Abstract
Currently there is debate on whether hypoglycemia is an independent risk factor for atherosclerosis, but little attention has been paid to the effects of recovery from hypoglycemia. In normal control individuals and in people with type 1 diabetes, recovery from a 2-h induced hypoglycemia was obtained by reaching normoglycemia or hyperglycemia for another 2 h and then maintaining normal glycemia for the following 6 h. Hyperglycemia after hypoglycemia was also repeated with the concomitant infusion of vitamin C. Recovery with normoglycemia is accompanied by a significant improvement in endothelial dysfunction, oxidative stress, and inflammation, which are affected by hypoglycemia; however, a period of hyperglycemia after hypoglycemia worsens all of these parameters, an effect that persists even after the additional 6 h of normoglycemia. This effect is partially counterbalanced when hyperglycemia after hypoglycemia is accompanied by the simultaneous infusion of vitamin C, suggesting that when hyperglycemia follows hypoglycemia, an ischemia–reperfusion-like effect is produced. This study shows that the way in which recovery from hypoglycemia takes place in people with type 1 diabetes could play an important role in favoring the appearance of endothelial dysfunction, oxidative stress, and inflammation, widely recognized cardiovascular risk factors.
Recent evidence suggests that hypoglycemia may play an important role in the vascular complications of diabetes (1). Hypoglycemia causes oxidative stress (2), inflammation (3), and endothelial dysfunction (4). Oxidative stress is considered the key player in the pathogenesis of diabetes complications (5). During hyperglycemia, oxidative stress is produced at the mitochondrial level (5), similarly as in hypoglycemia (2). Therefore, oxidative stress might be considered the common factor linking hyperglycemia, hypoglycemia, and the vascular complications of diabetes. Consistent with this hypothesis is the evidence that hyperglycemia (6) and hypoglycemia both produce endothelial dysfunction and inflammation through the generation of oxidative stress (4,7). Endothelial dysfunction and inflammation are well-recognized pathogenic factors for vascular disease, particularly in diabetes (8).
There is, however, evidence that free radical production rises, not only during hypoglycemia but particularly during glucose reperfusion after hypoglycemia (9). In both mice and cultured neurons, hypoglycemia, followed by different concentrations of glucose reperfusion, has been linked to a degree of superoxide production and neuronal death that increased proportionally with glucose concentrations during the reperfusion period (9).
Until now, little attention has been given to studying the effects of recovery from hypoglycemia. The aim of this study was to evaluate these effects and, in particular, to determine if the level of glycemia reached during recovery could have a different impact, in vivo, on oxidative stress generation, inflammation, and endothelial function.
