Abstract
We examined the role of vascular function and inflammation in the development and failure to heal diabetic foot ulcers (DFUs). We followed 104 diabetic patients for a period of 18.4 ± 10.8 months. At the beginning of the study, we evaluated vascular reactivity and serum inflammatory cytokines and growth factors. DFUs developed in 30 (29%) patients. DFU patients had more severe neuropathy, higher white blood cell count, and lower endothelium-dependent and -independent vasodilation in the macrocirculation. Complete ulcer healing was achieved in 16 (53%) patients, whereas 13 (47%) patients did not heal. There were no differences in the above parameters between the two groups, but patients whose ulcers failed to heal had higher tumor necrosis factor-α, monocyte chemoattractant protein-1, matrix metallopeptidase 9 (MMP-9), and fibroblast growth factor 2 serum levels when compared with those who healed. Skin biopsy analysis showed that compared with control subjects, diabetic patients had increased immune cell infiltration, expression of MMP-9, and protein tyrosine phosphatase-1B (PTP1B), which negatively regulates the signaling of insulin, leptin, and growth factors. We conclude that increased inflammation, expression of MMP-9, PTP1B, and aberrant growth factor levels are the main factors associated with failure to heal DFUs. Targeting these factors may prove helpful in the management of DFUs.
Diabetic foot ulcers (DFUs) are one of the most common and serious complications of diabetes and affects 15% of all diabetic patients, leading to >80,000 amputations per year in the U.S. and results in a high financial burden (1,2). Neuropathy, peripheral vascular disease, and reduced resistance to infection are recognized risk factors leading to the development of DFUs, which have all the characteristics of a chronic wound (3,4).
In the current study, we have well characterized and prospectively followed-up a large number of diabetic patients, the majority of whom were at risk for developing foot ulceration. Our main hypothesis was that changes in the peripheral nerve function and the diabetes-associated proinflammatory state are related not only to the development of DFUs but also to wound-healing failure.
RESEARCH DESIGN AND METHODS
We enrolled 108 diabetic patients and 36 healthy control subjects. The diabetic subjects were divided into those at low risk for developing foot ulceration and those at high risk, according to their neuropathic status (5). The exclusion criteria were presence of foot ulceration at the time of recruitment, clinically present peripheral arterial disease, end-stage renal disease (patients on renal dialysis or kidney transplantation), and any other serious chronic disease that can affect wound healing. For the comparison between diabetic patients with and without foot ulceration, we used a group of seven diabetic patients with active foot ulceration (6 male subjects, aged 55 ± 11 years). The protocol was approved by the institutional review board of the Beth Israel Deaconess Medical Center. All participants gave written informed consent.
Baseline visit.
All subjects attended the Joslin-Beth Israel Deaconess Foot Center and the General Clinical Research Center where they had a full physical examination and all tests described below. All diabetic patients received education about foot care and were seen regularly by their podiatrist as required.
Exit visit.
All diabetic patients were asked to return for a follow-up visit at ~18–24 months, whereas the healthy control subjects only were seen during the baseline visit. The same types of tests that were performed during the baseline also were performed at the exit visit.
DFU development and treatment.
Participants were asked to contact the study coordinator in case of the development of foot ulceration or any other adverse event. In addition, they were contacted over regular periods of time by the study coordinator. Treatment for any ulceration that occurred during the study was provided at the Joslin-Beth Israel Deaconess Foot Center according to standard guidelines (4). The cause of the foot ulceration and its progression were recorded.
Clinical and laboratory assessments.
Serum was analyzed for the measurements of inflammatory cytokines, growth factors, and biochemical markers of endothelial function using a Luminex 200 apparatus (Luminex, Austin, TX) and Millipore multiplex immunoassay panels (Millipore, Chicago, IL).
Evaluation of diabetic neuropathy and peripheral vascular disease.
The symptoms were evaluated by using the Neuropathy Symptom Score (NSS) and the clinical signs by using the Neuropathy Disability Score (NDS) and the assessment of vibration perception threshold (VPT) and cutaneous perception threshold using a set of 12 Semmes-Weinstein monofilaments ranging from 2.83 to 10.0 g. Patients who were defined at high risk of foot ulceration had an NDS ≥5 and were unable to feel a 5.07 Semmes-Weinstein monofilament (5). Detailed description of these standard tests has been provided elsewhere (5).
Vascular reactivity tests.
All measurements were performed with the subjects in the fasting state. The vascular reactivity of the forearm-skin microcirculation was evaluated by laser Doppler perfusion imaging measurements before and after the iontophoresis of acetylcholine chloride (Ach; endothelium-dependent vasodilation) and sodium nitroprusside (SNP; endothelium-independent vasodilation), as previously described (6). The nerve axon reflex–related vasodilation (NARV) was performed by using a single-point laser probe and the Moor DRT4 System (6). The flow-mediated brachial artery dilation (FMD; endothelium dependent) and nitroglycerine-induced dilation (NID; endothelium independent) were measured in accordance with published guidelines (7).
Evaluation of oxy- and deoxyhemoglobin.
Data were collected using the HyperMed System (HyperMed, Burlington, MA), as previously described (8).
Skin biopsies
Forearm-skin biopsies.
One 2-mm skin-punch biopsy was taken from the volar aspect of the forearm.
Foot-skin biopsies.
Discarded skin specimens were obtained from subjects who underwent foot surgery for various reasons. The biopsy staining was performed under the supervision of a trained pathologist with experience in dermatopathology (A.K.) using standard techniques. To evaluate the percentage of cells expressing factor XIIIa and matrix metallopeptidase-9 (MMP-9), a score of 1 was entered when 1–5 of the cells were expressing the antibody, a score of 2 for 5–10 of the cells, and a score of 3 for >10 of the cells. For protein tyrosine phosphatase-1B (PTP1B), a score of 1 was entered for 1–5 cells, 2 for 5–10 cells, 3 for 10–20 cells, and 4 for >20 positive cells. The intensity of each antibody expression was scored as follows: 1 for weak, 2 for moderate, and 3 for strong expressions for all utilized antibodies. Western blot analysis was performed using standard techniques, and protein quantification was normalized with tubulin.
Statistical analysis.
Data analysis was performed in collaboration with a biostatistician (C.G.) using Minitab (Minitab, State College, PA). The analysis was undertaken by univariate techniques and modeling the data through multiple logistic regression. ANOVA was used for normally distributed data. Nonparametrical data were analyzed through Kruskal-Wallis ANOVA. The comparison between baseline and exit-visit measurements was made by using the paired t test for parametrically distributed data and the Wilcoxon signed rank test for nonnormal data. A logistic regression model was run having as an outcome variable the development of DFUs, whereas the NDS (low = NDS ≤5, high = NDS >5), forearm and dorsum of the foot NARV, and FMD were used as explanatory variables. The model was fitted to data after controlling for the age and sex of the study participants.
