Abstract
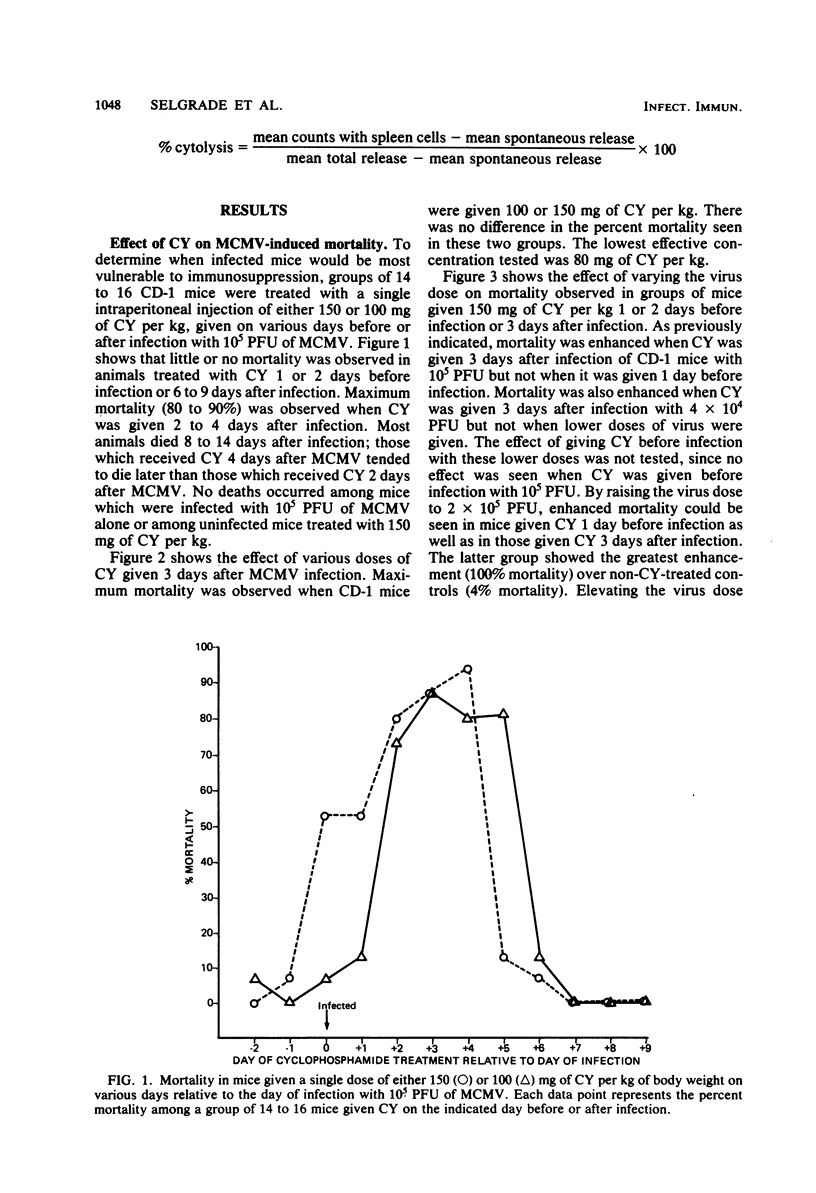
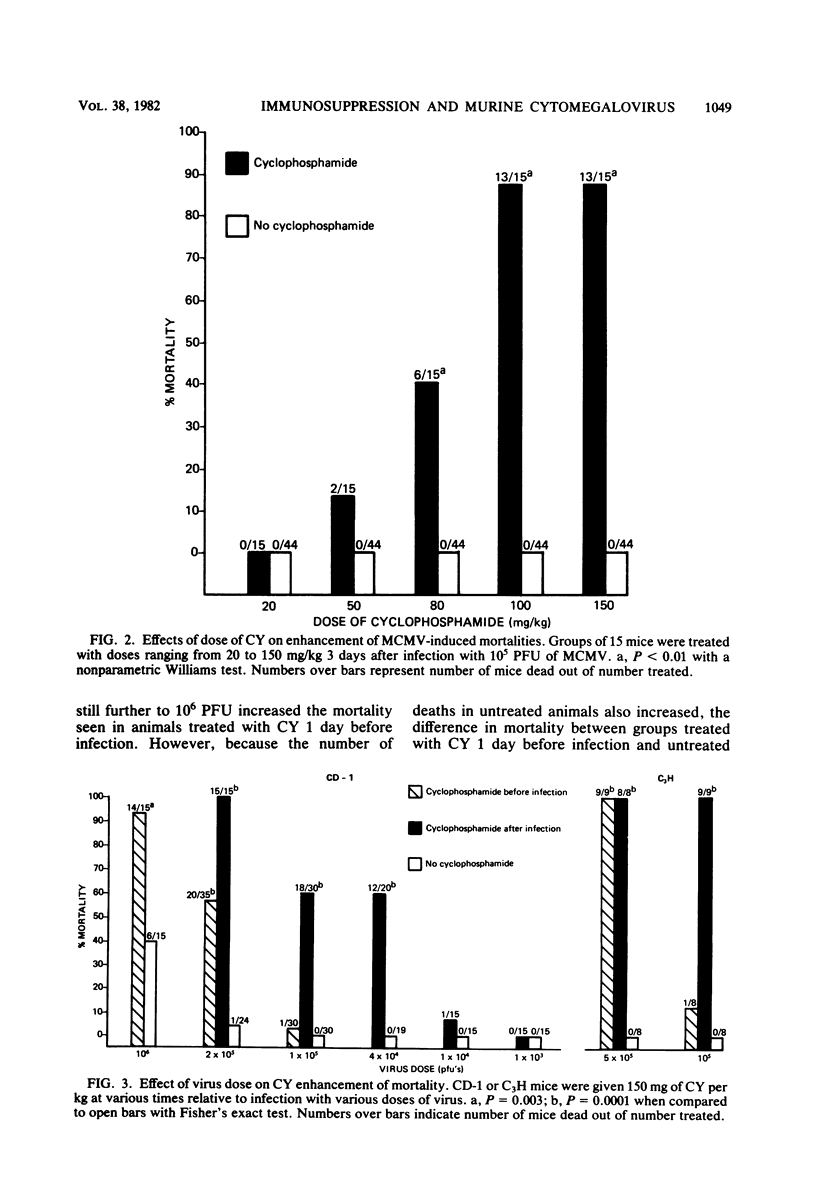
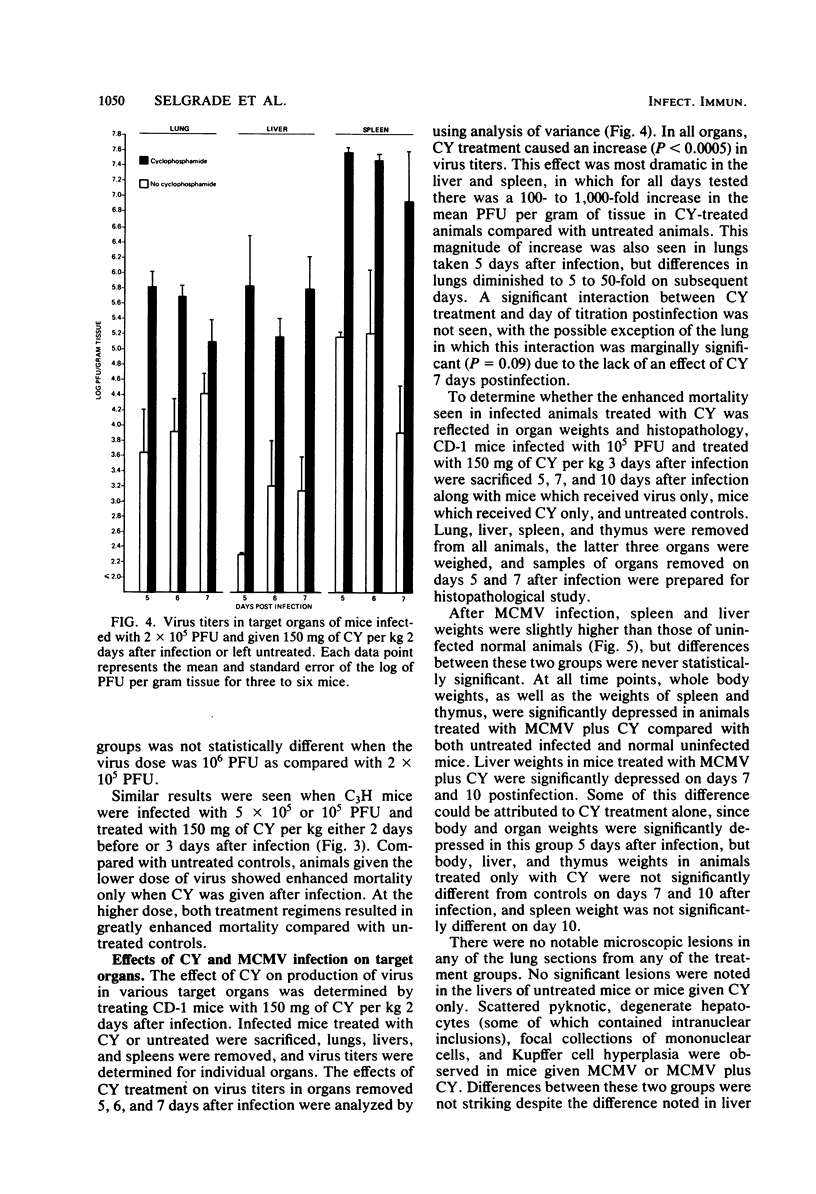
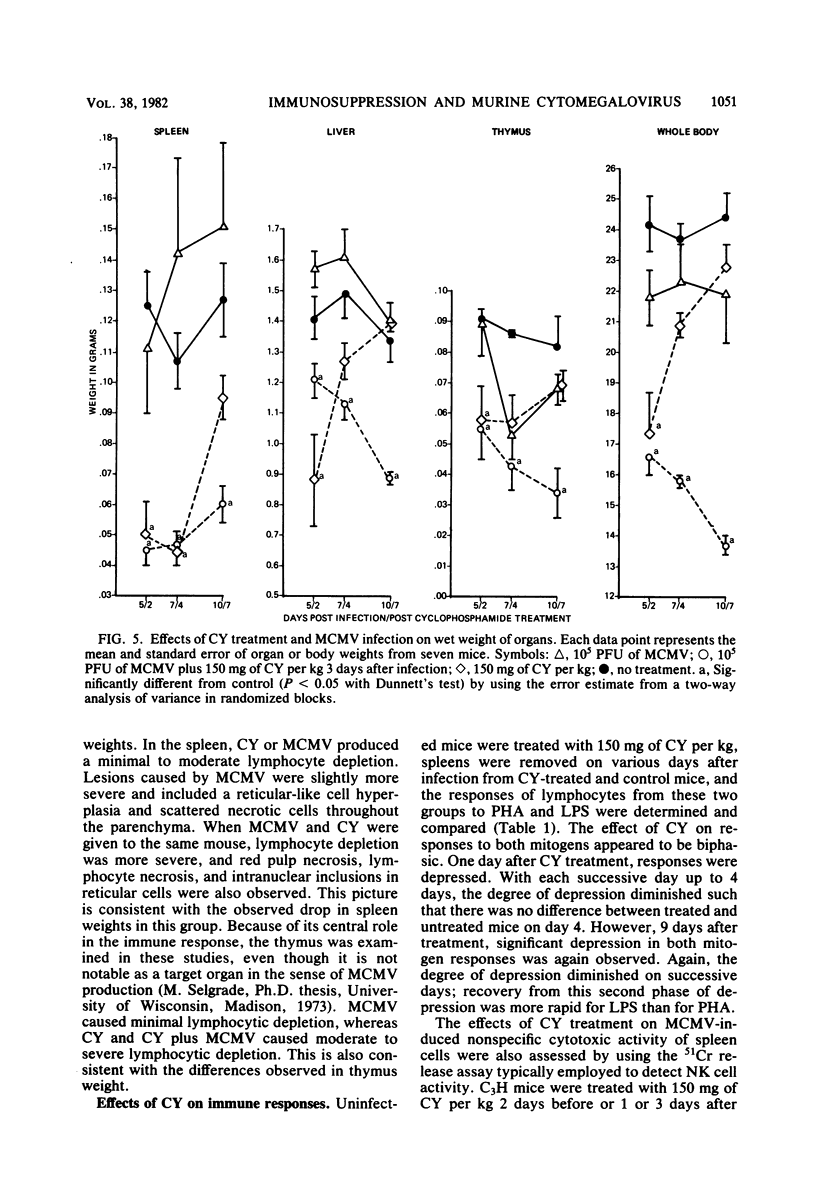
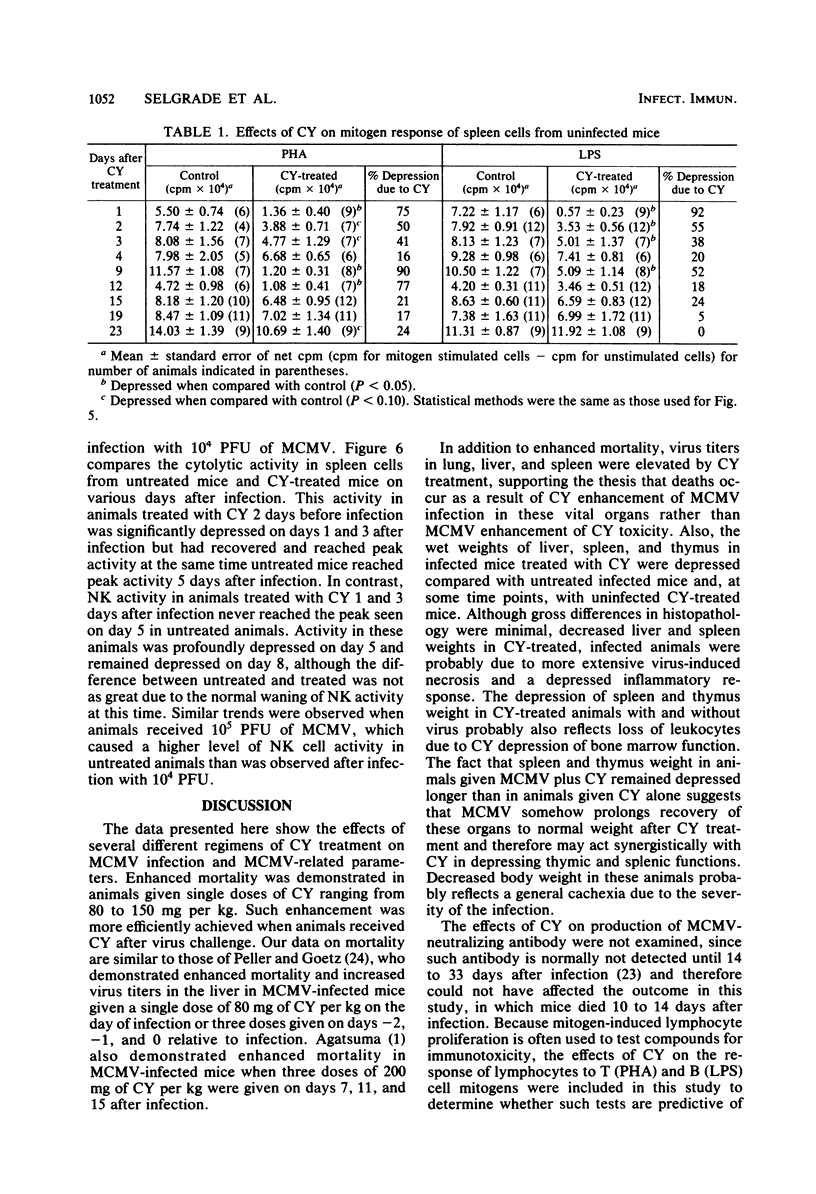
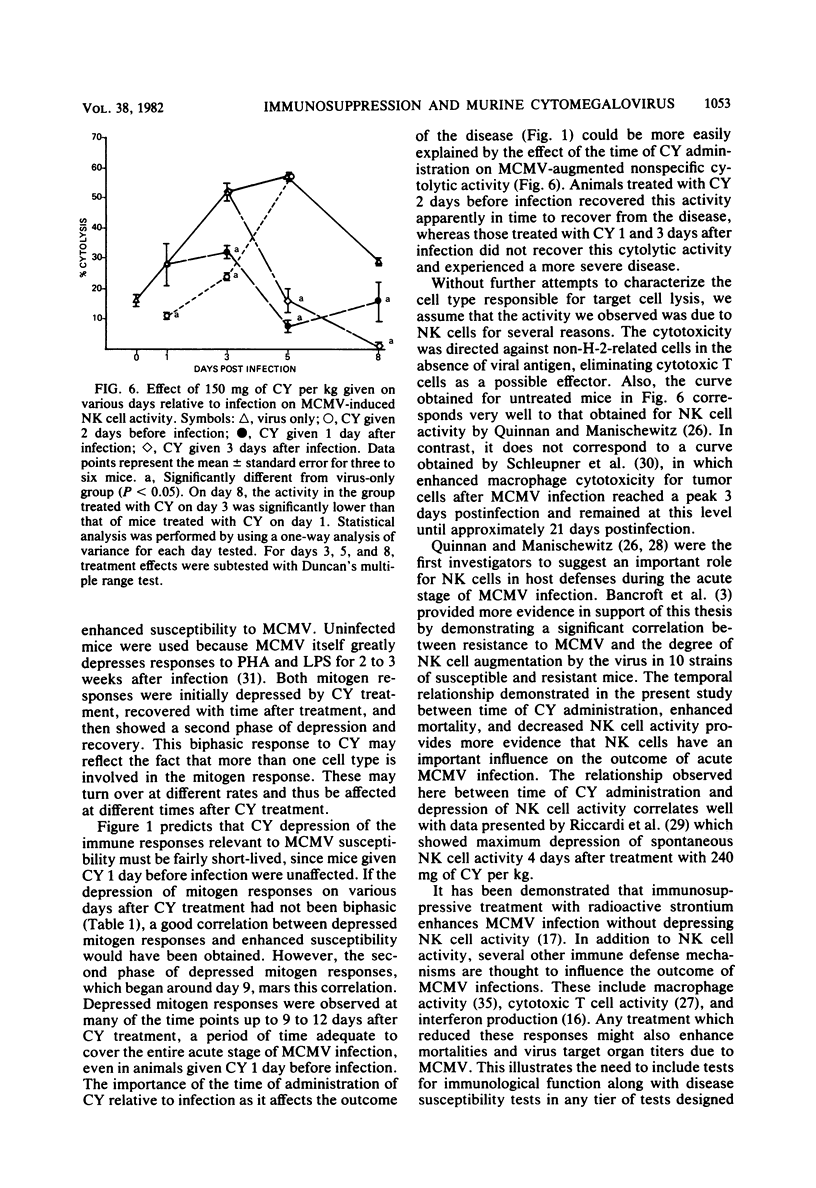
The effects of cyclophosphamide (CY) treatment on acute murine cytomegalovirus (MCMV) infection were studied to explore the potential usefulness of MCMV as a means of detecting immune dysfunction and to identify host defense mechanisms important for protection against MCMV. Conditions found optimal for enhancing MCMV infection with CY included infecting adult mice with 2 X 10(5) PFU or more of virus and administering 80 mg or more of CY per kg 1 to 3 days later. In addition to enhanced mortality, virus titers in lung, liver, and spleen were elevated in CY-treated mice, and wet weights of liver, spleen, and thymus were depressed when compared with those of infected but untreated mice. Treatment with CY before MCMV challenge was not as efficient a means of enhancing mortality as treatment after virus challenge. The effect that the time of CY administration relative to infection had on mortality correlated with the effect of such timing on natural killer cell activity. Animals treated before infection exhibited depressed natural killer cell activity initially. However, they rapidly recovered this response, and by 5 days postinfection they had the same level of virus-augmented activity seen