Abstract
Background
For living donor liver transplantation, the genetic association of CYP3A5 genotype of recipient's native intestine and donor's liver allograft with tacrolimus pharmacokinetics has not been explained completely considering liver regeneration time. The goal of study was to investigate the longitudinal effects of recipient-donor combinational CYP3A5 genotypes on tacrolimus dose-normalized concentration (C/D ratio) in blood.
Methods
Tacrolimus blood concentrations were measured for fifty-eight Korean adult living donor liver transplant recipients on tacrolimus-based immunosuppressants during 4 years of follow-up. CYP3A5 was genotyped for both recipient and donor, and the recipient-donor combinational genetic effect on tacrolimus C/D ratios were evaluated as a function of time after adjusting for covariates including demographics and clinical variables.
Results
CYP3A5 expresser recipients grafted from CYP3A5 expresser donors consistently had the least C/D ratio throughout the entire study period, whereas CYP3A5 expresser recipients grafted from CYP3A5 nonexpresser donors had an intermediate, and CYP3A5 nonexpresser recipients grafted from CYP3A5 nonexpresser donors had the largest C/D ratio (all p < 0.01). The CYP3A5 nonexpresser recipients grafted from CYP3A5 expresser donors showed a significant decrease from the largest to the intermediate in C/D ratio for the first month.
Conclusions
CYP3A5 genotypes of both recipient and donor were important factors influencing pharmacokinetic variability of tacrolimus. The recipient-donor combinational genetic effect on C/D ratio changed over time after transplantation.
Keywords: tacrolimus, living donor liver transplantation, CYP3A5, donor, recipient
The calcineurin inhibitor tacrolimus is an immunosuppressive drug that has a narrow therapeutic index with high interindividual variations in its pharmacokinetics, which makes it difficult to establish an empirical dosage regimen in organ transplant recipients (1). Therefore, routine therapeutic drug monitoring (TDM) of tacrolimus is recommended to optimize the therapy for prevention of allograft rejections and adverse effects (2). Among the potential causes for large variability, recent pharmacogenetic studies have suggested genetic association of CYP3A5 genotype and tacrolimus pharmacokinetics (3, 4). Solid-organ recipients with at least one wild-type allele CYP3A5*1, cytochrome P450 (CYP) 3A5 expressers, exhibited the lower tacrolimus dose-normalized concentration (C/D ratio) and required a higher dose to reach the same blood concentration than the recipients with homozygous CYP3A5*3 allele, CYP3A5 nonexpressers (5, 6). Furthermore, dose-normalized tacrolimus exposure almost doubled in CYP3A5 nonexpressers compared with CYP3A5 expressers (7).
As tacrolimus is metabolized by both intestinal and hepatic CYP3A5 enzymes, the combined contribution of CYP3A5 expressions in native intestine and liver allograft should be considered for the pharmacokinetics of tacrolimus in liver transplant recipients (4, 6). Therefore, in the case of patients receiving liver transplantation the CYP3A5 genotypes of both the recipients' native intestine themselves and donors' liver allograft, recipient-donor combinational effect of CYP3A5 genotype, could be essential to the marked inter-individual variation in postoperative tacrolimus pharmacokinetics.
However, the recipient-donor combinational effect of CYP3A5 genotype on tacrolimus dose and blood C/D ratio in living donor liver transplantation (LDLT) remains to be elucidated. LDLT is becoming increasingly important strategy in reducing the waiting time mortality in liver failure patients (8, 9). For LDLT, the physiologic recovery of liver allograft should be different from a full sized liver transplantation since only a partial liver is transplanted (10). That is, in LDLT the systemic clearance of tacrolimus by the partial graft liver will gradually increase with postoperative time as the grafted liver regenerates its mass. Therefore, in order to understand inter- and intra-individual variations in tacrolimus pharmacokinetics over time in LDLT recipients, it is critically important to evaluate time-dependent tacrolimus clearance as a function of CYP3A5 genotypes of both native intestine and the graft liver. To our knowledge, however, this time trend on tacrolimus pharmacokinetics has not been clearly demonstrated. The previous studies were limited to relatively large graft size from deceased donor liver transplantation (DDLT) (11, 12), short study period (13), or analysis without consideration of continuous time trend and repeated measurements of tacrolimus concentration (14).
Based on this background, we hypothesized that tacrolimus clearance would change over time by recipient-donor combinational effect of CYP3A5 genotypes in patients after LDLT, and aimed to evaluate longitudinal effect of recipient-donor combinational CYP 3A5 genotypes on blood tacrolimus C/D ratio in Korean adults LDLT recipients.