RESULTS
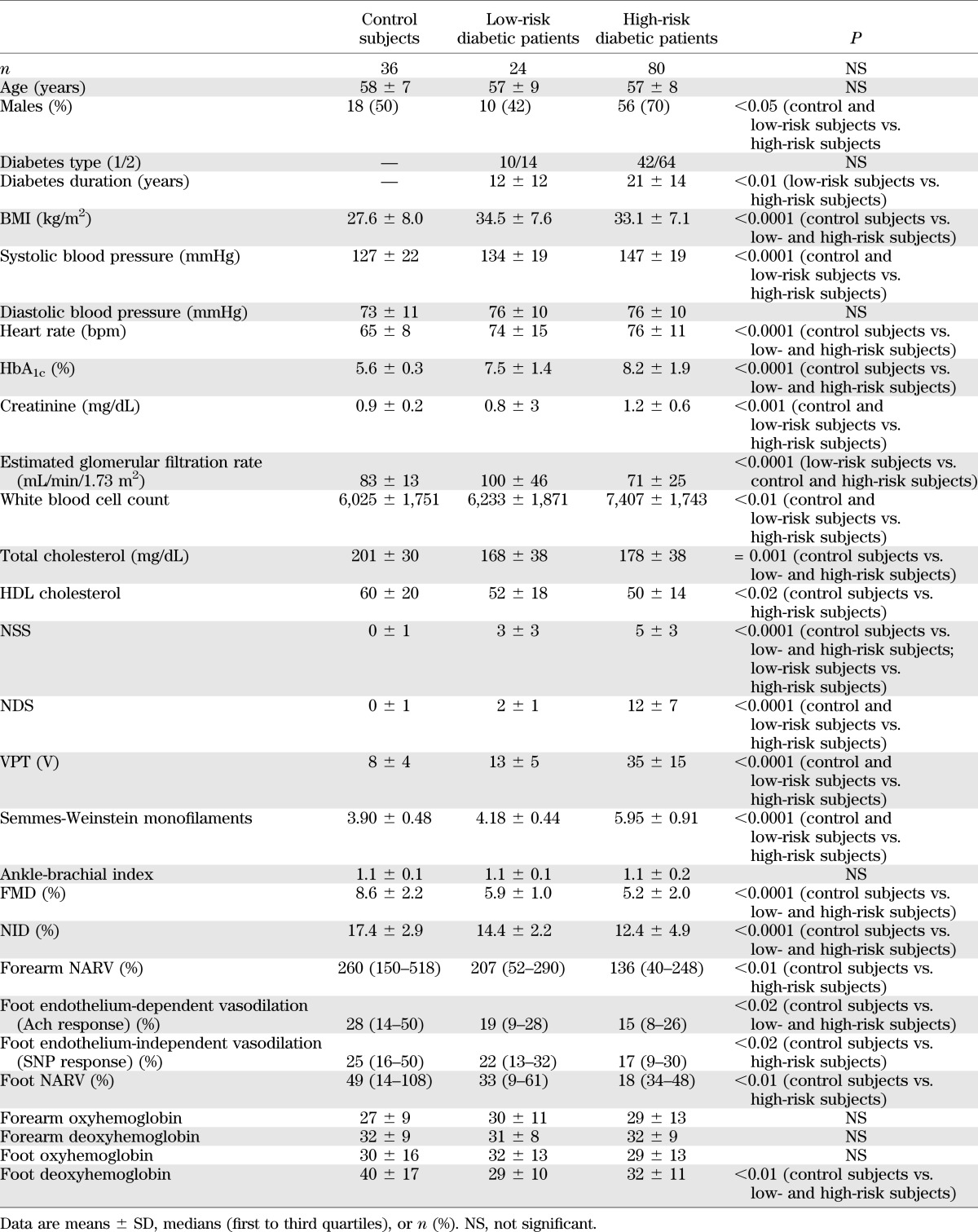
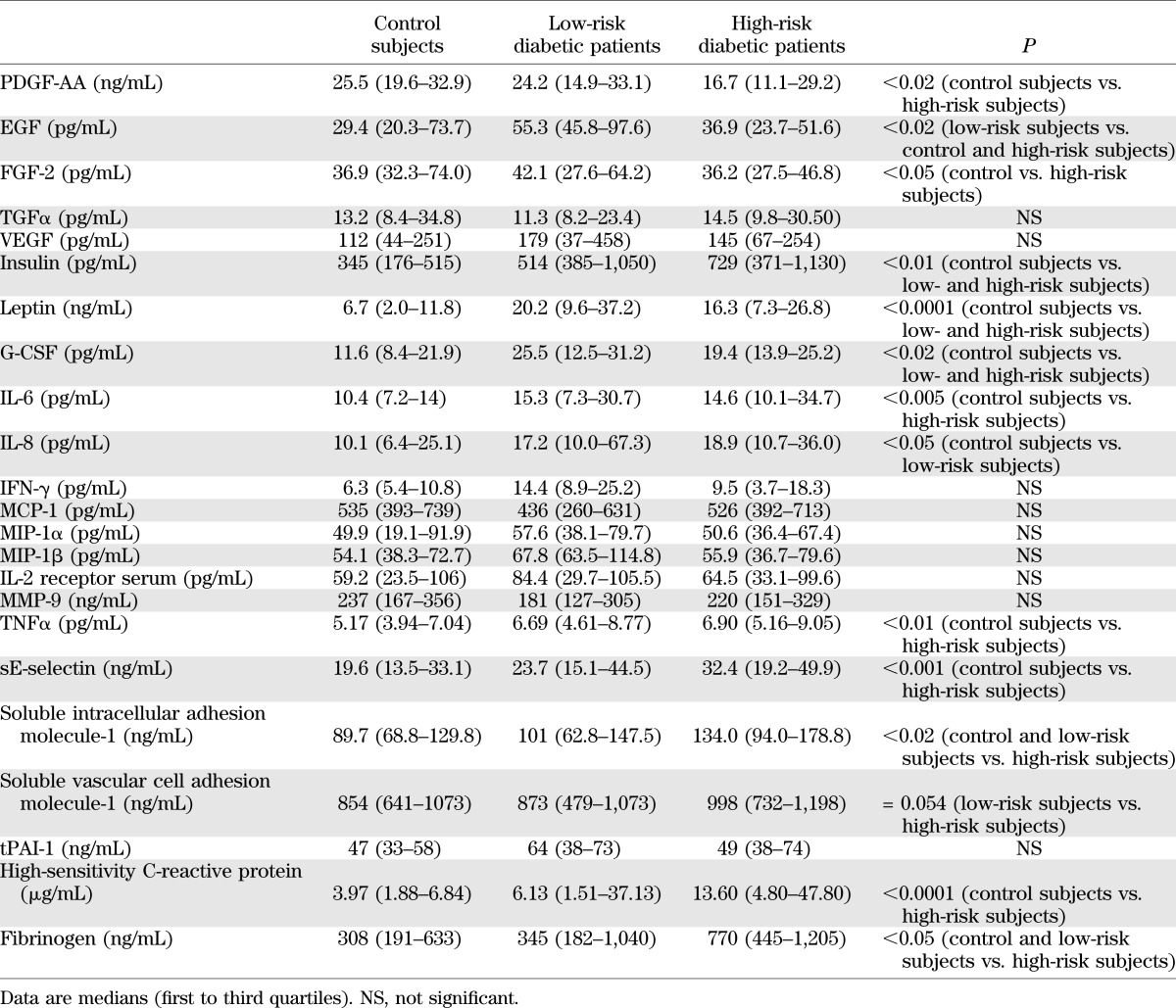
The demographics of the patients at low and high risk for DFUs and the control subjects are presented in Table 1. Four (4%) diabetic patients withdrew consent or were lost to follow-up and were excluded from the study. Twenty-four diabetic patients were characterized as being at low risk for developing foot ulceration and 80 as being at high risk. As expected, diabetic patients at high risk had different measurements of nerve function (NSS, NDS, VPT, and Semmes-Weinstein monofilaments). Furthermore, FMD and NID and foot-skin endothelium-dependent vasodilation were reduced in both diabetic groups, whereas the foot-skin SNP response and the NARV response at both the forearm and dorsum of the foot were reduced in the diabetic patients at high risk. The differences in the serum cytokines and growth factors in the three groups are shown in Table 2. In general, diabetic patients at high risk had higher levels of inflammatory cytokines.
