R with IL2-IC is that essentially only the desired antigen-specific CD8+ T cells are driven into the memory pool.
IL-2 has been used with varied results to promote Teff and T memory responses (10, 12, 13, 45). Notably, application of IL-2 during the course of an immune response to viral infections did not lead to striking preferential development into CD8+ T memory cells (10, 13). Our findings provide several explanations that may account for the variable results in past studies. One point is that IL2-dependent amplified CD8+ Teff and T memory responses did not strikingly occur in the context of LM-OVA infection, suggesting that an active infection represents a setting that does not readily support substantial amplified memory production due to more persist antigen and inflammatory signals. The other point is that the improved pharmacokinetics of IL2-IC with its long half-life generates consistent and prolonged IL-2R signaling that is difficult to replicate with free IL-2 with its very short half-life (30 min). Our findings raise the possibility that a selective and optimal enhancement of IL-2R signaling over 2–3 days in the context of peptide immunizations represent a unique set of conditions that yield potent cell-mediated antigen-specific CD8+ T cell immunity. However, this point will require more systemic testing of varied conditions of immunization, including other peptides and vaccine formulations.
Considering our results in the context a physiological immune response, CD8+ TEM cells are predicted to be favored when they are in a microenvironment rich in IL-2, but low in antigen and inflammatory mediators. Moreover, there is a limited time for such TEM development to occur as expression of IL-2 and IL-2Rα are highly transient during most immune responses in vivo (7, 8). During immune responses to peptide antigen in vivo without application of IL-2, the selective and short duration of IL-2Rα expression and IL-2 production ensures development of TEM cells, but at limited numbers. Indeed when IL-2R signaling is deliberately limited in this context, TEM but not TCM development is impaired (7). Thus, enhanced IL-2R signaling early after priming or booster injections favors the development of CD8+ TEM memory cells.
Molecular analysis is consistent with transient IL-2R signaling in vivo primarily favoring activation of STAT5 in supporting amplified CD8+ T memory development. Minimal activation of pS6, downstream of PI3K/Akt and mTOR, was seen when IL-2-treated OVA/LPS-primed OT-I were stimulated with IL-2 ex vivo. We believe this result is noteworthy because recent work indicates that lower mTOR signaling favors CD8+ T memory development (46, 47). Thus, IL2-IC in the context of OVA/LPS intrinsically promotes intracellular signaling that favors CD8+ T memory. IL2-IC substantially altered gene expression initially that favor growth, metabolism, and gene programming while reducing cell death. IL2-IC increased many transcripts associated with the mitochondria suggests that our regimen of IL2-IC may lead to increased mitochondrial function that has been suggest to favor memory development (46, 48, 49). Another consequence of IL2-IC is that the balance of key transcription factors, including Blimp-1, IRF4, Eomes, Id2, Id3 and Tcf7, are altered, which likely favors the expanding antigen-responsive CD8+ T cells to develop into TEM cells.
This immunization scheme used a defined class I binding peptide, i.e. OVA257-264, that does not activate antigen-specific CD4+ T cells. Even though the IL2-IC caused a transient increase in CD4+ Treg cells, this approach should not yield an increase in antigen-specific natural or induced Treg cells to a class I peptide. Importantly, the generalized transient increase in Treg cells did not prevent a substantial immune response not only by OT-I T cells but also by non-TCR transgenic polyclonal OVA-specific CD8+ T cells. In addition, CD4+ T “help” was not provided for these CD8+ T cell responses. Other studies have shown that CD8+ T cells generate “helpless” immune responses and persistent memory cells, but their recall responses are impaired (50–52). CD4+ T help influences CD8+ T memory during the initial priming that in part is dependent upon IL-2R signaling (28). In this study, amplified TEM development by OT-I T cells was dependent on a relatively high frequency of naïve OT-I T cells and IL2-IC. We speculate that the high number of antigen-specific CD8+ T cells in the context of IL2-IC provides their own “helper” signals that support amplified TEM memory development and effective recall responses. At this time we have not defined the intrinsic “helper” signal associated with IL2-IC peptide-primed primed CD8 T cells. Many studies have implicated CD4+ T cells in providing help for CD8-dependent T cell responses through licensing of dendritic cells and secreting IL-2 (53). Lack of IL-2 readily explains the low memory responses by OT-I T cells at a high precursor frequency when mice were immunized with OVA257-264 in the absence of IL2-IC because enhancing IL-2R signaling was sufficient for amplified CD8+ TEM cells. However, lack of IL-2 does not explain the inability to generate amplified TEM cells at a low precursor frequency of OT-I T cells because we supplied excess IL-2 in the form of IL2-IC. However, CD40L or other molecules such as OX40 or 4-1BB, which promote CD8 memory development and survival (54–56), remain potential candidates as each were increased as assessed by gene profiling of OT-I T cells that developed into amplified TEM cells (Fig. 6C and not shown).
