Abstract
Although enriched in normal intestines, the role of CD4+ Th17 cells in regulation of the host response to microbiota, and whether and how they contribute to intestinal homeostasis is still largely unknown. It is also unclear whether Th17 cells regulate intestinal IgA production, which is also abundant in the intestinal lumen and plays a crucial role as the first defense line in host response to microbiota. In this study, we found that intestinal polymeric Ig receptor (pIgR) and IgA production was impaired in T cell-deficient TCRβxδ−/− mice. Repletion of TCRβxδ−/− mice with Th17 cells from CBir1 flagellin TCR transgenic mice, which are specific for a commensal antigen, increased intestinal pIgR and IgA. The levels of intestinal pIgR and IgA in B6.IL-17 receptor (IL-17R−/−) mice were lower than wild-type mice. Treatment of colonic epithelial HT-29 cells with IL-17 increased pIgR expression. IL-17R−/− mice demonstrated systemic anti-microflora antibody response. Consistently, administering dextran sulfate sodium (DSS) to C57BL/6 mice after treatment with IL-17-neutralizing antibody resulted in more severe intestinal inflammation as compared to control antibody. Administering DSS to IL-17R−/− mice resulted in increased weight loss and more severe intestinal inflammation compared to wild-type mice, indicating a protective role of Th17 cells in intestinal inflammation. Individual mice with lower levels of pIgR and intestinal secreted IgA correlated with increased weight loss at the end of DSS administration. Collectively, our data reveal that microbiota-specific Th17 cells contribute to intestinal homeostasis by regulating intestinal pIgR expression and IgA secretion.
Introduction
T helper 17 (Th17) cells, a subset of CD4+ T cells that primarily secrete Interleukin-17 (IL-17)A (also referred to as IL-17), IL-17F, IL-21, and IL-22, have been shown to be present in the intestinal lamina propria (LP), where they encounter a large number and diverse array of microbiota, commensal fungi, and food antigens1. Although accumulating evidence demonstrates that Th17 cells play a pathogenic role in a variety of inflammatory conditions2, there is considerable controversy as to whether they also contribute to the maintenance of intestinal immune homeostasis. Both protective and pathogenic functions of the Th17 cytokine IL-17 have been reported in patients with inflammatory bowel diseases (IBD) and in experimental colitis. IBD patients often have increased levels of IL-17 in inflamed tissues3, 4. Specific inhibition of IL-17-producing Th17 cells by anti-IL-23p19 monoclonal antibody prevents, as well as treats, colitis in an adoptive T cell transfer model, further confirming a role for the IL-23/Th17 pathway in the pathogenesis of colitis5. Furthermore, IL-17 deficiency results in resistance to TNBS-induced colitis4. However, IL-17- and/or IL-17F-deficiency does not prevent colitis mediated by transfer of naive CD4+ T cells. Adoptive transfer of IL-17−/− CD45RBhi T cells, compared to wild type counterparts, induced a more severe wasting disease when transferred into RAG−/− mice, indicating a protective role of IL-176. DSS-induced colitis has also provided conflicting reports of IL-17 involvement in intestinal inflammation7, 8. However, whether and how Th17 cells protect against chronic intestinal inflammation is still not understood.
IgA is enriched in mucosal secretions of the intestine9. Both T cell-dependent and T cell-independent mechanisms regulate intestinal IgA production10. IgA functions in the neutralization and clearance of extracellular pathogens by preventing adherence and access to epithelial surfaces9. Notably, germ-free mice that lack microbiota exhibit very low levels of intestinal IgA. Colonization with commensal microbiota restores IgA production. In particular, colonization with segmented filamentous bacteria (SFB) selectively increases IgA production and secretion11,12. It has been separately reported that colonization of germ-free mice with SFB also selectively increases levels of Th17 cells in the intestines13, 14. The observations that SFB can induce both Th17 cells and IgA indicate that there could be a link between Th17 cells and IgA production/secretion. Produced by plasma cells in the mucosa, IgA secretion relies on transport across the intestinal epithelium, which is mediated by the polymeric Ig receptor (pIgR) expressed on the basolateral surface of epithelial cells15. After translocation, a portion of the pIgR is covalently linked to IgA and secreted in the form of secretory IgA (sIgA), thereby improving stability of the complex16. Expression of the pIgR is vital to IgA-mediated innate protection17. The rate of IgA secretion is limited by the rate in which IgA binds to the pIgR, and is therefore ultimately dictated by the expression levels of the pIgR15. Reductions in pIgR expression lead to decreased IgA-mediated protection against luminal antigens17. Previous studies inflicting epithelial injury and colitis revealed that secretory antibodies significantly contribute to protection of the intestinal mucosa and that mice deficient in the pIgR displayed greater disease than did wild-type mice18. A recent study further demonstrated that Th17 cells increase pIgR expression in the bronchial epithelium in response to inhaled antigen19. However, whether and how Th17 cells regulate intestinal IgA and pIgR expression, and whether the Th17-IgA axis contributes to intestinal homeostasis are unknown. In this report, we demonstrate that Th17 cells contribute to the maintenance of host immune homeostasis against microbiota at least partially via IL-17 induction of epithelial pIgR expression, thereby increasing IgA secretion into the lumen. In the context of intestinal inflammation, mice that lack IL-17 signaling displayed more severe inflammation than their counterparts, correlating with decreased pIgR expression and subsequent IgA secretion.
Materials and Methods
Mice
C57BL/6 and TCRβxδ−/− mice were obtained from Jackson Laboratory. IL-17R−/− mice were kindly provided by Amgen. CBir1 flagellin-specific TCR transgenic (CBir1-Tg) mice were maintained in the Animal Facilities at University of Texas Medical Branch. 8–12 week-old mice were used for all experiments. All experiments were reviewed and approved by the Institutional Animal Care and Use Committees of the UTMB. All the mice strains were bred in the UTMB animal facility, and housed together from 3 weeks of age. All mice contain SFB as verified via PCR.