RESEARCH DESIGN AND METHODS
Subjects.
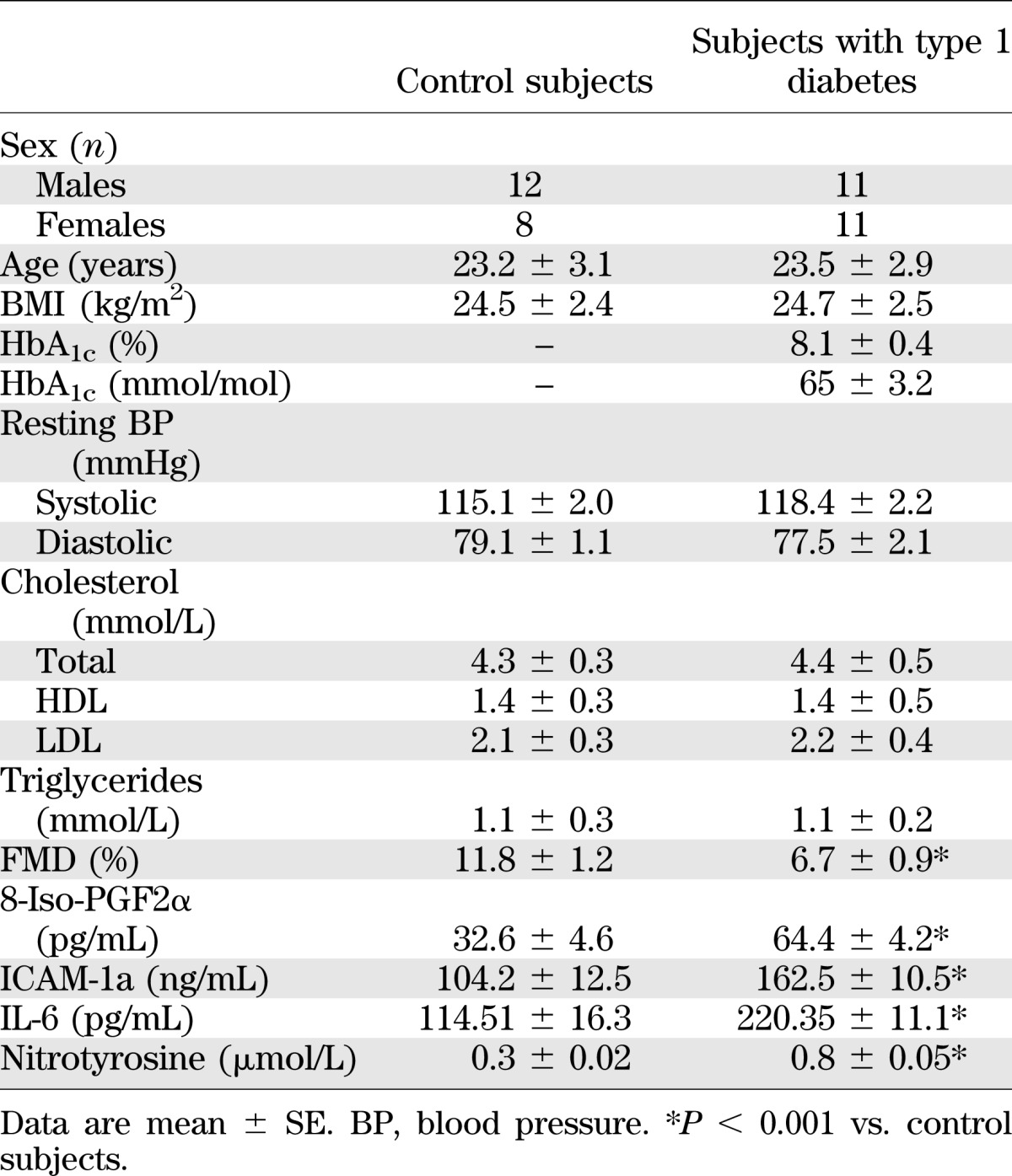
Twenty healthy subjects and 22 matched individuals with type 1 diabetes were studied (Table 1). Results of bedside tests of autonomic function in individuals with type 1 diabetes were within normal reference ranges (10), and they did not exhibit hypoglycemia unawareness, as based on the methods of Gold et al. (11). Type 1 diabetic individuals had no major macro- or microcomplications of diabetes. The study excluded individuals with type 1 diabetes who had had at least one major episode of hypoglycemia in the preceding 2 years. Type 1 diabetic individuals were treated with multiple daily insulin injections. All subjects were nonsmokers and had a normal blood count, plasma lipids, plasma electrolytes, liver and renal function, and blood pressure. No subject was taking medications known to affect neuroendocrine responses to hypoglycemia or inflammation. Studies were approved by the ethical committees of the respective research institutions involved, and all participants gave written informed consent.
TABLE 1.
Baseline characteristics of control subjects and of type 1 diabetic patients
All subjects were admitted to the Research Center on the evening before each experiment and ate a meal. Individuals with type 1 diabetes received a continuous low-dose infusion of insulin to normalize plasma glucose. The insulin infusion was adjusted overnight to maintain blood glucose between 4.4 and 7.2 mmol/L.
Hypoglycemia experiments.
Three different experiments, each following a 2-h period of induced hypoglycemia and lasting for 2 hours, were planned for each subject in a randomized order: reaching and maintaining normoglycemia, reaching and maintaining hyperglycemia, or reaching and maintaining hyperglycemia with simultaneous infusion of vitamin C. Each subject underwent the experiments at intervals of 2 weeks or longer.
All subjects fasted overnight for 10 h. In all experiments, an initial period of 120 min was allowed to elapse, followed by a 120-min hyperinsulinemic hypoglycemic experimental period. At time 120 min, a primed constant (9.0 pmol · kg−1 · min−1) infusion of insulin (Actrapid, Novo Nordisk, Copenhagen, Denmark) began and continued for 240 min. The rate of glucose decrease was controlled (∼0.08 mmol/min), and glucose nadir (2.9 mmol/L), a level that likely elicits minimal counterregulatory effect, was achieved using a modification of the glucose clamp technique. During the clamp period, plasma glucose was measured every 5 min, and a 20% dextrose infusion was adjusted so that plasma glucose levels were held constant at 2.9 ± 0.1 mmol/L (12). Potassium chloride (20 mmol/L) was infused during the clamp to reduce insulin-induced hypokalemia.
