Abstract
CD4+ T helper type 2 (TH2) cytokine responses promote the development of allergic inflammation and are critical for immunity to parasitic helminth infection. Recent studies highlighted that basophils can promote TH2 cytokine-mediated inflammation and that phenotypic and functional heterogeneity exists between classical IL-3-elicited basophils versus TSLP-elicited basophils. However, whether distinct basophil populations develop following helminth infection, and their relative contributions to anti-helminth immune responses remain to be defined. Following Trichinella spiralis infection of mice, we show that basophil responses are rapidly induced in multiple tissue compartments, including intestinal-draining lymph nodes. Trichinella-induced basophil responses were IL-3-IL-3R-independent but critically dependent on TSLP-TSLPR interactions. Selective depletion of basophils following Trichinella infection impaired infection-induced CD4+ TH2 cytokine responses, suggesting that TSLP-dependent basophils augment TH2 cytokine responses following helminth infection. The identification and functional classification of TSLP-dependent basophils in a helminth infection model, coupled with their recently-described role in promoting atopic dermatitis, suggests these cells may be a critical population in promoting TH2 cytokine-associated inflammation in a variety of inflammatory or infectious settings. Collectively, these data suggest that the TSLP-basophil pathway may represent a new target in the design of therapeutic intervention strategies to promote or limit TH2 cytokine-dependent immunity and inflammation.
INTRODUCTION
CD4+ T helper type 2 (TH2) cells, characterized by expression of IL-4, IL-5, IL-9 and IL-13, are required for pathogen clearance and tissue repair following exposure to helminth parasites (1–5). However, TH2 cytokine responses can also promote the pathological changes associated with asthma and allergic diseases at multiple barrier surfaces (6, 7). Recent studies have identified that in addition to their well-established role as late-phase effector cells, basophils can express MHC class II, secrete IL-4, migrate into lymph nodes (LNs) and promote optimal TH2 cytokine-mediated immune responses following exposure to some, but not all, allergens or helminth parasites (8–17).
The predominately T cell-derived cytokine IL-3 is a primary factor involved in basophil maturation, activation and trafficking (9, 18–20). However, since IL-3 is not required for basophil development (18), it was hypothesized that other factors could regulate basophil development and/or activation. Consistent with this, recent studies indicate that basophils are a heterogeneous population of cells, whose differentiation can be promoted by the epithelial cell-derived cytokine thymic stromal lymphopoietin (TSLP) cooperatively or independently of IL-3-IL-3R interactions (21). Further, TSLP-elicited basophils exhibit a distinct pattern of gene expression compared to classical IL-3-elicited basophils, respond more robustly to stimulation by IL-1 family cytokines IL-18 and IL-33 and produce higher levels of IL-4 and IL-6 (21). The identification of functional heterogeneity within the basophil lineage may, in part, explain the differential requirements for basophils in promoting optimal TH2 cytokine responses depending on the pathogen or allergen examined. Although TSLP-dependent basophils promote TH2 cytokine-associated inflammation in a mouse model of atopic dermatitis (21), the functional potential of IL-3-dependent versus TSLP-dependent basophils to helminth-induced TH2 cytokine-mediated inflammation is unknown.
In the present study we demonstrate that following infection with the intestinal helminth parasite Trichinella spiralis, IL-3-IL-3R-independent, TSLP-dependent basophils are rapidly recruited into multiple tissue compartments, including intestinal-draining lymph nodes (LNs). Critically, depletion of basophils diminished the magnitude of infection-induced CD4+ TH2 cytokine responses, suggesting that the rapid generation of “early-responder” TSLP-dependent basophil populations contributes to an environment permissive for optimal TH2 cell differentiation that is required for immunity to invading helminths.
MATERIALS AND METHODS
Animals, parasitological techniques and cell isolations
WT C57BL/6 mice were ordered from Jackson ImmunoResearchLaboratories and TSLPR−/− mice were obtained from Amgen. IL-3R−/− mice (Csf2rb2tm1Cgb Csf2rbtm1Clsc) and BaS-TRECK mice were bred at the University of Pennsylvania and maintained in a specific-pathogen free environment. All experiments were performed with age, gender and genetic background-matched mice, to minimize variations in infection-induced immune responses. All experiments were performed according to guidelines from University of Pennsylvania Institutional Animal Care and Use Committee-approved protocols. Methods for maintenance, recovery, infection and isolation of Trichinella Ag were previously described (22, 23). Mice were infected with 300 Trichinella muscle larvae by oral gavage and were sacrificed at d 2, 4, 7 or 12 p.i. for assessment of basophil responses, or were sacrificed at d 12 p.i. for analysis of peak infection-induced TH2 cytokine responses, worm burdens or humoral responses. At necropsy, single cell suspensions of mLN were prepared by passing through 70 μm nylon mesh filter. Splenocytes were isolated by homogenization followed by RBC lysis. Blood was collected by cardiac puncture, serum was isolated and peritoneal exudate cells were recovered by lavage with 10mL cold PBS. Bone marrow was isolated from femurs and single cell suspensions made by filtration through 70 μm nylon mesh filters and RBC lysis.
Neutralizing Ab treatments and cell depletions
Mice were treated with neutralizing mAb against mouse TSLP (obtained from Amgen) or mouse IL-3 (34D.11) by i.p. injection with 0.25 mg of Ab 4 h prior to infection and every 3 d after infection. Control mice received equivalent amounts of rat Ig (Control Ig). Mice were depleted of basophils by i.p. injection of 10 μg anti-FcεRI Ab (MAR-1, eBioscience) or were given control hamster Ig (eBioscience) on d 0, 1, 2, 7, 8 and 9 after infection. BaS-TRECK mice were given i.p. injections of Diphtheria toxin (500 ng) on d -1, 4 and 9 after Trichinella infection.