in untreated mice. In contrast, animals treated after infection did not recover natural killer cell activity and were more likely to die. A similar correlation was not obtained when the effects of CY on lymphocyte responses to B and T cell mitogens were examined, nor were there striking differences in pathology between the treatment groups. The data suggest an important role for natural killer cells in host defense against MCMV. Also, increased susceptibility to MCMV may provide a useful indicator of deficits in the natural killer cell response.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft G. J., Shellam G. R., Chalmer J. E. Genetic influences on the augmentation of natural killer (NK) cells during murine cytomegalovirus infection: correlation with patterns of resistance. J Immunol. 1981 Mar;126(3):988–994. [PubMed] [Google Scholar]
- Dean J. H., Padarathsingh M. L., Jerrells T. R. Assessment of immunobiological effects induced by chemicals, drugs or food additives. I. Tier testing and screening approach. Drug Chem Toxicol. 1979;2(1-2):5–17. doi: 10.3109/01480547908993178. [DOI] [PubMed] [Google Scholar]
- Dowling J. N., Saslow A. R., Armstrong J. A., Ho M. Cytomegalovirus infection in patients receiving immunosuppressive therapy for rheumatologic disorders. J Infect Dis. 1976 Apr;133(4):399–408. doi: 10.1093/infdis/133.4.399. [DOI] [PubMed] [Google Scholar]
