Abstract
Although obesity rates are rapidly rising, caloric restriction remains one of the few safe therapies. Here we tested the hypothesis that obesity-associated disorders are caused by increased adipose tissue as opposed to excess dietary lipids. Fat mass (FM) of lean C57B6 mice fed a high-fat diet (HFD; FMC mice) was “clamped” to match the FM of mice maintained on a low-fat diet (standard diet [SD] mice). FMC mice displayed improved glucose and insulin tolerance as compared with ad libitum HFD mice (P < 0.001) or SD mice (P < 0.05). These improvements were associated with fewer signs of inflammation, consistent with the less-impaired metabolism. In follow-up studies, diet-induced obese mice were food restricted for 5 weeks to achieve FM levels identical with those of age-matched SD mice. Previously, obese mice exhibited improved glucose and insulin tolerance but showed markedly increased fasting-induced hyperphagia (P < 0.001). When mice were given ad libitum access to the HFD, the hyperphagia of these mice led to accelerated body weight gain as compared with otherwise matched controls without a history of obesity. These results suggest that although caloric restriction on a HFD provides metabolic benefits, maintaining those benefits may require lifelong continuation, at least in individuals with a history of obesity.
Caloric restriction (CR) has become a popular recommendation to decrease body weight (BW) (1), achieve metabolic benefits, and potentially increase life expectancy (2–4). Although the benefits of weight loss are undeniable, the optimal method to achieve a healthy weight is the subject of continuing debate. Obesity is accompanied by many comorbidities (5), and it is widely accepted as a causal factor. A current hypothesis suggests that obesity provokes inflammation in adipose tissue (6), leading to the release of proinflammatory mediators into systemic circulation. Rodent studies suggest that consumption of a high-fat diet (HFD) leads to infiltration by T cells (7) and macrophages (8), which likely contributes to inflammation in adipose tissue and the development of obesity-associated diseases such as type 2 diabetes (9).
Alternative explanations for the obesity-related comorbidities are based on specific harmful effects of HFDs per se. High-fat foods induce stress in the intestinal epithelium (10) and promote inflammation by absorption of antigenic material from the gut (11,12). Furthermore, HFD feeding increases reactive oxygen species production (13) that preceded obesity (14), suggesting that like sucrose and fructose, dietary lipids may contribute to obesity-associated diseases. In the present experiments we tested the hypothesis that obesity-associated disorders are caused by increased adipose tissue, as opposed to the obesogenic diet. To dissect these possibilities, we isolated body fat mass (FM) from fat intake using a “FM clamp” mouse model (Fig. 1A). In related studies, we asked whether a history of obesity affects future energy metabolism. Published reports suggest that counter-regulatory mechanisms that develop during CR lead to enhanced metabolic efficiency and rapid weight regain (15,16). We thus aimed to distinguish if this metabolic reprogramming is related to prior obesity, dietary fat intake, or having been calorically restricted (Fig. 1A).
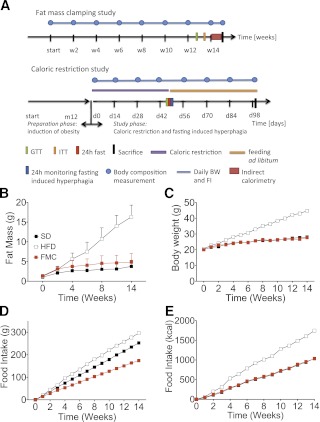
FIG. 1.
A: Study design and FM clamping. B: FM was measured by nuclear magnetic resonance technology and did not differ between SD and FMC mice. C: BW of SD, HFD fed, and FMC mice was measured daily. Mice from the SD and HFD group were fed with SD or HFD ad libitum. Mice from the FMC group were fed defined amounts of HFD to match FM to the SD group. D: Absolute food intake was lowest in FMC mice (P < 0.001). E: Caloric intake was similar in FMC and SD mice and highest in mice of the HFD group. All mice are male (n = 16 per group). All data are represented as mean ± SEM. ITT, insulin tolerance test; FI, food intake.
RESEARCH DESIGN AND METHODS
Animals.
Six- to 7-week- (FM clamp study) or 12-month-old (CR study) male, C57Bl/6J mice (Jackson Laboratories) were fed a standard diet (SD; D12329; 16.4 kcal% protein; 73 kcal% carbohydrate; 10.5% kcal% fat) or a matched HFD (D12331; 16.4 kcal% protein; 25.5 kcal% carbohydrate; 58.0 kcal% fat; Research Diets, New Brunswick, NJ). Mice were single or group-housed on a 12:12-h light-dark cycle (light on from 0600 to 1800 h) at 22°C with free access to water. All studies were approved by and performed according to the guidelines of the Institutional Animal Care and Use Committee of the University of Cincinnati.
FM clamping.