Antibody and reagents
Antibodies against IL-17A, CD45.2, and avidin were purchased from Biolegend. Neutralizing antibody to IL-17A was kindly provided by Merck. Mouse recombinant IL-6, IL-12, and human recombinant IL-17A, TNFα, TGFβ1 were purchased from R&D Systems. Antibodies against IgA were purchased from Kirkegaard & Perry Labs. Antibodies against pIgR and Actin were purchased from Santa Cruz Biotechnology. Anti-μ was purchased from Jackson ImmunoResearch Laboratories. Antibodies against phosphorylated NF-κB-p65 and total NF-κB-p65 were purchased from Cell Signaling. NF-κB inhibitor Bay11-7082, PI3K inhibitor LY294002, and all-trans-retinoic acid were purchased from Sigma-Aldrich.
Polarization of Th17 and Th1 cells
CD4+ T cells were isolated from spleens of CBir1 Tg mice using anti-mouse CD4-magnetic beads (BD Biosciences) as previously described20. To polarize Th17 cells, CBir1-Tg CD4+ T cells were cultured with 10ng/ml TGFβ1, 20ng/ml IL-6, 10μg/ml anti-IFNγ, and 10μg/ml anti-IL-421 with irradiated splenic APC. After 5 days, cells were stimulated with PMA (50 ng/ml) and ionomycin (750 ng/ml), and isolated with a capture complex of avidin, biotinylated-CD45.2, and biotinylated-IL-17A antibodies. Cells were counterstained with fluorescence-labeled antibodies for IL-17A, CD4, and CD45.2, and sorted by flow cytometry with >97% purity. To polarize Th1 cells, CBir1-Tg CD4+ T cells were cultured with 10ng/ml IL-12 and 10μg/ml anti-IL-4.
Fecal pellet preparation
Fecal pellets were homogenized in PBS containing 0.04mg/ml soybean trypsin inhibitor, 20mM EDTA, and 2mM PMSF and centrifuged to remove bacteria and insoluble debris as described previously22. Commensal bacterial lysate was prepared by homogenizing cecal contents and centrifuging to remove insoluble debris as described previously22.
ELISA
96-well plates (Nunc) were coated with 1μg/ml anti-IgA (Kirkegaard & Perry Labs) or 0.5μg/ml anti-pIgR (R&D Systems) or 1μg/ml of commensal bacterial lysate overnight at 4°. The plates were washed in PBS/Tween and blocked in PBS with 1% BSA. Fecal samples were diluted 1/100 and a 2-fold serial dilution was made. Samples were incubated at room temperature for 2 hours. 0.25μg/ml of biotinylated anti-IgA (KPL) was added for one hour, followed by HRP-conjugated streptavidin (KPL) for one hour. Plates were developed using a two-component TMB substrate (KPL) according to the manufacturer’s instructions, and plate was analyzed at 450nm. Results were quantified by normalizing to standard concentrations of IgA (Southern Biotechnology Associates).
Quantitative Real-Time PCR
RNA was extracted with TRIzol (Invitrogen) and followed by cDNA synthesis with Revertaid reverse transcriptase (Fermentas). Quantitative PCR was performed using TaqMan Gene Expression Assays. Predesigned primers and probes for PIGR and GAPDH were ordered from Applied Biosystems, and data were normalized to GAPDH mRNA expression.
Dextran sulfate sodium induction of colitis
As described previously23, dextran sulfate sodium (DSS) (MP Biomedicals) was dissolved into drinking water and administered to mice ad libitum. For acute colitis, 2.5% w/v DSS was administered over seven days, followed by 3 days of fresh water. For chronic colitis, 1.75% DSS was administered for seven days, followed by 3 days of fresh water and repeated over 60 days.
Histopathologic assessment
At necropsy, the small intestine, cecum, and colon were separated and Swiss rolls of each prepared. Tissues were fixed in 10% buffered formalin and paraffin embedded. 5μm sections were sliced, stained with H&E, and blindly scored by an experienced pathologist. Histological scoring was performed using a modification of scoring system reported previously24. In brief, longitudinal sections were examined for crypt epithelial hyperplasia, degeneration, loss; goblet cell loss; crypt exudate; LP and submucosal inflammatory cell accumulation; submucosal edema; mucosal ulceration; and transmural inflammation. Each lesion component was scored 1, 2, or 3 for mild, moderate, or severe, respectively (intensity), and 0 for absent, or 1, 2, 3, or 4 for 25, 50, 75, or 100% of the tissue affected, respectively (extent). The total lesion severity score was calculated by summation of the products of extent and intensity scores for each individual lesion component.
TGF-β bioassay
As described previously25, MFB-F11 cells are embryonic fibroblasts from Tgfb1−/− mice that are stably transfected with a reporter plasmid consisting of TGF-β responsive Smad-binding elements coupled to a secreted alkaline phosphatase reporter gene. Secreted alkaline phosphatase activity shown as chemiluminescence units was measured using Great EscApe SEAP Chemiluminescence kit 2.0 (Clontech) following the manufacturer’s instructions and represents biologically active TGF-β activity.
Bacterial enumeration
Mesenteric lymph nodes were isolated and homogenized in 500μL PBS. 10μL was spotted onto blood agar plates (BD Biosciences) in serial dilution and incubated at 37°C under aerobic and anaerobic conditions. Anaerobic cultures were placed in a sealed jar with a lit candle to induce a microaerophilic environment.
Statistical analysis
For comparisons between samples, levels of significance were determined by Student’s t test in Prism 5.0 (Graphpad). Where appropriate, mean ± SEM is represented on graphs. *p < 0.05; **p < 0.01.
Results
1. Low levels of intestinal IgA and pIgR in IL-17 receptor deficient mice
Analysis of fecal content in mice deficient in IL-17R (IL-17R−/−) revealed that the level of IgA was significantly decreased in the absence of IL-17 signaling as compared to wild-type mice (Figure 1A). It has been shown that the pIgR mediates the translocation of IgA into intestinal lumen, and a portion of the pIgR is secreted with IgA to improve stability16. Further analysis of fecal content revealed that the level of the pIgR was also significantly reduced to a similar level as IgA in IL-17R−/− mice (Figure 1B), indicating that the deficiency in intestinal IgA is partially due to a decrease in secretion. Pigr mRNA was also decreased in both the small intestines and large intestines of IL-17R−/− mice (Figure 1C), indicating that the reduction in fecal pIgR levels was not from variable levels of protein degradation. While TLR signaling on epithelial cells can regulate pIgR expression26, 27, the large intestines contain significantly greater numbers of microflora than the small intestines. These data indicate that IL-17 signaling regulates pIgR expression independent of microbiota.