- Fiala M., Payne J. E., Berne T. V., Moore T. C., Henle W., Montgomerie J. Z., Chatterjee S. N., Guze L. B. Epidemiology of cytomegalovirus infection after transplantation and immunosuppression. J Infect Dis. 1975 Oct;132(4):421–433. doi: 10.1093/infdis/132.4.421. [DOI] [PubMed] [Google Scholar]
- Gardner M. B., Officer J. E., Parker J., Estes J. D., Rongey R. W. Induction of disseminated virulent cytomegalovirus infection by immunosuppression of naturally chronically infected wild mice. Infect Immun. 1974 Oct;10(4):966–969. doi: 10.1128/iai.10.4.966-969.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehrz R. C., Marker S. C., Knorr S. O., Kalis J. M., Balfour H. H., Jr Specific cell-mediated immune defect in active cytomegalovirus infection of young children and their mothers. Lancet. 1977 Oct 22;2(8043):844–847. doi: 10.1016/s0140-6736(77)90782-6. [DOI] [PubMed] [Google Scholar]
- Henson D., Smith R. D., Gehrke J., Neapolitan C. Effect of cortisone on nonfatal mouse cytomegalovirus infection. Am J Pathol. 1967 Dec;51(6):1001–1011. [PMC free article] [PubMed] [Google Scholar]
- Herberman R. B., Ortaldo J. R. Natural killer cells: their roles in defenses against disease. Science. 1981 Oct 2;214(4516):24–30. doi: 10.1126/science.7025208. [DOI] [PubMed] [Google Scholar]
- Jordan M. C. Adverse effects of cytomegalovirus vaccination in mice. J Clin Invest. 1980 Apr;65(4):798–803. doi: 10.1172/JCI109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan M. C., Shanley J. D., Stevens J. G. Immunosuppression reactivates and disseminates latent murine cytomegalovirus. J Gen Virol. 1977 Nov;37(2):419–423. doi: 10.1099/0022-1317-37-2-419. [DOI] [PubMed] [Google Scholar]