Mice in the SD and HFD groups had ad libitum access to food. Mice in the clamped group (FM clamped [FMC]) were fed HFD every day, 3 h after light onset. BW and food intake were measured daily with a laboratory scale (Metler, Toledo, OH). FM and lean mass were determined every other week using noninvasive nuclear magnetic resonance technology (EchoMRI, Houston, TX). Because FM was measured every other week, allocated food portions were adjusted daily based on the daily changes in BW.
CR study.
Lean (SD group), obese (HFD group), and FMC mice were generated as described above. Additionally, age-matched diet-induced obese (DIO) mice were partitioned into two CR groups, one fed SD diet and the other HFD. CR continued for 45 days, when both groups of CR mice had attained a mean FM comparable with that of the lean SD control group. All groups were then given ad libitum access to SD or HFD for the remainder of the experiment (58 days). At this time the SD mice were divided such that half remained on SD and the other half were switched to HFD.
Fasting-induced hyperphagia.
All mice were fasted for 24 h on day 45 of the CR. Food was removed at 0900 h. At 0900 h the following day, mice were given free access to SD (SD and caloric restricted fed standard diet [CR-SD] groups) or HFD (HFD, FMC, and caloric restricted fed high-fat diet [CR-HFD] groups) and food intake was monitored for 24 h.
Energy expenditure and locomotor activity.
Energy intake, expenditure, and home-cage activity were assessed using a combined indirect calorimetry system (TSE Systems). O2 consumption and CO2 production were measured every 45 min for a total of 120 h (including 12 h of adaptation) to determine the respiratory quotient (RQ) and energy expenditure.
Glucose and insulin tolerance tests.
Intraperitoneal glucose and insulin tolerance tests were performed by injection of glucose (2 g/kg, 20% wt/vol d-glucose [Sigma] in 0.9% wt/vol saline) or human regular insulin (1 unit/kg [Lilly Humolog] in 0.9% wt/vol saline) to 6-h fasted mice. Blood samples were collected immediately before and 15, 30, 60, and 120 min after injection. Blood glucose was determined with a TheraSense Freestyle Glucometer. C-peptide was measured via radioimmunoassay (Millipore, Billerica, MA) in plasma collected at the 30-min time point of glucose tolerance test (GTT).
Plasma parameters.
Leptin, insulin, ghrelin, triglyceride, and cholesterol levels in 16-h fasted mice were measured at the termination of the study. TG and cholesterol were measured by enzymatic assay (Thermo Electron, Waltham, MA). Four lipoprotein pooled samples (0.35 mL) per group were subjected to fast-performance liquid chromatography (gel filtration on two Superose 6 columns connected in series. Leptin and insulin were quantified simultaneously via multiplex assay, and ghrelin was quantified via ELISA (Millipore).
Low-density gene array.
Mice were fasted for 16 h and decapitated without anesthesia, and trunk blood and tissues were collected. Tissues were frozen on dry ice and stored at −80°C. RNA was isolated using the RNeasy Lipid Mini-Kit (Qiagen) and reverse transcription PCR was performed using SuperScriptIII, DNase, and anti-RNase treatment (all Invitrogen, Carlsbad, CA). Custom-made low-density gene arrays and single-gene quantitative PCR were performed according to the manufacturer’s instructions on a 7900 real-time PCR machine (Applied Biosystems, Foster City, CA). Data were normalized to the housekeeping gene 18s and expressed as fold change.
Statistics.
All data are represented as mean and SEM. One-way or two-way ANOVA with Bonferroni's multiple comparison post-test was performed as appropriate using GraphPad Prism version 5.0c (GraphPad Software, San Diego, CA). Statistical significance was assumed when P < 0.05.
RESULTS
To test the hypothesis that increased body FM, rather than ingestion of excess lipid, is the predominant pathogenic effect of HFD feeding, we determined the metabolic phenotype of lean and obese mice that were chronically exposed to HFD. The ad libitum HFD group developed severe DIO, as expected, while leanness in FMC mice was maintained equivalent to SD mice. This allowed for separation of the metabolic consequences of excess dietary lipids (FMC group) from the effects of increased body fat induced by excess dietary lipids (HFD ad libitum group).
FM is dictated by caloric intake.
At study initiation all mice had identical BW (Fig. 1C). Predictably, the HFD group gained BW quickly and achieved significant DIO within 14 weeks. FM of clamped mice did not differ from SD mice (Fig. 1B). Food intake was lower in FMC mice than in SD controls, presumably because of the higher caloric density of the HFD (Fig. 1D). However, caloric intake was similar in both lean groups and appreciably lower than HFD-fed mice (Fig. 1E). These findings suggest that energy metabolism and food efficiency were not altered by exposure to the HFD per se. To test this possibility more directly, we analyzed energy expenditure.
FM clamping normalizes energy expenditure and enhances metabolic flexibility.
