Abstract
Recent evidence suggests that the fat mass and obesity-associated gene (FTO) genotype may interact with dietary intakes in relation to adiposity. We tested the effect of FTO variant on weight loss in response to 2-year diet interventions. FTO rs1558902 was genotyped in 742 obese adults who were randomly assigned to one of four diets differing in the proportions of fat, protein, and carbohydrate. Body composition and fat distribution were measured by dual-energy x-ray absorptiometry and computed tomography. We found significant modification effects for intervention varying in dietary protein on 2-year changes in fat-free mass, whole body total percentage of fat mass, total adipose tissue mass, visceral adipose tissue mass, and superficial adipose tissue mass (for all interactions, P < 0.05). Carriers of the risk allele had a greater reduction in weight, body composition, and fat distribution in response to a high-protein diet, whereas an opposite genetic effect was observed on changes in fat distribution in response to a low-protein diet. Likewise, significant interaction patterns also were observed at 6 months. Our data suggest that a high-protein diet may be beneficial for weight loss and improvement of body composition and fat distribution in individuals with the risk allele of the FTO variant rs1558902.
The prevalence of overweight and obesity has increased substantially in the U.S. and worldwide, and the health burden of obesity-related complications has grown accordingly (1–3). Obesity is primarily determined by both genetic and lifestyle factors, including diet, as well as their interactions (4). In the past few years, genome-wide association studies (GWASs) have identified a group of genetic loci associated with BMI and obesity risk (5–7). Among them, the fat mass and obesity-associated gene (FTO) locus shows the strongest effect (5,8). Accumulating evidence has suggested that this locus is involved in the hypothalamic regulation of appetite and dietary energy intake (9,10).
Recently, several studies have examined the effect of the FTO-diet interaction on body weight, but the results are not entirely consistent. Several cross-sectional studies showed that dietary factors such as low fat intake might modify the genetic effect of FTO on BMI or fat distribution (11–13). However, the gene by diet interaction was not univocally observed in randomized intervention trials (13–17), although one study found that a Mediterranean diet intervention modified the association between the FTO variant and weight changes in a population with high cardiovascular risk (18). These intervention trials, however, largely are limited by relatively small sample size or the short term of follow-up. In addition, animal studies have suggested that FTO might differentially affect various body compositions and fat distribution at different depots (19–21). Few studies have evaluated systematically the effect of the FTO variant on these measurements.
The POUNDS LOST Trial thus far is the largest 2-year randomized intervention trial that tested the effect of four diets varying in proportions of fat, protein, and carbohydrate on weight loss in overweight or obese subjects (22). By use of the data from this trial, we evaluated whether various weight-loss diets might modify the effect of the FTO variant on weight loss and long-term changes in body composition and fat distribution.
RESEARCH DESIGN AND METHODS
Study population.
The POUNDS LOST Trial was conducted from October 2004 through December 2007 at two sites as follows: Harvard School of Public Health and Brigham & Women’s Hospital in Boston, Massachusetts; and the Pennington Biomedical Research Center of Louisiana State University System, Baton Rouge, Louisiana. The design and sample collection have been described previously in detail (22). In brief, the study population was composed of 811 overweight or obese (BMI ranged from 25 to 40 kg/m2) participants aged 30–70 years. Major criteria for exclusion were the presence of diabetes or unstable cardiovascular disease, the use of medications that affect body weight, and insufficient motivation as assessed by interview and questionnaire. Among the 742 participants who were genotyped successfully, 61% were women, 80% were white, 15% were black, 3% were Hispanic, and 2% were Asian or other ethnic groups by self-report. The participants were assigned randomly to one of four diets constituting a two-by-two factorial design; the target percentages of energy derived from fat, protein, and carbohydrate in the four diets were 20, 15, and 65%; 20, 25, and 55%; 40, 15, and 45%; and 40, 25, and 35%. After 2 years, 645 participants (80% of total population) completed the trial. The study was approved by the human subjects committee at each institution and by a data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute. All participants provided written informed consent.
Measurements.
In the morning before breakfast, body weight and waist circumference (WC) were measured on 2 days at baseline; 6, 12, and 18 months; and 2 years. BMI was calculated as weight by height squared (kg/m2). A dual-energy X-ray absorptiometry (DEXA) scan was performed on 50% of a random sample from the total study participants (n = 424), including 242 (57.1%) women, using a Hologic QDR 4500A (Waltham, MA) (23). Total fat mass (kg), total fat-free mass (FFM; kg), whole body total percentage of fat mass (FM%) and percentage of trunk fat were obtained once at baseline, 6 months, and 2 years. Computed tomography (CT) was used in 50% of a random sample from those participants who had DEXA scans, resulting in a sample of 25% of the total participants (n = 195), including 113 (58.2%) women. Total adipose tissue (TAT) mass, visceral adipose tissue (VAT) mass, deep subcutaneous adipose tissue (DSAT) mass, and superficial adipose tissue (SAT) mass within the abdomen were measured by standard methods (24), once at baseline, 6 months, and 2 years. Because of radiation exposure, premenopausal women would not subject themselves to CT scans. A series of eight single-slice images were obtained every 10 cm from 2 below and 5 above the fourth and fifth lumbar vertebrae interspaces. These contiguous cross-sectional images were analyzed, and then the total volume was calculated from the individual slices. In this analysis, we only included data at baseline, 6 months, and 2 years with all the outcomes because the DEXA and CT scans were only performed at these three time points.