- Kelsey D. K., Overall J. C., Jr, Glasgow L. A. Correlation of the suppression of mitogen responsiveness and the mixed lymphocyte reaction with the proliferative response to viral antigen of splenic lymphocytes from cytomegalovirus-infected mice. J Immunol. 1978 Aug;121(2):464–470. [PubMed] [Google Scholar]
- Kern E. R., Olsen G. A., Overall J. C., Jr, Glasgow L. A. Treatment of a murine cytomegalovirus infection with exogenous interferon, polyinosinic-polycytidylic acid, and polyinosinic-polycytidylic acid-poly-L-lysine complex. Antimicrob Agents Chemother. 1978 Feb;13(2):344–346. doi: 10.1128/aac.13.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A., Bennett M. Murine cytomegalovirus stimulates natural killer cell function but kills genetically resistant mice treated with radioactive strontium. Infect Immun. 1981 Dec;34(3):970–979. doi: 10.1128/iai.34.3.970-979.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattsson D. M., Howard R. J., Balfour H. H., Jr Immediate loss of cell-mediated immunity to murine cytomegalovirus upon treatment with immunosuppressive agents. Infect Immun. 1980 Dec;30(3):700–708. doi: 10.1128/iai.30.3.700-708.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayo D., Armstrong J. A., Ho M. Activation of latent murine cytomegalovirus infection: cocultivation, cell transfer, and the effect of immunosuppression. J Infect Dis. 1978 Dec;138(6):890–896. doi: 10.1093/infdis/138.6.890. [DOI] [PubMed] [Google Scholar]