TABLE 1.
Clinical characteristics of the three studied groups
TABLE 2.
Growth factor and cytokine results
DFU development and healing.
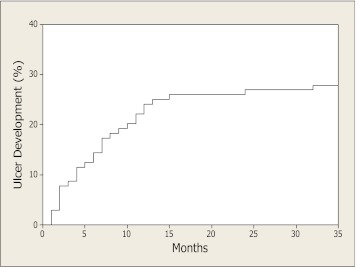
The mean follow-up time was (means ± SD) 18.4 ± 10.8 months. DFUs developed in 30 (29%) diabetic patients. The average period of time between enrollment in the study and the development of the first foot ulceration was 8.0 ± 7.1 months (Fig. 1). All subjects who developed ulceration belonged to the high-risk group, resulting in an annual rate of 24% in this group. The results of logistic regression analysis identifying the risk factors for the development of DFUs are shown in Supplementary Table 1. In short, high NDS (>5) was the most important predictor, whereas age, forearm NARV, and FMD also were associated with the risk of DFUs.
FIG. 1.
Cumulative incidence of DFUs.
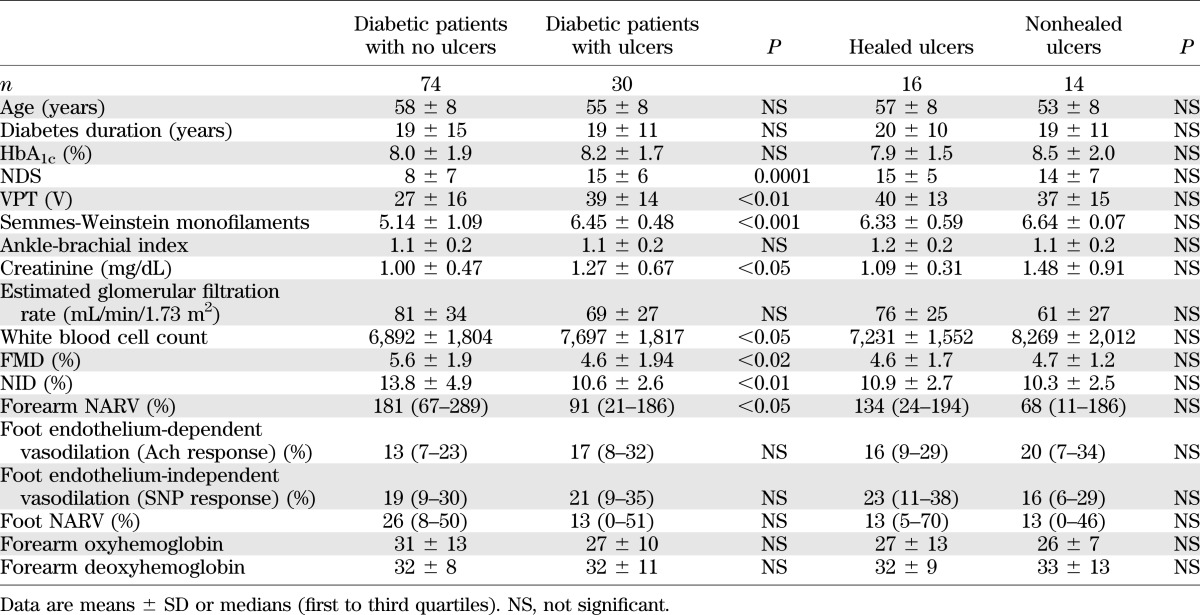
Complete wound healing during the first 12 weeks after ulceration occurred in 16 (53%) patients, whereas the remaining 14 (47%) went on to develop chronic, nonhealed foot ulcers. The differences in the clinical characteristics between the diabetic patients who did not develop foot ulcers and those who did are shown in Table 3. Table 3 also contains the comparisons between the patients who had complete ulcer healing versus those who did not heal. Patients who developed DFUs had more severe neuropathy, as seen by the NDS, VPT, and Semmes-Weinstein monofilament measurements; higher creatinine and white blood cell count; and lower FMD and NID than those who did not. No differences were observed in any of the above measurements between the patients whose ulcers healed when compared with those whose ulcers did not.
TABLE 3.