Genotyping.
DNA was extracted from the buffy coat fraction of centrifuged blood using the QIAmp Blood Kit (Qiagen, Chatsworth, CA). Single nucleotide polymorphism (SNP) rs1558902 was selected because it had emerged as the top variant of FTO locus for BMI and WC in recent obesity-related GWAS (25,26). The SNP was genotyped successfully in 742 of 811 total participants and 603 of 645 participants who completed the trial using the OpenArray SNP Genotyping System (BioTrove, Woburn, MA). Of the 424 participants who received DEXA scans, 391 were genotyped at baseline, and 224 participants who completed the trial were genotyped. Of the 195 participants who received CT scans, 175 were genotyped at baseline and 105 participants who completed the trial were genotyped. The genotype success rate was 99% in available DNA samples. Replicated quality control samples (10%) were included in every genotyping plate with greater than 99% concordance (27). The allele frequency in two major ethnic groups (white and black) was compatible with Hardy-Weinberg equilibrium (P > 0.05).
Statistical analysis.
The primary outcomes were changes in body weight and WC. Secondary outcomes were changes in body composition including total fat mass, FFM, FM% and percentage of trunk fat, and fat distribution (TAT, VAT, SAT, and DSAT). Data were pooled from the diets for the two factorial comparisons: low protein versus high protein and low fat versus high fat (22). Because the majority of the study population were white (80%), we also analyzed the main effects and interactions among white participants separately. The Hardy-Weinberg equilibrium and comparison of categorical variables at baseline were assessed with χ2 test. Differences in continuous variables at baseline were tested using ANCOVA, with adjustment for age, sex, and ethnicity. The main effects of genotype and diet intervention on outcome changes at 6 months and 2 years were analyzed using general linear regression models, with adjustment for covariates including age, sex, ethnicity, carbohydrate, the baseline value for the respective outcome, and baseline BMI. We excluded individuals with missing measures at each time point in the analysis. Moreover, to analyze the potential interactions between genotype and diet intervention, an interaction product term of genotype-diet was included in the models. In a secondary analysis, we used linear mixed models, with time as a repeated measurement factor, to test genetic associations with the trajectory of changes in outcomes in the participants who provided measurements at baseline, 6 months, and 2 years in each of four diet groups over the 2-year intervention by including genotype-time interaction terms. Because an additive genetic effect was reported in the original large-scale GWAS in which the SNP was identified (25,26), additive models were analyzed for genotype. All reported P values were two-sided and a P value of 0.05 was considered statistically significant. All data were analyzed with SAS version 9.1 (SAS Institute, Inc., Cary, NC).
RESULTS
Characteristics of study population.
Baseline characteristics of participants according to the FTO rs1558902 genotype are presented in Table 1. The minor allele frequency (MAF; A allele) was 0.402 in the total population. The genotype frequencies were significantly different among the sexes and ethnicities. After adjustment for age, sex, and ethnicity, all variables such as weight, BMI, WC, body composition, and fat distribution had no association with genotype at baseline. Baseline characteristics were similar among participants in the four diet groups (Supplementary Table 1). Likewise, no associations between the FTO genotypes and these measures were observed in the white participants (data not shown).
TABLE 1.
Baseline characteristics of the study participants according to FTO rs1558902 genotype
Effects of FTO rs1558902 genotype on weight and waist: overall and two-factorial analysis.