After this period of induced hypoglycemia, common to all the experiments, recovery from hypoglycemia was achieved for 120 min, in random order, by reaching and maintaining normoglycemia (4.5 mmol/L), by reaching and maintaining hyperglycemia (15 mmol/L), or by reaching and maintaining hyperglycemia with the simultaneous infusion of vitamin C (30 mg/min) (13). Therefore, glycemia was normalized for the next 360 min.
At baseline (120 min from the start of the experiment) and after 2, 4, and 10 h, blood samples were taken for biochemical assays of glycemia, and plasma levels of nitrotyrosine and 8-iso prostaglandin F2α (8-iso-PGF2α), both markers of oxidative stress, as well as intracellular adhesion molecule-1a (ICAM-1a) and interleukin-6 (IL-6), markers of inflammation. Endothelial function was measured by flow-mediated dilatation (FMD).
Biochemical and clinical measurements.
Cholesterol, triglycerides, HDL and LDL cholesterol, and plasma nitrotyrosine were measured as previously described (14). Plasma glucose was measured by the glucose-oxidase method, HbA1c by high-performance liquid chromatography, and insulin by microparticle enzyme immunoassay (Abbott Laboratories, Wiesbaden, Germany). Plasma 8-iso-PGF2α (Cayman Chemical, Ann Arbor, MI), soluble (s)ICAM-1 (British Biotechnology, Abington, Oxon, U.K.), and IL-6 (R&D Systems, Minneapolis, MN) were determined using commercially available kits.
Endothelial function was evaluated measuring the FMD of the brachial artery (15). At the end of each test, sublingual nitroglycerin (0.3 mg) was administered and 3-min endothelium-independent vasodilatation measured.
Statistical analysis.
Sample size was selected according to previous studies (3). Data are expressed as means ± SE. The Kolmogorov-Smirnov algorithm was used to determine whether each variable had a normal distribution. Comparisons of baseline data among the groups were performed using the unpaired Student t test or Mann-Whitney U test, where indicated. The changes in variables during the tests were assessed by two-way ANOVA with repeated measures or Kolmogorov-Smirnov test, where indicated. If differences reached statistical significance, post hoc analyses with the two-tailed paired t test or Wilcoxon signed rank test for paired comparisons were used to assess differences at individual time periods in the study. Statistical significance was defined as P < 0.05.
RESULTS
Basal FMD was significantly decreased in people with type 1 diabetes compared with control subjects, whereas 8-iso-PGF2α, nitrotyrosine, sICAM-1, and IL-6 were significantly increased (Table 1). Similarly to previous studies (3,4), FMD significantly decreased after 2 h of hypoglycemia, whereas sICAM-1, 8-iso-PGF2α, nitrotyrosine, and IL-6 significantly increased compared with basal values in control subjects and in subjects with type 1 diabetes (Figs. 1 and 2).
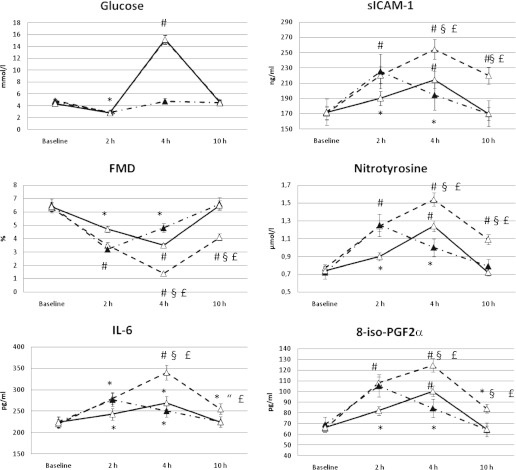
FIG. 1.