Figure 1. Intestinal IgA secretion and pIgR expression is decreased in IL-17R−/− mice.
(A, B) Fecal pellets were collected from age-matched 8 week old wild-type or IL-17R−/− mice which were co-housed from 3 weeks old. IgA (A) and pIgR (B) levels were quantified through ELISA and normalized to total protein. *p<0.05. (C) Pigr mRNA was analyzed from intestinal tissue from wild-type or IL-17R−/− mice by RT-PCR. Pigr expression values were normalized to Gapdh expression. Significant differences are compared between respective tissues. *p<0.05 compared to wild-type mice. SB: small bowel: LB: large bowel.
2. Transfer of Th17 cells results in increased pIgR and IgA in TCRβxδ−/− mice
Although both T cell-dependent and -independent pathways are involved in regulation of IgA production, CD4+ T cells play a significant role in the induction of the pIgR and secretion of IgA into the intestine, as TCRβxδ−/− mice have significantly lower amounts of fecal IgA20 (Figure 2A), as well as pIgR (Figure 2B). Because IL-17 is predominantly produced by Th17 cells which are enriched in the intestine, we asked whether the presence of Th17 cells could influence pIgR expression and intestinal IgA secretion. We generated Th17 cells by polarizing CD4+ T cells from CBir1 Tg mice, which are specific for an immunodominant microbiota antigen20, 28, under standard Th17 conditions with TGFβ and IL-6, and transferred them into TCRβxδ−/− mice. Th17 cells were also generated from OTII transgenic mice, which are specific for the model antigen ovabumin (OVA) that is not present in intestinal lumen, and transferred into TCRβxδ−/− mice to serve as a control for antigen-specific stimulation in the intestines. The mice were sacrificed and Pigr mRNA expression was measured in intestinal tissue 30 days later. Intestines displayed significant increases in Pigr mRNA after transfer of CBir1 Th17 cells, as compared to native TCRβxδ−/− mice receiving only PBS or OVA-specific OTII Th17 cells (Figure 2C). Increases in fecal IgA and pIgR were apparent after approximately 1 week and continued to increase for the duration of the experiment (Figure 2D). This is consistent with a recent report that revealed that specific antigen-stimulation was required for Th17 cells to induce pIgR from the bronchial epithelium19. T regulatory cells (Treg) have been shown to promote intestinal IgA production through production of TGFβ20. As Th17 cells are not stable and are able to convert into Treg cells29, 30, we measured TGFβ production in the intestine of TCRβxδ−/− mice that received CBir1 Th17 cells or PBS to determine if TGFβ was involved in Th17 cell promotion of intestinal IgA. The intestines from both groups of mice produced TGFβ at a comparable level (Figure 2E). Neutralization of IL-17A significantly decreased the amount of IgA present in the fecal content (Figure 2F). Adoptive transfer of CBir1 Th1 cells slightly increased total IgA and antigen-specific IgA, but not to the extent seen in the transfer of CBir1 Th17 cells. Furthermore, neutralization of IL-17A decreased fecal IgA levels comparable to the transfer of CBir1 Th1 cells, signifying that the increases in intestinal IgA as a result of Th17 cell transfer is not solely due to the presence of T cell-mediated help, but that IL-17A contributes to IgA secretion as well. Collectively, our data indicates that Th17 cells increases pIgR expression and IgA secretion in vivo.
Figure 2. TCRβxδ−/− mice have lower intestinal pIgR and IgA production, and transfer of Th17 cells into TCRβxδ−/− mice increases pIgR expression and IgA secretion.
(A, B) Fecal pellets were collected from age-matched 8 week old wild-type and TCRβxδ−/− mice. IgA (A) and pIgR (B) levels were quantified through ELISA and normalized to total protein. *p<0.05, **p<0.01. N=5 mice per group. (C) In vitro-polarized Th17 cells from CBir1 or OTII TCR transgenic mice were transferred IV into TCRβxδ−/− mice. After 30 days, intestinal tissue was obtained from Th17 recipients or control TCRβxδ−/− mice receiving PBS, and Pigr mRNA was analyzed from intestinal tissue by RT-PCR. mRNA was normalized to Gapdh mRNA. Significant differences are compared to respective tissues. *p<0.05. SB: small bowel: LB: large bowel. (D) Fecal pellets were collected from Th17 cell recipients during the course of the experiment. IgA and pIgR levels were quantified through ELISA and normalized to total protein. Changes in expression over time are expressed as a fold change from individuals pre-transfer. *p<0.05, **p<0.01. N=4 mice. (E) Intestinal biopsies from CBir1 Th17 recipients or control TCRβxδ−/− were cultured for 24 hours. Supernatant was collected and cultured with MFB-F11 cells. Secreted alkaline phosphatase was measured as a reflection of TGFβ bioactivity. (F) Th17 and Th1 cells from CBir1-Tg mice were transferred IV into TCRβxδ−/− mice. Recipient mice were subsequently injected with a neutralizing antibody to IL-17A, or isotype control. Fecal pellets were collected from recipient mice, and total IgA and CBir1-antigen-specific IgA were quantified through ELISA. *p<0.05, **p<0.01. N=4 mice per group.
3. Th17 cells induced B cell IgA production in vitro
To determine whether Th17 cells directly induce B cell IgA production in vitro, splenic IgD+ B cells were cultured with in vitro polarized CBir1 Tg Th17, Th1, and unpolarized T cells (Th0). B cells were cultured with anti-μ, CD40L, TGFβ, and retinoic acid (RA) to serve as positive control31. B cells were also cultured with in vitro polarized OTII Th17, Th1, and Th0 cells, without the presence of OVA. Total IgA in the supernatant was measured at day 5. As shown in Figure 3, CBir1 Th17 cell greatly promoted IgA production, whereas CBir1 Th1 and Th0 cells only slightly enhanced IgA production. However, OTII T cells did not promote IgA production in the absence of their cognate antigen, indicating that the T cell activation and production of effector cytokines are required for Th17 cell-mediated induction of IgA. Taken together, Th17 cells were more adept at promoting IgA secretion in an antigen-specific manner, both by directly inducing IgA production as well as pIgR expression.