An important aspect of this study is that we have utilized the information obtained from OT-I T cells as a model to generate potent protective CD8+ T cell memory responses by polyclonal CD8+ T cells. Our ability to replicate amplified memory development for rare naïve antigen-specific CD8+ T cells required the use of a homologous prime-boost immunization strategy where the initial priming increased the number of antigen-reactive CD8+ T cells to a critical number for TEM development. We readily detected a substantial fraction of antigen-specific T cells 1–3 weeks after the boost in the PBL, spleen, and non-lymphoid tissues and these cells persisted for at least 45 day after the boost as assessed by protection against a lethal challenge with LM-OVA. TEM cells were the main OVA-specific polyclonal T cells that persisted after the boost with IL2-IC. However, TEM cells did not strikingly dominate this memory response when compared to OT-I T cells. This difference likely reflects heterogeneity by the responding polyclonal T cells. Nevertheless, the success of this homologous-primed boost immunization in generating protective CD8+ T cell responses after a lethal infectious challenge indicates that this approach represents a potentially general means to design vaccines where robust cell-mediated immune memory is a desired component. Prophylactic immunity to any infectious agent, including malaria, HIV/AIDS and tuberculosis, may be possible to appropriate MHC class I epitopes by transiently increasing IL-2R signaling. Although peptide based vaccines have been disappointing in various therapeutic settings, the transient enhancement of IL-2R signaling that amplifies CD8+ T memory responses offers a new approach to induce potent long-lasting immune responses in the context of infection or tumor immunotherapy.
Acknowledgments
We thank Dr. Oliver Umland of the University of Miami Diabetes Research Institute Flow Cytometry Core for skilled assistance with FACS and flow panel design for nine color staining.
This work was supported by National Institutes of Health Grants AI040114 and CA109094.
Abbreviations used in this paper
- Teff
T effector
- TEM
T effector-memory
- TCM
T central-memory
- IL2-IC
IL-2/anti-IL-2 complexes
- MLN
mesenteric lymph node
- LP
laminia propria
- PP
Peyer’s patch
- BM
bone marrow
- LM-OVA
Listeria monocytogenes-expressing OVA
Footnotes
Disclosures
A provisional patent application has been filed based on the information in this report.
References
- 1.Zhang N, Bevan MJ. CD8+ T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Lanzavecchia A, Araki K, Ahmed R. From vaccines to memory and back. Immunity. 2010;33:451–463. doi: 10.1016/j.immuni.2010.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27:393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stemberger C, Huster KM, Koffler M, Anderl F, Schiemann M, Wagner H, Busch DH. A single naive CD8+ T cell precursor can develop into diverse effector and memory subsets. Immunity. 2007;27:985–997. doi: 10.1016/j.immuni.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 5.Chang JT, V, Palanivel R, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–1691. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]
- 6.Gerlach C, van Heijst JW, Swart E, Sie D, Armstrong N, Kerkhoven RM, Zehn D, Bevan MJ, Schepers K, Schumacher TN. One naive T cell, multiple fates in CD8+ T cell differentiation. J Exp Med. 2010;207:1235–1246. doi: 10.1084/jem.20091175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro I, Yu A, Dee MJ, Malek TR. The basis of distinctive IL-2- and IL-15-dependent signaling: Weak CD122-dependent signaling favors CD8+ T central-memory cell survival but not T effector-memory cell development. J Immunol. 2011;187:5170–5182. doi: 10.4049/jimmunol.1003961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged interleukin-2Rα expression on virus-specific CD8+ T cells favors terminal- effector differentiation in vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 9.Malek TR, Castro I. Interleukin-2 receptor signaling: at the interface between tolerance and immunity. Immunity. 2010;33:153–165. doi: 10.1016/j.immuni.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 11.Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J Immunol. 2009;183:4904–4912. doi: 10.4049/jimmunol.0900284. [DOI] [PubMed] [Google Scholar]
- 12.Chang J, Choi SY, Jin HT, Sung YC, Braciale TJ. Improved effector activity and memory CD8 T cell development by IL-2 expression during experimental respiratory syncytial virus infection. J Immunol. 2004;172:503–508. doi: 10.4049/jimmunol.172.1.503. [DOI] [PubMed] [Google Scholar]