- Neiman P. E., Reeves W., Ray G., Flournoy N., Lerner K. G., Sale G. E., Thomas E. D. A prospective analysis interstitial pneumonia and opportunistic viral infection among recipients of allogeneic bone marrow grafts. J Infect Dis. 1977 Dec;136(6):754–767. doi: 10.1093/infdis/136.6.754. [DOI] [PubMed] [Google Scholar]
- Neiman P., Wasserman P. B., Wentworth B. B., Kao G. F., Lerner K. G., Storb R., Buckner C. D., Clift R. A., Fefer A., Fass L. Interstitial pneumonia and cytomegalovirus infection as complications of human marrow transplantation. Transplantation. 1973 May;15(5):478–485. [PubMed] [Google Scholar]
- Notkins A. L., Mergenhagen S. E., Howard R. J. Effect of virus infections on the function of the immune system. Annu Rev Microbiol. 1970;24:525–538. doi: 10.1146/annurev.mi.24.100170.002521. [DOI] [PubMed] [Google Scholar]
- Osborn J. E., Blazkovec A. A., Walker D. L. Immunosuppression during acute murine cytomegalovirus infection. J Immunol. 1968 Apr;100(4):835–844. [PubMed] [Google Scholar]
- Peller P., Goetz O. immunsuppression und Cytomegalievirusinfektion. Tierexperimentelle Untersuchungen mit Cyclophosphamid. Res Exp Med (Berl) 1974;162(4):267–280. doi: 10.1007/BF01851699. [DOI] [PubMed] [Google Scholar]