Flow cytometry
Cell preparations were surface stained with anti-mouse fluorochrome-conjugated mAbs against CD3ε (145-2C11), CD4 (GK1.5), CD11c (N418), CD19 (1D3), CD49b (DX5), CD123 (5B11), MHC class II (AF6-120.1), FcεRIα (MAR-1), c-kit (2B8), IgE (R35-72) and TSLPR (obtained from Amgen). For staining with intracellular cytokines, cells were stimulated for 4 h with 50 ng/mL PMA, 500 ng/mL ionomycin and 10 μg/mL brefeldin A (Sigma Aldrich), stained with cell surface Abs, fixed with paraformaldehyde, permeabilized in saponin and then stained with fluorochrome-labeled anti-IL-4 (11B11) and anti-IL-13 (eBio13A) antibodies. All Abs were from eBioscience unless specified otherwise. Cells were analyzed by flow cytometry using a FACSCanto or LSRII (BD Biosciences) and further analysis was performed using FlowJo software (Tree Star, Inc.).
Trichinella Ag-specific cell stimulations and ELISAs
Single cell suspensions of mLN from naive or infected mice were plated at 6 million cells/ml in complete medium (DMEM; Life Technologies) supplemented with 10% heat-inactivated FBS, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, and 50 μM β-mercaptoethanol and stimulated for 48 h with 50 μg/mL Trichinella Ag. Supernatants or serum samples were assayed for IL-3, IL-4, IL-5, IL-13 and IgE using standard sandwich ELISA protocols (eBioscience).
RNA isolations and real time quantitative PCR
RNA from 1 cm sections of small intestine was isolated by homogenization in TRIzol using a TissueLyzer (Qiagen) followed by phenol-chloroform extraction and isopropanol precipitation. cDNA was generated per standard protocol with Superscript reverse transcriptase (Invitrogen) and used as input for real-time PCR. Real time data were analyzed using the ΔΔCT method whereby actin served as the endogenous gene. All reactions were run on ABI 7500 Fast Real-Time PCR System (Applied Biosystems). Samples are normalized to naïve controls
Statistics
Groups of animals were compared using Mann Whitney U tests or Student’s t-tests where applicable. P values ≤0.05 were considered significant.
RESULTS
Trichinella infection elicits rapid basophil responses in multiple compartments
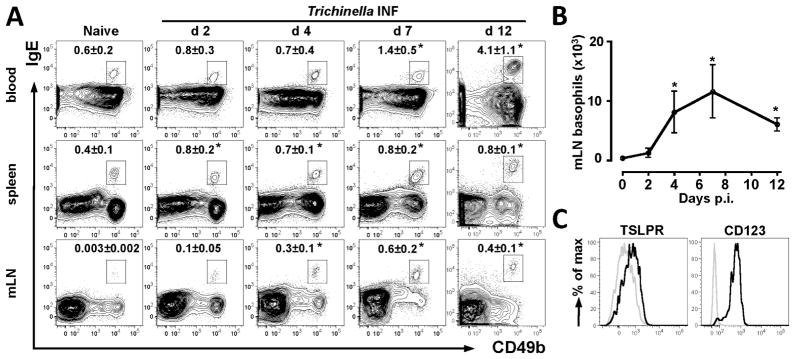
Basophils have been implicated as important regulators and effectors of TH2 cytokine responses and the recent identification of heterogeneity in the basophil lineage has provoked renewed interest in the factors that regulate basophil development, activation and function. In the present study we employed infection with the helminth parasite Trichinella spiralis to investigate regulation of basophil responses. Trichinella is a self-limiting natural intestinal nematode parasite of mice that undergoes a transient intestinal phase where the parasite occupies a partially intracellular niche within intestinal epithelial cells (24). Trichinella infection elicits peripheral basophilia (25) and CD4+ TH2 cytokine responses coincident with expulsion of the intestinal stage of the parasite (26), however the temporal and spatial kinetics of Trichinella-induced basophilia have not been reported. To address this, basophil responses in the blood, spleen and mesenteric LN (mLN) of WT mice were examined in the first 12 d post-infection (p.i.). Trichinella infection resulted in significant increases in frequencies of basophils (identified as CD3−, CD4−, CD19−, c-kit−, CD49b+, IgE+ cells) in the blood and spleen between d 2 and d 12 p.i. compared to naïve mice (Fig. 1A). Most strikingly, while basophils were found in very low frequencies within mLN of naïve mice, Trichinella infection resulted in a 10-fold increase in frequencies of basophils in the mLN by d 4 p.i., correlating with significant increases in total numbers of basophils in the mLN, which persisted until d 12 p.i. (Fig. 1A, 1B). Phenotypic analysis of basophils in the mLN demonstrated expression of subunits of the receptors for both TSLP and IL-3 (TSLPR and CD123) (Fig. 1C). Together, these data demonstrate that Trichinella infection elicits the rapid population expansion of IL-3 and/or TSLP-responsive basophil populations.
Figure 1. Trichinella infection elicits rapid basophil responses in multiple compartments.
WT mice were infected with Trichinella. (A) Representative plots displaying mean frequencies ± SEM of blood, spleen and mLN basophils in naïve mice and on d 2, 4 or 7 or 12 p.i. (B) Total numbers of basophils in mLN ± SEM. (C) Expression of TSLPR and CD123 on basophils isolated from the mLN of d 4 infected mice (open black histograms). Shaded histograms for TSLPR staining represent basophils from TSLPR−/− mice and for CD123 staining represent CD4+ T cells. Data are representative of 2 independent experiments, Naïve (n=2), d 2, 4, 7, 12 INF (n=3). *P<0.05 compared to Naïve (A), or day 0 (B).