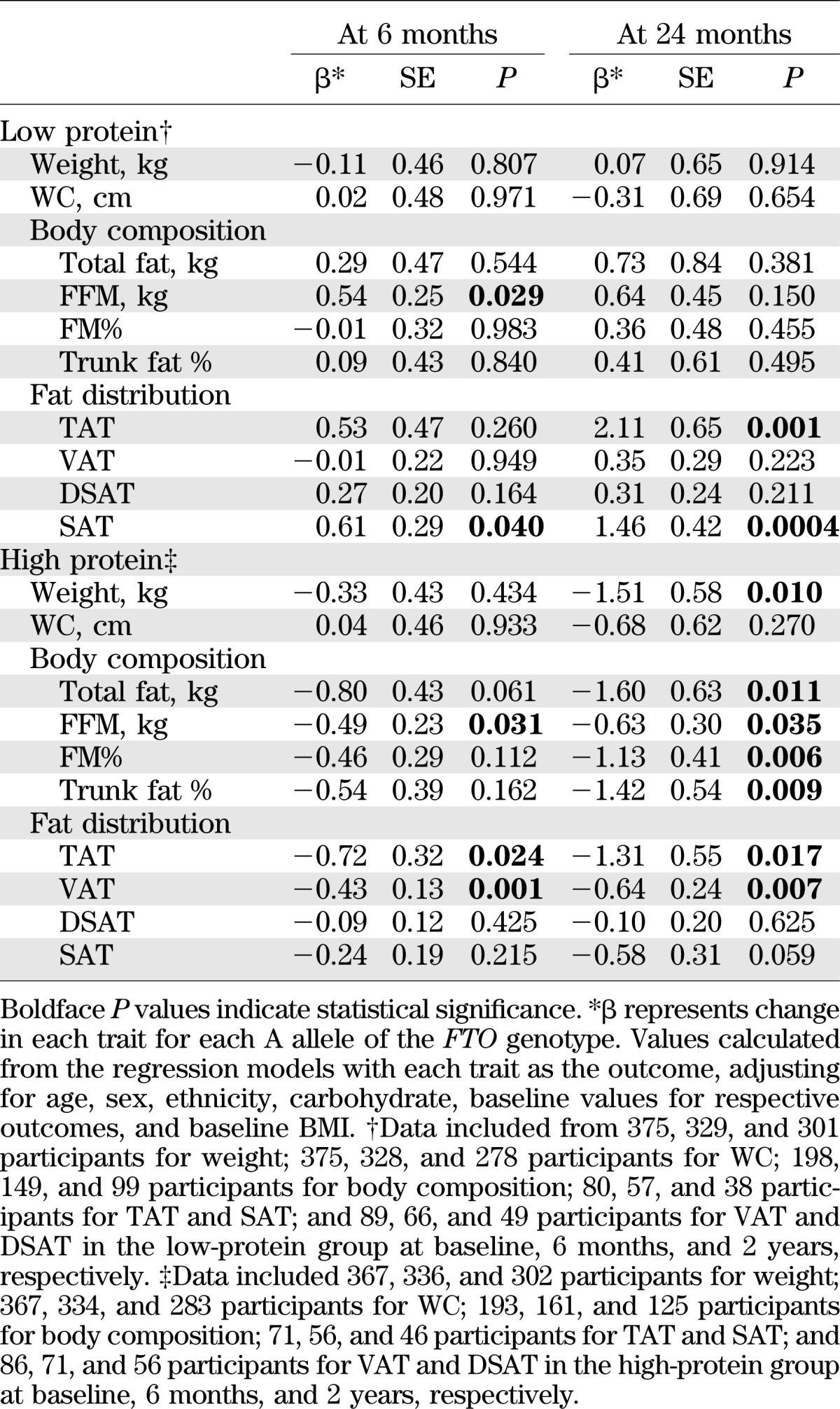
After adjustment for age, sex, ethnicity, baseline BMI, and diet groups, no main effects of the FTO rs1558902 genotype on changes in weight or WC were found in any participants at 6 months and 2 years (data not shown). We next examined the genetic effects on changes in weight and WC following a two-factorial design: low versus high fat and low versus high protein. We found that the risk allele (A) was significantly associated with a 1.51-kg greater weight loss in the high-protein group (P = 0.010), but not in the low-protein group, by the end of intervention (2 years). The changes in weight and WC were less significant at 6 months (Table 2). In subgroups treated by different proportions of dietary fat, we did not find significant genetic effects on changes in weight and WC (all P > 0.05; Supplementary Table 2).Similarly, in the white participants, we found the risk allele was associated with a 1.38-kg greater weight loss in the high-protein group at 2 years (P = 0.028), but not in other subgroups (data not shown).
TABLE 2.
The effects of the FTO rs1558902 genotype on weight, body composition, and fat distribution response to dietary protein intervention
The FTO rs1558902 genotype and changes in body composition by diet intervention.