RESULTS
Characteristics of the Study Participants
A total of 58 de novo ethnically Korean adult LDLT recipients administering tacrolimus were stratified into four study groups according to the CYP3A5 genotype of recipients and donors: CYP3A5 expresser recipient grafted from CYP3A5 expresser donor (REDE, n=10); CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor (REDN, n=13); CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor (RNDE, n=8); CYP3A5 nonexpresser recipient grafted from CYP3A5 nonexpresser donor (RNDN, n=27). The demographic and baseline clinical characteristics of the study participants were compatible among 4 genotype groups (all p>0.05), except for recipient's age (p<0.05) (Table 1). REDN were youngest (44.5±8.8 years) while RNDN were oldest (52.6±7.0 years) (p=0.049). The mean length of hospital stay for transplantation was 27±18 days ranging from 13 to 127. The most prevalent primary indication of transplantation was hepatitis B viral cirrhosis, which accounted for 75.0% of all cases, followed by alcoholic cirrhosis (8.3%), hepatitis C viral cirrhosis (6.7%), biliary cirrhosis (5.0%), fulminant hepatitis (3.3%), and cryptogenic cirrhosis (1.7%). Incidental hepatocellular carcinoma in addition to the primary disease was present in 29 of the recipients (49.2%). Six patients died during the study (at 1 and 8 months, and 1.5, 2, 3, 4 years post operation). Child-Pugh scores consisted of 13.6% of grade A (5–6), 22.0% of grade B (7–9), and 64.4% of grade C (10–15). A total of 23 cases of acute cellular rejection (ACR) were observed during the study (19 cases within the first 4 weeks while the others occurred at 0.5, 0.7, 1 and 3.6 years, respectively). Severity of ACR based on the rejection activity index (RAI) (15) were 73.9% of mild (RAI 2–4), 26.1% of moderate (RAI 5–6), and none of severe (RAI 7–9).
TABLE 1.
Demographic and baseline characteristics of study participants
| Characteristics | Overall | REDE | REDN | RNDE | RNDN | p |
|---|---|---|---|---|---|---|
| n (%) | 58(100) | 10 (17.2) | 13 (22.4) | 8 (13.8) | 27 (46.6) | |
| Recipient | ||||||
| Male, n (%) | 46 (79.3) | 8 (80) | 9 (69.2) | 8 (100) | 21 (77.8) | |
| Age (years) | 49.2±8.7 (19 to 65) | 49.4±6.7 (38 to 57) | 44.5±8.8 (32 to 62) | 45.1±12.1 (19 to 58) | 52.6±7.0 (35 to 65) | 0.018a |
| Body weight (kg) | 66.4±10.2 (45.3 to 86.3) | 63.4±10.1 (50.2 to 80.3) | 68.3±11.9 (46.7 to 86.3) | 67.1±8.2 (51.8 to 76.3) | 66.4±10.1 (45.3 to 86.0) | 0.719 |
| Donor | ||||||
| Male, n (%) | 41 (70.7) | 8 (80) | 9 (69.2) | 5 (62.5) | 19 (70.4) | |
| Age (years) | 30.0±9.1 (17 to 52) | 28.8±8.6 (17 to 44) | 31.9±9.3 (18 to 47) | 33.9±11.5 (17 to 52) | 28.4±8.5 (18 to 50) | 0.398 |
| Body weight (kg) | 67.1±11.3 (46.4 to 98.1) | 60.4±7.8 (46.4 to 71.3) | 69.7±10.7 (54.0 to 91.0) | 64.0±10.4 (52.5 to 82.6) | 69.2±12.3 (50.5 to 98.1) | 0.128 |
| GRWR (%) | 1.07±.24 (.59 to 1.60) | 1.13±.21 (.84 to 1.39) | 1.17±.23 (.82 to 1.48) | .95±.20 (.59 to 1.19) | 1.04±.25 (.59 to 1.60) | 0.137 |
| Hematocrit (%) | 24.1±3.9 (17.8 to 36.3) | 23.9±4.8 (19.5 to 36.3) | 24.0±3.7 (19.6 to 30.7) | 24.5±4.3 (20.9 to 34.4) | 24.1±3.7 (17.8 to 33.7) | 0.991 |
| Albumin (g/dL) | 3.1±.3 (2.4 to 3.6) | 3.0±.3 (2.7 to 3.5) | 3.1±.2 (2.8 to 3.6) | 3.0±.4 (2.4 to 3.6) | 3.1±.3 (2.5 to 3.6) | 0.945 |
| T.Bil (mg/dL) | 3.2±2.8 (.7 to 12.0) | 3.4±3.6 (.7 to 11.0) | 2.7±2.0 (.8 to 7.3) | 4.1±3.2 (.9 to 10.6) | 3.1±2.7 (1.0 to 12.0) | 0.706 |
| ALT (IU/L) | 161±117 (28 to 570) | 163±111 (48 to 396) | 231±164 (83 to 570) | 141±60 (77 to 274) | 133±96 (28 to 502) | 0.205 |
Age of REDN was younger than that of RNDN (p=0.049) by Dunnett's T3 post hoc tests.
Data are presented as mean ± SD (range).
SD, standard deviation; REDE, CYP3A5 expresser recipient grafted from CYP3A5 expresser donor; REDN, CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor; RNDE, CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor; RNDN, CYP3A5 nonexpresser recipient grafted from CYP3A5 nonexpresser donor; GRWR, graft to recipient body weight ratio; T. Bil, total bilirubin; ALT, alanine transaminase.