Trichinella-induced basophil and CD4+ TH2 cytokine responses are significantly diminished following anti-TSLP mAb treatment but not anti-IL-3 mAb treatment
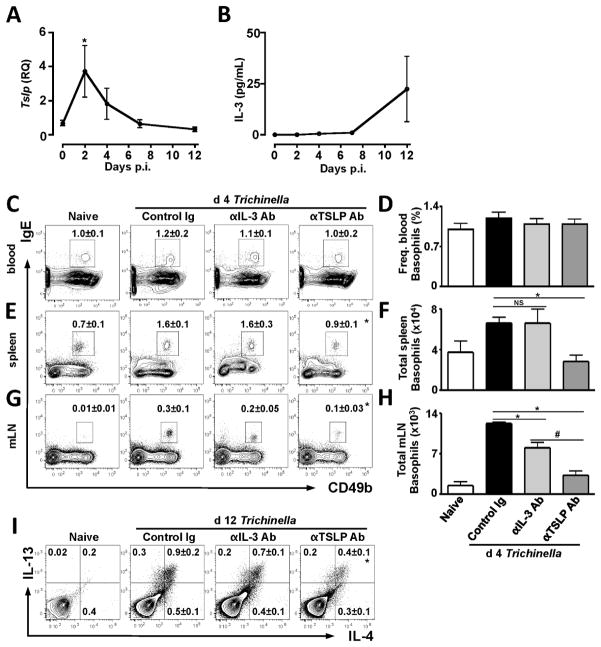
Given that both IL-3 and TSLP can regulate basophil responses (18, 21), we sought to test the relative contributions of each of these cytokines to Trichinella-induced basophilia and CD4+ TH2 cytokine responses. First, WT mice were infected with Trichinella and the expression of TSLP mRNA and IL-3 were assessed in the small intestine or serum at d 2, 4, 7 and d 12 p.i.. Trichinella infection elicited a 4-fold increase in TSLP mRNA expression by d 2 p.i. and TSLP expression returned to basal levels by day 7 p.i. (Fig. 2A). In contrast, serum IL-3 levels were not elevated above those seen in naïve animals until d 12 p.i. (Fig. 2B). Collectively, these data demonstrate that Trichinella infection induces rapid and transient TSLP expression but more delayed increases in IL-3 levels. Next, WT mice were infected with Trichinella and received either isotype control or neutralizing anti-IL-3 or anti-TSLP mAbs and basophil responses were assessed at d 4 p.i., a time point at which significant splenic and mLN basophilia were observed (Fig. 1A). Trichinella-infected mice treated with control Ig did not exhibit increases in the frequencies of basophils in the blood, consistent with data presented in Figure 1A, and these responses were not affected by ablation of TSLP or IL-3 (Fig. 2C, 2D). However, frequencies and absolute numbers of splenic basophils were increased in Trichinella-infected mice treated with control Ig or anti-IL-3 mAb (Fig. 2E, 2F). In contrast, treatment of mice with anti-TSLP mAb completely abolished infection-induced splenic basophil population expansion (Fig. 2E, 2F). While anti-IL-3 mAb treatment did partially reduce frequencies and total numbers basophils in the mLN (Fig. 2G, 2H), consistent with a role for IL-3 in mediating basophil homing into LNs during infection with other helminth species (9), anti-TSLP mAb treatment had a greater effect and reduced mLN basophil numbers to those seen in naive animals (Fig. 2G, 2H). Collectively, these data suggest that Trichinella-induced basophil responses are highly dependent on TSLP-TSLPR signaling.
Figure 2. Trichinella-induced basophil responses are abolished by anti-TSLP mAb treatment but not by anti-IL-3 mAb treatment.
WT mice were infected with Trichinella and (A) Tslp mRNA expression was assessed in small intestinal tissue homogenates by RT-PCR and (B) IL-3 protein levels were measured in serum by ELISA. (C–I) WT mice were treated with either control Ig or mAb to IL-3 or TSLP and infected with Trichinella and basophil responses were assessed at d 4 p.i. and CD4+ TH2 cytokine responses were assessed at d 12 p.i.. Representative plots displaying (C–D) frequencies of blood basophils, (E) frequencies and (F) total numbers of splenic basophils and (G) frequencies and (H) total numbers of mLN basophils ± SEM at d 4 p.i. (I) Ex vivo intracellular IL-4 and IL-13 staining in mLN CD4+ cells at d 12 p.i., numbers ± SEM indicate frequency of cells in each quadrant. Data are representative of 2–3 independent experiments, Naïve (n=1–2), Control Ig (n=4–5), anti-IL-3 (n=3–4), anti-TSLP (n=4–6). *P<0.05 compared to Control Ig. #P<0.05 compared to anti-IL-3 Ab.
Critically, when infection-induced CD4+ TH2 cytokine responses were assessed at d 12 p.i., mice treated with either control Ig or anti-IL-3 mAb displayed increases in frequencies of mLN CD4+ T cells that co-express the TH2 cytokines IL-4 and IL-13, while mice treated with anti-TSLP mAb exhibited a significantly diminished response (Fig. 2I). Together, these data suggest that while both TSLP and IL-3 may be required for optimal basophil responses, TSLP appears to play a dominant role in regulating basophil responses and the magnitude of CD4+ TH2 cytokine responses following Trichinella infection.