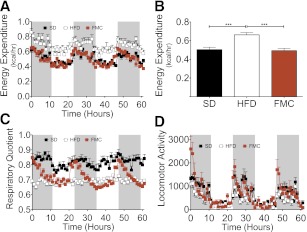
Energy expenditure and locomotor activity were measured using indirect calorimetry during week 14. Consistent with prior observations (17), EE was increased (P < 0.0001) in the HFD group compared with the SD control group (Fig. 2A and B). Intriguingly, EE of the FMC mice was similar to that of the SD control group, suggesting that HFD exposure per se did not compromise EE.
FIG. 2.
FM clamping normalizes energy expenditure. Absolute (A) and average energy expenditure (B), RQ (C), and locomotor activity (D) of SD, HFD, and FMC mice was measured hourly. Mice from the SD and HFD group were fed with SD or HFD ad libitum. Mice from the FMC group were fed defined amounts of HFD to match FM to the SD group. All mice are male (n = 16 per group). All data are represented as mean ± SEM. ***P < 0.001.
RQ (Fig. 2C) of the HFD group did not change markedly between the light and dark cycles (0.69 ± 0.00), implying that fat oxidation was constantly high. Conversely, the RQ of the SD group averaged 0.82 ± 0.01 over the course of the day, reflecting increased carbohydrate oxidation. The RQ of the FMC mice varied greatly between the light and dark. During the light, FMC RQ was similar to that of the HFD group, suggesting fat oxidation. At the beginning of the dark phase, the mean RQ increased considerably, reaching levels similar to those of the SD group.
Total locomotor activity (Fig. 2D) was comparable in the SD and FM-clamped groups, and both were higher than HFD mice. These data imply that body fat and/or adipose tissue–derived adipokines or other factors, rather than high proportion of fat in the HFD, may compromise both energy homeostasis and locomotor activity.
FM clamping improves glucose and insulin tolerance.
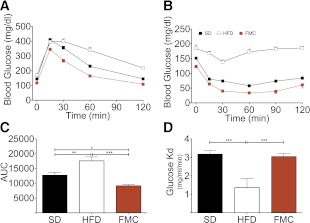
Mice were challenged with GTT and insulin tolerance tests in week 13. Fasting blood glucose of the FM-clamped mice was slightly below all other groups, and, as anticipated, DIO mice were glucose intolerant (Fig. 3A and C). Glucose tolerance was markedly better in the FMC mice than in the HFD group. Surprisingly, glucose tolerance was also markedly improved as compared with the SD group (Fig. 3A and C). This improved glucose homeostasis was matched by enhanced insulin sensitivity in FMC mice (Fig. 3B). Analysis of the rate of disappearance in the first 30 min of the challenge revealed similar sensitivity in SD and FMC mice, which were both improved over the HFD group (Fig. 3D). Thus, consumption of HFD has beneficial effects on glucose and insulin responsiveness, when FM is constrained at a normal level.
FIG. 3.
FM clamping improves glucose tolerance and insulin sensitivity. Glucose tolerance was assessed at week 13 via intraperitoneal GTT. After 6 h of fasting mice were injected with 2.0 g/kg glucose. FMC mice had significantly lower blood glucose excursion (A) and area under the curve (AUC; C) compared with lean SD and obese HFD mice. Insulin tolerance was assessed at week 13 via intraperitoneal insulin challenge (1 unit/kg). Insulin tolerance was significantly improved in FMC mice as compared with HFD mice when assessed by either blood glucose excursion (B) or rate of disappearance (Kd) over the initial 30 min (D). All data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
FM clamping prevents obesity-associated inflammation.
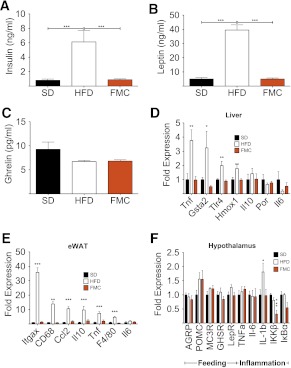
After 14 weeks of FM clamping, mice were killed and tissues collected to gain mechanistic insight into the beneficial metabolic effects of FM clamping. HFD mice exhibited elevated plasma leptin and insulin, but no change in ghrelin (Fig. 4A–C). Expression of key metabolic genes in skeletal muscle, hypothalamus, liver, and adipose tissue was conducted. Markers of both fatty acid and glucose transport were suppressed in the skeletal muscle of FMC mice as compared with the HFD group (Supplementary Table 1). However, two genes recently implicated in enhanced glucose transport, Nr4a3 and Ucp3, were both elevated in FMC as compared with both control groups (Supplementary Table 1). In adipose and liver, as in skeletal muscle, canonical regulators of glucose homeostasis, glucose transporter expression in epididymal WAT, and gluconeogenic genes of the liver were not different between groups (Supplementary Tables 2 and 3). Epididymal WAT and liver of HFD mice exhibited increased gene expression levels for tumor necrosis factor-α (TNFα), cluster of differentiation 68 (CD68), integrin αx (ITGAX), chemokine (C-C motif) ligand 2, toll-like receptor 4, glutathione S-transferase A2, and heme oxygenase 1. FM clamping dramatically prevented expression of these markers in both liver (Fig. 4D) and WAT (Fig. 4E), resulting in levels similar to those in SD mice. Additionally, hypothalamic expression of IL-6 and IKKβ were also suppressed as compared with that in HFD mice (Fig. 4F). These data suggest that DIO-associated inflammation is related to expanded FM and not dietary lipids. Furthermore, they provide additional evidence for central and peripheral inflammation as a component of diet-induced glucose intolerance.