- 13.Cheng LE, Greenberg PD. Selective delivery of augmented IL-2 receptor signals to responding CD8+ T cells increases the size of the acute antiviral response and of the resulting memory T cell pool. J Immunol. 2002;169:4990–4997. doi: 10.4049/jimmunol.169.9.4990. [DOI] [PubMed] [Google Scholar]
- 14.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 15.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. PNAS. 2010;107:2171–2176. doi: 10.1073/pnas.0909384107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamilton SE, Schenkel JM, Akue AD, Jameson SC. IL-2 complex treatment can protect naive mice from bacterial and viral infection. J Immunol. 2010;185:6584–6590. doi: 10.4049/jimmunol.1001215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang Q, Adams JY, Penaranda C, Melli K, Piaggio E, Sgouroudis E, Piccirillo CA, Salomon BL, Bluestone JA. Central role of defective interleukin-2 production in the triggering of islet autoimmune destruction. Immunity. 2008;28:687–697. doi: 10.1016/j.immuni.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Webster KE, Walters S, Kohler RE, Mrkvan T, Boyman O, Surh CD, Grey ST, Sprent J. In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med. 2009;206:751–760. doi: 10.1084/jem.20082824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badovinac VP, Porter BB, Harty JT. CD8+ T cell contraction is controlled by early inflammation. Nat Immunol. 2004;5:809–817. doi: 10.1038/ni1098. [DOI] [PubMed] [Google Scholar]
- 21.Blair DA, Turner DL, Bose TO, Pham QM, Bouchard KR, Williams KJ, McAleer JP, Cauley LS, Vella AT, Lefrancois L. Duration of antigen availability influences the expansion and memory differentiation of T cells. J Immunol. 2011;187:2310–2321. doi: 10.4049/jimmunol.1100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prlic M, Hernandez-Hoyos G, Bevan MJ. Duration of the initial TCR stimulus controls the magnitude but not functionality of the CD8+ T cell response. J Exp Med. 2006;203:2135–2143. doi: 10.1084/jem.20060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carrio R, Bathe OF, Malek TR. Initial antigen encounter programs CD8+ T cells competent to develop into memory cells that are activated in an antigen-free, IL-7- and IL-15-rich environment. J Immunol. 2004;172:7315–7323. doi: 10.4049/jimmunol.172.12.7315. [DOI] [PubMed] [Google Scholar]
- 24.Obar JJ, Jellison ER, Sheridan BS, Blair DA, Pham QM, Zickovich JM, Lefrancois L. Pathogen-induced inflammatory environment controls effector and memory CD8+ T cell differentiation. J Immunol. 2011;187:4967–4978. doi: 10.4049/jimmunol.1102335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joshi NS, Kaech SM. Effector CD8 T cell development: a balancing act between memory cell potential and terminal differentiation. J Immunol. 2008;180:1309–1315. doi: 10.4049/jimmunol.180.3.1309. [DOI] [PubMed] [Google Scholar]
- 26.Mitchell DM, Ravkov EV, Williams MA. Distinct Roles for IL-2 and IL-15 in the Differentiation and Survival of CD8+ Effector and Memory T Cells. J Immunol. 2010;184:6719–6730. doi: 10.4049/jimmunol.0904089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obar JJ, Molloy MJ, Jellison ER, Stoklasek TA, Zhang W, Usherwood EJ, Lefrancois L. CD4+ T cell regulation of CD25 expression controls development of short-lived effector CD8+ T cells in primary and secondary responses. PNAS. 2010;107:193–198. doi: 10.1073/pnas.0909945107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and inflammation induce distinct transcriptional programs that promote the differentiation of effector cytolytic T cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki H, Kundig TM, Furlonger C, Wakeham A, Timms E, Matsuyama T, Schmits R, Simard JJL, Ohashi PS, Griesser H, Taniguchi T, Paige CJ, Mak TW. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor β. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 31.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 32.Foulds KE, Zenewicz LA, Shedlock DJ, Jiang J, Troy AE, Shen H. Cutting edge: CD4 and CD8 T cells are intrinsically different in their proliferative responses. J Immunol. 2002;168:1528–1532. doi: 10.4049/jimmunol.168.4.1528. [DOI] [PubMed] [Google Scholar]
- 33.Walunas TL, Bruce DS, Dustin L, Loh DY, Bluestone JA. Ly-6C is a marker of memory CD8+ T cells. J Immunol. 1995;155:1873–1883. [PubMed] [Google Scholar]