Genotype Frequencies
CYP3A5 genotype frequencies for recipients and donors of liver grafts are shown in Table 2. As a feature of liver transplantation, there were cases in which the CYP3A5 genotype of the recipient was different from that of donor, however, there was no difference in CYP3A5 genotype frequencies between recipients and donors (p=0.610). The allele frequency of the CYP3A5*3 variant of the whole recipient and donor was 79.7%. The observed CYP3A5 genotype frequency distributions for recipients and donors were consistent with Hardy-Weinberg equilibrium (HWE) (all p >0.05).
TABLE 2.
Genotype frequencies of CYP3A5
| Donor (graft liver) |
||||||
|---|---|---|---|---|---|---|
| Expresser | Nonexpresser | |||||
|
|
||||||
| *1/*1 | *1/*3 | *3/*3 | Total | |||
| Recipient (Native intestine) | Expresser | *1/*1 | 1 (1.7) | 1 (1.7) | 2 (3.4) | 4 (6.9) |
| *1/*3 | 1 (1.7) | 7 (12.1) | 11 (19.0) | 19 (32.8) | ||
| Nonexpresser | *3/*3 | - | 8 (13.8) | 27 (46.6) | 35 (60.3) | |
| Total | 2 (3.4) | 16 (27.6) | 40 (69.0) | 58 (100) | ||
Data are expressed as n (%).
Time Trend of the C/D Ratio Stratified by Recipient-Donor Combinational CYP3A5 Genotype
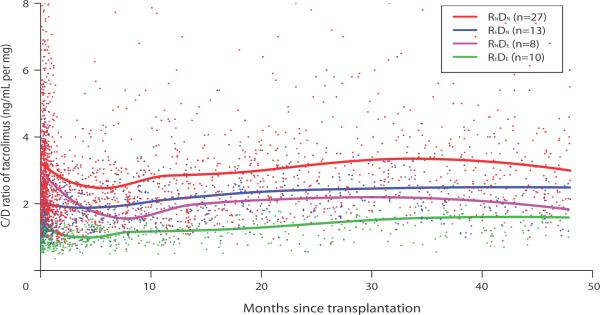
Figure 1 presents tacrolimus C/D ratio over 48 months post transplantation stratified by four recipient-donor combinational CYP3A5 genetic groups. The C/D ratios for all 4 groups tended to decline, though with different degree, during the first 6 months post transplantation, and remained fairly stable thereafter. Approximately after 4 months post transplantation, C/D ratios of CYP3A5 expresser liver (donor expresser) were lower than those of nonexpresser regardless of recipients' genotype (REDE, RNDE < REDN, RNDN). Given the same donor genotype, C/D ratios of CYP3A5 expresser intestine (recipient expresser) were lower than those of nonexpresser (REDE < RNDE given the same DE and REDN < RNDN given the same DN). However, during the early period until about 4 months post transplantation, C/D ratios of CYP3A5 expresser intestine were lower than those of nonexpresser independent of donors' genotype (REDE, REDN < RNDE, RNDN). The overall C/D ratio during the entire study period was nearly 2-folds greater in both intestinal and hepatic nonexpressers (RNDN) than both expressers (REDE).
FIGURE 1.
Tacrolimus dose-normalized concentration (C/D ratio, ng/mL per mg) stratified by the four donor-recipient combinational CYP3A5 genotype groups over 48 months after transplantation. REDE, CYP3A5 expresser recipient grafted from CYP3A5 expresser donor; REDN, CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor; RNDE, CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor; RNDN, CYP3A5 nonexpresser recipient grafted from CYP3A5 nonexpresser donor
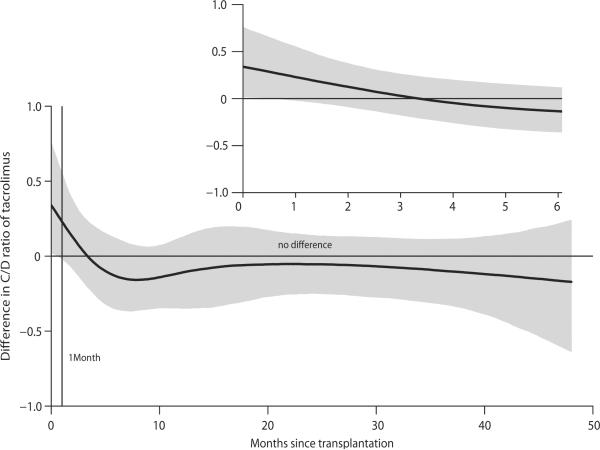
Since C/D ratio of RNDE changed dramatically over time, we further analyzed the difference between RNDE and REDN over time as illustrated in Figure 2. As the 95% confidence band (shaded area) suggested (i.e., zero excluded), C/D ratio between the two groups was significantly different until approximately 1 month of transplantation, but the difference was diminished thereafter. This motivated performing separate analyses for comparison of C/D ratios among 4 genotype groups by two different time periods, until one month and after one month.
FIGURE 2.
Differences in log-transformed tacrolimus dose-normalized concentration (C/D ratio, mg/mL per mg) between RNDE and REDN over 48 months after transplantation. The C/D ratios were logarithmically transformed as the distribution was skewed to the right. RNDE, CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor; REDN, CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor.