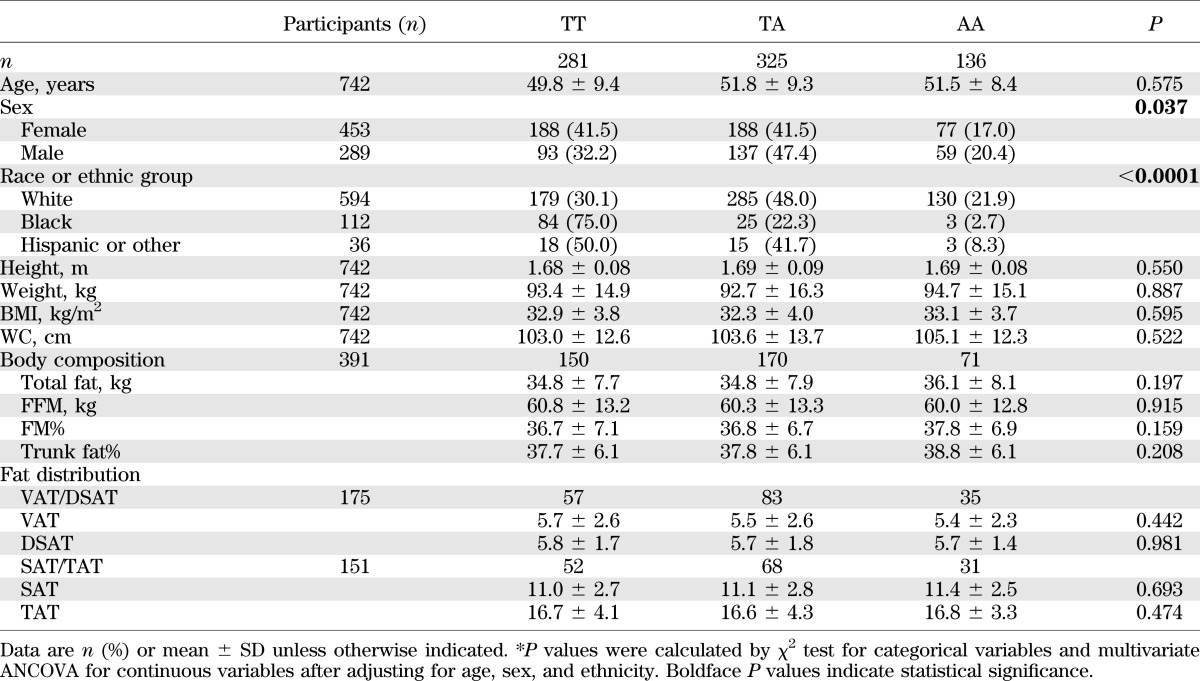
Consistent with the observations of change in body weight, we found that the rs15589002 risk allele (A) was associated with greater loss of total fat, FFM, FM%, and percentage of trunk fat at 2 years in the high-protein group, but not in the low-protein group (Table 2). Tests for genotype-diet protein interaction were significant on changes in FFM and FM% (for interactions, P = 0.034 and 0.049, respectively) adjusted for age, sex, ethnicity, carbohydrate, baseline BMI, and the baseline value of body composition (Fig. 1). At 6 months, we only observed gene by protein diet interaction on changes in FFM (P = 0.008 for interaction; Fig. 1). The risk allele carriers in the high-protein group had greater loss of FFM than noncarriers, but those in the low-protein group had less loss of FFM compared with noncarriers (Table 2).
FIG. 1.
Interaction between the FTO rs1558902 genotype and dietary protein intervention on changes in total fat (A), FFM (B), FM% (C), and percentage of trunk fat (D) at 6 months and 2 years. P values are adjusted for age, sex, ethnicity, carbohydrate, baseline values for respective outcomes, and baseline BMI. Data included 52 and 60 (TT), 66 and 73 (TA), and 31 and 28 (AA) participants in the low-protein group and the high-protein group at 6 months, respectively (total n = 310), and 34 and 44 (TT), 46 and 61 (TA), and 19 and 20 (AA) participants in the low-protein group and the high-protein group at 2 years, respectively (total n = 224).
We did not find significant genetic effect and interactions between the FTO variant and dietary fat intake on changes in body composition in total participants (Supplementary Table 2 and Supplementary Fig. 1). The results in the white participants were similar (data not shown).
The FTO rs1558902 genotype and changes in fat distribution by diet intervention.
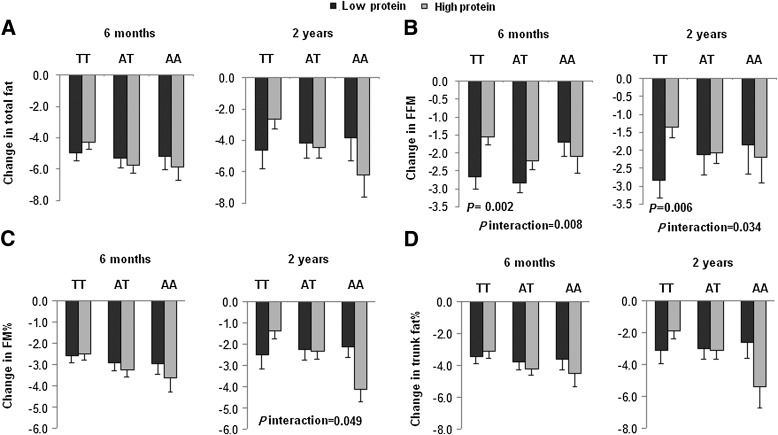
We further analyzed body fat distribution measured by CT. At 2 years, we found significant interactions between the FTO rs1558902 genotype and protein diet intervention on changes in TAT, VAT, and SAT (for interactions, P = 0.001, 0.012, and 0.002, respectively; Fig. 2). The risk allele (A) was associated with greater loss of TAT and VAT in the high-protein group but with less loss of TAT and SAT in the low-protein group (Table 2). At 6 months, gene-protein interactions were observed on changes in TAT and SAT (for interactions, P = 0.026 and 0.050, respectively; Fig. 2), and the risk allele carriers in the high-protein group had greater loss of TAT and VAT than noncarriers, but those in the low-protein group had less loss of SAT than noncarriers (Table 2).