Levels for glucose, sICAM-1, FMD, nitrotyrosine, IL-6, and 8-iso-PGF2α in type 1 diabetes are shown for hypoglycemia + hyperglycemia (solid line), hypoglycemia + normoglycemia (dashed line), and hypoglycemia + hyperglycemia + vitamin C (dashed-dotted line). *P < 0.05 vs. basal. #P < 0.01 vs. basal. §P < 0.01 vs. hypoglycemia + normoglycemia. “P < 0.05 vs. hypoglycemia + normoglycemia. P < 0.01 vs. hypoglycemia + hyperglycemia + vitamin C.
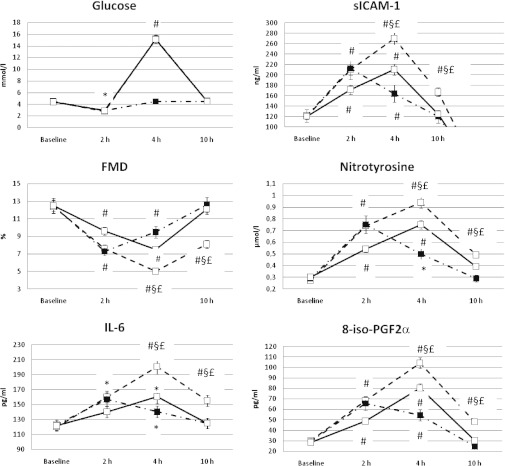
FIG. 2.
Levels for glucose, sICAM-1, FMD, nitrotyrosine, IL-6, and 8-iso-PGF2α in normal healthy control subjects are shown for hypoglycemia + hyperglycemia (solid line), hypoglycemia + normoglycemia (dashed line), and hypoglycemia + hyperglycemia + vitamin C (dashed-dotted line). *P < 0.05 vs. basal. #P < 0.01 vs. basal. §P < 0.01 vs. hypoglycemia + normoglycemia. P < 0.01 vs. hypoglycemia + hyperglycemia + vitamin C.
Recovery from hypoglycemia had a dramatically different effect when obtained reaching normoglycemia or hyperglycemia.
After 2 h of recovery in normoglycemia, FMD was still significantly decreased in control subjects and in people with type 1 diabetes, whereas sICAM-1, 8-iso-PGF2α, nitrotyrosine, and IL-6 were still significantly increased compared with basal values, reaching the baseline values after 6 h of normal glycemia (Figs. 1 and 2).
After 2 h of recovery in hyperglycemia, FMD was even more significantly decreased compared with basal, than recovery in normoglycemia, in both control subjects and people with type 1 diabetes (Figs. 1 and 2). The decrease was more pronounced in control subjects than in participants with diabetes (41 vs. 16%). Levels of sICAM-1, 8-iso-PGF2α, nitrotyrosine, and IL-6 were significantly increased in a consistent manner, in both control subjects and people with type 1 diabetes, compared with basal, than after recovery in normoglycemia (Figs. 1 and 2).
After 6 h of glucose normalization after recovery in hyperglycemia, FMD was still decreased compared with basal in normal control subjects and people with diabetes (Figs. 1 and 2). Compared with basal, sICAM-1, 8-iso-PGF2α, and nitrotyrosine were significantly increased in a consistent manner in control subjects and people with type 1 diabetes (Figs. 1 and 2).
These phenomena were significantly attenuated in control subjects and in people with type 1 diabetes when recovery in hyperglycemia was accompanied by the simultaneous infusion of vitamin C: FMD decreased less, whereas sICAM-1, 8-iso-PGF2α, nitrotyrosine, and IL-6 were less increased (Figs. 1 and 2). Endothelial-independent vasodilatation was not affected in any of the experiments.
DISCUSSION
This study confirms that hypoglycemia induces endothelial dysfunction, oxidative stress, and inflammation. However, it also shows that the way in which recovery from hypoglycemia takes place may also have an effect on cardiovascular risk. When recovery from hypoglycemia is obtained reaching normoglycemia, the deleterious effects of the previous hypoglycemia are mainly counterbalanced, whereas when recovery is obtained reaching hyperglycemia, endothelial function, oxidative stress, and inflammation are further worsened.
In recent years, hyperglycemia and hypoglycemia have both been confirmed as affecting atherothrombosis and inflammation (1,6). However, the role of hypoglycemia in this setting has received particular attention. Two epidemiologic studies have determined that hypoglycemia results in an increased risk of cardiovascular disease and all-cause mortality (16,17), whereas the increase in cardiovascular mortality in people with type 2 diabetes in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (18) and possibly in the Veterans Affairs Diabetes Trial (VADT) (19) and Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) (20) studies has caused concern, because intensive treatment had tripled the frequency of severe hypoglycemia.