Figure 3. Th17 cells induce IgA production from B cells.
In vitro-polarized Th1, Th17 or unpolarized (Th0) cells from CBir1 Tg or OTII mice were co-cultured with splenic IgD+ B cells in the presence of CBir1 antigen. B cells were also cultured with anti-μ, CD40L, with or without TGFβ and retinoic acid. Five days later, supernatant was collected and total IgA production was quantified by ELISA. *p<0.05.
4. IL-17 directly induces pIgR expression from epithelial cells through NF-κB and PI3K
To further elucidate the role of IL-17 on the induction of pIgR, we asked if IL-17 signaled directly upon intestinal epithelial cells to produce pIgR, or whether there was another intermediate. Treatment of HT-29 human colon epithelial cells with human IL-17A resulted in an increase of PIGR mRNA, in a time and dose-dependent manner, appearing as soon as two hours after IL-17A treatment (Figure 4A). This induction of PIGR mRNA also mirrors the induction by TNFα (referred to as TNF), which is also produced by Th17 cells32, and is known to be a potent stimulator of pIgR33. Most notably, the combination of human IL-17A and TNF resulted in very strong induction of PIGR at all time points (Figure 4A and B). This increase in pIgR expression was greater than expected from the two cytokines alone, and suggests a strong synergism between IL-17A and TNF. The effect of IL-17A and synergism of IL-17A and TNF appeared to last beyond 24 hours, as PIGR mRNA steadily increased, whereas the effect of TNF began to decline at 24 hours (Figure 4A).
Figure 4. IL-17 upregulates pIgR in epithelial cells through activation of PI3K and NF-κB pathway.
(A) HT-29 cells were treated with human TNF (10ng/ml), IL-17A (20ng/ml), or TNF and IL-17A for the hours indicated. PIGR mRNA expression was analyzed by RT-PCR, and normalized to GAPDH mRNA. Significant differences are compared to non-treated controls. *p<0.05 compared to non-treated cells. Data reflects three independent experiments. (B) HT-29 cells were treated with TNF, IL-17A, or TNF and IL-17A for 48 hours. PIgR expression was detected by Western blot, with Actin as a loading control. One of two experiments with similar results was shown. (C, D) HT-29 cells were treated with TNF, IL-17A, or TNF and IL-17A for the time indicated. Phosphorylated NF-κB p65 was detected by western blot (C), with total NF-κB p65 as a loading control. (D) Relative increase of phosphorylated p65 over non-treated cells, as a percentage of total NF-κB p65. One of two experiments with similar results was shown. *p<0.05 of IL-17 treated cells compared to non-treated cells. (E) HT-29 cells were treated with PI3K inhibitor LY249002 (10uM) or NF-κB inhibitor Bay11-7082 (10uM) for one hour, then treated with TNF, IL-17A, or TNF and IL-17A for four hours. PIGR mRNA expression was analyzed by RT-PCR, and normalized to GAPDH mRNA. Significant differences are compared to nontreated controls. *p<0.05 compared to non-treated cells. Data reflects five independent experiments.
Previous reports have detailed that IL-17 can stimulate a number of cytokines and antimicrobial peptides, and that this upregulation occurs through NF-κB2, 34 as well as PI3 kinase activation34. In order to ascertain the mechanisms of IL-17A-mediated PIGR mRNA induction, we examined the effect of IL-17A and the synergism of IL-17A and TNF on NF-κB activation. IL-17A was able to rapidly induce phosphorylation of p65, indicative of activated NF-κB signaling. (Figure 4C and D).
Next we questioned whether IL-17-induced pIgR was mediated through the NF-κB and PI3K pathways. We included inhibitors specific for NF-κB (Bay11-7082, 10μM) and PI3K (LY294002, 10 μM) pathways to HT-29 cells cultured with IL-17A and TNF and PIGR mRNA was measured 4 h later. Blocking NF-κB activity greatly reduced levels of PIGR mRNA induced by IL-17A, TNF, or the combination of both IL-17A and TNF (Figure 4E). However, inhibition of either pathway alone does not result in significant abrogation of PIGR transcription, which could be due to the short treatment time as it has been demonstrated that PIGR mRNA response to TNF stimulation in HT-29 cells peaks at 24 h35, 36. Blocking both pathways at once resulted in significant downregulation of PIGR mRNA under all treatments, however did not completely shut down PIGR transcription, therefore signifying that while NF-κB and PI3K signaling may be identified as the major pathways involved, they do not appear to be the only pathways activated.
