Abstract
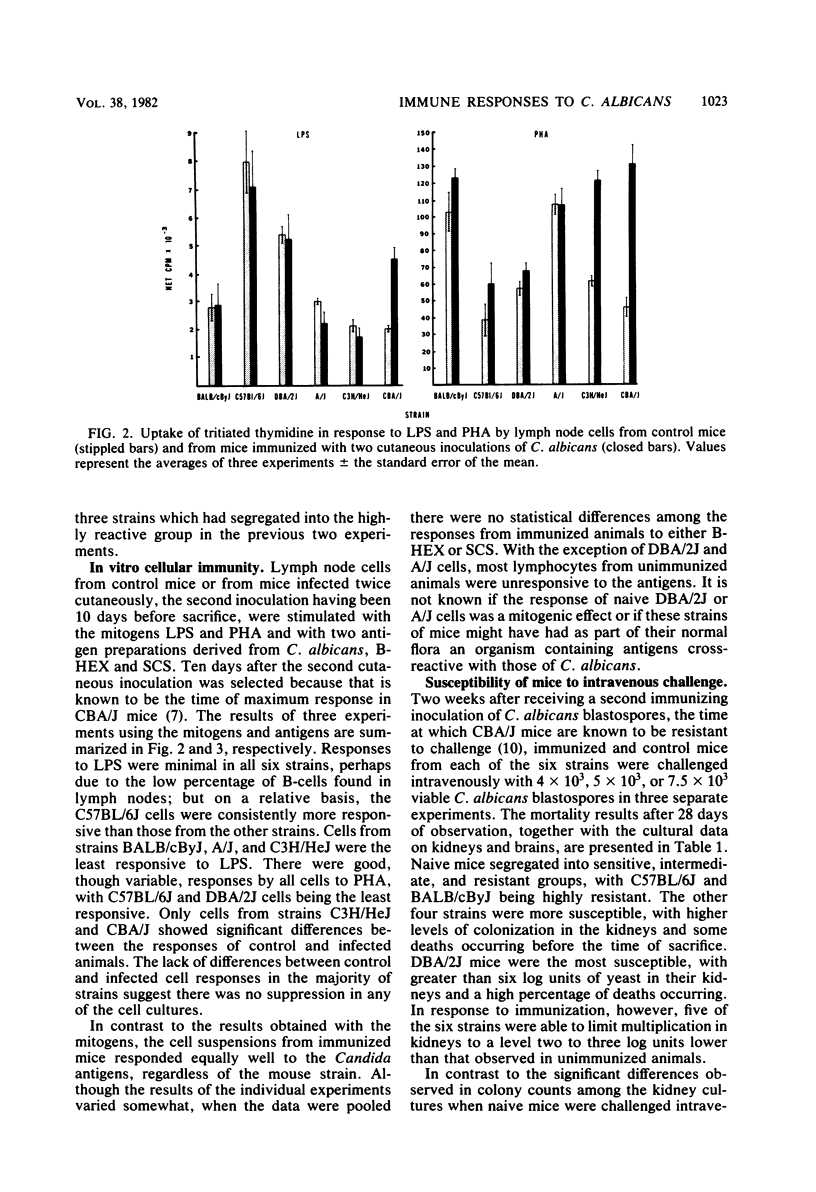
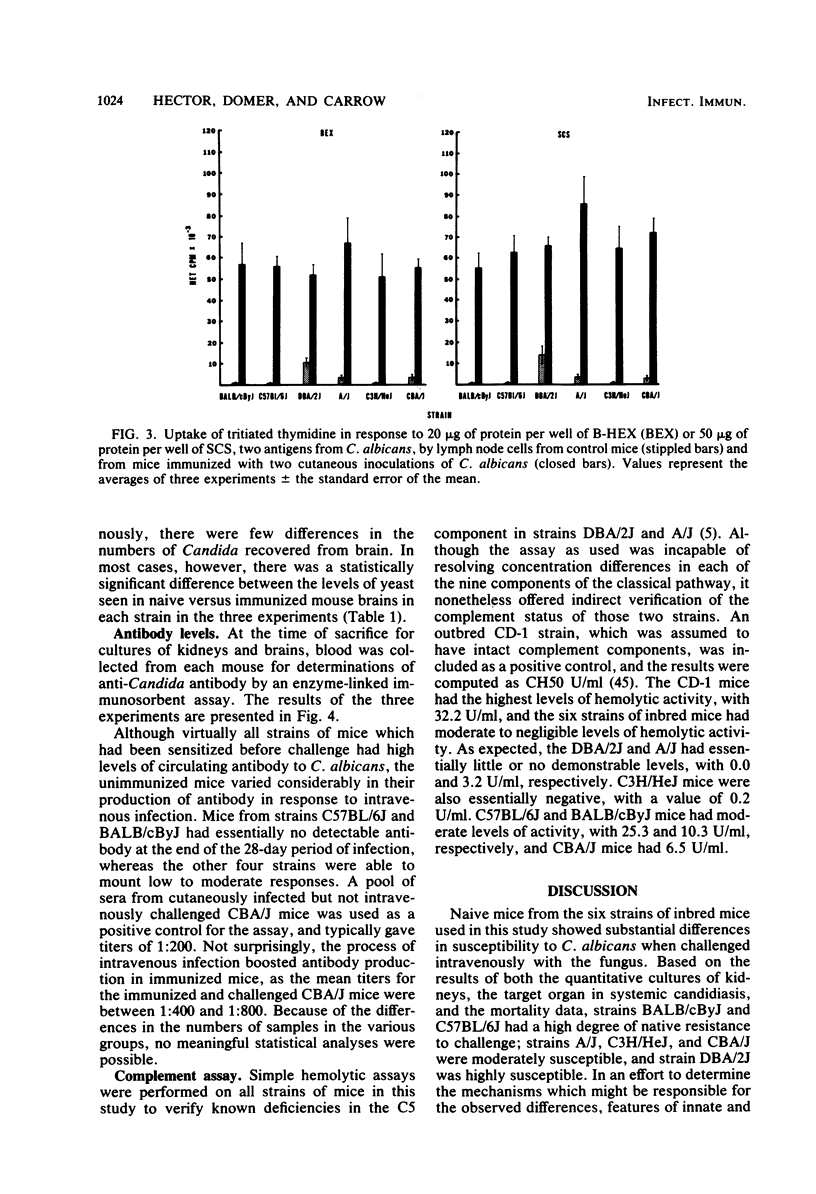
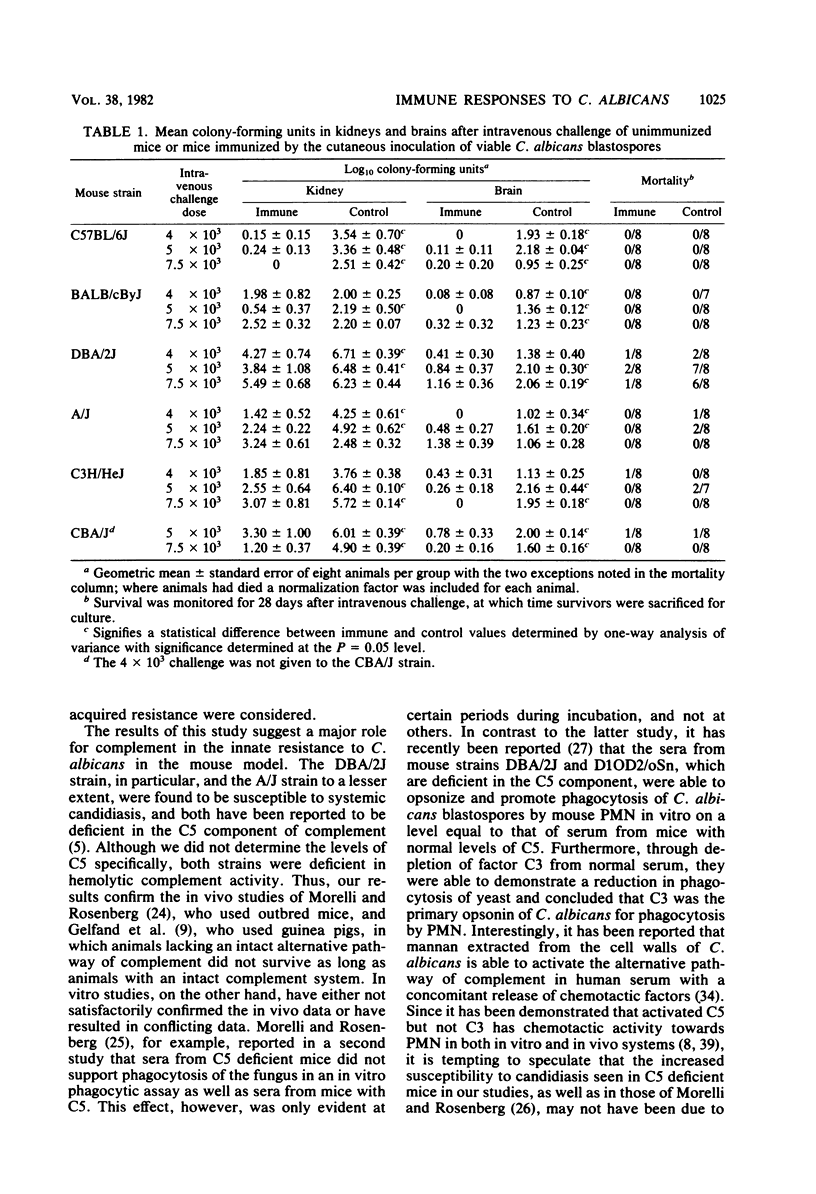
Mice from six genetically distinct strains were examined for their immune responses to Candida albicans in in vitro and in vivo assays, and naive mice and mice immunized with the fungus were challenged intravenously with three different doses of C. albicans to determine differences in susceptibility. Naive mice from the six groups showed substantial differences in resistance to challenge based on mortalities and quantitative cultures of kidneys, with mice from strains C57BL/6J and BALB/cByJ showing the most resistance; mice from strains A/J, C3H/HeJ, and CBA/J showing moderate susceptibility; and mice from strain DBA/2J showing the highest degree of susceptibility to challenge. Unimmunized mice from strains C57BL/6J and BALB/cByJ did not produce detectable levels of Candida-specific antibody by the end of the 28-day observation period when challenged intravenously, but the other strains did. Immunized mice showed a degree of protection to challenge, with all groups except mice from strain BALB/cByJ showing a reduction of two to three log units in the level of colonization in their kidneys and all strains producing significant levels of antibody. Additionally, the immunized mice of all strains developed substantial levels of delayed-type hypersensitivity and demonstrated nearly identical lymphocyte proliferative responses to Candida antigens. The results indicate that resistance to systemic candidiasis is dependent upon a combination of innate factors, predominately an intact complement system, and the acquisition of an immune response, most likely of a cell-mediated type. Additionally, the findings suggest that genetic control of acquired resistance to C. albicans may not be associated with the H-2 complex.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. Differences in resistance to Trypanosoma musculi infection among strains of inbred mice. Infect Immun. 1981 Aug;33(2):364–371. doi: 10.1128/iai.33.2.364-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaman L., Benjamini E., Pappagianis D. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun. 1981 Nov;34(2):347–353. doi: 10.1128/iai.34.2.347-353.