- Pien F. D., Smith T. F., Anderson C. F., Webel M. L., Taswell H. F. Herpesviruses in renal transplant patients. Transplantation. 1973 Nov;16(5):489–495. doi: 10.1097/00007890-197311000-00014. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Manischewitz J. F., Kirmani N. Involvement of natural killer cells in the pathogenesis of murine cytomegalovirus interstitial pneumonitis and the immune response to infection. J Gen Virol. 1982 Jan;58(Pt 1):173–180. doi: 10.1099/0022-1317-58-1-173. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E., Ennis P. A. Role of cytotoxic T lymphocytes in murine cytomegalovirus infection. J Gen Virol. 1980 Apr;47(2):503–508. doi: 10.1099/0022-1317-47-2-503. [DOI] [PubMed] [Google Scholar]
- Quinnan G. V., Manischewitz J. E. The role of natural killer cells and antibody-dependent cell-mediated cytotoxicity during murine cytomegalovirus infection. J Exp Med. 1979 Dec 1;150(6):1549–1554. doi: 10.1084/jem.150.6.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riccardi C., Barlozzari T., Santoni A., Herberman R. B., Cesarini C. Transfer to cyclophosphamide-treated mice of natural killer (NK) cells and in vivo natural reactivity against tumors. J Immunol. 1981 Apr;126(4):1284–1289. [PubMed] [Google Scholar]
- Schleupner C. J., Olsen A., Glasgow L. A. Activation of reticuloendothelial cells following infection with murine cytomegalovirus. J Infect Dis. 1979 Jun;139(6):641–652. doi: 10.1093/infdis/139.6.641. [DOI] [PubMed] [Google Scholar]