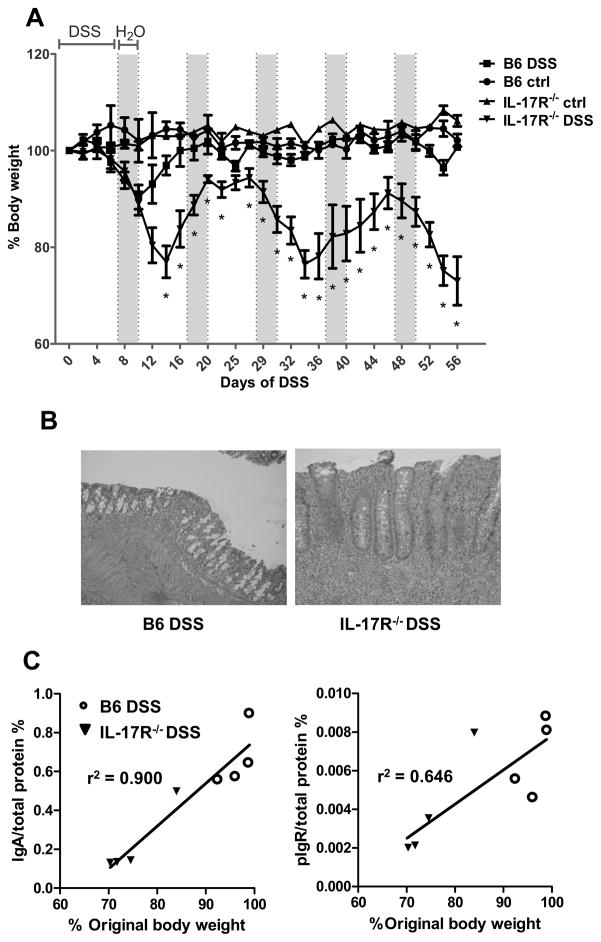
5. More severe colitis in IL-17R−/− mice with chronic DSS
Previous reports have presented conflicting results on the role of IL-17 in IBD. Some reports have suggested a pathogenic role for IL-17 in the development of colitis4, 8, whereas other work details that IL-17 may alleviate disease7. Next, we wanted to assess if there was a functional deficiency in epithelial protection in the absence of IL-17 signaling. We subjected IL-17R−/− mice to intestinal injury through DSS administration to determine if the decrease in intestinal IgA played a significant role in protecting the epithelium. Therefore we settled upon a sub-optimal dose of DSS that would not inflict significant injury in wild-type mice, but still injure the IL-17R−/− mice. Fecal pellets were collected and IgA and pIgR levels were quantified before colitis induction. Administration of 1.75% DSS induced colitis after five days in the IL-17R−/− and control mice, and continued over six cycles of seven days of DSS administration, followed by three days of fresh water. Disease progression was characterized by weight loss and visual examination of loose/bloody stool every 48 hours. As shown in Figure 5A, the IL-17R−/−mice displayed more significant disease as witnessed by increased weight loss, loose, mucoidal, and bloody stool. Weight loss and recovery in the control mice were responsive shortly after the switch from DSS to water. IL-17R−/− mice showed a delayed recovery in weight at the end of the first cycle, and continued to display irregular responses to the treatment cycles. As a whole, IL-17R−/− mice suffered from a more severe colitis than the control mice (Figure 5B), detailing that IL-17 provides significant protection in chronic DSS colitis. Whereas the control mice recovered their weight after the initial cycle of DSS, the IL-17R−/− mice repeatedly lost over 10% of their body weight with each cycle. Interestingly, mice that expressed the lowest levels of fecal IgA and pIgR under healthy conditions before DSS administration went on to exhibit a more severe disease and more severe weight loss than mice that expressed higher levels of IgA and the pIgR (Figure 5C).
Figure 5. IL-17R−/− mice suffer worsened colitis as a result of decreased pIgR and IgA secretion.
(A) Age-matched wild-type and IL-17R−/− mice, which had been co-housed from 3 weeks old, were administered 1.75% DSS in drinking water and weight was measured every 2 days. After 7 days of DSS, drinking water was replaced with fresh water for 3 days, and the cycle was repeated over 60 days. Weights are shown as a percentage of individual weight on Day 0. Significant differences are compared between strains on DSS. *p<0.05 compared to wild-type mice. N=4 mice per group. (B) Colonic histopathology of DSS-treated mice after 60 days of DSS administration. (C) IgA and pIgR in fecal pellets were quantified from mice by ELISA before DSS administration, and plotted against their individual body weight after 54 days.
6. Blockade of IL-17 increases severity of acute colitis in response to DSS
To further address the nature of IL-17 in the context of IBD, we injected a neutralizing antibody to IL-17A into C57BL/6 mice, followed by DSS administration. As shown in Figure 6A–C, mice that received neutralizing antibody to IL-17A demonstrated more severe colitis than mice receiving a control antibody after ten days, as measured by weight loss and histological examination. The differences were seen in weight loss after six days of DSS administration although it did not reach statistical significance (Figure 6A), and the histopathological scores (Figure 6B–C), confirming a protective role of IL-17 in DSS-induced intestinal inflammation.
Figure 6. Blockade of IL-17 induces more severe colitis from DSS administration and bacterial translocation is increased in IL-17R−/− mice.
(A) C57BL/6 mice were IP injected with a neutralizing antibody to IL-17A, or isotype control, and administered DSS for seven days. Weights are shown as a percentage of body weight on Day 0. (B) Pathological score of colitis was examined by blind histological observation 10 days after DSS administration. N=4 mice per group. **p<0.01 compared to the mice treated with control mAb. (C) Colonic histopathology of the DSS-treated mice after 10 days of DSS administration. (D) Mesenteric lymph nodes were harvested from wild-type or IL-17R−/− mice under aseptic conditions. MLN homogenates were cultured onto blood agar plates and incubated in aerobic and anaerobic conditions at 37°C. Aerobic cultures were incubated overnight, anaerobic cultures were incubated for 3 days. *p<0.05 compared to wild-type mice. N=3 mice. (E) Serum IgG against commensal bacterial lysate were quantified from wild-type or IL-17R−/− mice by ELISA. N=4 mice per group. *p<0.05 compared to wild-type mice. Wild-type mice were injected i.v. with 200 μg of A4 bacteria to indicate relative amount of serum IgG.
7. Increased commensal bacterial stimulation in IL-17 receptor deficient mice
Our data indicates a role of IL-17 in maintenance of intestinal homeostasis. We then questioned if the lack of IL-17 signaling would result in more commensal bacterial translocation with increased systemic response to commensal bacterial activities. There were more bacteria in the MLN of IL-17R−/− mice compared to that in wild-type mice (Figure 6D). Consistent with our previous observations37, there was no serum IgG against commensal bacterial antigens in wild-type mice, but significant serum IgG against the bacterial antigens was observed in wild-type mice immunized i.v. with commensal A4 bacteria38. In contrast, analysis of serum antibody titers revealed detectable levels of IgG specifically directed against commensal bacterial antigens in IL-17R−/− mice (Figure 6E). This signifies an important role for IL-17 signaling in the prevention of bacterial translocation across the epithelium, thereby limiting the activation of inflammatory responses against innocuous commensal antigens, both in the intestinal tract as well as systemically.