FIG. 2.
Interaction between the FTO rs1558902 genotype and dietary protein intervention on changes in TAT (A), VAT (B), DSAT (C), and SAT (D) at 6 months and 2 years. P values are adjusted for age, sex, ethnicity, carbohydrate, baseline values for respective outcomes, and baseline BMI. Data included 17, 18, 18, and 17 (TT); 30, 35, 35, and 30 (TA); and 10, 13, 13, and 10 (AA) participants in the low-protein group and 18, 22, 22, and 18 (TT); 26, 35, 35, and 26 (TA); and 12, 14, 14, and 12 (AA) in the high-protein group for TAT, VAT, DSAT, and SAT at 6 months (total n = 137); and 12, 15, 15, and 12 (TT); 18, 25, 25, and 18 (TA); and 8, 9, 9, and 8 (AA) participants in the low-protein group and 15, 17, 17, and 15 (TT); 23, 30, 30, and 23 (TA); and 8, 9, 9, and 8 (AA) in the high-protein group for TAT, VAT, DSAT, and SAT at 2 years (total n = 105).
We did not find significant genetic effects and interactions on changes in fat distribution in subgroups treated by different dietary fat components (all P > 0.05; Supplementary Table 2 and Supplementary Fig. 2).Similar results were found in the white participants (data not shown).
The trajectory of changes in body composition and fat distribution by FTO rs1558902 in response to protein diet.
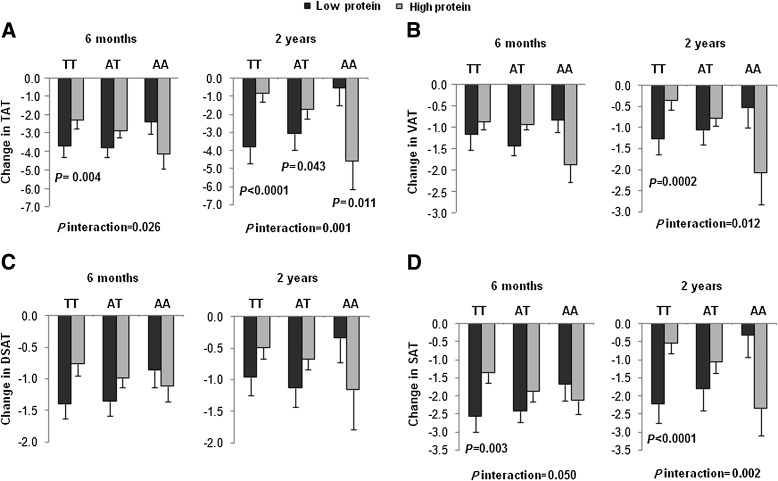
In a secondary analysis, we used linear mixed models to assess the genotype by time effect over the 2-year trial in those treated by the four dietary compositions. We observed significant genotype-time interactions on changes in total fat, FM%, and percentage of trunk fat in response to the high-protein diet. When assigned to the high-protein diet, participants who carried the AA genotype had greater loss in total fat, FM%, and percentage of trunk fat than those without this genotype. No genotype-time interaction on changes in body composition was found in the low-protein group (Fig. 3).
FIG. 3.
Changes in total fat (A), FFM (B), FM% (C), and percentage of trunk fat (D) in the low-protein and the high-protein diet groups according to the FTO rs1558902 genotype from baseline to 6 months and 2 years. P values are adjusted for age, sex, ethnicity, carbohydrate, baseline values for respective outcomes, and baseline BMI. Data included 198, 149, and 99 in the low-protein group and 193, 161, and 125 in the high-protein group for body composition at baseline, 6 months and 2 years, respectively. (A high-quality color representation of this figure is available in the online issue.)
We also observed significant genotype-time interactions on changes in fat distribution in response to the low- and high-protein diets. Participants with the AA genotype had greater decrease in fat distribution in response to the high-protein diet compared with those without this genotype. In contrast, participants with the AA genotype were associated with less loss in TAT and SAT in response to the low-protein diet (Fig. 4).
FIG. 4.
Changes in TAT (A), VAT (B), DSAT (C), and SAT (D) in the low-protein and high-protein diet groups according to the FTO rs1558902 genotype from baseline to 6 months and 2 years. P values are adjusted for age, sex, ethnicity, carbohydrate, baseline values for respective outcomes, and baseline BMI. Data included values at baseline and at 6 months and 2 years for 80, 57, and 38 participants, respectively, for TAT and SAT and 89, 66, and 49 participants, respectively, for VAT and DSAT in the low-protein group; and 71, 56, and 46 participants, respectively, for TAT and SAT and 86, 71, and 56 participants, respectively, for VAT and DSAT in the high-protein group. (A high-quality color representation of this figure is available in the online issue.)