Effects of CYP3A5 Genotype on the Tacrolimus C/D Ratio
Table 3 presents the results for fixed effects in the final model for examining the association between log-transformed tacrolimus C/D ratio and the recipient-donor combinational CYP3A5 genotype groups after adjusting for covariates. After adjustment of the covariates, CYP3A5 genotypes remained as the most important factor affecting tacrolimus C/D ratio. The nonexpression of CYP3A5 was significantly associated with higher tacrolimus C/D ratio. For example, both intestinal and hepatic nonexpressers, RNDN, was associated with an increase of 0.89 and 0.8 in log-transformed tacrolimus C/D ratio compared to both expressers, REDE, until and after one month, respectively (p<0.001). If either intestine or liver was CYP3A5 nonexpresser (RNDE or REDN), C/D ratio increased by a range of ~0.5 when compared with both expresseres (REDE) (p≤0.01) whereas it decreased by approximately 0.3 in comparison with both nonexpressers (RNDN) (all p<0.01 except p=0.178 for RNDE vs. RNDN until 1 month). Among covariates in the model, after adjusting for the genotypes, albumin was negatively associated with the log-transformed tacrolimus C/D ratio during the entire study period (p<0.001). Time after transplantation is an important variable in the changing tacrolimus C/D ratio.
TABLE 3.
Results for fixed effects in the mixed-effects model for examining the association between log-transformed tacrolimus C/D ratio and the recipient-donor combinational CYP3A5 genotype groups
| Until 1 Month | After 1 Month | |||
|---|---|---|---|---|
|
| ||||
| Covariatea | Coefficientb (95% CI) | p | Coefficient (95% CI) | p |
| Genotype (RNDN vs. REDE) | .89 (.62,1.16) | < 0.001 | .80 (.60,1.00) | < 0.001 |
| Genotype (REDN vs. REDE) | .40 (.10, .71) | 0.010 | .52 (.29, .74) | < 0.001 |
| Genotype (RNDE vs. REDE) | .68 (32,1.03) | < 0.001 | .47 (.21, .73) | < 0.001 |
| Genotype (REDN vs. RNDN)c | −.48 (−.75, −.22) | < 0.001 | −.28 (−0.48, −.09) | 0.005 |
| Genotype (RNDE vs. RNDN)c | −.21 (−.52, .10) | 0.178 | −.33 (−.55, −.10) | 0.004 |
| Timed | .001 (.0007, .002) | < 0.001 | .00004 (.00003, .00005) | < 0.001 |
| Time2 | −2.03×10−6 (−2.62×10−6,−1.44×10−0) | < 0.001 | −8.48×10−10 (−1.01×10−9,−6.88×10−10) | < 0.001 |
| Albumin (g/dL) | −.13 (−.21,−.05) | .001 | −.09 (−.13, −.04) | < 0.001 |
| Hematocrit (%) | .01 (.003, .02) | .010 | −.001 (−.006, .003) | .556 |
| Body weight (kg) | .007 (−.0002, .01) | .058 | −.005 (−.01, .001) | .081 |
| T. Bil (mg/dL) | .02 (−.002, .04) | .084 | −.006 (−.03, .04) | .766 |
| ALT (IU/L) | .00003 (−.0002, .0003) | .836 | .0004 (.0001, .0006) | 0.002 |
| GRWR (%)e | .22 (−.22, .66) | .324 | −.07 (−.39, .25) | .686 |
| Age (years) | .0008 (−.01, .01) | .894 | .001 (−.008, .01) | .810 |
All covariates except genotype and GRWR were measured at the same time when tacrolimus concentrations were measured.
For coefficients with a positive value, the covariate results in an increase in log-transformed tacrolimus C/D ratio, whereas a negative values results in a decrease in log-transformed tacrolimus C/D ratio.
Obtained using RNDN as a reference group.
Time since transplant
Missing values of GRWR for two subjects were imputed using the median of GRWR from fifty-six subjects.
C/D ratio, dose-normalized concentration; CI, confidence interval; REDE, CYP3A5 expresser recipient grafted from CYP3A5 expresser donor; REDN, CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor; RNDE, CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor; RNDN, CYP3A5 nonexpresser recipient grafted from CYP3A5 nonexpresser donor; T. Bil, total bilirubin; ALT, alanine transaminase; GRWR, graft to recipient body weight ratio.
DISCUSSION
To the best of our knowledge, this prospective study is the first study to examine time-dependent effect of combinational CYP3A5 genotype in graft liver and native intestine on the C/D ratio of tacrolimus in adult LDLT recipients. Our major finding was that recipients' native intestinal CYP3A5 genotype played more important role than donors' hepatic genotype in early stage after transplantation, and the roles would be gradually changed to be switched in later stage. Thus, C/D ratio of RNDE was significantly higher than that of REDN shortly after transplantation, but decreased rapidly during the first month of post-transplantation, so that there was no difference between two groups after 1 month. In addition, as expected, our study confirmed that C/D ratio was consistently the greatest in RNDN whereas it was the lowest in REDE throughout the entire study period.