- 34.Malek TR, Ashwell JD. Interleukin 2 upregulates expression of its receptor on a T cell clone. J Exp Med. 1985;161:1575–1580. doi: 10.1084/jem.161.6.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998;70:1–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 36.Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. doi: 10.1038/nri1901. [DOI] [PubMed] [Google Scholar]
- 37.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 38.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, Mullen AC, Gasink CR, Kaech SM, Miller JD, Gapin L, Ryan K, Russ AP, Lindsten T, Orange JS, Goldrath AW, Ahmed R, Reiner SL. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 39.Ji Y, Pos Z, Rao M, Klebanoff CA, Yu Z, Sukumar M, Reger RN, Palmer DC, Borman ZA, Muranski P, Wang E, Schrump DS, Marincola FM, Restifo NP, Gattinoni L. Repression of the DNA-binding inhibitor Id3 by Blimp-1 limits the formation of memory CD8+ T cells. Nat Immunol. 2011;12:1230–1237. doi: 10.1038/ni.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang CY, Best JA, Knell J, Yang E, Sheridan AD, Jesionek AK, Li HS, Rivera RR, Lind KC, D’Cruz LM, Watowich SS, Murre C, Goldrath AW. The transcriptional regulators Id2 and Id3 control the formation of distinct memory CD8+ T cell subsets. Nat Immunol. 2011;12:1221–1229. doi: 10.1038/ni.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Yu S, Zhao DM, Harty JT, Badovinac VP, Xue HH. Differentiation and persistence of memory CD8+ T cells depend on T cell factor 1. Immunity. 2010;33:229–240. doi: 10.1016/j.immuni.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jenkins MK, Chu HH, McLachlan JB, Moon JJ. On the composition of the preimmune repertoire of T cells specific for Peptide-major histocompatibility complex ligands. Annu Rev Immunol. 2009;28:275–294. doi: 10.1146/annurev-immunol-030409-101253. [DOI] [PubMed] [Google Scholar]
- 43.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 44.Joshi NS, Cui W, Chandele A, Lee HK, Urso DR, Hagman J, Gapin L, Kaech SM. Inflammation directs memory precursor and short-lived effector CD8+ T cell fates via the graded expression of T-bet transcription factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nguyen CL, Salem ML, Rubinstein MP, Demcheva M, Vournakis JN, Cole DJ, Gillanders WE. Mechanisms of enhanced antigen-specific T cell response following vaccination with a novel peptide-based cancer vaccine and systemic interleukin-2 (IL-2) Vaccine. 2003;21:2318–2328. doi: 10.1016/s0264-410x(03)00096-3. [DOI] [PubMed] [Google Scholar]
- 46.Araki K, Turner AP, Shaffer VO, Gangappa S, Keller SA, Bachmann MF, Larsen CP, Ahmed R. mTOR regulates memory CD8 T-cell differentiation. Nature. 2009;460:108–112. doi: 10.1038/nature08155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim EH, Sullivan JA, Plisch EH, Tejera MM, Jatzek A, Choi KY, Suresh M. Signal integration by akt regulates CD8 T cell effector and memory differentiation. J Immunol. 2012;188:4305–4314. doi: 10.4049/jimmunol.1103568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pearce EL, Walsh MC, Cejas PJ, Harms GM, Shen H, Wang LS, Jones RG, Choi Y. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460:103–107. doi: 10.1038/nature08097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, Pearce EJ, Pearce EL. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36:68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen EM, Lemmens EE, Wolfe T, Christen U, von Herrath MG, Schoenberger SP. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature. 2003;421:852–856. doi: 10.1038/nature01441. [DOI] [PubMed] [Google Scholar]
- 51.Shedlock DJ, Shen H. Requirement for CD4 T cell help in generating functional CD8 T cell memory. Science. 2003;300:337–339. doi: 10.1126/science.1082305. [DOI] [PubMed] [Google Scholar]
- 52.Sun JC, Bevan MJ. Defective CD8 T cell memory following acute infection without CD4 T cell help. Science. 2003;300:339–342. doi: 10.1126/science.1083317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Swain SL, McKinstry KK, Strutt TM. Expanding roles for CD4+ T cells in immunity to viruses. Nat Rev Immunol. 2012;12:136–148. doi: 10.1038/nri3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4-1BB and 4-1BBL: complexities and challenges. Immunol Rev. 2009;229:192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez MG, Shen L, Rock KL. CD40 on APCs is needed for optimal programming, maintenance, and recall of CD8+ T cell memory even in the absence of CD4+ T cell help. J Immunol. 2008;180:4382–4390. doi: 10.4049/jimmunol.180.7.4382. [DOI] [PubMed] [Google Scholar]