We found genotype-time interactions on changes in total fat, FM%, and percentage of trunk fat in response to the low-fat diet (Supplementary Fig. 3), but no genotype-time interactions on changes in fat distribution were found in response to the low- or high-fat diets (Supplementary Fig. 4).A similar trend was observed in the white population (data not shown).
DISCUSSION
In the POUNDS LOST Trial, a 2-year, randomized weight-loss intervention, we found that dietary protein intake significantly modified the effect of an FTO variant on changes in body composition and fat distribution. Carriers of the risk allele (A allele) of the rs1558902 genotype had a greater loss of weight and regional fat in response to a high-protein diet compared with noncarriers, whereas an opposite genetic effect was observed regarding changes in fat distribution in response to a low-protein diet. Our data indicate that the modification effects of dietary treatment were more evident with prolonged intervention. We did not observe significant modification of dietary fat intake on the genotype effects.
The rs1558902 genotype was reported to show the strongest association with obesity in the European (25,26) and other ethnic populations (28), and it has strong linkage disequilibrium with other obesity-associated FTO variants such as the rs9939609 genotype. In this study, the MAF of the polymorphism in all participants was similar to those in the HapMap CEU population (0.45). At baseline, no significant difference was observed in anthropometrics and metabolic estimates, body composition, or fat distribution across genotypes. The lack of association with baseline BMI is probably largely due to the fact that the participants were all overweight or obese, so that the groups had relatively smaller variances in BMI than the general population.
Several cross-sectional studies showed that diets might modify the effect of the FTO variant on obesity, but the data from randomized diet intervention trials are conflicting and limited by small sample size or short term of follow-up (Supplementary Table 3). Two lifestyle intervention studies with follow-up periods of 9 and 12 months did not find significant influence of FTO polymorphisms (rs8050136 and rs9939609) on changes in body weight or fat distribution related to diet among 200 overweight and obese individuals treated by diets with reduced fat and increased fiber or reduced fat and sugar (13,14). In another 10-week, hypo-energetic diet intervention with either low fat or high fat content, the FTO variant had an effect on only changes in resting energy expenditure, insulin release, and sensitivity, not on weight loss (15). Similarly, in the Finnish Diabetes Prevention Study, the FTO variant did not modify weight change by individualized diet intervention with reduced fat and increased fiber during the 4-year follow-up of 255 individuals with impaired glucose tolerance (16). In our study, when macronutrient components of diets were not considered, we found no main effects of the FTO variant on changes in weight and body composition during the intervention.
Grau et al. (15,29) reported that dietary fat/carbohydrate content interacted with some genetic variants including the FTO variant on weight reduction or change in obesity-related phenotypes. In our study, we also found significant gene-diet interactions on changes in body composition and fat distribution. However, our data indicate that it is the dietary protein component, rather than dietary fat, that might drive the observed interactions. In previous studies, high-protein intervention has been found to result in a greater weight loss and abdominal fat mass (30–32). Our results suggest that individuals with a certain genetic background may benefit more in weight loss by following a high-protein diet. The mechanism of how protein intake interacts with FTO genotype is unclear.
Our data indicate that the genetic effects on certain fat compositions or depots may be more evident than the effects on overall adiposity. Functional studies have shown that the loss or overexpression of FTO in mice led to different changes in fat distribution at different dissected sites (19–21). FTO mRNA expression was fat depot–specific and was found to differ significantly in subcutaneous fat and in visceral fat (33–35). Epidemiological studies also have shown that FTO variants are significantly associated with distribution of fat depots (13,36,37). Taken together, these data suggest that genetic effects of FTO on the change of fat mass at various sites may be different, and changes in anthropometrics may not adequately reflect the effects of an FTO variant.
The genetic effect in our study seemed to be more evident at 2 years than that at 6 months. The results were in line with a recent study by Razquin et al. (18) in which it was found that FTO risk allele carriers had the highest weight reduction after 3 years of intervention with a Mediterranean diet compared with several short-term diet interventions (less than 1 year) in which no influence of an FTO variant on weight loss or change in fat distribution was found (13–15,17). Of note, between 6 months and 2 years of intervention in our trial, the participants regained weight. Therefore, it seems that the genetic variant might affect both the reduction and regain of the adiposity measures. These data suggest that the modification effects of diet treatment on an FTO genotype effect are more likely to be identified in long-term interventions.
Several limitations need to be considered when interpreting our findings. Even though our study is thus far the largest and longest diet intervention weight-loss trial, the relatively small sample size of the subgroups may limit the power to detect very moderate genetic effects or interactions. We did not adjust for multiple testing according to the recommendation by Rothman (38) and Lai et al. (39) because outcomes and the repeated measurements at 6 months and 2 years were highly correlated in our study. Overadjustment for multiple comparisons may increase the type II error and reduce power to detect significant differences. In addition, the majority of the total participants were white, and further studies are needed to determine whether our findings are generalizable to other ethnic groups. Even though the randomized clinical trial is thought to be the best model to test gene-environment interactions, we acknowledge that replication in diverse populations is needed to verify our findings.