As hepatic metabolism by CYP3A enzymes is considered a major eliminating process of tacrolimus, liver regeneration from the ischemia reperfusion injury as well as enlarging the liver mass after transplantation in LDLT may have influence on the total clearance of tacrolimus. In LDLT, tacrolimus metabolism is reduced during the liver regeneration period (16) due to the recovery of liver mass to the standard liver volume (17, 18) as well as the down-regulation of the CYP3A enzyme system (19). In the present study, the significant difference in C/D ratio between the RNDE and REDN groups was not distinctive after the first month of transplantation indicating the recovery of hepatic metabolism along with the regeneration of partial liver allograft takes about one month. Meanwhile, this recovery time in DDLT can be conjectured to be shorter due to receipt of a full-sized liver graft instead of partial grafted liver such as in LDLT (11, 12).
Donor's CYP3A5 genotype not recipient's exhibited genetic effect on tacrolimus pharmacokinetics in the studies of DDLT subjects (11, 12, 20), using one point tacrolimus concentration(21) and excluding recipient's CYP3A5 genotype (22, 23). During the first month of our study in those CYP3A5 nonexpresser recipients, donor's CYP3A5 genotype had minimal influence on tacrolimus metabolism similar to what was previously reported (24). Uesugi et al. compared the C/D ratio of tacrolimus according to the CYP3A5 genotype combination of the donor and recipient for the first 35 days after LDLT (13). They reported a gradual decline of the C/D ratio, no hepatic influence in intestinal non-expressers, and significant hepatic influence in intestinal expressers; however, they observed no difference between RNDE and REDN, and no prolonged difference between REDE and RNDE after 1 month. This may be attributable to the characteristics of their study population, which was comprised of both pediatric and adult transplant recipients. As previously described for DDLT, it may be difficult to detect the influence of intestinal metabolism in pediatric LDLT patients receiving relatively larger graft size than adults. Fukudo et al. found a significant impact of intestinal CYP3A5 expression on tacrolimus oral clearance only during the first 4 weeks after LDLT, whereas hepatic CYP3A5 expression significantly influenced tacrolimus dose and C/D ratio after the first month until one year post transplantation (14). The different results of these two studies might be derived from the present study design for time as a continuous variable and tacrolimus concentration as repeated measurement, which is reported for the first time in this study.
Tacrolimus is extensively distributed to red blood cells and bound to plasma protein, and the physiological status of the liver transplanted is expected to influence the pharmacokinetics of tacrolimus. Thus, covariates that have been known to affect tacrolimus disposition such as time since transplantation, hematocrit, albumin, total bilirubin, alanine transaminase (ALT), body weight, and graft-to-recipient body weight ratio (GRWR) as well as demographics (1) were adjusted to analyze the association of CYP3A5 genetic variants with the pharmacokinetics of tacrolimus over time in the current study. The overall C/D ratio, which had stabilized at 6 months post-transplantation, gradually increased until 2–3 years after transplantation (Figure 1). This trend of increasing C/D ratio might be explained mostly by less intensive and frequent sampling of the tacrolimus trough blood levels due to prolonged or delayed outpatient follow-ups, poor adherence to tacrolimus treatment and blood sampling, and relatively low dose of tacrolimus as well as inevitable gradual deterioration of liver function over time.
The allele frequency of the CYP3A5*3 variant was 79.7% in our study, which is consistent with the reported frequencies of 76.5 to 81.4% for this variant in the Korean population (21, 25–27). The CYP3A5 allele was speculated to be the wild type (*1) when the CYP3A5 6986G variant was not detected, as the CYP3A5*6 allele is not expressed to any significant degree among the Korean population (26, 27). Even though the allele frequencies are different across the different ethnic groups, similar contributions from CYP3A5 to hepatic drug metabolism have been reported (4, 6).
In conclusion, CYP3A5 genotypes of both recipient and donor were independently important factors influencing inter-individual pharmacokinetic variability of tacrolimus. And the recipient-donor combinational genetic effect on tacrolimus C/D ratio changed over time since transplantation. To individualize tacrolimus therapy, however, quantitative guidelines should be developed for tacrolimus dosage regimens according to CYP3A5 genotype of donor and recipient pairs considering time after transplantation in adult LDLT.
METHODS
Subjects
Between 9 July 2004 and 7 August 2006, a total of 103 adult patients received LDLT at a tertiary hospital affiliated with a university in Seoul, South Korea. Fifty-nine of these patients were recruited into this prospective 4-year follow-up study. One patient was excluded because of severe hepatic impairment, which was never resolved. Inclusion criteria were: (1) patients on tacrolimus-based immunosuppressive therapy; (2) corresponding donor participation; and (3) patients older than 18 years of age. Exclusion criteria were: patients with (1) multi-organ transplantation; (2) retransplantation; and (3) administration of any investigational or immunosuppressive agent not included as part of the study regimen during the study period. The study protocol was approved by the Institutional Review Board (C-0505-148-005). All procedures were performed in accordance with the recommendations of the Declaration of Helsinki on Biomedical Research Involving Human Subjects, as well as the International Conference on the Harmonization of the Technical Requirements for the Registration of Pharmaceuticals for Human Use–Good Clinical Practice guidelines. All participants provided written informed consent before enrollment in the study.