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bistoni F., Marconi P., Frati L., Bonmassar E., Garaci E. Increase of mouse resistance to Candida albicans infection by thymosin alpha 1. Infect Immun. 1982 May;36(2):609–614. doi: 10.1128/iai.36.2.609-614.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer E., Vris T. W., Lawrence H. S. A microculture system for the measurement of antigen-induced murine lymphocyte proliferation: advantages of 5% horse serum and 5 X 10(-5) M mercaptoethanol. J Immunol Methods. 1977;17(3-4):319–327. doi: 10.1016/0022-1759(77)90114-4. [DOI] [PubMed] [Google Scholar]
- CINADER B., DUBISKI S., WARDLAW A. C. DISTRIBUTION, INHERITANCE, AND PROPERTIES OF AN ANTIGEN, MUB1, AND ITS RELATION TO HEMOLYTIC COMPLEMENT. J Exp Med. 1964 Nov 1;120:897–924. doi: 10.1084/jem.120.5.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Moser S. A. Experimental murine candidiasis: cell-mediated immunity after cutaneous challenge. Infect Immun. 1978 Apr;20(1):88–98. doi: 10.1128/iai.20.1.88-98.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez H. N., Henson P. M., Otani A., Hugli T. E. Chemotactic response to human C3a and C5a anaphylatoxins. I. Evaluation of C3a and C5a leukotaxis in vitro and under stimulated in vivo conditions. J Immunol. 1978 Jan;120(1):109–115. [PubMed] [Google Scholar]
- Gelfand J. A., Hurley D. L., Fauci A. S., Frank M. M. Role of complement in host defense against experimental disseminated candidiasis. J Infect Dis. 1978 Jul;138(1):9–16. doi: 10.1093/infdis/138.1.9. [DOI] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses to cutaneous inoculation with Candida albicans. Infect Immun. 1978 Feb;19(2):499–509. doi: 10.1128/iai.19.2.499-509.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giger D. K., Domer J. E., Moser S. A., McQuitty J. T., Jr Experimental murine candidiasis: pathological and immune responses in T-lymphocyte-depleted mice. Infect Immun. 1978 Sep;21(3):729–737. doi: 10.1128/iai.21.3.729-737.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gros P., Skamene E., Forget A. Genetic control of natural resistance to Mycobacterium bovis (BCG) in mice. J Immunol. 1981 Dec;127(6):2417–2421. [PubMed] [Google Scholar]