FIG. 4.
FM clamping rescues hyperinsulinemia and hyperleptinemia and normalizes expression of inflammatory markers. Plasma hormone levels measured after 16-h fast at week 14 (A–C) are shown. All mice are male (n = 14 to 15 per group). Expression of inflammatory markers (D), liver (E), epididymal white adipose tissue (eWAT), and (F) hypothalamic tissues was suppressed in FMC mice. All mice are male (n = 8 per group). All data are represented as mean ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
Post-obesity studies.
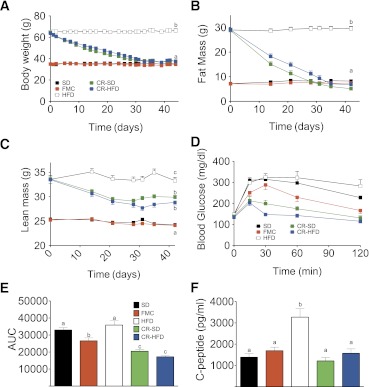
To investigate the metabolic impact of prior obesity, cohorts of DIO mice were placed on a defined CR paradigm and fed either HFD or SD until FM was reduced to that of otherwise matched SD mice. Ad libitum HFD and SD and FMC mice were maintained as controls. CR mice received 50% of calories consumed on average by the SD ad libitum group. Within 1 month mice subjected to CR lost excess BW (Fig. 5A) and FM (Fig. 5B), independent of the diet. Lean mass of previously obese mice remained significantly greater than that of the always-lean controls (Fig. 5C).
FIG. 5.
CR restores leanness independent of the diet. A: BW of mice that were fed with SD or HFD ad libitum in comparison with calorically restricted mice on SD or HFD. The SD and HFD groups were fed ad libitum, whereas the groups CR-SD and CR-HFD were calorically restricted and fed 50% of calories (given as SD or HFD) that were consumed on average in the SD group. FMC mice were fed defined amounts of HFD to match FM to the SD group. FM (B) and lean mass (C) curves of mice fed ad libitum or that were calorically restricted are shown. D: intraperitoneal GTT conducted after 6 h of fasting at day 45 of CR. E: Area-under-the-curve (AUC) analysis of GTT. F: C-peptide secretion at 30 min after intraperitoneal glucose challenge. All mice are male (n = 10–15 per group). All data are represented as mean ± SEM. Lowercase letters above bars (A–F) denote statistically similar (P > 0.05) groups.
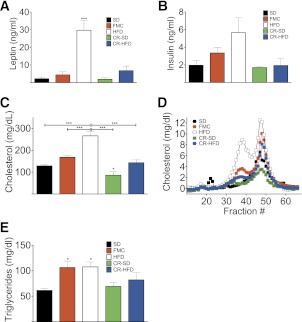
At day 44, 10 days after achieving FM levels equal to those of the SD-fed mice, all mice were subjected to a GTT and plasma lipids were analyzed. Although BW and FM were the same in all lean groups (SD, FMC, CR-SD, and CR-HFD), CR mice were most glucose tolerant as reflected by glucose excursion (Fig. 5D) and area-under-the-curve analysis (Fig. 5E). Similar to FMC mice, glucose tolerance was greatest in the CR-HFD group, indicating that dietary fat might have a beneficial effect on glucose tolerance when FM is strictly maintained. Furthermore, assessment of C-peptide 30 min after glucose challenge suggests that this enhanced tolerance occurs in the presence of normal insulin secretion (Fig. 5F). Analysis of plasma from fasted mice revealed elevated plasma leptin and trend for increased insulin in HFD mice after 16 h fast, with similar levels in all other groups (Fig. 6A and B). A complete ablation of diet-induced hypercholesterolemia cholesterol was observed in FMC and CR mice (Fig. 6C). Fast-performance liquid chromatography analysis of cholesterol identified a cholesterol profile similar to that of SD mice and greatly reduced from those of HFD mice (Fig. 6D). Furthermore, CR mice fed a HFD and FMC mice exhibited a slight increase in the LDL and HDL peaks compared with SD-fed mice. Intriguingly, when compared with SD fed mice, CR-SD mice had a marked decrease in the main HDL peak and in the shoulder, where HDL-1 and LDL elute. In addition to these effects on cholesterol, a trend for normalization of triglycerides (Fig. 6E) was observed in CR, but not FMC mice. Together these data suggest that CR after prior obesity induces beneficial effects on glucose and lipid metabolism independent of FM.