Discussion
Despite enormous bacterial challenge, the host intestine establishes a mutualistic relationship with the microbiota. Multiple mechanisms have evolved to regulate this relationship. The intestinal tract has been shown as a natural site for Th17 cell development, which is stimulated by specific species of microbiota14, with SFB being recently identified as one of such stimulators13. Although both pro- and anti-inflammatory functions of Th17 have been demonstrated in different experimental systems4–8, the enrichment of Th17 cells in the intestine suggests a role for these cells in mucosal homeostasis and more specifically in the containment of the vast local microbiota. In consistency with this argument, our data demonstrated that Th17 cells are able to promote intestinal IgA secretion via induction of epithelial cell pIgR expression, thereby contributing to the maintenance of host immune homeostasis to microbiota.
One of the most important strategies to generate immune protection and maintain intestinal homeostasis is the production of IgA9, which is the primary antibody in the gut. IgA regulates the microbiota, and bacteria in turn adapt to IgA by altering their gene expression patterns39. Although IgA also plays a role in host resistance to infection, it has been argued that the major role of IgA in the intestine is in maintaining the balance between the host and its microbiota40. In the absence of pathogen exposure, specific pathogen free mice have abundant levels of IgA, whereas germ-free mice have very low levels of IgA9. B cell IgA production can be stimulated by DC-B cell or epithelial cell-B cell interactions via BAFF, APRIL, iNOS, and TLR ligands, or utilizing T cell help and a number of cytokines including TGFβ, IL-4, IL-6, and IL-1010. Although the relative contribution of T cell-dependent and –independent regulation to intestinal IgA production is still not completely understood, decreased levels of intestinal IgA in T cell-deficient TCRβxδ−/− mice compared to wild-type mice indicates a predominant role of the T cell-dependent pathway20, 40. However, which T cells provide help and the sources of cytokines needed for intestinal IgA production in the mucosa is still largely unclear. Although TGFβ has been shown as a crucial cytokine in promoting IgA class switching10, and Treg cell production of TGFβ greatly contributes to intestinal IgA production20, it cannot completely explain why high levels of IgA are present only in the intestine, but not other lymphoid tissues even though TGFβ are also present in those sites. Our data indicated that repletion of Th17 cells promoted intestinal IgA secretion in the TCRβxδ−/− mice. Blockade of Th17 cytokine IL-17 decreased intestinal IgA (Figure 2). Additionally, IL-17R deficiency resulted in lower intestinal IgA secretion compared to wild-type mice (Figure 1), indicating that Th17 cells and their signature cytokine IL-17 greatly contribute to intestinal IgA secretion. Promotion of IgA secretion is not due to Treg cells that were converted from Th17 cells as the intestinal tissues produced TGFβ at a similar level. Several types of innate cells have been recently identified in the intestines that could also provide sources of IL-17 to promote intestinal IgA production41–43. Indeed, a previous report showed that RORγt+ LTi cells but not RORγt+ CD4+ T cells induced T cell-independent LP IgA production in the absence of Peyer’s Patches44. In RORγt deficient mice, transfer of RORγt+ LTi cells induced isolated lymphoid follicle (ILF) formation as well as LP IgA. However, transfer of RORγt+ CD4+ T cells did not induce ILF or PP formation, nor intestinal IgA, indicating that in the absence of PP and ILF, Th17 cells would not be activated, thus would not produce cytokines required for induction of intestinal IgA. Several recent elegant studies demonstrated that communal microbiota greatly impact intestinal Treg, Th17 cells and IgA responses. SFB preferably induces intestinal Th17 cells13 and IgA12, 13, whereas colonization with Clostridium species and Schaedler flora which contain 8 known commensal bacteria including Clostridium induces Tregs45, 46. Interestingly, failure to activate Treg cells results in the induction of Th17 cells, thus commensal bacteria regulate the balance between Tregs and Th17 cells. As Tregs have been shown to promote intestinal IgA response20, and we now show that Th17 cells are also able to upregulate intestinal IgA, the microbiota greatly influence intestinal IgA responses at least partially through regulation of Treg and Th17 cells.
IgA translocation across the intestinal epithelium is mediated by the polymeric immunoglobulin receptor (pIgR)9. IgA function in the intestinal lumen is dependent upon pIgR expression and thus, reduction in pIgR expression has been shown to lead to decreased IgA-mediated protection against luminal antigens15. Intestinal pIgR expression was lower in TCRβxδ−/− mice compared to wild-type mice, indicating a role for T cells in the induction of pIgR (Figure 2). Consistent with a previous report describing IL-17-mediated pIgR expression in airway epithelial cells19, repletion of Th17 cells restored intestinal pIgR expression in TCRβxδ−/− mice and IL-17R deficiency resulted in lower expression of intestinal pIgR, demonstrating that Th17 and IL-17 signaling regulate intestinal epithelial pIgR expression. Indeed, treatment with IL-17 greatly increased HT-29 epithelial cell expression of pIgR, alone or synergistically with TNF. IL-17 was able to activate NF-κB p65 signaling in intestinal epithelial cells (Figure 4). Blockade of NF-κB signaling and PI3 kinase activity with selective chemical inhibitors inhibited IL-17 induction of pIgR. Interestingly, both pathways work independently in IL-17 signaling as the inhibition of either pathway did not result in strong abrogation of PIGR transcription; only blockade of both pathways resulted in significant downregulation of PIGR mRNA. Intestinal Th17 cells require cognate luminal antigen stimulation to produce effector cytokines. Once cytokines are produced by the activated T cells, they regulate intestinal IgA production in an antigen non-specific manner.