Genotype Identification
Genomic deoxyribonucleic acid (DNA) was extracted from peripheral whole-blood samples of each subject using a Qiagen DNA extraction kit (Qiagen, Hilden, Germany). The presence of the CYP3A5*3 allele (6986A>G, rs 776746) was identified by mismatch polymerase chain reaction (PCR)-restriction fragment length polymorphism analysis, as reported previously (25). The oligonucleotides used for PCR were synthesized commercially (Bioneer Co. Ltd., Daejeon, Korea). PCR was carried out in a GeneAmp PCR system 2400 (Perkin Elmer, Boston, MA). Digested PCR products were analyzed by electrophoretic separation on agarose gels containing ethidium bromide. Ten percent of the DNA samples were directly sequenced for validation, which confirmed the results. Laboratory staffs were blinded to the patients' clinical data.
Immunosuppression Protocol
Our institutional immunosuppression protocol, a triple or double regimen including tacrolimus and corticosteroids with or without mycophenolate mofetil (MMF), was applied to all study participants. The initial tacrolimus dosage was 0.03 mg/kg for the triple regimen or 0.05 mg/kg for the double regimen, administered twice daily at 10:00 and 22:00 on an empty stomach starting on the post operational day (POD) 1. The following doses were adjusted based on the measured blood concentration in order to achieve the following target trough concentrations: 8–13 ng/mL until 2 weeks, 7–10 ng/mL between 2 weeks and 3 months, 5–7 ng/mL between 3 and 6 months, and approximately 5 ng/mL thereafter for the triple regimen; and 13–17 ng/mL, 8–13 ng/mL, 5–10 ng/mL, and approximately 5 ng/mL during the respective periods for the double regimen. Clinical staffs adjusting tacrolimus dosage were blinded to the individual patient's genotyping results. Methylprednisolone was administered intravenously at a dose of 500 mg before and after reperfusion, then tapered gradually, switched to oral prednisolone at 20 mg on POD 7, and then tapered to discontinuation approximately 6 months after transplantation. MMF was administered at 0.5 g with white blood cells (WBC) count of >3500/L or 0.25 g with WBC count of 2500–3500/L twice a day with tacrolimus. Patients did not receive any other medications known to interact significantly with tacrolimus. Prophylactic fluconazole of 50 mg daily was allowed due to its small amount and application to all participants.
Data Collection and TDM
Tacrolimus C/D ratio was calculated as tacrolimus trough blood concentration in ng/mL divided by a dose administered prior to blood sampling in mg. Tacrolimus trough concentrations from the routine TDM were adopted to calculate C/D ratio. Blood samples for tacrolimus routine TDM were collected daily at about 09:00 in the morning, beginning the day after the first dose and continuing until the day of discharge. Subsequent samples were obtained at each outpatient visit. Whole-blood tacrolimus concentrations were determined by microparticle enzyme immunoassay using an IMx analyzer and tacrolimus II reagent (Abbott Laboratories Diagnostic Division, Abbott Park, IL). Clinical laboratory test markers were measured simultaneously.
The written and electronic medical records of each subject were reviewed for abstraction of basic demographic information, concomitant medications and laboratory test results associated with tacrolimus concentrantion with corresponding dose and body weight, hematocrit, albumin, total bilirubin, ALT and pathological data. The amount of methylprednisolone was converted to prednisolone by multiplying by 1.25.
Statistics
We performed an exploratory analysis to examine the time trends of C/D ratio changes for the four groups stratified by the CYP3A5 genotype of recipients and donors. To further examine the differences in the time trends of C/D ratio between RNDE and REDN, cluster bootstrap analysis was performed with 5000 bootstraps, and the median and 95% confidence band were constructed for the difference. The distribution of C/D ratio was skewed to the right, and therefore logarithmically transformed C/D ratio values were used in all analyses to reduce the skewness of the distribution.
The exploratory analyses motivated performing separate analyses for two periods, from the first day until one month and thereafter. The differences in C/D ratio among 4 groups were examined separately in these two periods, using mixed effects models to take into account the correlation due to repeated measurements. The following covariates were selected a priori based on their potential effects on C/D ratio and adjusted in the analyses: age, body weight at each tacrolimus measurement, GRWR, hematocrit, albumin, total bilirubin, and ALT. To model linear and nonlinear time trends, we included a continuous time variable as well as its quadratic function in the models. The GRWR values for two subjects were missing, which were imputed using the median of GRWR values from 56 subjects. HWE was tested using Pearson's χ2 test for HWE in the R package genetics and our own R program. Data were presented as means, standard deviations or ranges.
All analyses were performed using STATA 11.0 (Stata Corp., College Station, TX) and the statistical programming language R version 2.10.0.(28)
ACKNOWLEDGEMENTS
The authors thank Jiyung Jun for her help in laboratory genotyping.