FIG. 6.
CR normalizes plasma cholesterol. Plasma leptin (A) and insulin levels (B) measured after 16-h fast at day 44 are shown. Total plasma cholesterol (C), cholesterol profile (D), and total plasma triglycerides (E) of mice that were fed with SD or HFD ad libitum in comparison with FMC or CR-SD or CR-HFD are shown. All mice are male (n = 6–10 per group). All data are represented as mean ± SEM. *P < 0.05; ***P < 0.001.
CR induces persistent hyperphagia leading to rebound of obesity.
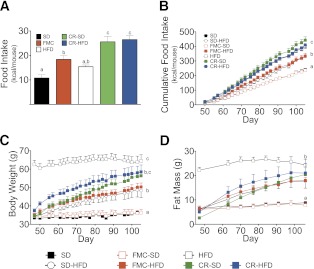
To test if CR disturbs metabolic programming and consequently facilitates the rebound of obesity, we measured fasting-induced hyperphagia and BW gain during ad libitum feeding. After 45 days of CR, both CR and control mice were fasted for 24 h. All mice were then given free access to SD or HFD, and food intake was monitored for 24 h. SD-fed control mice consumed the least amount of calories after the fast (Fig. 7A). HFD-fed mice ate slightly, but not significantly, more than the SD control group. Caloric intake in FMC mice was significantly elevated over SD mice; yet this was not associated with expression of orexigenic signals in the hypothalamus (Fig. 4F). CR mice ate significantly more calories after food deprivation than all other groups. This effect was independent of the diet since the CR-SD group ate the same amount of calories as the CR-HFD group after refeeding.
FIG. 7.
CR induces persistent hyperphagia. A: At day 45 CR was ended and mice of all groups were fasted for 24 h. After the fast mice were given free access to SD or HFD and food intake was measured after 15 h. B: After the fasting-induced hyperphagia experiment mice were allowed to eat ad libitum to study rebound of obesity. Additional control groups (FMC-SD and previously SD-fed ad libitum and switched to HFD ad libitum [SD-HFD]) were introduced. FMC-SD mice were previously fed HFD and were switched to SD, whereas SD-HFD mice were previously fed SD and were switched to HFD. C: BW curves of the ad libitum eating period. D: FM during the period of ad libitum eating. All mice are male (n = 7 to 8 per group). All data are represented as mean ± SEM. Lowercase letters denote statistically similar (P > 0.05) groups.
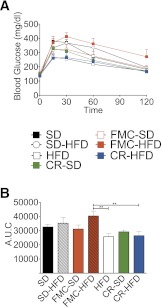
After CR was ended, previously obese CR mice were given HFD ad libitum to characterize the effects, if any, on return to obesity. SD and FMC groups were split into two ad libitum groups each, with half allowed to consume SD and half HFD. These groups were introduced to control for feeding behavior in mice without prior obesity. SD mice maintained on the SD diet displayed relatively constant food intake and BW for the duration of the study (Fig. 7B and C). Food intake significantly increased in SD and FMC mice switched to ad libitum HFD, leading to concomitant increases in BW and FM (Fig. 7B–D). These effects, however, were intermediate to previously obese CR groups. Previously, CR HFD-fed mice increased food intake, achieving levels similar to those of obese HFD-fed mice (Fig. 7B). This exaggerated hyperphagia led to accelerated BW gain such that CR-HFD mice attained BWs and FMs similar to those of DIO mice after ∼50 days of free eating (Fig. 7B–D). CR-SD mice increased food intake (Fig. 7B) and FM (Fig. 7D) in a similar manner to CR-HFD mice; however, initial BW gain was delayed (Fig. 7C). The rapid accumulation of FM was associated with a deterioration of glucose tolerance, especially in the FMC-HFD group (Fig. 8A and B). These data collectively suggest that prior obesity induces a drive to defend the BW achieved before CR, and this phenomenon is independent of diet.
FIG. 8.
Rebound from CR induces glucose intolerance. Intraperitoneal GTT (A) and area-under-the-curve (AUC) analysis (B) are shown. GTT was conducted after 6 h of fasting following 58 days of rebound. All mice are male (n = 6–10 per group). All data are represented as mean ± SEM. **P < 0.01.