The situation remains unclear, however. In the ACCORD trial, epidemiologic analyses were perplexing; for example, earlier severe hypoglycemic episodes were a risk factor for death and occurred three times as often as in the intensive glycemic treatment group, but the excess of deaths in that group was not accounted for by those subjects who had suffered earlier episodes of severe hypoglycemia (21). In the ADVANCE trial, severe hypoglycemia was strongly associated with increased risks of a range of adverse clinical outcomes; however, although it is possible that severe hypoglycemia contributes to adverse outcomes, the data indicate that hypoglycemia is just as likely to be a marker of vulnerability to such events (20). None of these studies devoted attention to how patients recovered from hypoglycemia, and these kinds of data are probably not available. For the first time, our data raise the interesting hypothesis that the way in which recovery from hypoglycemia takes place may, on the one hand, have a role in determining the outcomes, and, on the other, in confusion regarding the interpretation of results.
The way by which recovery from hypoglycemia through hyperglycemia worsens the situation is, convincingly, an increase in oxidative stress. When hyperglycemia after hypoglycemia was accompanied by the simultaneous infusion of the antioxidant vitamin C, the expected improvement in oxidative stress was accompanied by a simultaneous improvement in endothelial function and inflammation. This finding is consistent with the evidence reported in cells and animals (9), suggesting that hyperglycemia after hypoglycemia produces a “reperfusion-like” effect (22).
In our opinion, it is interesting that recovery from hypoglycemia with normoglycemia in control subjects and in people with type 1 diabetes was not completely without negative effects. In both groups, even improved endothelial function, inflammation, and oxidative stress were not completely recovered, suggesting that hypoglycemia regardless might produce a sort of legacy effect, even for a short period. The concept of “legacy” or “metabolic memory” is well recognized in hyperglycemia and indicates the possibility of a long-lasting deleterious effect persisting for some time after glucose normalization (23). This concept is not new at all to hypoglycemia; for example, baroreflex sensitivity and sympathetic response to hypotension are attenuated after previous hypoglycemia (24). Interestingly, oxidative stress is the mediator of the hyperglycemia-induced metabolic memory (23). Moreover, the “memory” phenomenon, after heart ischemia–reperfusion, is also well known: alterations of metabolism in the heart may last for even days after a period of ischemia–reperfusion (22). It is possible that after hypoglycemia, alterations similar to those occurring in the reperfused heart are produced in endothelial cells. This is supported by the similarity of the pathways activated in the two situations, as reported in basic experiments (25).
In conclusion, our study shows for the first time that the way in which recovery from hypoglycemia takes place could have a dramatic impact on cardiovascular risk in diabetes, suggesting that in clinical practice, a gradual correction of blood glucose in patients with hypoglycemia may be preferred to a more rapid correction and hyperglycemia. This hypothesis, of course, needs to be confirmed in large-scale, specifically designed clinical trials. However, because hypoglycemia as a risk factor for cardiovascular disease has become increasingly evident (1) and because hypoglycemia is a frequent event in the life of people with diabetes, we believe that our study may already give a rationale for a more careful treatment of hypoglycemia in daily practice.
ACKNOWLEDGMENTS
No potential conflicts of interest relevant to this article were reported.