This research was supported by the grant from Korea Food & Drug Administration in 2005–2006 (06132KFDA412) and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000185). Additional grant support included R21 AG034412 by the National Institute of Health, U.S.A.
Grants J.O. was supported by the grant from Korea Food & Drug Administration (06132KFDA412) and by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MEST) (No. 2012-0000185). And L.C. was supported by a research grant by the National Institute of Health, U.S.A. (R21 AG034412)
ABBREVIATIONS
- ACR
acute cellular rejection
- ALT
alanine transaminase
- C/D ratio
dose-normalized concentration
- CYP
cytochrome P450
- DDLT
deceased donor liver transplantation
- GRWR
graft-to-recipient body weight ratio
- HWE
Hardy-Weinberg equilibrium
- LDLT
living donor liver transplantation
- MMF
mycophenolate mofetil
- PCR
polymerase chain reaction
- POD
post operational day
- RAI
rejection activity index
- REDE
CYP3A5 expresser recipient grafted from CYP3A5 expresser donor
- REDN
CYP3A5 expresser recipient grafted from CYP3A5 nonexpresser donor
- RNDE
CYP3A5 nonexpresser recipient grafted from CYP3A5 expresser donor
- RNDN
CYP3A5 nonexpresser recipient grafted from CYP3A5 nonexpresser donor
- TDM
therapeutic drug monitoring
- WBC
white blood cells
Footnotes
Authorship E.J. participated in research design, writing of the paper, performance of the research, and data analysis; L.C. participated in writing of the paper and data analysis; K.S., J.C and N.H. participated in performance of the research; J.O participated in research design, writing of the paper.
Conflict of interest All authors declared no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Venkataramanan R, Swaminathan A, Prasad T, et al. Clinical pharmacokinetics of tacrolimus. Clin Pharmacokinet. 1995;29:404. doi: 10.2165/00003088-199529060-00003. [DOI] [PubMed] [Google Scholar]
- 2.Jusko WJ, Thomson AW, Fung J, et al. Consensus document: therapeutic monitoring of tacrolimus (FK-506) Ther Drug Monit. 1995;17:606. doi: 10.1097/00007691-199512000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Zheng H, Webber S, Zeevi A, et al. Tacrolimus dosing in pediatric heart transplant patients is related to CYP3A5 and MDR1 gene polymorphisms. Am J Transplant. 2003;3:477. doi: 10.1034/j.1600-6143.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 4.Huang W, Lin YS, McConn DJ, 2nd, et al. Evidence of significant contribution from CYP3A5 to hepatic drug metabolism. Drug Metab Dispos. 2004;32:1434. doi: 10.1124/dmd.104.001313. [DOI] [PubMed] [Google Scholar]
- 5.Kuehl P, Zhang J, Lin Y, et al. Sequence diversity in CYP3A promoters and characterization of the genetic basis of polymorphic CYP3A5 expression. Nat Genet. 2001;27:383. doi: 10.1038/86882. [DOI] [PubMed] [Google Scholar]
- 6.Masuda S, Inui K. An up-date review on individualized dosage adjustment of calcineurin inhibitors in organ transplant patients. Pharmacol Ther. 2006;112:184. doi: 10.1016/j.pharmthera.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Kuypers DR, de Jonge H, Naesens M, Lerut E, Verbeke K, Vanrenterghem Y. CYP3A5 and CYP3A4 but not MDR1 single-nucleotide polymorphisms determine long-term tacrolimus disposition and drug-related nephrotoxicity in renal recipients. Clin Pharmacol Ther. 2007;82:711. doi: 10.1038/sj.clpt.6100216. [DOI] [PubMed] [Google Scholar]
- 8.Olthoff KM, Merion RM, Ghobrial RM, et al. Outcomes of 385 adult-to-adult living donor liver transplant recipients: a report from the A2ALL Consortium. Ann Surg. 2005;242:314. doi: 10.1097/01.sla.0000179646.37145.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berg CL, Gillespie BW, Merion RM, et al. Improvement in survival associated with adult-to-adult living donor liver transplantation. Gastroenterology. 2007;133:1806. doi: 10.1053/j.gastro.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schiano TD, Kim-Schluger L, Gondolesi G, Miller CM. Adult living donor liver transplantation: the hepatologist's perspective. Hepatology. 2001;33:3. doi: 10.1053/jhep.2001.21489. [DOI] [PubMed] [Google Scholar]
- 11.Wei-lin W, Jing J, Shu-sen Z, et al. Tacrolimus dose requirement in relation to donor and recipient ABCB1 and CYP3A5 gene polymorphisms in Chinese liver transplant patients. Liver Transpl. 2006;12:775. doi: 10.1002/lt.20709. [DOI] [PubMed] [Google Scholar]