DISCUSSION
To distinguish between the metabolic consequences of dietary fat intake and increased body fat, we studied three groups of age-matched, randomized male C57BL/6J mice. The first was fed ad libitum with a low-fat diet (SD), matched in protein and micronutrient composition to the HFD. The second consisted of mice that had ad libitum access to HFD, and the third (FM-clamped mice) was fed the calculated amount of HFD each day that would effectively “clamp” their FM to that of the SD group. It is noteworthy that this did not require decreasing caloric intake of FMC mice below that of the SD mice. This clamping technique revealed that BW and body composition is primarily determined by calories ingested and not by the source of calories. In the present paradigm, ingestion of a high proportion of dietary fat per se did not favor accumulation of excess body fat. Rather, DIO occurred only when excess calories were consumed, as occurred in the HFD ad libitum group.
It has been reported that as rodents become obese, total EE also increases (15,16,18). Consistent with these findings, we found that EE of DIO mice was increased and that this effect was related to the amount of body fat, i.e., exposure to HFD in the FMC group resulted in EE that was similar to SD mice. Therefore, implying that increased ingestion of fat, in and of itself, does not necessarily have adverse effects on energy homeostasis when BW, FM, and/or caloric intake are maintained at normal levels. Moreover, clamping FM to that of normal weight control mice restored metabolic flexibility. Metabolic flexibility describes the ability of an organism or tissue to adapt to substrate availability by switching between the oxidation of carbohydrates and lipids (19). As reflected by the RQ, FMC mice were able to alternate between carbohydrate and fat oxidation, whereas mice fed the HFD ad libitum mainly used fat as the primary fuel source. This fluctuation of fuel choice within the FM-clamped group was likely related to the time of feeding. Although the two control groups had access to food around the clock, FMC mice were fed only once a day, 3 h after light onset. These FMC mice typically ate their daily ration within 3 h, leaving them fasted for the remaining 21 h of the day. This pattern presumably explains why the RQ decreased during the beginning of light phase when these mice were maximally fasted. Their RQ remained low during food ingestion but began to increase around 3 h after feeding. This suggests that they preferentially used fat as the primary fuel source to most efficiently use stored energy. However, when carbohydrates became available during the prandial period, these mice were able to quickly switch to carbohydrate oxidation.
It is noteworthy that exposure to limited amounts of HFD had no adverse effect on glucose homeostasis. Conversely, and perhaps counter-intuitively, it significantly improved glucose tolerance and insulin sensitivity. This finding suggests that in moderation dietary lipids are beneficial to glucose tolerance. It should be noted that both standard and HFD contained high amounts of sucrose. However, mice in the FMC group consumed less sucrose than SD mice because the HFD contains more fat than sucrose and the absolute caloric intake in both groups was similar. In fact, carbohydrate consumed in SD mice was about three times greater than that in FMC mice during the study period.
Carbohydrates, including sucrose, play a role in the pathogenesis of insulin resistance and are essential to induce hyperglycemia (20). Meta-analysis in humans (21) showed that increased consumption of sugar-sweetened beverages is strongly associated with obesity and type 2 diabetes. A proposed mechanism for the diabetes development after high-sugar consumption is pancreatic β-cell dysfunction and inflammation (22). Moreover, increased intake of fructose-sweetened beverages stimulates visceral adiposity and hepatic de novo lipogenesis (23).
To identify potential mechanisms responsible for the improved glucose homeostasis of FMC mice, we measured candidate genes in liver, skeletal muscle, hypothalamus, and white adipose tissue. Many of the expected regulators of glucose homeostasis were not differentially expressed. However, altered expression of two recently described modulators of glucose uptake, Nr4a3 and Ucp3, was identified in skeletal muscle. Intriguingly, both Nr4a3 (24) and Ucp3 (25) enhance insulin signaling, resulting in increased glucose uptake. Likewise, cytokine expression in liver was significantly increased in DIO mice. This effect is likely influenced by the amount of body fat and not the ingestion of fatty food per se since expanding body fat increases markers of inflammation (26). In contrast, mice fed HFD, but with clamped FM, had markers of inflammation comparable with that of SD mice. Therefore, a possible explanation for the preservation of glucose tolerance in FMC mice despite high-fat feeding could be the absence of inflammation. Our group has recently shown that DIO induces hypothalamic inflammation in rodents and humans after only 4 weeks exposure to HFD (27). This HFD induced inflammation results in neuronal damage at the arcuate nucleus of the hypothalamus and possibly contributes to diabetogenesis. This hypothalamic inflammatory response to HFD is mediated by activation of Jnk (28) and the IKKβ/NF-κB pathway (29). It is noteworthy that we found that hypothalamic IKKβ expression is not upregulated in the HFD group but significantly downregulated in FMC mice. Thus, reduced activation of the hypothalamic IKKβ/NF-κB pathway could play an important role in not only maintaining, but further improving, insulin sensitivity of FMC mice compared with SD- and HFD-fed mice.