- Hurtrel B., Langrange P. H., Michel J. C. Absence of correlation between delayed-type hypersensitivity and protection in experimental systemic candidiasis in immunized mice. Infect Immun. 1981 Jan;31(1):95–101. doi: 10.1128/iai.31.1.95-101.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakoniuk P., Borowski J., Jabłońska-Strynkowska W., Badmajew W. The effects of lipids from Listeria monocytogenes on bacterial and fungal infections in mice. Arch Immunol Ther Exp (Warsz) 1978;26(1-6):637–640. [PubMed] [Google Scholar]
- Kagaya K., Fukazawa Y. Murine defense mechanism against Candida albicans infection. II. Opsonization, phagocytosis, and intracellular killing of C. albicans. Microbiol Immunol. 1981;25(8):807–818. doi: 10.1111/j.1348-0421.1981.tb00084.x. [DOI] [PubMed] [Google Scholar]
- Kagaya K., Shinoda T., Fukazawa Y. Murine defense mechanism against Candida albicans infection. I. Collaboration of cell-mediated and humoral immunities in protection against systemic C. albicans infection. Microbiol Immunol. 1981;25(7):647–654. doi: 10.1111/j.1348-0421.1981.tb00068.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laforce F. M., Mills D. M., Iverson K., Cousins R., Everett E. D. Inhibition of leukocyte candidacidal activity by serum from patients with disseminated candidiasis. J Lab Clin Med. 1975 Oct;86(4):657–666. [PubMed] [Google Scholar]
- Lehrer R. I. Measurement of candidacidal activity of specific leukocyte types in mixed cell populations I. Normal, myeloperoxidase-deficient, and chronic granulomatous disease neutrophils. Infect Immun. 1970 Jul;2(1):42–47. doi: 10.1128/iai.2.1.42-47.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine R. F., Mansfield J. M. Genetics of resistance to African trypanosomes: role of the H-2 locus in determining resistance to infection with Trypanosoma rhodesiense. Infect Immun. 1981 Nov;34(2):513–518. doi: 10.1128/iai.34.2.513-518.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra S., Balish E. Immunity to Candida albicans induced by Listeria monocytogenes. Infect Immun. 1974 Jul;10(1):72–82. doi: 10.1128/iai.10.1.72-82.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake T., Takeya K., Nomoto K., Muraoka S. Cellular elements in the resistance to candida infection in mice. I. Contribution of T lymphocytes and phagocytes at various stages of infection. Microbiol Immunol. 1977;21(12):703–725. doi: 10.1111/j.1348-0421.1977.tb00339.x. [DOI] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. Role of complement during experimental Candida infection in mice. Infect Immun. 