Abstract
Survival in type 1 diabetes has improved, but the impact on life expectancy in the U.S. type 1 diabetes population is not well established. Our objective was to estimate the life expectancy of the Pittsburgh Epidemiology of Diabetes Complications (EDC) study cohort and quantify improvements by comparing two subcohorts based on year of diabetes diagnosis (1950–1964 [n = 390] vs. 1965–1980 [n = 543]). The EDC study is a prospective cohort study of 933 participants with childhood-onset (aged <17 years) type 1 diabetes diagnosed at Children’s Hospital of Pittsburgh from 1950 to 1980. Mortality ascertainment was censored 31 December 2009. Abridged cohort life tables were constructed to calculate life expectancy. Death occurred in 237 (60.8%) of the 1950–1964 subcohort compared with 88 (16.2%) of the 1965–1980 subcohort. The life expectancy at birth for those diagnosed 1965–1980 was ∼15 years greater than participants diagnosed 1950–1964 (68.8 [95% CI 64.7–72.8] vs. 53.4 [50.8–56.0] years, respectively) (P < 0.0001); this difference persisted regardless of sex or pubertal status at diagnosis. This improvement in life expectancy emphasizes the need for insurance companies to update analysis of the life expectancy of those with childhood-onset type 1 diabetes because weighting of insurance premiums is based on outdated estimates.
Several worldwide studies have shown that survival in type 1 diabetes has improved over time (1–9). However, formal assessments of life expectancy of people with type 1 diabetes are relatively rare, and the most recent we found was published in 2001, where Brown et al. (10) reported a life expectancy at birth of 59.7 years in a subset of the Canterbury Diabetes Registry (New Zealand) cohort diagnosed with diabetes when aged younger than 30 years and that began insulin therapy within 12 months of diagnosis. In 1999, Borch-Johnsen (3) reported an increase in life expectancy of 15 years over a 50-year period up to 1982 in a Danish type 1 diabetes cohort. The life expectancy of individuals with type 1 diabetes in the U.S. seems to have been last formally assessed in 1975 by Goodkin (11), who reported that life expectancy in type 1 diabetes (diagnosis age <15 years) was reduced 27 years compared with individuals without diabetes in a life insurance cohort. Using National Health Interview Survey data from 1984 to 2000, however, Narayan et al. (12) estimated that U.S. children diagnosed with diabetes at age 10 years lose an average of ∼19 life-years. Similarly, the estimated life expectancy for people with diabetes was 13 years less than people without diabetes in Ontario, Canada; however, this estimate included type 1 and type 2 diabetes (13).
The Pittsburgh Epidemiology of Diabetes Complications (EDC) study cohort provides a unique opportunity to examine mortality and life-expectancy changes over time in a U.S. cohort with long-term (>30 years) follow-up, because the participants were all diagnosed with childhood-onset type 1 diabetes between 1950 and 1980. To determine if, and to what degree, life expectancy has improved, this article compares two subcohorts based on year of type 1 diabetes diagnosis (1950–1964 vs. 1965–1980). We further assess the representativeness of the EDC cohort by comparing the 1965–1980 subcohort with the population-based Allegheny County Type 1 Diabetes Registry (ACR) of childhood-onset type 1 diabetes.
RESEARCH DESIGN AND METHODS
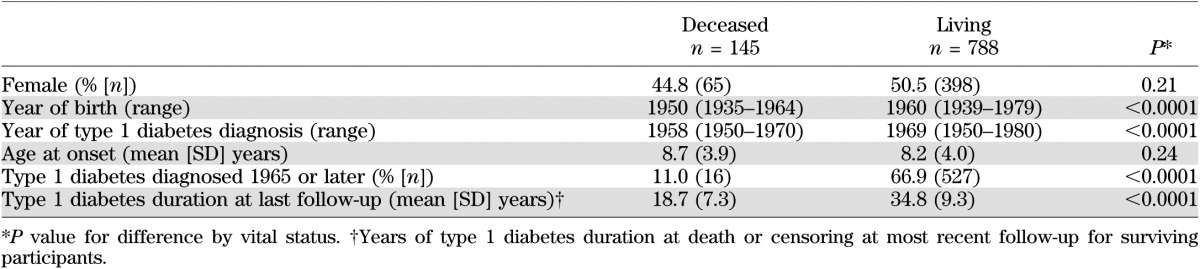
The Pittsburgh EDC study is a prospective cohort study of childhood-onset (age <17 years) type 1 diabetes. All participants were diagnosed or seen within 1 year of diagnosis at Children’s Hospital of Pittsburgh between 1950 and 1980. Potential participants were identified using hospital records and were considered eligible for the study if the record noted a clinical diagnosis of type 1 diabetes. The cohort has been described in detail elsewhere (6). Briefly, 933 individuals were studied, with 658 participating in the EDC study baseline examination between 1986 and 1988 and 130 completing questionnaires only. The remaining 145 participants died before the baseline examination in 1986. A comparison of these 145 individuals and those who survived and participated in the study baseline assessment is provided in Table 1. Mortality status ascertainment was censored at 31 December 2009. As of that date, vital status was known for 878 individuals (>94%). The 55 individuals with unknown status were censored at the last date each was known to be living. Death certificates and hospital, autopsy, and coroner reports were obtained, as appropriate, to document mortality for all participants who died during the follow-up period, including the 145 who died before the EDC study baseline examination, and were reviewed by a physician mortality classification committee. The correlation between age and duration of type 1 diabetes at time of study baseline was assessed using Pearson correlation. The EDC study protocol was approved by the University of Pittsburgh institutional review board. Informed consent was obtained in writing from the participants.