- Selgrade M. J., Osborn J. E. Divergence of mouse brain interferon responses following virulent or avirulent Newcastle disease virus inoculation. Proc Soc Exp Biol Med. 1973 May;143(1):12–18. doi: 10.3181/00379727-143-37243. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Ahmed A., Sell K. W., Gershwin M. E., Steinberg A. D. Effect of murine cytomegalovirus on the in vitro responses of T and B cells to mitogens. J Immunol. 1976 May;116(5):1459–1465. [PubMed] [Google Scholar]
- Selgrade M. K., Mole M. L., Miller F. J., Hatch G. E., Gardner D. E., Hu P. C. Effect of NO2 inhalation and vitamin C deficiency on protein and lipid accumulation in the lung. Environ Res. 1981 Dec;26(2):422–437. doi: 10.1016/0013-9351(81)90218-8. [DOI] [PubMed] [Google Scholar]
- Selgrade M. K., Nedrud J. G., Collier A. M., Gardner D. E. Effects of cell source, mouse strain, and immunosuppressive treatment on production of virulent and attenuated murine cytomegalovirus. Infect Immun. 1981 Sep;33(3):840–847. doi: 10.1128/iai.33.3.840-847.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selgrade M. K., Osborn J. E. Role of macrophages in resistance to murine cytomegalovirus. Infect Immun. 1974 Dec;10(6):1383–1390. doi: 10.1128/iai.10.6.1383-1390.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos J. G. Immune suppression as related to toxicology. CRC Crit Rev Toxicol. 1977 May;5(1):67–101. doi: 10.3109/10408447709101342. [DOI] [PubMed] [Google Scholar]