A.C. researched the data, contributed to discussion, and wrote, reviewed, and edited the manuscript. A.N., E.O., R.T., A.R.B., and K.E. researched the data, contributed to discussion, and reviewed and edited the manuscript. L.L.S. and G.P. researched the data and contributed to discussion. D.G. contributed to discussion and reviewed and edited the manuscript. A.C. is the guarantor of this work, and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
REFERENCES
- 1.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycaemia an aggravating factor? Diabetes Metab Res Rev 2008;24:353–363 [DOI] [PubMed] [Google Scholar]
- 2.Singh P, Jain A, Kaur G. Impact of hypoglycemia and diabetes on CNS: correlation of mitochondrial oxidative stress with DNA damage. Mol Cell Biochem 2004;260:153–159 [DOI] [PubMed] [Google Scholar]
- 3.Gogitidze Joy N, Hedrington MS, Briscoe VJ, Tate DB, Ertl AC, Davis SN. Effects of acute hypoglycemia on inflammatory and pro-atherothrombotic biomarkers in individuals with type 1 diabetes and healthy individuals. Diabetes Care 2010;33:1529–1535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang J, Alexanian A, Ying R, et al. Acute exposure to low glucose rapidly induces endothelial dysfunction and mitochondrial oxidative stress: role for AMP kinase. Arterioscler Thromb Vasc Biol 2012;32:712–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceriello A. Hyperglycaemia and the vessel wall: the pathophysiological aspects on the atherosclerotic burden in patients with diabetes. Eur J Cardiovasc Prev Rehabil 2010;17(Suppl. 1):S15–S19 [DOI] [PubMed] [Google Scholar]
- 7.Razavi Nematollahi L, Kitabchi AE, Stentz FB, et al. Proinflammatory cytokines in response to insulin-induced hypoglycemic stress in healthy subjects. Metabolism 2009;58:443–448 [DOI] [PubMed] [Google Scholar]
- 8.Nandish S, Wyatt J, Bailon O, Smith M, Oliveros R, Chilton R. Implementing cardiovascular risk reduction in patients with cardiovascular disease and diabetes mellitus. Am J Cardiol 2011;108(Suppl.):42B–51B [DOI] [PubMed] [Google Scholar]
- 9.Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest 2007;117:910–918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years experience in diabetes. Diabetes Care 1985;8:491–498 [DOI] [PubMed] [Google Scholar]
- 11.Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994;17:697–703 [DOI] [PubMed] [Google Scholar]
- 12.Amiel SA, Tamborlane WV, Simonson DC, Sherwin RS. Defective glucose counterregulation after strict glycemic control of insulin-dependent diabetes mellitus. N Engl J Med 1987;316:1376–1383 [DOI] [PubMed] [Google Scholar]
- 13.Mullan BA, Ennis CN, Fee HJ, Young IS, McCance DR. Pretreatment with intravenous ascorbic acid preserves endothelial function during acute hyperglycaemia (R1). Clin Exp Pharmacol Physiol 2005;32:340–345 [DOI] [PubMed] [Google Scholar]
- 14.Ceriello A, Mercuri F, Quagliaro L, et al. Detection of nitrotyrosine in the diabetic plasma: evidence of oxidative stress. Diabetologia 2001;44:834–838 [DOI] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265 [DOI] [PubMed] [Google Scholar]
- 16.Wei M, Gibbons LW, Mitchell TL, Kampert JB, Stern MP, Blair SN. Low fasting plasma glucose level as a predictor of cardiovascular disease and all-cause mortality. Circulation 2000;101:2047–2052 [DOI] [PubMed] [Google Scholar]
- 17.Johnston SS, Conner C, Aagren M, Smith DM, Bouchard J, Brett J. Evidence linking hypoglycemic events to an increased risk of acute cardiovascular events in patients with type 2 diabetes. Diabetes Care 2011;34:1164–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Action to Control Cardiovascular Risk in Diabetes Study Group. Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duckworth W, Abraira C, Moritz T, et al. VADT Investigators Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 20.Zoungas S, Patel A, Chalmers J, et al. ADVANCE Collaborative Group Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 21.Genuth S, Ismail-Beigi F. Clinical implications of the ACCORD trial. J Clin Endocrinol Metab 2012;97:41–48 [DOI] [PubMed] [Google Scholar]
- 22.Taegtmeyer H, Dilsizian V. Imaging myocardial metabolism and ischemic memory. Nat Clin Pract Cardiovasc Med 2008;5(Suppl. 2):S42–S48 [DOI] [PubMed] [Google Scholar]
- 23.Ceriello A, Ihnat MA, Thorpe JE. Clinical review 2: The “metabolic memory”: is more than just tight glucose control necessary to prevent diabetic complications? J Clin Endocrinol Metab 2009;94:410–415 [DOI] [PubMed] [Google Scholar]
- 24.Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009;58:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewald O, Sharma S, Adrogue J, et al. Downregulation of peroxisome proliferator-activated receptor-alpha gene expression in a mouse model of ischemic cardiomyopathy is dependent on reactive oxygen species and prevents lipotoxicity. Circulation 2005;112:407–415 [DOI] [PubMed] [Google Scholar]