Infection-induced basophil responses are primarily IL-3-IL-3R independent but critically dependent on TSLP
In a mouse model of atopic dermatitis, TSLP-elicited basophil responses were independent of IL-3-IL-3R interactions (21). We sought to test whether TSLP-dependent basophil responses following Trichinella infection were dependent or independent of the IL-3-IL-3R pathway. To test this, WT or mice deficient in both beta chains of the IL-3R (Csf2rb2−/− Csf2rb−/−) were infected with Trichinella and basophil responses examined. Critically, both WT and Csf2rb2−/− Csf2rb−/− mice exhibited similar Trichinella-induced increases in frequencies of basophils in the blood (Fig. 3A, 3D), spleen (Fig. 3B) and mLN (Fig. 3C). In addition, both WT and Csf2rb2−/− Csf2rb−/− mice exhibited increases in total numbers of spleen and mLN basophils at d 4 p.i. (Fig. 3E, 3F), indicating that IL-3-IL-3R interactions are not required for Trichinella-induced basophil responses.
Figure 3. Infection-induced basophil responses are primarily IL-3-IL-3R independent but critically dependent on TSLP.
WT or IL-3R−/− mice (Csf2rb2−/−/Csf2rb−/−) were infected with Trichinella. Representative plots displaying frequencies of (A, D) blood (B) spleen and (C) mLN basophils ± SEM at d 4 p.i. Total numbers of (E) spleen and (F) mLN basophils ± SEM. Csf2rb2−/−/Csf2rb−/− mice were treated with either control Ig or anti-TSLP mAb and infected with Trichinella and (G) frequencies of blood basophils and total numbers of (H) spleen and (I) mLN basophils were enumerated at d 4 p.i. Data shown for A–F and G–I are representative of 3 independent experiments, Naïve A–F (n=1–2), WT and Csf2rb2−/−/Csf2rb−/− INF A–F (n=4), Naïve G–I (n=2), Control Ig, anti-TSLP INF G–I (n=3). *P<0.05 compared to Control Ig.
To directly test whether the IL-3R-independent basophil responses that develop following Trichinella infection were dependent on TSLP-TSLPR interactions, Trichinella-infected Csf2rb2−/− Csf2rb−/− mice were treated with either control Ig or neutralizing anti-TSLP mAbs. While infection of Csf2rb2−/− Csf2rb−/−mice treated with control Ab resulted in pronounced basophil population expansion in the blood (Fig. 3G), spleen (Fig. 3H) and mLN (Fig. 3I), treatment with anti-TSLP mAb significantly diminished these responses, suggesting that TSLP directly promotes basophilia independently of IL-3-IL-3R interactions. Taken together, these data indicate that Trichinella infection is a potent stimulus for the rapid development of TSLP-dependent, IL-3-independent basophil responses.
Basophil and CD4+ TH2 cytokine responses following Trichinella infection are dependent on TSLP-TSLPR interactions
To examine the influence of TSLP-TSLPR interactions on the induction of Trichinella-induced basophilia and TH2 cytokine responses, we employed mice genetically deficient in TSLPR. WT or TSLPR−/− mice were infected with Trichinella and basophil responses and TH2 cytokine responses were examined at d 4 or d 12 p.i., respectively. While blood basophil responses in WT and TSLPR−/− mice were comparable (Fig. 4A), infected TSLPR−/− mice exhibited reduced frequencies of basophils in the bone marrow (Fig. 4B), spleen (Fig. 4C) and mLN (Fig. 4E), and total numbers of basophils in the spleen (Fig. 4D) and mLN (Fig. 4F) compared to WT mice, consistent with results observed following antibody-mediated TSLP ablation (see Figure 2). Further, TSLPR−/− mice exhibited significantly diminished CD4+ TH2 cytokine responses in the mLN compared to WT mice as measured by ex vivo intracellular cytokine staining (Fig. 4G, 4H) and production of IL-4, IL-5 and IL-13 by mLN cells following restimulation with Trichinella antigen (Fig 4I, 4J, 4K). These data indicate that TSLP-TSLPR interactions are critically important for both Trichinella-induced basophil responses and TH2 cytokine responses, provoking the hypothesis that TSLP may also regulate TH2 cytokine responses by eliciting basophil populations.
Figure 4. Basophil and CD4+ TH2 cytokine responses following Trichinella infection are dependent on TSLP-TSLPR interactions.
WT or TSLPR−/− mice were infected with Trichinella. Representative plots displaying (A) frequency of blood basophils, (B) frequency of bone marrow basophils, (C) frequencies and (D) total spleen basophils and (E) frequencies and (F) total numbers of mLN basophils ± SEM at d 4 p.i. (G) Ex vivo intracellular IL-4 and IL-13 staining in mLN CD4+ cells at d 12 p.i., numbers ± SEM indicated frequency of cells in each quadrant. (H) Total IL-4 and IL-13 double-positive CD4+ cells ± SEM. (I) IL-4, (J) IL-5 and (K) IL-13 concentrations in cell-free supernatants following 48 h culture of mLN cells with Trichinella Ag, measured by ELISA. Data are representative of 4 independent experiments, Naïve (n=1), WT A–F (n=4), TSLPR−/− A–F (n=3), WT, TSLPR−/− G–K (n=5). *P<0.05 compared to WT INF.