Clinical characteristics of diabetic patients with and without foot ulceration
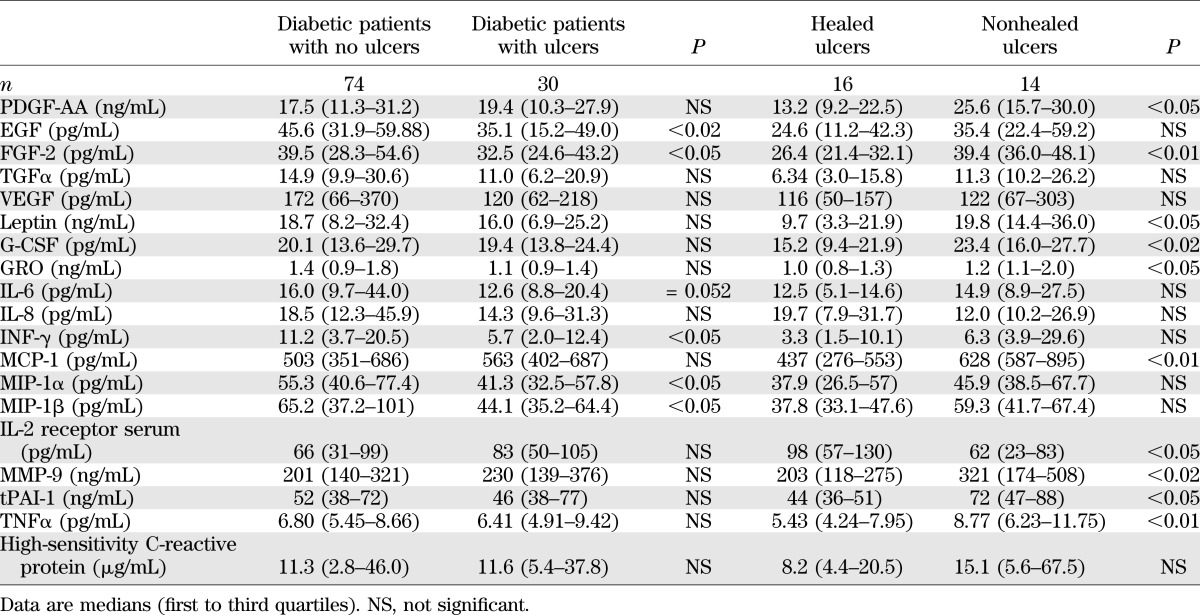
The differences in growth factors and cytokines between the patients who did not develop DFUs and those who did are shown in Table 4. The most notable differences included a reduction in epidermal growth factor (EGF), fibroblast growth factor (FGF), γ-interferon (IFN-γ), macrophage inflammatory protein 1 α (MIP-1α), and MIP-1β and a marginal reduction in interleukin (IL)-6 serum levels at baseline in the patients who developed DFUs when compared with those who did not. The differences between the patients whose ulcers healed and those whose ulcers did not also are shown in Table 4. The main differences included an increase in the platelet-derived growth factor (PDGF)-AA, FGF, leptin, granulocyte colony-stimulating factor (G-CSF), GRO, monocyte chemoattractant protein 1 (MCP-1), MMP-9, and tissue plasminogen activator-1 (tPAI-1) serum levels in the patients whose ulcers failed to heal (Fig. 2).
TABLE 4.
Results of growth factors and cytokines in diabetic patients with and without foot ulceration
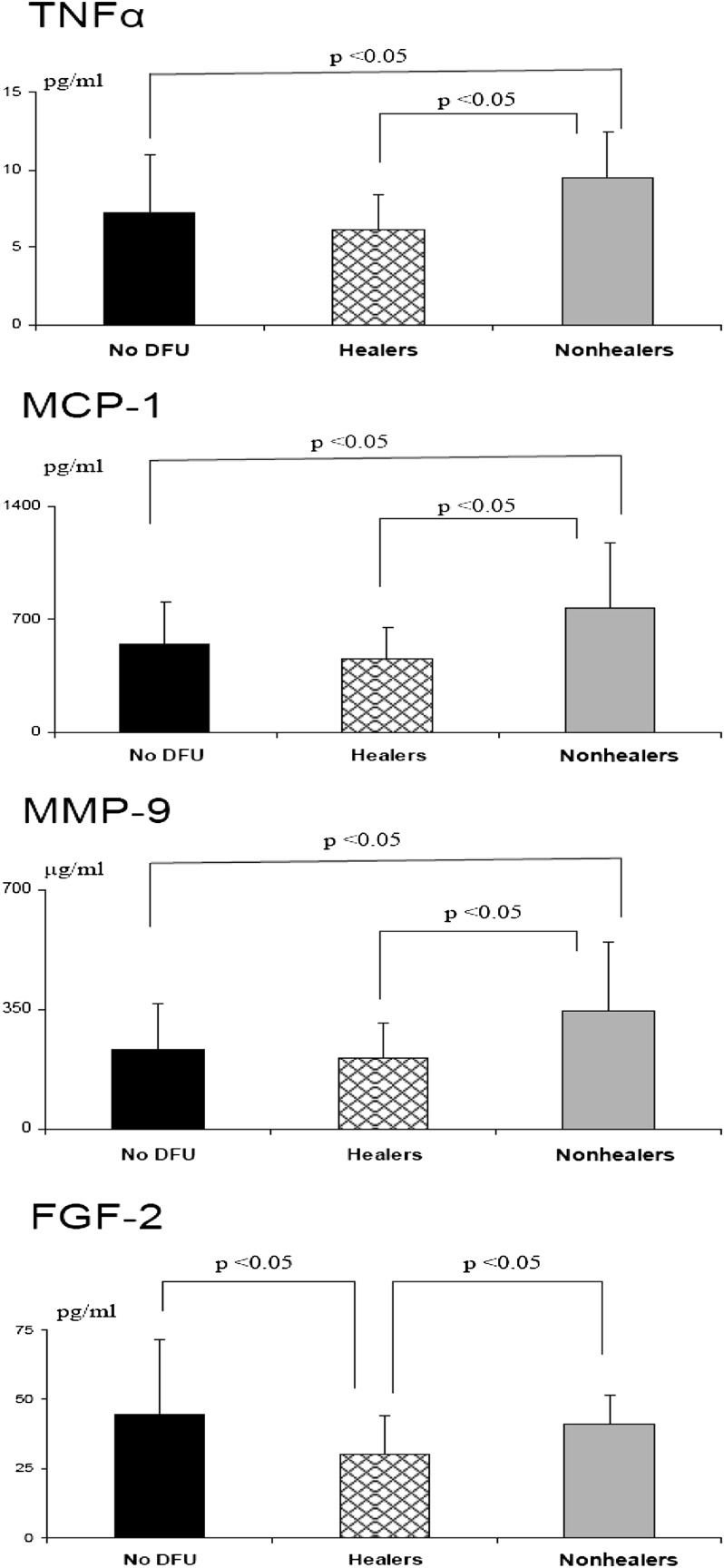
FIG. 2.
Differences in serum TNFα, MCP-1, MMP-9, and FGF-2 among diabetic patients who did not develop DFUs, those who developed foot ulceration and completely healed over a 12-week period (Healers), and those who developed foot ulceration and failed to heal over the same time period (Nonhealers).