1971 Apr;3(4):521–523. doi: 10.1128/iai.3.4.521-523.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli R., Rosenberg L. T. The role of complement in the phagocytosis of Candida albicans by mouse peripheral blood leukocytes. J Immunol. 1971 Aug;107(2):476–480. [PubMed] [Google Scholar]
- Morozumi P. A., Halpern J. W., Stevens D. A. Susceptibility differences of inbred strains of mice to blastomycosis. Infect Immun. 1981 Apr;32(1):160–168. doi: 10.1128/iai.32.1.160-168.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser S. A., Domer J. E. Effects of cyclophosphamide on murine candidiasis. Infect Immun. 1980 Feb;27(2):376–386. doi: 10.1128/iai.27.2.376-386.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourad S., Friedman L. Passive immunization of mice against Candida albicans. Sabouraudia. 1968 Feb;6(2):103–105. [PubMed] [Google Scholar]
- Mukherji A. K., Mallick K. C. Disseminated candidosis in cyclophosphamide induced leucopenic state: an experimental study. Indian J Med Res. 1972 Nov;60(11):1584–1591. [PubMed] [Google Scholar]
- Niederkorn J. Y., Shadduck J. A., Schmidt E. C. Susceptibility of selected inbred strains of mice to Encephalitozoon cuniculi. J Infect Dis. 1981 Sep;144(3):249–253. doi: 10.1093/infdis/144.3.249. [DOI] [PubMed] [Google Scholar]
- Pearsall N. N., Adams B. L., Bunni R. Immunologic responses to Candida albicans. III. Effects of passive transfer of lymphoid cells or serum on murine candidiasis. J Immunol. 1978 Apr;120(4):1176–1180. [PubMed] [Google Scholar]
- Plant J., Glynn A. A. Genetics of resistance to infection with Salmonella typhimurium in mice. J Infect Dis. 1976 Jan;133(1):72–78. doi: 10.1093/infdis/133.1.72. [DOI] [PubMed] [Google Scholar]
- Ray T. L., Hanson A., Ray L. F., Wuepper K. D. Purification of a mannan from Candida albicans which activates serum complement. J Invest Dermatol. 1979 Oct;73(4):269–274. doi: 10.1111/1523-1747.ep12531641. [DOI] [PubMed] [Google Scholar]