After severe CR, all mice underwent the anticipated acute period of hyperphagia relative to nonrestricted mice. However, CR mice that had previously been obese exhibited significantly greater hyperphagia than all other groups, including the FM-clamped group. This finding indicates that individuals in a postobese metabolic state are reprogrammed to favor relative overeating and rapid weight regain. The susceptibility of weight-reduced subjects to hyperphagia and regain of lost weight is well known and implicated in the obesity rebound (30). Studies in mice highlight that the magnitude of hyperphagia after CR is not proportional to the duration of restriction, indicating that hunger does not diminish during the time course of CR (31). However, in our paradigm, the degree of weight regain seems to be influenced in large part by the degree of prior obesity and not by the CR per se. The never-obese FMC mice that can be considered as mildly CR for a long period of time best depicted this effect. These mice responded with significantly less hyperphagia than mice with a history of obesity. Therefore, prior obesity, rather than CR, is likely to contribute a greater component of the drive to overeat. This finding could be explained by the relative leptin deficiency of weight-reduced previously obese mice. Substantial weight loss leads to a reduction in leptin concentrations beyond that predicted by loss of FM (32).
This drive to overeat may be directly related to the propensity for quick rebound in previously obese individuals. It is of interest, however, that prior obesity may also result in beneficial glucose metabolism. Furthermore, prior obesity also leads to changes in lipoprotein profiles that vary dependent on the diet consumed. Ad libitum consumption of a HFD after CR seems to have a beneficial effect on lipoprotein metabolism since it considerably normalizes the profile. In contrast, there was a marked reduction of HDL cholesterol levels in mice changed to the low-fat SD after CR compared with mice that had always been fed the SD. Previous studies with mice overexpressing scavenger receptor class B type 1 in liver demonstrated increased reverse cholesterol transport and reduced HDL cholesterol levels (33), resulting in reduction of atherosclerosis (34). Hence, consuming a low-fat diet after CR may have beneficial effects on atherogenesis independent of increased HDL. These observations in both FM-clamped and previously obese mice suggest that previously unknown benefits of mild CR, especially in the context of prior obesity. Taken together, these data suggest that CR from an obese state provides metabolic benefits, including improved glucose and lipid homeostasis. Furthermore, these benefits are equally observed when restricted on a HFD or low-fat diet. However, the data also indicate that CR (or pharmaceutical intervention) may have to be continued lifelong to prevent consequences of chronically altered metabolic programming, which may lead to an even worse state of metabolic disease.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grant R01-DK-077975, Neuroendocrine Regulation of Adipocyte Metabolism (M.H.T.), and the University of Cincinnati Training Program in Neuroendocrinology of Homeostasis Grant T32-DK-059803 (K.M.H.).
M.H.T. is a consultant for Roche Pharmaceuticals and receives research funds from Roche. No other potential conflicts of interest relevant to this article were reported.
H.K. and K.M.H. were responsible for the conception, implementation, and design of the study; the analyses and interpretation of data; and drafting and critically revising the article. S.M.H. was responsible for interpretation of data and drafting and critically revising the article. A.F.-R. was responsible for the implementation of the study and the analyses and interpretation of data. J.H., W.A., N.O., E.D., and R.K. were responsible for the implementation of the study. S.C.W., T.D.M., J.S., D.P.-T., and P.T.P. were responsible for interpretation of data and drafting and critically revising the article. M.H.T. was responsible for the conception and design of the study, the analyses and interpretation of data, and drafting and critically revising the article. All authors approved the final version of the manuscript to be published. K.M.H. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db11-1621/-/DC1.