TABLE 1.
Characteristics of the Pittsburgh EDC cohort by vital status at the 1986–1988 assessment (study baseline)
To explore changes in survival, before analyses, the participants were divided into two groups by year of type 1 diabetes diagnosis: 1950–1964 and 1965–1980. This method of division was chosen because it divides the period into two equal halves, and data would become sparse if smaller time periods, such as by year, were used. The difference in observed survival between the two subcohorts was visually assessed using Kaplan-Meier curves and the log-rank statistic. Abridged life tables were constructed using the cohort approach, where individuals in a group, in this case, individuals diagnosed with type 1 diabetes during two specific periods of time, are followed up through their lifetime to describe the mortality experience of the group. Life-table intervals were defined as the age groups 0–1 year, 1–5 years, and by 5-year intervals thereafter. The information used to calculate the life-table statistics includes the total population alive at the beginning of each interval, the number of deaths occurring in each interval, and the number of persons censored within the interval. These values are then used to calculate the probability of death and survival by the end of each interval, conditional on being alive at the beginning of the interval. By definition, this cohort has survived to the age of type 1 diabetes diagnosis and, therefore, had no prior death. Therefore, a key assumption of this analysis is that the life tables and resulting life expectancy estimates are conditional on living to childhood-onset type 1 diabetes diagnosis.
Because the EDC study has a large proportion of individuals who are currently living, the true maximum life span of the cohort has not been observed. Therefore, before the life tables were constructed, the terminal age was estimated by extrapolation of Weibull accelerated-failure time curves based on observed mortality patterns to the age at which the probability of survival in this study cohort approximates zero. The Weibull distribution was chosen to estimate the terminal age because it is a flexible distribution used to model survival times and life-span data. The terminal age was estimated to be ∼85 years old for the total cohort. It is necessary to use the same terminal age for both subcohorts because setting this age at different values would lead to an overestimated difference in life expectancy.
Another consequence of having surviving study participants is that the entire survival curve has not been observed, and thus, the survival function for the age intervals with censored observations must be estimated. Therefore, the computation of these life tables was based on the methodology described by Chiang (14), using the maximum likelihood exponential adjustment of the probability of death for censored data (15). When this method is used, individuals are censored at the age they were last known to be living, and the event rates for incomplete segments (i.e., the age intervals with censored observations) are assumed to have an underlying exponential distribution, using Chiang’s (14) maximum likelihood formula and including the information from the observed deaths within the interval (15).
Conditional life expectancy for each age interval (i.e., the average number of years of life remaining in participants who attained the age at the beginning of the interval) was calculated and compared using a two-sided paired Ztest across the diagnosis subcohorts. This report focuses on conditional life expectancy at birth because this is the most frequently cited statistic derived from life-table analysis due to its intuitive interpretation of mean age in years at death. In addition, life expectancy at various ages is presented in the tables. Comparisons of life expectancy were made across the two diagnosis subcohorts by sex and by puberty status at diagnosis because pubertal onset of type 1 diabetes has been shown to be associated with an increased risk of death compared with prepubertal onset (16). Pubertal onset of type 1 diabetes was defined as an age of diagnosis of ≥11 years for female and ≥12 years for male participants. A significance level of α = 0.05 was used for all statistical tests. All life table and life expectancy calculations were performed using Survival 10.0 software (17).