Basophils contribute to optimal TH2 cytokine responses following Trichinella infection
Basophils can promote TH2 cytokine-mediated inflammation and act as late-stage effector cells in some models of helminth infection or allergy (10, 11, 13, 27), but not in others (8, 12, 14). Paradoxical reports on the requirement of basophils for promoting optimal TH2 cell responses may be explained by previously unrecognized functional heterogeneity between IL-3 and TSLP-elicited basophils (21). Because early Trichinella-induced basophil responses are TSLP-dependent, we tested the contribution of basophils to Trichinella-induced TH2 cytokine responses. We utilized two commonly used methods for depleting basophils, anti-FcεRI mAb treatment or Diptheria toxin-(DT) mediated depletion in BaS-TRECK mice. Treatment of mice with anti-FcεRI mAb depleted splenic and mLN basophils in infected mice (Fig. 5A). Analysis of Trichinella-induced CD4+ TH2 cytokine responses at d 12 p.i. revealed that anti-FcεRI mAb-treated mice displayed significantly reduced frequencies and total numbers of CD4+ T cells that co-express IL-4 and IL-13 in the mLN compared to control Ig-treated mice (Fig. 5B, 5C). It has been reported that anti-FcεRI mAb treatment can target mast cell or DC populations in some settings (8, 27, 28). While no alteration of peritoneal cavity mast cell or mLN FcεRI+ DC responses was observed following anti-FcεRI mAb treatment (Fig. S1A, S1C, S1D), anti-FcεRI mAb-treated mice did exhibit reduced frequencies of tissue-resident mast cells in the small intestine following Trichinella infection (Fig. S1B). Therefore as an alternative approach to selectively deplete basophils, BaS-TRECK (BaS-DTR+) mice were employed that allow lineage-specific basophil deletion via expression of the human DTR under the control of the proximal 3’UTR element in the mouse il4 locus (21, 29). These studies were critical to avoid the potential off-target effects of anti-FcεRI Ab treatment on mast cell populations (Fig. S1B). Treatment of Trichinella-infected BaS-DTR+ and littermate BaS-DTR− mice with DT resulted in complete ablation of basophils in the spleen and mLN (Fig. 5D). Critically, basophil-depleted mice exhibited significantly reduced frequencies and total numbers of CD4+ T cells that co-express IL-4 and IL-13 in the mLN (Fig. 5E, 5F), indicating impaired induction of TH2 cytokine responses. Basophil-depleted mice also exhibited significantly reduced IL-4, IL-5 and IL-13 production by mLN cells following restimulation with Trichinella antigen compared to control mice (Fig. 5G, 5H, 5I) and reduced serum IgE titers (Fig. 5J). Consistent with previous studies demonstrating that the intestinal phase of Trichinella infection is self-limiting and that mice naturally expel the parasite even in the absence of lymphocytes (30), basophil depletion by either anti-FcεRI Ab-treatment or DT-mediated ablation did not significantly affect the rate of intestinal worm expulsion: (d 12 p.i: Control Ig: 20±5 worms vs anti-FcεRI mAb: 39±13), or DT-mediated basophil depletion (BaS-DTR− worms: 17±5 vs BaS-DTR+ 23±6). Together, these data indicate that TSLP-dependent basophil responses are a key contributor to the promotion of optimal CD4+ TH2 cytokine responses following Trichinella infection.
Figure 5. Basophils contribute to optimal TH2 cytokine responses following Trichinella infection.
WT mice were treated with either control Ig or anti-FcεRI Ab during Trichinella infection. (A) Representative plots displaying depletion of spleen and mLN basophils at d 12 p.i. in anti-FcεRI Ab treated mice. (B) Ex vivo intracellular IL-4 and IL-13 staining in mLN CD4+ T cells, numbers ± SEM indicates frequency of cells in each quadrant. (C) Total IL-4 and IL-13 positive CD4+ cells ± SEM from naïve and infected mice. BaS-TRECK diptheria toxin receptor (BaS-DTR)− and BaS-DTR+ mice were treated with DT i.p and infected with Trichinella. (D) Representative plots displaying DT-mediated depletion of spleen and mLN basophils in BaS-DTR+ mice at d 12 p.i. (E) Ex vivo intracellular IL-4 and IL-13 staining in mLN CD4+ cells, numbers ± SEM indicates frequency of cells in each quadrant. (F) Total IL-4 and IL-13 double-positive CD4+ cells ± SEM from naïve and infected mice. (G) IL-4, (H) IL-5 and (I) IL-13 concentrations in cell-free supernatants following 48 h culture of mLN cells with Trichinella Ag, measured by ELISA. (J) Total serum IgE levels in infected mice. Data are representative of at least 2 independent experiments, Naïve A–C (n=2), Control Ig and anti-FcεRI Ab INF (n=4). Naïve D–J (n=1), BaS-DTR− INF (n=5), BaS-DTR+ INF (n=3). *P<0.05 compared to Control Ig. **P<0.05 compared to BaS-DTR INF.
DISCUSSION
The generation of CD4+ TH2 cytokine responses is critical for immunity to parasitic helminths and is also responsible for the chronic inflammation associated with many allergic diseases (1, 6, 7, 31), however the early events that drive TH2 cytokine production remain incompletely understood. Recent evidence that basophils can contribute to optimal TH2 cytokine-mediated immune responses following infection with some, but not all, parasitic helminth species and allergens (8–17), and that functional heterogeneity exists in the basophil lineage (21), provoked the question as to whether differences in the phenotype of responding basophil populations may explain the paradoxical roles for these cells in regulating TH2 cytokine responses. Data from the present study demonstrate that following gastrointestinal helminth infection, TSLP rapidly elicits the population expansion of TSLP-dependent, IL-3-independent basophils. TSLP-dependent basophils are rapidly recruited into LNs and depletion of basophils results in impaired CD4+ TH2 cytokine responses following infection. These data indicate that TSLP-dependent basophil populations are critical for promoting optimal CD4+ TH2 cytokine responses following infection with a gastrointestinal helminth.