- Restrepo-Moreno A., Schneidau J. D., Jr Nature of the skin-reactive principle in culture filtrates prepared from Paracoccidioides brasiliensis. J Bacteriol. 1967 Jun;93(6):1741–1748. doi: 10.1128/jb.93.6.1741-1748.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes J. C., Wicker L. S., Urba W. J. Genetic control of susceptibility to Cryptococcus neoformans in mice. Infect Immun. 1980 Aug;29(2):494–499. doi: 10.1128/iai.29.2.494-499.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers T. J., Balish E. The role of activated macrophages in resistance to experimental renal candidiasis. J Reticuloendothel Soc. 1977 Oct;22(4):309–318. [PubMed] [Google Scholar]
- Ruthe R. C., Andersen B. R., Cunningham B. L., Epstein R. B. Efficacy of granulocyte transfusions in the control of systemic candidiasis in the leukopenic host. Blood. 1978 Sep;52(3):493–498. [PubMed] [Google Scholar]
- Snyderman R., Shin H. S., Phillips J. K., Gewurz H., Mergenhagen S. E. A neutrophil chemotatic factor derived from C'5 upon interaction of guinea pig serum with endotoxin. J Immunol. 1969 Sep;103(3):413–422. [PubMed] [Google Scholar]
- Sohnle P. G., Frank M. M., Kirkpatrick C. H. Mechanisms involved in elimination of organisms from experimental cutaneous Candida albicans infections in guinea pigs. J Immunol. 1976 Aug;117(2):523–530. [PubMed] [Google Scholar]
- Staples P. J., Boujak J., Douglas R. G., Jr, Leddy J. P. Disseminated candidiasis in a previously healthy girl: implication of a leukocyte candidacidal defect. Clin Immunol Immunopathol. 1977 Mar;7(2):157–167. doi: 10.1016/0090-1229(77)90044-7. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Kongshavn P. A., Skamene E. Genetic linkage of resistance to Listeria monocytogenes with macrophage inflammatory responses. J Immunol. 1981 Aug;127(2):402–407. [PubMed] [Google Scholar]
- Tanowitz H. B., Minato N., Lalonde R., Wittner M. Trypanosoma cruzi: correlation of resistance and susceptibility in infected bred mice with the in vivo primary antibody response to sheep red blood cells. Exp Parasitol. 1981 Oct;52(2):233–242. doi: 10.1016/0014-4894(81)90078-3. [DOI] [PubMed] [Google Scholar]
- Williams D. L., Cook J. A., Hoffmann E. O., Di Luzio N. R. Protective effect of glucan in experimentally induced candidiasis. J Reticuloendothel Soc. 1978 Jun;23(6):479–490. [PubMed] [Google Scholar]
- Williams D. M., Grumet F. C., Remington J. S. Genetic control of murine resistance to Toxoplasma gondii. Infect Immun. 1978 Feb;19(2):416–420. doi: 10.1128/iai.19.2.416-420.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