The growth factor and cytokine levels of patients with healed and nonhealed ulcers also were compared with a group of diabetic patients who had an active foot ulcer when blood tests were performed, and the results are shown in Supplementary Table 2. Of interest, when compared with patients whose ulcers healed, patients whose ulcers failed to heal had measurements in most cytokines, including PDGF-AA, FGF, transforming growth factor (TGF) α, GRO, and MMP-9, that were closer to the group with the active ulcers.
Skin biopsy analysis
Forearm-skin biopsies.
Eighty-three subjects (12 healthy control subjects and 71 diabetic patients) underwent forearm-skin biopsy. The diabetic patients were subdivided to 12 low-risk and 59 high-risk diabetic patients. The high-risk group included 10 subjects who developed a foot ulcer that healed and 13 who developed a chronic wound. There were no major differences between the subjects who underwent a skin biopsy and those who did not. Because there were no major differences among the various diabetic groups, the main comparisons were made between the control and diabetic group and are presented in Table 5. The results of the diabetic patients who did not develop DFUs, those who developed DFUs, and the two subgroups whose DFUs healed or failed to heal are shown in the Supplementary Table 3.
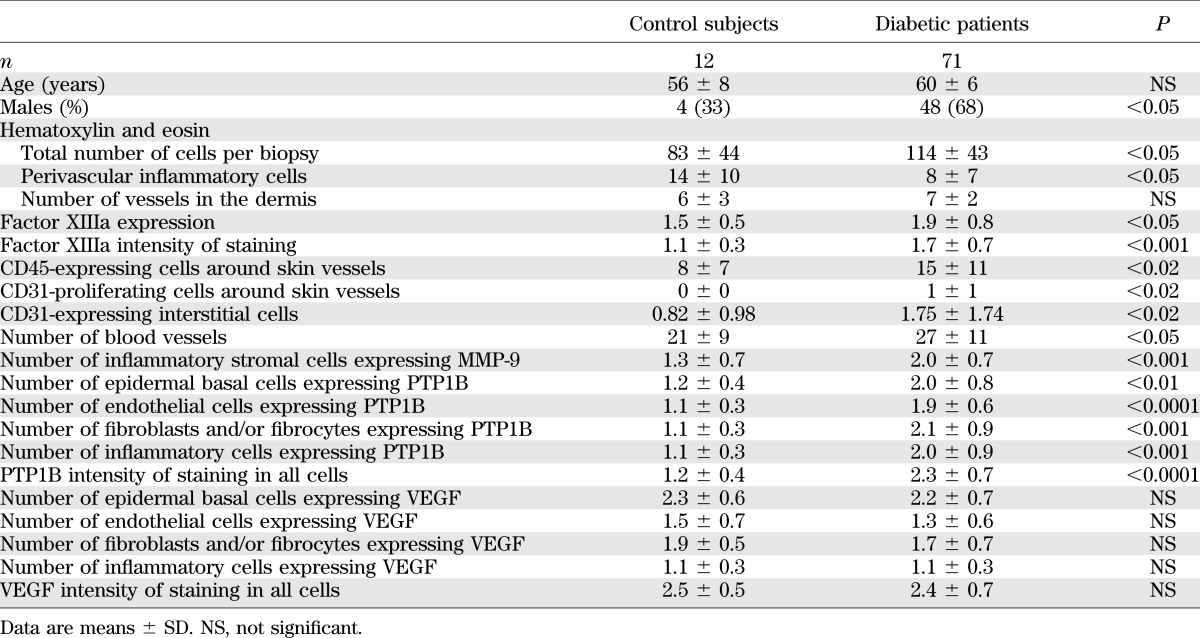
TABLE 5.
Results of the forearm-skin biopsy analysis
Hematoxylin and eosin analysis.
The total number of cells per biopsy in the dermis of the forearm-skin biopsies was higher in the diabetic patients when compared with the control subjects (Table 5). The number of inflammatory cells around vessels, a strong indication of inflammation, also was increased in diabetic patients (Fig. 3A and B). The number of blood vessels was higher in the patients who developed an ulcer that healed compared with those whose ulcer failed to heal.
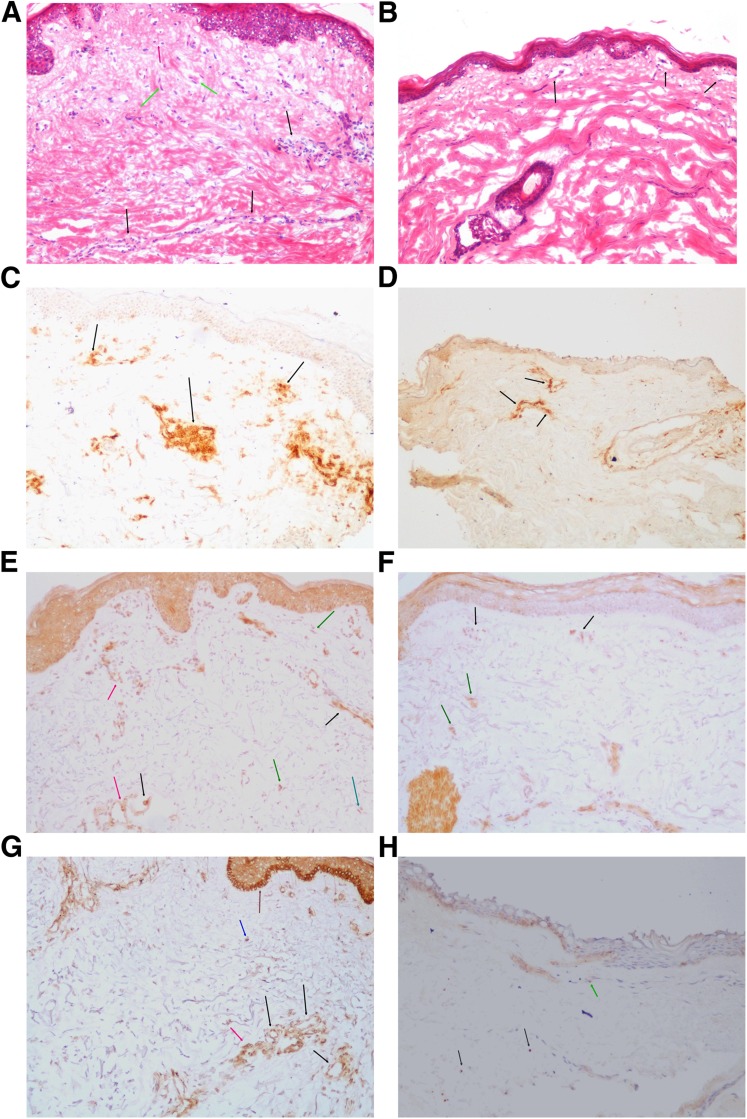
FIG. 3.