TSLP is a primarily epithelial-derived cytokine that, along with other epithelial-derived cytokines IL-25 and IL-33 (32–35), has been implicated in regulating TH2 cytokine responses following exposure to infectious or allergic stimuli (36–38). For example, TSLP-TSLPR signaling is important for TH2 cytokine responses following infections with some helminth species (37, 39). However, the mechanisms by which TSLP promotes TH2 cytokine responses are not fully understood and TSLP can have effects on diverse cell types, including DCs via limitation of IL-12 p40 expression and upregulation of OX40L, which creates a more TH2-permissive environment (38, 40). Data from the present study implicate TSLP-TSLPR interactions as critically important for Trichinella-induced basophil responses in the bone marrow, spleen and intestinal draining lymph nodes and also the magnitude of the infection-induced TH2 cytokine response. This provokes the hypothesis that TSLP regulates TH2 cytokine responses in part by eliciting basophil populations that can be rapidly recruited into intestinal-draining LNs to influence developing TH2 cell responses. Since Trichinella infection elicits rapid TSLP expression at the site of infection, which precedes any increase in circulating IL-3 levels, this may explain how TSLP is more important in regulating the acute basophil response elicited following infection. Given that TSLP-elicited basophils are more potent IL-4 producers than classical IL-3-elicited basophils and more responsive to stimulation with the epithelial-derived cytokine IL-33 (21), these studies suggest that tissue-resident epithelial cells may be central for rapidly regulating the differentiation, mobilization and activation of TSLP-dependent basophils.
T-cell derived IL-3 is an important factor involved in basophil maturation, activation and trafficking (9, 18, 19, 41–43). While basophil responses following Trichinella infection and in a model of atopic dermatitis are largely IL-3-IL-3R-independent (21), IL-3 ablation did result in reduced recruitment of basophils into intestinal-draining lymph nodes following Trichinella infection. Collectively, these data provoke the hypothesis that TSLP and IL-3 may cooperate to regulate optimal basophil responses. However, additional studies are needed to further interrogate the contributions of TSLP and/or IL-3 to basophil responses in the context of health and disease.
In addition to TSLP and IL-3, IL-18 (44), IL-33 (45, 46), GM-CSF (45), IgE (47, 48), IgD (49), C5a (50) and immune complexes (17, 51) have also been demonstrated to regulate basophil activation, development or homing. Consistent with this, TSLPR−/−IL-3R−/− mice still exhibit circulating basophil populations (21) and in the present study, TSLP ablation in IL-3R−/− mice failed to completely inhibit basophil homing to the mLN. The mechanisms by which basophil responses can be regulated are complex and are most likely regulated by multiple factors. Further research is required to classify the molecular and cellular factors involved in regulation of distinct IL-3 or TSLP-elicited basophil populations during different modes of inflammation. The development of genetically modified mice with cell-specific deletions in either IL-3R or TSLPR will provide new tools to interrogate the relative contribution of these distinct granulocyte populations in regulation of inflammation and immunity.
In conclusion, data from the present study provokes a model of the initial cellular immune response to a helminth infection, whereby epithelial cells at the site of infection produce TSLP which elicits an “early responder” population of basophils that are immediately mobilized prior to T cell activation. Later during infection, when the effector CD4+ T cell response has been established, levels of IL-3 are elevated and a “late responder” population of classical basophils can be elicited and maintained by IL-3 (9, 18–20). The identification and functional classification of TSLP-dependent basophils in a natural helminth infection model, coupled with their role in promoting atopic dermatitis (21), suggests these cells may be a critical population in promoting TH2 cytokine-associated inflammation in a variety of inflammatory or infectious settings. As such, this cell population could represent a new target in the design of therapeutic intervention strategies to promote or limit TH2 cytokine-dependent immunity and inflammation.
Supplementary Material
Acknowledgments
We thank members of the Artis laboratory for discussions and critical reading of the manuscript. We also thank the Matthew J. Ryan Veterinary Hospital Pathology Lab, the Penn Microarray Facility, and the Mucosal Immunology Studies Team of the NIAID for expertise and resources. The authors would also like to thank the Abramson Cancer Center Flow Cytometry and Cell Sorting Resource Laboratory for technical advice and support. Michael R. Comeau is employed by Amgen and supported by Amgen Inc.
Abbreviations
- DTR
Diptheria toxin receptor
- mLN
mesenteric lymph node
- TH2
T helper type 2
- TSLP
Thymic stromal lymphopoietin
Footnotes
Grant support: Research in the Artis lab is supported by the National Institutes of Health (AI061570, AI087990, AI074878, AI083480, AI095466, AI095608 and AI097333 to D.A.) and the Burroughs Wellcome Fund Investigator in Pathogenesis of Infectious Disease Award (D.A.), F32-AI085828 (M.C.S), the National Health and Medical Research Council Overseas Biomedical Fellowship 613718 (P.R.G.) and the American Australian Association Education Fund (P.R.G.). The Abramson Cancer Center Flow Cytometry and Cell Sorting Shared Resource is partially supported by NCI Comprehensive Cancer Center Support Grant (#2-P30 CA016520). R.K.G is supported by the Wellcome Trust. This work was supported by the NIH/NIDDK P30 Center for Molecular Studies in Digestive and Liver Diseases (P30-DK050306), its pilot grant program and scientific core facilities (Molecular Pathology and Imaging, Molecular Biology, Cell Culture and Mouse), as well as the Joint CHOP-Penn Center in Digestive, Liver and Pancreatic Medicine and its pilot grant program.
The authors declare no other competing financial interests.