REFERENCES
- 1.Tsai AG, Wadden TA. The evolution of very-low-calorie diets: an update and meta-analysis. Obesity (Silver Spring) 2006;14:1283–1293 [DOI] [PubMed] [Google Scholar]
- 2.McCay CM, Crowell MP, and Maynakd LA The effect of retarded growth upon the length of the life span and upon the ultimate body size. J Nutr 1935;10:63–79 [PubMed] [Google Scholar]
- 3.Cheney KE, Liu RK, Smith GS, Leung RE, Mickey MR, Walford RL. Survival and disease patterns in C57BL/6J mice subjected to undernutrition. Exp Gerontol 1980;15:237–258 [DOI] [PubMed] [Google Scholar]
- 4.Masoro EJ. Dietary restriction-induced life extension: a broadly based biological phenomenon. Biogerontology 2006;7:153–155 [DOI] [PubMed] [Google Scholar]
- 5.Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health 2009;9:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuncman G, Hirosumi J, Solinas G, Chang L, Karin M, Hotamisligil GS. Functional in vivo interactions between JNK1 and JNK2 isoforms in obesity and insulin resistance. Proc Natl Acad Sci USA 2006;103:10741–10746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kintscher U, Hartge M, Hess K, et al. T-lymphocyte infiltration in visceral adipose tissue: a primary event in adipose tissue inflammation and the development of obesity-mediated insulin resistance. Arterioscler Thromb Vasc Biol 2008;28:1304–1310 [DOI] [PubMed] [Google Scholar]
- 8.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kvietys PR, Specian RD, Grisham MB, Tso P. Jejunal mucosal injury and restitution: role of hydrolytic products of food digestion. Am J Physiol 1991;261:G384–G391 [DOI] [PubMed] [Google Scholar]
- 11.Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009;50:90–97 [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Ghoshal S, Ward M, de Villiers W, Woodward J, Eckhardt E. Chylomicrons promote intestinal absorption and systemic dissemination of dietary antigen (ovalbumin) in mice. PLoS ONE 2009;4:e8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bournat JC, Brown CW. Mitochondrial dysfunction in obesity. Curr Opin Endocrinol Diabetes Obes 2010;17:446–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matsuzawa-Nagata N, Takamura T, Ando H, et al. Increased oxidative stress precedes the onset of high-fat diet-induced insulin resistance and obesity. Metabolism 2008;57:1071–1077 [DOI] [PubMed] [Google Scholar]
- 15.MacLean PS, Higgins JA, Johnson GC, et al. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R1306–R1315 [DOI] [PubMed] [Google Scholar]
- 16.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 1995;332:621–628 [DOI] [PubMed] [Google Scholar]
- 17.Felig P, Cunningham J, Levitt M, Hendler R, Nadel E. Energy expenditure in obesity in fasting and postprandial state. Am J Physiol 1983;244:E45–E51 [DOI] [PubMed] [Google Scholar]
- 18.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Peters JC, Hill JO. Metabolic adjustments with the development, treatment, and recurrence of obesity in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 2004;287:R288–R297 [DOI] [PubMed] [Google Scholar]
- 19.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes 2000;49:677–683 [DOI] [PubMed] [Google Scholar]
- 20.Jürgens HS, Neschen S, Ortmann S, et al. Development of diabetes in obese, insulin-resistant mice: essential role of dietary carbohydrate in beta cell destruction. Diabetologia 2007;50:1481–1489 [DOI] [PubMed] [Google Scholar]
- 21.Malik VS, Popkin BM, Bray GA, Després JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care 2010;33:2477–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulze MB, Liu S, Rimm EB, Manson JE, Willett WC, Hu FB. Glycemic index, glycemic load, and dietary fiber intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr 2004;80:348–356 [DOI] [PubMed] [Google Scholar]
- 23.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest 2009;119:1322–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu Y, Luo L, Luo N, Zhu X, Garvey WT. NR4A orphan nuclear receptors modulate insulin action and the glucose transport system: potential role in insulin resistance. J Biol Chem 2007;282:31525–31533 [DOI] [PubMed] [Google Scholar]
- 25.Huppertz C, Fischer BM, Kim YB, et al. Uncoupling protein 3 (UCP3) stimulates glucose uptake in muscle cells through a phosphoinositide 3-kinase-dependent mechanism. J Biol Chem 2001;276:12520–12529 [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 27.Thaler JP, Yi CX, Schur EA, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 2012;122:153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Souza CT, Araujo EP, Bordin S, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 2005;146:4192–4199 [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008;135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pankevich DE, Teegarden SL, Hedin AD, Jensen CL, Bale TL. Caloric restriction experience reprograms stress and orexigenic pathways and promotes binge eating. J Neurosci 2010;30:16399–16407 [DOI] [PMC free article] [PubMed]
- 31.Hambly C, Mercer JG, Speakman JR. Hunger does not diminish over time in mice under protracted caloric restriction. Rejuvenation Res 2007;10:533–542 [DOI] [PubMed] [Google Scholar]
- 32.Rosenbaum M, Murphy EM, Heymsfield SB, Matthews DE, Leibel RL. Low dose leptin administration reverses effects of sustained weight-reduction on energy expenditure and circulating concentrations of thyroid hormones. J Clin Endocrinol Metab 2002;87:2391–2394 [DOI] [PubMed] [Google Scholar]
- 33.Zhang Y, Da Silva JR, Reilly M, Billheimer JT, Rothblat GH, Rader DJ. Hepatic expression of scavenger receptor class B type I (SR-BI) is a positive regulator of macrophage reverse cholesterol transport in vivo. J Clin Invest 2005;115:2870–2874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arai T, Wang N, Bezouevski M, Welch C, Tall AR. Decreased atherosclerosis in heterozygous low density lipoprotein receptor-deficient mice expressing the scavenger receptor BI transgene. J Biol Chem 1999;274:2366–2371 [DOI] [PubMed] [Google Scholar]