In summary, we found that dietary protein intake might modify the FTO variant’s effect on changes in body composition and fat distribution. A high-protein diet may be beneficial for weight loss in individuals with the risk allele of an FTO variant. Further studies are warranted to verify our findings and explore the potential mechanisms.
ACKNOWLEDGMENTS
This study was supported by grants from the National Heart, Lung, and Blood Institute (HL071981), the National Institute of Diabetes and Digestive and Kidney Diseases (DK091718), the General Clinical Research Center (RR-02635), and the Boston Obesity Nutrition Research Center (DK46200). L.Q. was a recipient of the American Heart Association Scientist Development Award (0730094N). C.Z. was supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institutes of Health. X.Z. was supported by the National Natural Science Foundation of China (NNSFC 30972453).
No potential conflicts of interest relevant to this article were reported.
X.Z., Q.Q., and L.Q. contributed to study concept and design, acquisition of data, analysis and interpretation of the data, drafting of the manuscript, and critical revision of the manuscript. C.Z., F.B.H., and F.M.S. contributed to study concept and design and critical revision of the manuscript. X.Z., Q.Q., and C.Z. contributed to statistical analysis. F.M.S., F.B.H., and L.Q. contributed to administration, material support, and study supervision. X.Z., C.Z., and L.Q. contributed to obtaining funding. L.Q. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
The authors are particularly grateful to all participants in the trial for their dedication and contribution to the research.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1799/-/DC1.
REFERENCES
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999-2008. JAMA 2010;303:235–241 [DOI] [PubMed] [Google Scholar]
- 2.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA 1999;282:1523–1529 [DOI] [PubMed] [Google Scholar]
- 3.Kelly T, Yang W, Chen CS, Reynolds K, He J. Global burden of obesity in 2005 and projections to 2030. Int J Obes 2008;32:1431–1437 [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C. Gene-environment interactions in the etiology of obesity: defining the fundamentals. Obesity (Silver Spring) 2008;16(Suppl 3):S5–S10 [DOI] [PubMed] [Google Scholar]
- 5.Frayling TM, Timpson NJ, Weedon MN, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 2007;316:889–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scuteri A, Sanna S, Chen WM, et al. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet 2007;3:e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dina C, Meyre D, Gallina S, et al. Variation in FTO contributes to childhood obesity and severe adult obesity. Nat Genet 2007;39:724–726 [DOI] [PubMed] [Google Scholar]
- 8.Kathiresan S, Melander O, Guiducci C, et al. Six new loci associated with blood low-density lipoprotein cholesterol, high-density lipoprotein cholesterol or triglycerides in humans. Nat Genet 2008;40:189–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wardle J, Carnell S, Haworth CM, Farooqi IS, O’Rahilly S, Plomin R. Obesity associated genetic variation in FTO is associated with diminished satiety. J Clin Endocrinol Metab 2008;93:3640–3643 [DOI] [PubMed] [Google Scholar]
- 10.Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med 2008;359:2558–2566 [DOI] [PubMed] [Google Scholar]
- 11.Sonestedt E, Roos C, Gullberg B, Ericson U, Wirfält E, Orho-Melander M. Fat and carbohydrate intake modify the association between genetic variation in the FTO genotype and obesity. Am J Clin Nutr 2009;90:1418–1425 [DOI] [PubMed] [Google Scholar]
- 12.Ahmad T, Lee IM, Paré G, et al. Lifestyle interaction with fat mass and obesity-associated (FTO) genotype and risk of obesity in apparently healthy U.S. women. Diabetes Care 2011;34:675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haupt A, Thamer C, Machann J, et al. Impact of variation in the FTO gene on whole body fat distribution, ectopic fat, and weight loss. Obesity (Silver Spring) 2008;16:1969–1972 [DOI] [PubMed] [Google Scholar]
- 14.Müller TD, Hinney A, Scherag A, et al. ‘Fat mass and obesity associated’ gene (FTO): no significant association of variant rs9939609 with weight loss in a lifestyle intervention and lipid metabolism markers in German obese children and adolescents. BMC Med Genet 2008;9:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grau K, Hansen T, Holst C, et al. Macronutrient-specific effect of FTO rs9939609 in response to a 10-week randomized hypo-energetic diet among obese Europeans. Int J Obes (Lond) 2009;33:1227–1234 [DOI] [PubMed] [Google Scholar]