- 12.Yu S, Wu L, Jin J, et al. Influence of CYP3A5 gene polymorphisms of donor rather than recipient to tacrolimus individual dose requirement in liver transplantation. Transplantation. 2006;81:46. doi: 10.1097/01.tp.0000188118.34633.bf. [DOI] [PubMed] [Google Scholar]
- 13.Uesugi M, Masuda S, Katsura T, Oike F, Takada Y, Inui K. Effect of intestinal CYP3A5 on postoperative tacrolimus trough levels in living-donor liver transplant recipients. Pharmacogenet Genomics. 2006;16:119. doi: 10.1097/01.fpc.0000184953.31324.e4. [DOI] [PubMed] [Google Scholar]
- 14.Fukudo M, Yano I, Yoshimura A, et al. Impact of MDR1 and CYP3A5 on the oral clearance of tacrolimus and tacrolimus-related renal dysfunction in adult living-donor liver transplant patients. Pharmacogenet Genomics. 2008;18:413. doi: 10.1097/FPC.0b013e3282f9ac01. [DOI] [PubMed] [Google Scholar]
- 15.Demetris AJ, Batts KP, Dhillon AP, et al. Banff schema for grading liver allograft rejection: an international consensus document. Hepatology. 1997;25:658. doi: 10.1002/hep.510250328. [DOI] [PubMed] [Google Scholar]
- 16.Lodewijk L, Mall A, Spearman CW, Kahn D. Effect of liver regeneration on the pharmacokinetics of immunosuppressive drugs. Transplant Proc. 2009;41:379. doi: 10.1016/j.transproceed.2008.10.055. [DOI] [PubMed] [Google Scholar]
- 17.Kawasaki S, Makuuchi M, Ishizone S, Matsunami H, Terada M, Kawarazaki H. Liver regeneration in recipients and donors after transplantation. Lancet. 1992;339:580. doi: 10.1016/0140-6736(92)90867-3. [DOI] [PubMed] [Google Scholar]
- 18.Haga J, Shimazu M, Wakabayashi G, et al. Liver regeneration in donors and adult recipients after living donor liver transplantation. Liver Transpl. 2008;14:1718. doi: 10.1002/lt.21622. [DOI] [PubMed] [Google Scholar]
- 19.Starkel P, Laurent S, Petit M, Van Den Berge V, Lambotte L, Horsmans Y. Early down-regulation of cytochrome P450 3A and 2E1 in the regenerating rat liver is not related to the loss of liver mass or the process of cellular proliferation. Liver. 2000;20:405. doi: 10.1034/j.1600-0676.2000.020005405.x. [DOI] [PubMed] [Google Scholar]
- 20.Li D, Lu W, Zhu JY, Gao J, Lou YQ, Zhang GL. Population pharmacokinetics of tacrolimus and CYP3A5, MDR1 and IL-10 polymorphisms in adult liver transplant patients. J Clin Pharm Ther. 2007;32:505. doi: 10.1111/j.1365-2710.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 21.Jun KR, Lee W, Jang MS, et al. Tacrolimus concentrations in relation to CYP3A and ABCB1 polymorphisms among solid organ transplant recipients in Korea. Transplantation. 2009;87:1225. doi: 10.1097/TP.0b013e31819f117e. [DOI] [PubMed] [Google Scholar]
- 22.Goto M, Masuda S, Kiuchi T, et al. CYP3A5*1-carrying graft liver reduces the concentration/oral dose ratio of tacrolimus in recipients of living-donor liver transplantation. Pharmacogenetics. 2004;14:471. doi: 10.1097/01.fpc.0000114747.08559.49. [DOI] [PubMed] [Google Scholar]
- 23.Elens L, Capron A, Kerckhove VV, et al. 1199G>A and 2677G>T/A polymorphisms of ABCB1 independently affect tacrolimus concentration in hepatic tissue after liver transplantation. Pharmacogenet Genomics. 2007;17:873. doi: 10.1097/FPC.0b013e3282e9a533. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z, Zhang WX, Chen B, Mao AW, Cai WM. Stepwise regression analysis of the determinants of blood tacrolimus concentrations in Chinese patients with liver transplant. Med Chem. 2009;5:301. doi: 10.2174/157340609788185918. [DOI] [PubMed] [Google Scholar]
- 25.Yu KS, Cho JY, Jang IJ, et al. Effect of the CYP3A5 genotype on the pharmacokinetics of intravenous midazolam during inhibited and induced metabolic states. Clin Pharmacol Ther. 2004;76:104. doi: 10.1016/j.clpt.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Diczfalusy U, Miura J, Roh HK, et al. 4Beta-hydroxycholesterol is a new endogenous CYP3A marker: relationship to CYP3A5 genotype, quinine 3-hydroxylation and sex in Koreans, Swedes and Tanzanians. Pharmacogenet Genomics. 2008;18:201. doi: 10.1097/FPC.0b013e3282f50ee9. [DOI] [PubMed] [Google Scholar]
- 27.Park SY, Kang YS, Jeong MS, Yoon HK, Han KO. Frequencies of CYP3A5 genotypes and haplotypes in a Korean population. J Clin Pharm Ther. 2008;33:61. doi: 10.1111/j.1365-2710.2008.00879.x. [DOI] [PubMed] [Google Scholar]
- 28.Team RDC . R: A Language and Environment for Statistical Computing. Vienna, Austria: 2005. [Google Scholar]