For validation, data from the population-based ACR were used. The ACR (n = 1,075), which has been described in detail (18), includes all individuals diagnosed with childhood-onset (aged <18 years) type 1 diabetes in Allegheny County (Pittsburgh, PA) between 1965 and 1979 and prescribed insulin at diagnosis. Individuals were identified via hospital record review and validated by contacting pediatricians throughout the county (ascertainment >95%) (19). Only individuals diagnosed at age <17 years were included in this analysis to match the inclusion criteria of the EDC study. Children who developed diabetes from a secondary cause (i.e., cystic fibrosis, Down syndrome, or steroid-induced diabetes) were excluded. Vital status has been determined as of 1 January 2008, when a search of the National Death Index was conducted, and total (8) and cause-specific mortality (20) have been reported. The ACR includes 271 participants who are also participants in the EDC 1965–1980 diagnosis cohort.
For a descriptive comparison of the improvement in life expectancy between the EDC and U.S. general population, U.S. life tables were used (21). To obtain estimates for the general U.S. population during the same intervals (1950–1964 and 1965–1980), the life expectancy at the midpoint year of each period was used (1957 and 1972, respectively). The life tables for Caucasians were used for comparability because the EDC cohort is 98% Caucasian. In addition, life expectancy at age 8 was used because this was approximately equivalent to the median year of type 1 diabetes diagnosis in the EDC cohort and the life-expectancy estimates are conditional on surviving to the age of diagnosis.
RESULTS
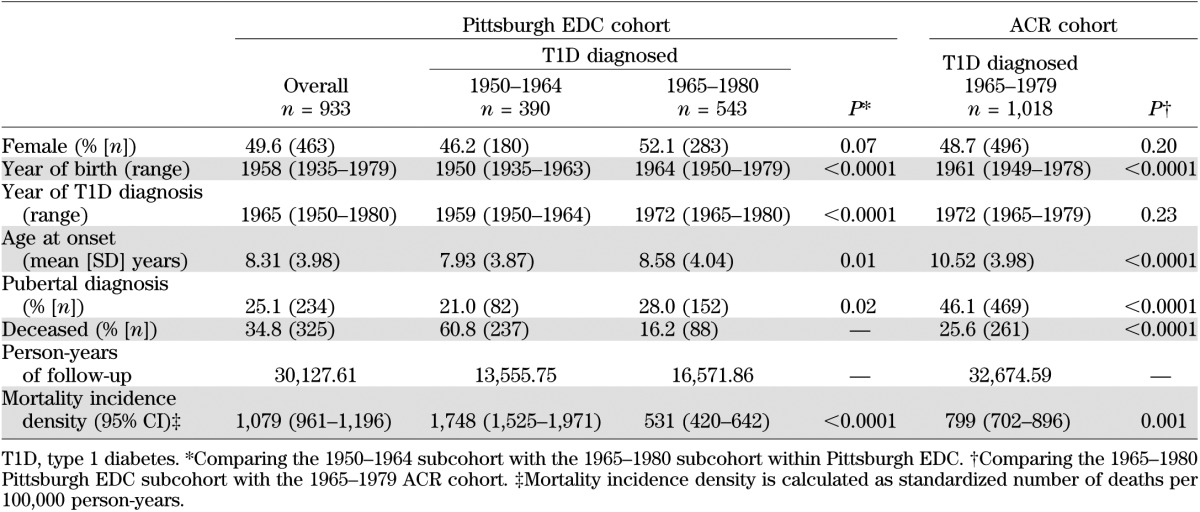
The characteristics of both EDC cohorts (overall and diagnosis years 1950–1964 and 1965–1980) and ACR (1965–1979) are presented in Table 2. The proportion of participants in the EDC who were female was slightly lower in the 1950–1964 subcohort, with 46.2% being female, compared with 52.1% in the 1965–1980 subcohort (P = 0.07). The mean age at onset was significantly younger in the 1950–1964 subcohort compared with the 1965–80 subcohort (7.9 vs. 8.6 years, respectively, P = 0.01). In the 1950–1964 subcohort, the distribution of the age at diagnosis was 26.4% at <5 years, 39.0% at 5–9 years, 32.3% at 10–14 years, and 2.3% at ≥15 years old. The distribution of age at diagnosis in the 1965–1980 subcohort was 21.2% at <5 years, 36.7% at 5–9 years, 38.1% at 10–14 years, and 4.1% at ≥15 years old. Likewise, the proportion of participants with pubertal onset of type 1 diabetes was lower in the 1950–1964 compared with the 1965–1980 subcohort (21 vs. 28%, respectively, P = 0.02). The overall EDC cohort was followed up for a total of 30,127.6 person-years, with 13,555.7 from the 1950–1964 subcohort and 16,571.9 person-years from the 1965–1980 subcohort. The mortality rate was three times greater in the 1950–1964 subcohort compared with the 1965–1980 subcohort (1,748 [95% CI 1,525–1,971] vs. 531 [420–642] per 100,000 person-years, respectively; P < 0.0001). The ACR showed a higher mean age at onset and death than the later EDC cohort.