- 16.Lappalainen TJ, Tolppanen AM, Kolehmainen M, et al. Finnish Diabetes Prevention Study Group The common variant in the FTO gene did not modify the effect of lifestyle changes on body weight: the Finnish Diabetes Prevention Study. Obesity (Silver Spring) 2009;17:832–836 [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, Hinney A, Toschke AM, Hebebrand J. Aggravating effect of INSIG2 and FTO on overweight reduction in a one-year lifestyle intervention. Arch Dis Child 2009;94:965–967 [DOI] [PubMed] [Google Scholar]
- 18.Razquin C, Martinez JA, Martinez-Gonzalez MA, Bes-Rastrollo M, Fernández-Crehuet J, Marti A. A 3-year intervention with a Mediterranean diet modified the association between the rs9939609 gene variant in FTO and body weight changes. Int J Obes (Lond) 2010;34:266–272 [DOI] [PubMed] [Google Scholar]
- 19.Fischer J, Koch L, Emmerling C, et al. Inactivation of the Fto gene protects from obesity. Nature 2009;458:894–898 [DOI] [PubMed] [Google Scholar]
- 20.Church C, Lee S, Bagg EA, et al. A mouse model for the metabolic effects of the human fat mass and obesity associated FTO gene. PLoS Genet 2009;5:e1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Church C, Moir L, McMurray F, et al. Overexpression of Fto leads to increased food intake and results in obesity. Nat Genet 2010;42:1086–1092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med 2009;360:859–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lovejoy JC, Smith SR, Rood JC. Comparison of regional fat distribution and health risk factors in middle-aged white and African American women: The Healthy Transitions Study. Obes Res 2001;9:10–16 [DOI] [PubMed] [Google Scholar]
- 24.Kvist H, Chowdhury B, Grangård U, Tylén U, Sjöström L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr 1988;48:1351–1361 [DOI] [PubMed] [Google Scholar]
- 25.Heard-Costa NL, Zillikens MC, Monda KL, et al. NRXN3 is a novel locus for waist circumference: a genome-wide association study from the CHARGE Consortium. PLoS Genet 2009;5:e1000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Speliotes EK, Willer CJ, Berndt SI, et al. MAGIC. Procardis Consortium Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet 2010;42:937–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qi Q, Bray GA, Smith SR, Hu FB, Sacks FM, Qi L. Insulin receptor substrate 1 gene variation modifies insulin resistance response to weight-loss diets in a 2-year randomized trial: the Preventing Overweight Using Novel Dietary Strategies (POUNDS LOST) trial. Circulation 2011;124:563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hotta K, Nakata Y, Matsuo T, et al. Variations in the FTO gene are associated with severe obesity in the Japanese. J Hum Genet 2008;53:546–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grau K, Cauchi S, Holst C, et al. TCF7L2 rs7903146-macronutrient interaction in obese individuals’ responses to a 10-wk randomized hypoenergetic diet. Am J Clin Nutr 2010;91:472–479 [DOI] [PubMed] [Google Scholar]
- 30.Larsen TM, Dalskov SM, van Baak M, et al. Diet, Obesity, and Genes (Diogenes) Project Diets with high or low protein content and glycemic index for weight-loss maintenance. N Engl J Med 2010;363:2102–2113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clifton PM, Bastiaans K, Keogh JB. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr Metab Cardiovasc Dis 2009;19:548–554 [DOI] [PubMed] [Google Scholar]
- 32.Te Morenga LA, Levers MT, Williams SM, Brown RC, Mann J. Comparison of high protein and high fiber weight-loss diets in women with risk factors for the metabolic syndrome: a randomized trial. Nutr J 2011;10:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Klöting N, Schleinitz D, Ruschke K, et al. Inverse relationship between obesity and FTO gene expression in visceral adipose tissue in humans. Diabetologia 2008;51:641–647 [DOI] [PubMed] [Google Scholar]
- 34.Zabena C, González-Sánchez JL, Martínez-Larrad MT, et al. The FTO obesity gene. Genotyping and gene expression analysis in morbidly obese patients. Obes Surg 2009;19:87–95 [DOI] [PubMed] [Google Scholar]
- 35.Terra X, Auguet T, Porras JA, et al. Anti-inflammatory profile of FTO gene expression in adipose tissues from morbidly obese women. Cell Physiol Biochem 2010;26:1041–1050 [DOI] [PubMed] [Google Scholar]
- 36.Hotta K, Nakamura M, Nakamura T, et al. Polymorphisms in NRXN3, TFAP2B, MSRA, LYPLAL1, FTO and MC4R and their effect on visceral fat area in the Japanese population. J Hum Genet 2010;55:738–742 [DOI] [PubMed] [Google Scholar]
- 37.López-Bermejo A, Petry CJ, Díaz M, et al. The association between the FTO gene and fat mass in humans develops by the postnatal age of two weeks. J Clin Endocrinol Metab 2008;93:1501–1505 [DOI] [PubMed] [Google Scholar]
- 38.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990;1:43–46 [PubMed] [Google Scholar]
- 39.Lai CQ, Demissie S, Cupples LA, et al. Influence of the APOA5 locus on plasma triglyceride, lipoprotein subclasses, and CVD risk in the Framingham Heart Study. J Lipid Res 2004;45:2096–2105 [DOI] [PubMed] [Google Scholar]