Forearm-skin biopsy immunohistochemistry analysis (frozen sections, ×100 magnification). A: Hematoxylin and eosin staining in a skin biopsy from a diabetic patient showing round cell inflammatory reaction around blood vessels (black arrows), round inflammatory cells in dermis far from vessels (red arrow), and fibrocytes/fibroblast (green arrows). B: Hematoxylin and eosin staining in a healthy control subject shows superficial blood vessels without perivascular round cell infiltration (black arrows). In the dermis, there are normal collagen bundles, without excess numbers of fibrocytes/fibroblasts or round single cells. C: CD45RO staining in a diabetic patient showing numerous positive lymphoid cells (round cells) around blood vessels (black arrows). D: CD45RO staining in a healthy subject showing a few positive lymphoid cells (round cells) around blood vessels (black arrows). E: MMP-9 staining in a diabetic patient showing intense expression by stromal cells (green arrows). In addition, the antibody was expressed by endothelial cells (black arrows) and reveals the basement membrane of blood vessels (red arrows). F: MMP-9 staining in a healthy control subject. There is faint expression by stromal cells (green arrows) and limited expression by endothelial cells (black arrows). G: PTP1B staining in a diabetic patient showing cytoplasmic, membranous, or paranuclear dot-like staining pattern. PTP1B is strongly expressed by endothelial cells (black arrows), inflammatory cells (red arrow), epidermal basal cells (brown arrow), and dermis cells, mainly fibroblast (blue arrow). H: PTP1B staining in a healthy control subject showing a faint stain pattern in fibrocytes/fibroblasts (black arrows) and endothelial cells (green arrow). (A high-quality digital representation of this figure is available in the online issue.)
Markers of inflammatory cells.
The percentage of cells expressing factor XIIIa, a marker of dermal dendrocytes, and the intensity of staining were higher in diabetic patients. The number of CD45RO-expressing cells around blood vessels, a marker of lymphocytes, also was higher in diabetic patients (Fig. 3C and D).
CD31 staining.
The number of CD31 (endothelial) proliferating cells around preexisting vessels, the number of CD31-positive cells in the dermis, and the number of blood vessels, including single-positive CD31 cells, was higher in diabetic patients.
MMP-9 staining.
The antibody was expressed at the basement membrane of the blood vessels by endothelial cells, fibroblasts/fibrocytes, and inflammatory cells. The number of stromal cells expressing the antibody was higher in diabetic patients (Fig. 3E and F).
PTP1B staining.
Expressing cells were observed at the basal layer of the epidermis where mitotically active cells exist, the dermis cells (fibrocytes or fibroblasts), the endothelial cells of blood vessels, and the inflammatory cells. The staining pattern was mainly membranous or paranuclear dot like. When compared with healthy control subjects, PTP1B was expressed by a higher number of epidermal basal cells, endothelial cells, fibrocytes and/or fibroblasts, and inflammatory cells in diabetic patients (Fig. 3G and H). The intensity of staining in all cells also was higher in diabetic patients. The number of endothelial cells expressing the antibody was marginally higher in the diabetic patients who developed and failed to heal their ulcer when compared with the subjects whose ulcer had healed (Supplementary Table 3). There were no differences between type 1 and type 2 diabetic patients.
Vascular endothelial growth factor staining.
No differences were observed in the number of epidermal basal cells, endothelial cells, fibrocytes, and/or fibroblasts that expressed the antibody.
Foot-skin biopsies.
Immunohistochemistry.
Immunohistochemistry was performed in dorsal foot discarded-skin specimens that were obtained during foot surgery from 8 nondiabetic healthy control subjects (aged 58 ± 18, 3 male subjects) and 7 diabetic patients (aged 58 ± 16, 5 male subjects). The results were similar to the ones observed in the forearm biopsies, although they failed to reach statistical significance in most cases because of the small number of participants. Thus, hematoxylin and eosin analysis showed that the total number of cells per biopsy in the dermis tended to be higher in diabetic patients (196 ± 78 vs. 107 ± 23, P = 0.10). The number of inflammatory cells around vessels also tended to be higher in diabetic patients (62 ± 39 vs. 43 ± 32, P = NS). MMP-9 expression by inflammatory cells also tended to be higher (2.2 ± 0.8 vs. 1.8 ± 0.4, P = NS). PTP1B expression by the endothelial cells in diabetic patients also tended to be higher (2.7 ± 0.6 vs. 2.0 ± 0.2, P = NS).
Western blot analysis.
We also measured the protein expression using Western blot analysis in foot-skin specimens from 11 healthy control subjects (aged 55 ± 18 years, 4 male subjects) and 9 diabetic patients (aged 62 ± 13, 6 male subjects) as described in the Supplemental Data. The majority of these subjects were similar with the ones above because, when feasible, specimens were tested for both immunohistochemistry and Western blot analysis. Protein expression was higher in diabetic patients than the control subjects regarding MMP-9 (140 ± 52 vs. 100 ± 35, P = 0.053), PTP1B (149 ± 72 vs. 100 ± 21, P < 0.05), and vascular endothelial growth factor (VEGF) (156 ± 72 vs. 100 ± 33, P < 0.05), whereas no differences were observed in PDGF-BB (122 ± 49 vs. 100 ± 29, P = NS) (Supplementary Fig. 1).
Second visit.
Fifty-eight diabetic patients (including 17 patients who developed a foot ulcer during the study) returned for a second (exit) visit 20 ± 8 months after the first visit. There were no major differences in any of the clinical characteristics between the patients who returned for a second visit and those who did not. The differences between baseline and exit visit in all 59 patients, the subgroup of the 41 patients who did not develop a foot ulcer, and the 17 patients who developed foot ulceration are shown in Supplementary Table 4 (clinical characteristics) and Supplementary Table 5 (growth factors and cytokines). No differences were observed in the analysis of biopsies that were taken in visits 1 and 2 (data not shown).
DISCUSSION
The main findings of the present prospective cohort study are that although neuropathy and vascular factors are associated with the development of DFUs, the main factors that are associated with failure to heal these ulcers are preexisting increased serum levels of inflammatory cytokines, MMP-9, and various growth factors. At the skin level, diabetes was associated with inflammation and increased expression of MMP-9 and PTP1B, factors that are associated with inflammation, can lead to resistance of the growth factor action, and may be responsible for the observed raised levels in the patients whose ulcers failed to heal.
As would be expected, only neuropathic patients developed DFUs at a rate that was similar to the one predicted by previous prospective studies, indicating that the selected subjects were representative of the general diabetic population (5,9). It also is of interest that the forearm NARV was a risk factor, indicating the existence of more severe peripheral neuropathy that affected the c-nociceptive fibers of the upper extremity in the DFU group. In addition, both endothelium-dependent and -independent vasodilation in the macrocirculation were reduced in the patients who developed DFUs, indicating that the same vascular factors that are associated with excess cardiovascular mortality in diabetes also are involved in foot ulceration (10).