References
- 1.Anthony RM, Rutitzky LI, Urban JF, Jr, Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Artis D, Grencis RK. The intestinal epithelium: sensors to effectors in nematode infection. Mucosal Immunol. 2008;1:252–264. doi: 10.1038/mi.2008.21. [DOI] [PubMed] [Google Scholar]
- 3.Finkelman FD, Shea-Donohue T, Goldhill J, Sullivan CA, Morris SC, Madden KB, Gause WC, Urban JF., Jr Cytokine regulation of host defense against parasitic gastrointestinal nematodes: lessons from studies with rodent models. Annu Rev Immunol. 1997;15:505–533. doi: 10.1146/annurev.immunol.15.1.505. [DOI] [PubMed] [Google Scholar]
- 4.Maizels RM, Pearce EJ, Artis D, Yazdanbakhsh M, Wynn TA. Regulation of pathogenesis and immunity in helminth infections. J Exp Med. 2009;206:2059–2066. doi: 10.1084/jem.20091903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urban JF, Jr, Madden KB, Svetic A, Cheever A, Trotta PP, Gause WC, Katona IM, Finkelman FD. The importance of Th2 cytokines in protective immunity to nematodes. Immunol Rev. 1992;127:205–220. doi: 10.1111/j.1600-065x.1992.tb01415.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim HY, DeKruyff RH, Umetsu DT. The many paths to asthma: phenotype shaped by innate and adaptive immunity. Nat Immunol. 2010;11:577–584. doi: 10.1038/ni.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maizels RM. Infections and allergy - helminths, hygiene and host immune regulation. Curr Opin Immunol. 2005;17:656–661. doi: 10.1016/j.coi.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Hammad H, Plantinga M, Deswarte K, Pouliot P, Willart MA, Kool M, Muskens F, Lambrecht BN. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 2010;207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Prout M, Ramshaw H, Lopez AF, LeGros G, Min B. Cutting edge: basophils are transiently recruited into the draining lymph nodes during helminth infection via IL-3, but infection-induced Th2 immunity can develop without basophil lymph node recruitment or IL-3. J Immunol. 2010;184:1143–1147. doi: 10.4049/jimmunol.0902447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 2010;33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 11.Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phythian-Adams AT, Cook PC, Lundie RJ, Jones LH, Smith KA, Barr TA, Hochweller K, Anderton SM, Hammerling GJ, Maizels RM, MacDonald AS. CD11c depletion severely disrupts Th2 induction and development in vivo. J Exp Med. 2010;207:2089–2096. doi: 10.1084/jem.20100734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sokol CL, Chu NQ, Yu S, Nish SA, Laufer TM, Medzhitov R. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, Allen CD, Locksley RM. Genetic analysis of basophil function in vivo. Nat Immunol. 2011;12:527–535. doi: 10.1038/ni.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Torrero MN, Hubner MP, Larson D, Karasuyama H, Mitre E. Basophils amplify type 2 immune responses, but do not serve a protective role, during chronic infection of mice with the filarial nematode Litomosoides sigmodontis. J Immunol. 2010;185:7426–7434. doi: 10.4049/jimmunol.0903864. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimoto T, Yasuda K, Tanaka H, Nakahira M, Imai Y, Fujimori Y, Nakanishi K. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 18.Lantz CS, Boesiger J, Song CH, Mach N, Kobayashi T, Mulligan RC, Nawa Y, Dranoff G, Galli SJ. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 19.Lantz CS, Min B, Tsai M, Chatterjea D, Dranoff G, Galli SJ. IL-3 is required for increases in blood basophils in nematode infection in mice and can enhance IgE-dependent IL-4 production by basophils in vitro. Lab Invest. 2008;88:1134–1142. doi: 10.1038/labinvest.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valent P, Schmidt G, Besemer J, Mayer P, Zenke G, Liehl E, Hinterberger W, Lechner K, Maurer D, Bettelheim P. Interleukin-3 is a differentiation factor for human basophils. Blood. 1989;73:1763–1769. [PubMed] [Google Scholar]
- 21.Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, Wherry EJ, Jessup HK, Siegel LA, Kambayashi T, Dudek EC, Kubo M, Cianferoni A, Spergel JM, Ziegler SF, Comeau MR, Artis D. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature. 2011;477:229–233. doi: 10.1038/nature10329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee TD, Grencis RK, Wakelin D. Specific cross-immunity between Trichinella spiralis and Trichuris muris: immunization with heterologous infections and antigens and transfer of immunity with heterologous immune mesenteric lymph node cells. Parasitology. 1982;84:381–389. doi: 10.1017/s0031182000044929. [DOI] [PubMed] [Google Scholar]
- 23.Wakelin D, Wilson MM. Transfer of immunity to Trichinella spiralis in the mouse with mesenteric lymph node cells: time of appearance of effective cells in donors and expression of immunity in recipients. Parasitology. 1977;74:215–224. doi: 10.1017/s0031182000047843. [DOI] [PubMed] [Google Scholar]
- 24.Despommier DD. Trichinella spiralis and the concept of niche. J Parasitol. 1993;79:472–482. [PubMed] [Google Scholar]
- 25.Arinobu Y, Iwasaki H, Gurish MF, Mizuno S, Shigematsu H, Ozawa H, Tenen DG, Austen KF, Akashi K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc Natl Acad Sci U S A. 2005;102:18105–18110. doi: 10.1073/pnas.0509148102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urban JF, Jr, Schopf L, Morris SC, Orekhova T, Madden KB, Betts CJ, Gamble HR, Byrd C, Donaldson D, Else K, Finkelman FD. Stat6 signaling promotes protective immunity against Trichinella spiralis through a mast cell- and T cell-dependent mechanism. J Immunol. 2000;164:2046–2052. doi: 10.4049/jimmunol.164.4.2046. [DOI] [PubMed] [Google Scholar]