Both intestinal pIgR and IgA have been implicated in maintenance of intestinal immune homeostasis, as deficiency of either pIgR or IgA results in greater commensal bacterial translocation across the intestinal epithelium, and more severe intestinal inflammation in response to DSS17, 18, 37. Thus, Th17 cell regulation of intestinal pIgR and IgA could play a crucial role in protection against intestinal inflammation induced by mucosal breach by commensal flora. Indeed, there was higher level of systemic anti-commensal bacterial IgG in IL-17R−/− mice but not in wild-type mice (Figure 6E), indicative of the presence of commensal bacteria in the systemic immune system. This revealed that deficiency of IL-17 signaling resulted in more commensal bacterial translocation from lumen, and sequentially, to more severe intestinal inflammation in response to DSS (Figure 5). Consistent with these observations, we also found higher numbers of bacteria in the mesenteric lymph nodes of IL-17R−/− mice (Figure 6D). This is likely due to impaired intestinal pIgR expression and IgA secretion, although the induction of a number of cytokines and anti-microbial peptides from epithelial cells by IL-17 could also contribute to IL-17-mediated protection against intestinal inflammation. However, we cannot exclude the possibility that wild-type and IL-17R−/− mice may have differences in the composition of their respective gut microbiota which could have contributed to our results.
In summary, our data demonstrate that enriched microbiota antigen-specific Th17 cells protect the host from chronic inflammation and contribute to intestinal immune homeostasis by regulating epithelial pIgR expression, thereby promoting intestinal IgA. However, it certainly does not mean that this is the only function of Th17 cells that contributes to intestinal immune homeostasis as Th17 cells and IL-17 have been shown to stimulate a number of cytokines and anti-microbial peptides which also contribute to the regulation of host immune responses to microbiota34. Treg cells have been shown to greatly promote intestinal IgA production via directly promoting B cell IgA class switching through production of TGFβ, now we show that Th17 cells promote IgA translocation across the intestinal epithelium via induction of pIgR by IL-17. Thus Treg and Th17 cells coordinately regulate intestinal IgA production and secretion (Figure 7). A deficiency in either pathway will result in decreased intestinal IgA, and disruption of intestinal immune homeostasis.
Figure 7. Coordinate regulation of intestinal IgA production and secretion by Treg and Th17 cells.
TGFβ produced by Treg cells drives naïve B cells to differentiate into IgA-producing cells. IL-21 from Th17 cells accentuates the effect of TGFβ and increases IgA+ B cell differentiation. Polymeric IgA then binds to pIgR expressed on IECs, causing transcytosis of pIgR-bound pIgA and the IgA complex is secreted into the lumen as sIgA. IL-17 from Th17 cells increases pIgR expression from IECs and thus increases the rate of sIgA secretion into the lumen.
Acknowledgments
Grant Support: This work was supported by research grants from NIH grants DK079918, AI083484, DK071176, and a start-up fund from University of Texas Medical Branch. ATC is a recipient of the J.W. McLaughlin Predoctoral Fellowship, UTMB.
Abbreviations used
- IL
interleukin
- Tg
transgenic
- IgA
immunoglobulin A
- pIgR
polymeric immunoglobulin receptor
Footnotes
No authors have conflicting financial interests.
References
- 1.Feng T, Elson CO. Adaptive immunity in the host-microbiota dialog. Mucosal Immunol. 2011;4:15–21. doi: 10.1038/mi.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28:454–67. doi: 10.1016/j.immuni.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm Bowel Dis. 2006;12:382–8. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 5.Elson CO, Cong Y, Weaver CT, Schoeb TR, McClanahan TK, Fick RB, Kastelein RA. Monoclonal anti-interleukin 23 reverses active colitis in a T cell-mediated model in mice. Gastroenterology. 2007;132:2359–70. doi: 10.1053/j.gastro.2007.03.104. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, Kolls JK, Flavell RA. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–9. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ogawa A, Andoh A, Araki Y, Bamba T, Fujiyama Y. Neutralization of interleukin-17 aggravates dextran sulfate sodium-induced colitis in mice. Clin Immunol. 2004;110:55–62. doi: 10.1016/j.clim.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Ito R, Kita M, Shin-Ya M, Kishida T, Urano A, Takada R, Sakagami J, Imanishi J, Iwakura Y, Okanoue T, Yoshikawa T, Kataoka K, Mazda O. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem Biophys Res Commun. 2008;377:12–6. doi: 10.1016/j.bbrc.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 9.Cerutti A, Rescigno M. The biology of intestinal immunoglobulin A responses. Immunity. 2008;28:740–50. doi: 10.1016/j.immuni.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerutti A. The regulation of IgA class switching. Nat Rev Immunol. 2008;8:421–34. doi: 10.1038/nri2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talham GL, Jiang HQ, Bos NA, Cebra JJ. Segmented filamentous bacteria are potent stimuli of a physiologically normal state of the murine gut mucosal immune system. Infect Immun. 1999;67:1992–2000. doi: 10.1128/iai.67.4.1992-2000.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klaasen HL, Van der Heijden PJ, Stok W, Poelma FG, Koopman JP, Van den Brink ME, Bakker MH, Eling WM, Beynen AC. Apathogenic, intestinal, segmented, filamentous bacteria stimulate the mucosal immune system of mice. Infect Immun. 1993;61:303–6. doi: 10.1128/iai.61.1.303-306.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–98. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ivanov II, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–49. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phalipon A, Corthesy B. Novel functions of the polymeric Ig receptor: well beyond transport of immunoglobulins. Trends Immunol. 2003;24:55–8. doi: 10.1016/s1471-4906(02)00031-5. [DOI] [PubMed] [Google Scholar]
- 16.Crottet P, Corthesy B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab′)2: a possible implication for mucosal defense. J Immunol. 1998;161:5445–53. [PubMed] [Google Scholar]