A major novel observation of this study is that although none of the above factors was associated with complete wound healing, the main factors involved were inflammatory cytokines and growth factors. Thus, failure of an ulcer to heal was associated with increased levels of various inflammatory cytokines, including tumor necrosis factor α (TNFα), G-CSF, GRO, MCP-1, and leptin, which are known to be increased in diabetes and diabetes complications (11,12). It should be emphasized that these increased inflammatory levels were observed in serum specimens taken at the baseline visit, which occurred on average ~8 months before the development of foot ulceration. Therefore, the observed raised levels cannot be attributed to mechanisms that are related with the healing process of an existing ulcer and clearly indicate that a pre-existing low-grade proinflammatory state, which already has been recognized as a major factor of metabolic and cardiovascular disease, has a negative impact of the healing of DFUs (10,13). Of interest, the soluble IL-2 receptor serum level, which increases considerably after acute trauma or burns and correlates with immunosuppression, was reduced in patents whose ulcers failed to heal (14).
The PDGF-AA and FGF-2 levels were higher in diabetic patients whose ulcers failed to heal compared with those whose did, whereas the EGF, TGFα, and VEGF levels tended to be higher but failed to reach statistical significance. Furthermore, patients whose ulcers failed to had growth factor levels closer to those observed in patients with active foot ulcers, whereas patients whose ulcers healed tended to have lower levels. These observations indicate a possible resistance to growth factor action. Glycation of various growth factors, along with increased MMP-9 and PTPB1 expression that were examined in this study, also may contribute to this resistance to growth factor function (15). This resistance may be one of the main reasons responsible for the failure of most clinical trials that have studied the effects of growth factors for DFU treatment (16).
MMP-9 is mainly released by inflammatory cells and is involved in the breaking down of matrix proteins and growth factors (17). Increased MMP-9 levels are present in various chronic nonhealing wounds, including DFUs (18). In the current study, increased serum levels long before the development of DFUs are associated with failure of an ulcer to heal. Of interest, patients with nonhealing ulcers also had higher levels of tPA, which is known to upregulate the expression of MMP-9 (19).
As all the above-mentioned changes were systemic, we also used forearm-skin biopsies in the same participants and at the same time points to focus on tissue-specific changes. Our results indicate that diabetic patients had higher dermis infiltration by inflammatory cells, a sign of chronic inflammation, which is compatible with the well-known diabetes generalized proinflammatory status. MMP-9 expression by inflammatory stromal cells also was higher in the diabetic patients, suggesting that these cells may be a source for the observed increased systemic levels in the same population. To the best of our knowledge, this is the first time that such skin changes are reported in diabetic patients.
Given the high levels of growth factors in patients whose ulcers failed to heal, indicating a resistance to their action, we explored possible mechanisms that could be related to this, and we focused on the role of PTP1B. PTP1B is a ubiquitously expressed protein tyrosine phosphatase that localizes in the endoplasmic reticulum, is upregulated by inflammation, and negatively regulates the signaling of insulin, leptin, and various growth factors that are involved in wound healing, such as VEGF, EGF, PDGF, and TGFβ (20). Ongoing animal studies in our unit indicate that both diabetic and nondiabetic PTP1B knockout mice have enhanced wound healing, increased healing response to topical leptin application, and increased periwound oxygen saturation (21). In humans, PTP1B overexpression and increased activity has been reported in the adipose tissue, especially the omental tissue (22,23). This is the first study to report increased expression in all prominent skin cell populations of diabetic patients, whereas patients whose ulcers failed to heal had marginally increased expression in the endothelial cells when compared with patients whose ulcers healed.
Taken in context, our results indicate that there is increased extracellular MMP-9 and intracellular PTP1B expression, leading to local inactivation and resistance to the action of various growth factors that are involved in wound healing. Furthermore, this leads to increased levels of circulating growth factors in a way that is similar to the increased insulin levels in situations of insulin resistance. Therefore, in case this hypothesis is correct, local wound inhibition of MMP-9 and/or PTP1B may have the potential to reverse these conditions and lead to promote wound healing of DFUs. Of note, PTP1B inhibitors already have been developed and currently are under consideration as a possible treatment of type 2 diabetes and may prove candidates for local treatment of DFUs (24,25).
The number of dermis vessels that were observed during the hematoxylin and eosin analysis was higher in the patients whose ulcers healed. Furthermore, CD31 staining showed a higher number of endothelial cells proliferating around preexisting vessels and blood vessels in diabetic patients. No changes were observed in the VEGF serum levels and skin immunohistochemistry studies, whereas Western blot analysis showed higher foot-skin expression in diabetic patients when compared with control subjects. Previous studies in our unit and elsewhere have reported an increase in serum VEGF in obese subjects and have hypothesized that this promotes revascularization in the adipose tissue that allows fat mass expansion (26,27). The results of the current study suggest that a similar increased vascularization exists at the skin level in diabetes, and its magnitude can affect complete wound healing. Furthermore, these results are in contrast with animal studies that have shown reduced VEGF expression and neovascularization, raising questions regarding the applicability of the existing animal models to the human condition (28).
Ulcer healing is heavily influenced by the quality of the provided care and the adherence to it by the patients. In the current study, all patients were followed at our unit by the same physicians who provided state-of-the-art care, including education and appropriate ulcer off loading, and also monitored the adherence to it (4). Because the provided care was the same in all participants, we do not believe that it had any influence in the observed results.
A large number of the participants returned for a second visit after a 20-month period. Because there were no major differences in both serum and skin biopsy measurements between these two visits, the data clearly indicate that the observed changes are chronic ones that develop over long periods of diabetes.
One limitation of the current study is that the skin biopsies were taken from the forearm and not the foot of the participating subjects. This was conducted because biopsies on the dorsum of the foot would carry an unacceptable high risk for complications, especially in the high-risk group. Furthermore, given the considerable variety of cells that are present in the skin and that play a role in wound healing, we based our main analysis on immunohistochemistry because it allows the study of each cell type separately. However, in order to address the possible above limitations, we also studied foot specimens from a different group of diabetic patients and the results were very similar to the ones observed at the forearm level, both when immunohistochemistry and Western blot analysis were used. Therefore, we feel very confident that the observed results at the forearm level indicate systemic skin changes and are representative to changes at the foot level.
In summary, in this cohort prospective study we have shown that systemic factors that are associated with foot ulceration and impaired wound healing are present long before the development of DFUs. Increased inflammation and skin expression of MMP-9, PTP1B, and serum growth factors were the main factors associated with failure to heal DFUs. Targeting these factors may prove helpful in the management of DFUs.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants R01-HL-075678, R01-DK-076937, and R01-NS-066205 (to A.V.). The project described was supported by the Clinical Translational Science Award (UL1RR025758) to Harvard University and Beth Israel Deaconess Medical Center from the National Center for Research Resources.