- 27.Ohnmacht C, Voehringer D. Basophils protect against reinfection with hookworms independently of mast cells and memory Th2 cells. J Immunol. 2010;184:344–350. doi: 10.4049/jimmunol.0901841. [DOI] [PubMed] [Google Scholar]
- 28.Obata K, Mukai K, Tsujimura Y, Ishiwata K, Kawano Y, Minegishi Y, Watanabe N, Karasuyama H. Basophils are essential initiators of a novel type of chronic allergic inflammation. Blood. 2007;110:913–920. doi: 10.1182/blood-2007-01-068718. [DOI] [PubMed] [Google Scholar]
- 29.Sawaguchi M, Tanaka S, Nakatani Y, Harada Y, Mukai K, Matsunaga Y, Ishiwata K, Oboki K, Kambayashi T, Watanabe N, Karasuyama H, Nakae S, Inoue H, Kubo M. Role of Mast Cells and Basophils in IgE Responses and in Allergic Airway Hyperresponsiveness. J Immunol. 2012 doi: 10.4049/jimmunol.1101746. [DOI] [PubMed] [Google Scholar]
- 30.Vallance BA, Croitoru K, Collins SM. T lymphocyte-dependent and - independent intestinal smooth muscle dysfunction in the T. spiralis-infected mouse. Am J Physiol. 1998;275:G1157–1165. doi: 10.1152/ajpgi.1998.275.5.G1157. [DOI] [PubMed] [Google Scholar]
- 31.Perrigoue JG, Marshall FA, Artis D. On the hunt for helminths: innate immune cells in the recognition and response to helminth parasites. Cell Microbiol. 2008;10:1757–1764. doi: 10.1111/j.1462-5822.2008.01174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neill DR, Wong SH, Bellosi A, Flynn RJ, Daly M, Langford TK, Bucks C, Kane CM, Fallon PG, Pannell R, Jolin HE, McKenzie AN. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature. 2010;464:1367–1370. doi: 10.1038/nature08900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paul WE, Zhu J. How are T(H)2-type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225–235. doi: 10.1038/nri2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saenz SA, Noti M, Artis D. Innate immune cell populations function as initiators and effectors in Th2 cytokine responses. Trends Immunol. 2010;31:407–413. doi: 10.1016/j.it.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Saenz SA, Siracusa MC, Perrigoue JG, Spencer SP, Urban JF, Jr, Tocker JE, Budelsky AL, Kleinschek MA, Kastelein RA, Kambayashi T, Bhandoola A, Artis D. IL25 elicits a multipotent progenitor cell population that promotes T(H)2 cytokine responses. Nature. 2010;464:1362–1366. doi: 10.1038/nature08901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 2010;11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramalingam TR, Pesce JT, Mentink-Kane MM, Madala S, Cheever AW, Comeau MR, Ziegler SF, Wynn TA. Regulation of helminth-induced Th2 responses by thymic stromal lymphopoietin. J Immunol. 2009;182:6452–6459. doi: 10.4049/jimmunol.0900181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Gros G, Ben-Sasson SZ, Conrad DH, Clark-Lewis I, Finkelman FD, Plaut M, Paul WE. IL-3 promotes production of IL-4 by splenic non-B, non-T cells in response to Fc receptor cross-linkage. J Immunol. 1990;145:2500–2506. [PubMed] [Google Scholar]
- 42.Ohnmacht C, Voehringer D. Basophil effector function and homeostasis during helminth infection. Blood. 2009;113:2816–2825. doi: 10.1182/blood-2008-05-154773. [DOI] [PubMed] [Google Scholar]
- 43.Shen T, Kim S, Do JS, Wang L, Lantz C, Urban JF, Le Gros G, Min B. T cell-derived IL-3 plays key role in parasite infection-induced basophil production but is dispensable for in vivo basophil survival. Int Immunol. 2008;20:1201–1209. doi: 10.1093/intimm/dxn077. [DOI] [PubMed] [Google Scholar]
- 44.Kroeger KM, Sullivan BM, Locksley RM. IL-18 and IL-33 elicit Th2 cytokines from basophils via a MyD88- and p38alpha-dependent pathway. J Leukoc Biol. 2009;86:769–778. doi: 10.1189/jlb.0708452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schneider E, Petit-Bertron AF, Bricard R, Levasseur M, Ramadan A, Girard JP, Herbelin A, Dy M. IL-33 activates unprimed murine basophils directly in vitro and induces their in vivo expansion indirectly by promoting hematopoietic growth factor production. J Immunol. 2009;183:3591–3597. doi: 10.4049/jimmunol.0900328. [DOI] [PubMed] [Google Scholar]
- 46.Suzukawa M, Iikura M, Koketsu R, Nagase H, Tamura C, Komiya A, Nakae S, Matsushima K, Ohta K, Yamamoto K, Yamaguchi M. An IL-1 cytokine member, IL-33, induces human basophil activation via its ST2 receptor. J Immunol. 2008;181:5981–5989. doi: 10.4049/jimmunol.181.9.5981. [DOI] [PubMed] [Google Scholar]
- 47.Hill DA, Siracusa MC, Abt MC, Kim BS, Kobuley D, Kubo M, Kambayashi T, LaRosa DF, Renner ED, Orange JS, Bushman FD, Artis D. Commensal bacterial–derived signals regulate basophil hematopoiesis and allergic inflammation. Nature Medicine. 2012 doi: 10.1038/nm.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ishizaka T, De Bernardo R, Tomioka H, Lichtenstein LM, Ishizaka K. Identification of basophil granulocytes as a site of allergic histamine release. J Immunol. 1972;108:1000–1008. [PubMed] [Google Scholar]
- 49.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, Cattalini M, Litzman J, BBJ, Huang B, Meini A, Riesbeck K, Cunningham-Rundles C, Plebani A, Cerutti A. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Siraganian RP, Hook WA. Complement-induced histamine release from human basophils. II. Mechanism of the histamine release reaction. J Immunol. 1976;116:639–646. [PubMed] [Google Scholar]
- 51.Charles N, Hardwick D, Daugas E, Illei GG, Rivera J. Basophils and the T helper 2 environment can promote the development of lupus nephritis. Nat Med. 2010;16:701–707. doi: 10.1038/nm.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.