- 17.Johansen FE, Pekna M, Norderhaug IN, Haneberg B, Hietala MA, Krajci P, Betsholtz C, Brandtzaeg P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J Exp Med. 1999;190:915–22. doi: 10.1084/jem.190.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy AK, Dubose CN, Banas JA, Coalson JJ, Arulanandam BP. Contribution of polymeric immunoglobulin receptor to regulation of intestinal inflammation in dextran sulfate sodium-induced colitis. J Gastroenterol Hepatol. 2006;21:1372–80. doi: 10.1111/j.1440-1746.2006.04312.x. [DOI] [PubMed] [Google Scholar]
- 19.Jaffar Z, Ferrini ME, Herritt LA, Roberts K. Cutting edge: lung mucosal Th17-mediated responses induce polymeric Ig receptor expression by the airway epithelium and elevate secretory IgA levels. J Immunol. 2009;182:4507–11. doi: 10.4049/jimmunol.0900237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cong Y, Feng T, Fujihashi K, Schoeb TR, Elson CO. A dominant, coordinated T regulatory cell-IgA response to the intestinal microbiota. Proc Natl Acad Sci U S A. 2009;106:19256–61. doi: 10.1073/pnas.0812681106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 Cells Induce Colitis and Promote Th1 Cell Responses through IL-17 Induction of Innate IL-12 and IL-23 Production. J Immunol. 2011;186:6313–8. doi: 10.4049/jimmunol.1001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cong Y, Brandwein SL, McCabe RP, Lazenby A, Birkenmeier EH, Sundberg JP, Elson CO. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–64. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elson CO, Sartor RB, Tennyson GS, Riddell RH. Experimental models of inflammatory bowel disease. Gastroenterology. 1995;109:1344–67. doi: 10.1016/0016-5085(95)90599-5. [DOI] [PubMed] [Google Scholar]
- 24.Iqbal N, Oliver JR, Wagner FH, Lazenby AS, Elson CO, Weaver CT. T helper 1 and T helper 2 cells are pathogenic in an antigen-specific model of colitis. J Exp Med. 2002;195:71–84. doi: 10.1084/jem.2001889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng T, Cong Y, Qin H, Benveniste EN, Elson CO. Generation of mucosal dendritic cells from bone marrow reveals a critical role of retinoic acid. J Immunol. 2010;185:5915–25. doi: 10.4049/jimmunol.1001233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruno ME, Frantz AL, Rogier EW, Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-kappaB pathways in intestinal epithelial cells. Mucosal Immunol. 2011;4:468–78. doi: 10.1038/mi.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneeman TA, Bruno ME, Schjerven H, Johansen FE, Chady L, Kaetzel CS. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: linking innate and adaptive immune responses. J Immunol. 2005;175:376–84. doi: 10.4049/jimmunol.175.1.376. [DOI] [PubMed] [Google Scholar]
- 28.Lodes MJ, Cong Y, Elson CO, Mohamath R, Landers CJ, Targan SR, Fort M, Hershberg RM. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become Th17 cells in the absence of exogenous TGF-beta. J Immunol. 2007;178:6725–9. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K, Sugai M, Nambu Y, Osato M, Hayashi T, Kawaguchi M, Komori T, Ito Y, Shimizu A. Requirement for Runx proteins in IgA class switching acting downstream of TGF-beta 1 and retinoic acid signaling. J Immunol. 2010;184:2785–92. doi: 10.4049/jimmunol.0901823. [DOI] [PubMed] [Google Scholar]
- 32.Infante-Duarte C, Horton HF, Byrne MC, Kamradt T. Microbial lipopeptides induce the production of IL-17 in Th cells. J Immunol. 2000;165:6107–15. doi: 10.4049/jimmunol.165.11.6107. [DOI] [PubMed] [Google Scholar]
- 33.Bruno ME, Frantz AL, Rogier EW, Johansen FE, Kaetzel CS. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-kappaB pathways in intestinal epithelial cells. Mucosal Immunol. 2011 doi: 10.1038/mi.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang F, Kao CY, Wachi S, Thai P, Ryu J, Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J Immunol. 2007;179:6504–13. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 35.Schjerven H, Tran TN, Brandtzaeg P, Johansen FE. De novo synthesized RelB mediates TNF-induced up-regulation of the human polymeric Ig receptor. J Immunol. 2004;173:1849–57. doi: 10.4049/jimmunol.173.3.1849. [DOI] [PubMed] [Google Scholar]
- 36.Bruno ME, Kaetzel CS. Long-term exposure of the HT-29 human intestinal epithelial cell line to TNF causes sustained up-regulation of the polymeric Ig receptor and proinflammatory genes through transcriptional and posttranscriptional mechanisms. J Immunol. 2005;174:7278–84. doi: 10.4049/jimmunol.174.11.7278. [DOI] [PubMed] [Google Scholar]
- 37.Konrad A, Cong Y, Duck W, Borlaza R, Elson CO. Tight mucosal compartmentation of the murine immune response to antigens of the enteric microbiota. Gastroenterology. 2006;130:2050–9. doi: 10.1053/j.gastro.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 38.Duck LW, Walter MR, Novak J, Kelly D, Tomasi M, Cong Y, Elson CO. Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm Bowel Dis. 2007;13:1191–201. doi: 10.1002/ibd.20237. [DOI] [PubMed] [Google Scholar]
- 39.Jarchum I, Pamer EG. Regulation of innate and adaptive immunity by the commensal microbiota. Curr Opin Immunol. 2011 doi: 10.1016/j.coi.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Feng T, Elson CO, Cong Y. Treg cell-IgA axis in maintenance of host immune homeostasis with microbiota. Int Immunopharmacol. 2010 doi: 10.1016/j.intimp.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 42.Takatori H, Kanno Y, Watford WT, Tato CM, Weiss G, Ivanov II, Littman DR, O’Shea JJ. Lymphoid tissue inducer-like cells are an innate source of IL-17 and IL-22. J Exp Med. 2009;206:35–41. doi: 10.1084/jem.20072713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Romani L, Fallarino F, De Luca A, Montagnoli C, D’Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–5. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 44.Tsuji M, Suzuki K, Kitamura H, Maruya M, Kinoshita K, Ivanov II, Itoh K, Littman DR, Fagarasan S. Requirement for lymphoid tissue-inducer cells in isolated follicle formation and T cell-independent immunoglobulin A generation in the gut. Immunity. 2008;29:261–71. doi: 10.1016/j.immuni.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–41. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Geuking MB, Cahenzli J, Lawson MA, Ng DC, Slack E, Hapfelmeier S, McCoy KD, Macpherson AJ. Intestinal bacterial colonization induces mutualistic regulatory T cell responses. Immunity. 2011;34:794–806. doi: 10.1016/j.immuni.2011.03.021. [DOI] [PubMed] [Google Scholar]