No potential conflicts of interest relevant to this article were reported.
T.D., T.E.L., and J.M.G. provided the clinical care to all participants. F.T., J.D., E.L., A.T., and L.P. obtained all the data. A.K. conducted the skin biopsy analysis. C.G. conducted the statistical analysis. A.V. was responsible for the study concept, design, and initial data analysis and wrote the manuscript. All authors participated in the data analysis and interpretation processes and reviewed and approved the final report. A.V. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db12-0227/-/DC1.
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the National Institutes of Health.
REFERENCES
- 1.Boulton AJ, Vileikyte L, Ragnarson-Tennvall G, Apelqvist J. The global burden of diabetic foot disease. Lancet 2005;366:1719–1724 [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association Economic costs of diabetes in the U.S. in 2007. Diabetes Care 2008;31:596–615 [DOI] [PubMed] [Google Scholar]
- 3.Dinh TL, Veves A. A review of the mechanisms implicated in the pathogenesis of the diabetic foot. Int J Low Extrem Wounds 2005;4:154–159 [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association Consensus Development Conference on Diabetic Foot Wound Care: 7–8 April 1999, Boston, Massachusetts. Diabetes Care 1999;22:1354–1360 [DOI] [PubMed] [Google Scholar]
- 5.Pham H, Armstrong DG, Harvey C, Harkless LB, Giurini JM, Veves A. Screening techniques to identify people at high risk for diabetic foot ulceration: a prospective multicenter trial. Diabetes Care 2000;23:606–611 [DOI] [PubMed] [Google Scholar]
- 6.Veves A, Akbari CM, Primavera J, et al. Endothelial dysfunction and the expression of endothelial nitric oxide synthetase in diabetic neuropathy, vascular disease, and foot ulceration. Diabetes 1998;47:457–463 [DOI] [PubMed] [Google Scholar]
- 7.Corretti MC, Anderson TJ, Benjamin EJ, et al. International Brachial Artery Reactivity Task Force Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol 2002;39:257–265 [DOI] [PubMed] [Google Scholar]
- 8.Greenman RL, Panasyuk S, Wang X, et al. Early changes in the skin microcirculation and muscle metabolism of the diabetic foot. Lancet 2005;366:1711–1717 [DOI] [PubMed] [Google Scholar]
- 9.Lavery LA, Higgins KR, Lanctot DR, et al. Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care 2007;30:14–20 [DOI] [PubMed] [Google Scholar]
- 10.de Jager J, Dekker JM, Kooy A, et al. Endothelial dysfunction and low-grade inflammation explain much of the excess cardiovascular mortality in individuals with type 2 diabetes: the Hoorn Study. Arterioscler Thromb Vasc Biol 2006;26:1086–1093 [DOI] [PubMed] [Google Scholar]
- 11.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation 2005;111:1448–1454 [DOI] [PubMed] [Google Scholar]
- 12.Doupis J, Lyons TE, Wu S, Gnardellis C, Dinh T, Veves A. Microvascular reactivity and inflammatory cytokines in painful and painless peripheral diabetic neuropathy. J Clin Endocrinol Metab 2009;94:2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hotamisligil GS. Inflammation and metabolic disorders. Nature 2006;444:860–867 [DOI] [PubMed] [Google Scholar]
- 14.Jobin N, Garrel D, Bernier J. Increased serum-soluble interleukin-2 receptor in burn patients: characterization and effects on the immune system. Hum Immunol 2000;61:233–246 [DOI] [PubMed] [Google Scholar]
- 15.Nass N, Vogel K, Hofmann B, Presek P, Silber RE, Simm A. Glycation of PDGF results in decreased biological activity. Int J Biochem Cell Biol 2010;42:749–754 [DOI] [PubMed] [Google Scholar]
- 16.Hinchliffe RJ, Valk GD, Apelqvist J, et al. A systematic review of the effectiveness of interventions to enhance the healing of chronic ulcers of the foot in diabetes. Diabetes Metab Res Rev 2008;24(Suppl. 1):S119–S144 [DOI] [PubMed] [Google Scholar]
- 17.Falanga V. Wound healing and its impairment in the diabetic foot. Lancet 2005;366:1736–1743 [DOI] [PubMed] [Google Scholar]
- 18.Liu Y, Min D, Bolton T, et al. Increased matrix metalloproteinase-9 predicts poor wound healing in diabetic foot ulcers. Diabetes Care 2009;32:117–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Lee SR, Arai K, et al. Lipoprotein receptor-mediated induction of matrix metalloproteinase by tissue plasminogen activator. Nat Med 2003;9:1313–1317 [DOI] [PubMed] [Google Scholar]
- 20.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 2008;283:14230–14241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zabolotny JM, Tellechea A, Leal EC, et al. Protein tyrosine phosphatase 1B (PTP1B) deficiency promotes wound healing (Abstract). Diabetologia 2011;54(Suppl. 1):S471 [Google Scholar]
- 22.Ahmad F, Considine RV, Goldstein BJ. Increased abundance of the receptor-type protein-tyrosine phosphatase LAR accounts for the elevated insulin receptor dephosphorylating activity in adipose tissue of obese human subjects. J Clin Invest 1995;95:2806–2812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, Hoffstedt J, Deeb W, et al. Depot-specific variation in protein-tyrosine phosphatase activities in human omental and subcutaneous adipose tissue: a potential contribution to differential insulin sensitivity. J Clin Endocrinol Metab 2001;86:5973–5980 [DOI] [PubMed] [Google Scholar]
- 24.Bence KK. Hepatic PTP1B Deficiency: the promise of a treatment for metabolic syndrome? J Clin Metab Diabetes 2010;1:27–33 [PMC free article] [PubMed] [Google Scholar]
- 25.Erbe DV, Klaman LD, Wilson DP, et al. Prodrug delivery of novel PTP1B inhibitors to enhance insulin signalling. Diabetes Obes Metab 2009;11:579–588 [DOI] [PubMed] [Google Scholar]
- 26.Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest 2007;117:2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doupis J, Rahangdale S, Gnardellis C, Pena SE, Malhotra A, Veves A. Effects of diabetes and obesity on vascular reactivity, inflammatory cytokines, and growth factors. Obesity (Silver Spring) 2011;19:729–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thangarajah H, Yao D, Chang EI, et al. The molecular basis for impaired hypoxia-induced VEGF expression in diabetic tissues. Proc Natl Acad Sci USA 2009;106:13505–13510 [DOI] [PMC free article] [PubMed] [Google Scholar]