TABLE 2.
Characteristics of overall cohort and diagnosis year subcohorts for the Pittsburgh EDC and ACR cohorts
As shown in the Kaplan-Meier curves in Fig. 1, crude survival was greater in the more recent (1965–1980) subcohort (log-rank test P < 0.0001) than in the earlier cohort. However, the later EDC and ACR cohorts had similar survival (log-rank test P = 0.10). Table 3 reports the observed probability of death and the life expectancy at various ages for the two EDC diagnosis subcohorts. The life expectancy at birth for the participants diagnosed with type 1 diabetes between 1950 and 1964 is 53.4 years compared with 68.8 years for participants diagnosed between 1965 and 1980, an increase of >15 years (P < 0.0001). A similar increase in life expectancy between the two diagnosis subcohorts persisted, regardless of sex, age at diagnosis, and pubertal status at diagnosis (Table 4). Table 3 shows the observed probability of death and life expectancy at various ages for the 1965–1980 EDC cohort and the ACR, for individuals diagnosed at age <17 years, as in EDC, during the same period of time. The life expectancy at birth in the population-based ACR cohort is estimated to be 67.2 years, which is 1.6 years less than the estimated life expectancy of the comparable EDC cohort; this difference did not reach statistical significance (P = 0.49).
FIG. 1.
Observed Kaplan-Meier survival function comparing EDC study type 1 diabetes diagnosis year subcohorts (1950–1964 vs. 1965–1980) and the ACR cohort. The small vertical lines represent censoring times of surviving individuals. EDC 1950–1964 vs. 1965–1980 log-rank P < 0.0001; EDC 1965–1980 vs. ACR log-rank P = 0.10. Remaining number at risk at each age: EDC 1950–1964: birth, 390; 20 years, 370; 40 years, 239; 60 years, 26; EDC 1965–1980: birth, 543; 20 years, 537; 40 years, 272; 60 years, 0; ACR: birth, 1,018; 20 years, 1,002; 40 years, 704; 60 years, 0.
TABLE 3.
Probability of death and life expectancy by age in the Pittsburgh EDC study by year of type 1 diabetes diagnosis subcohort (1950–1964 and 1965–1980) and the ACR cohort (1965–1979)
TABLE 4.
Life expectancy at birth by year of type 1 diabetes diagnosis subcohort stratified by sex, age at diabetes diagnosis, and pubertal status at diabetes diagnosis
The estimated life expectancy for the comparable cohort of the general U.S. population in 1957 and 1972 (the midpoint years for the two EDC subcohorts) was ∼71.5 and 72.4 years, respectively, an increase of <1 year.
DISCUSSION
This report describes changes in the life expectancy of the Pittsburgh EDC study by year of type 1 diabetes diagnosis (1950–1964 vs. 1965–1980). Crude survival was significantly higher in the more recent (1965–1980) diagnosis subcohort, as previously reported (6), and likewise, life expectancy at birth is now shown to have significantly increased by ∼15 years compared with the 1950–1964 subcohort. It should be noted that in the EDC study cohort, age is highly correlated with type 1 diabetes duration (r = 0.85); thus, the observed mortality patterns would be similar if diabetes duration were used as the time scale. The most recent report of life expectancy in type 1 diabetes estimated the life expectancy at birth was 59.7 years in the Canterbury Diabetes Registry’s 1984 prevalence database (10), which approximates the 61-year midpoint life expectancy of the two EDC subcohorts. The improvement in the EDC 1965–1980 subcohort was apparent in both sexes and persisted regardless of pubertal status at type 1 diabetes diagnosis. In a Romanian type 1 diabetes cohort, the mean age at death increased by ∼7 years between two similar intervals of diabetes diagnosis (1946–1965 vs. 1966–1985), and this improvement also did not differ by sex (22).
Although absolute mortality, expressed as mortality frequency and incidence density, was higher, the estimated life expectancy in the 1965–1980 EDC subcohort was similar to that of the population-based ACR cohort diagnosed in 1965–1979. The higher mortality rate, but similar life expectancy, reflects the somewhat older age of the ACR cohort. Accounting for the difference in age distribution, survival was similar between the two groups (Fig. 1). These results suggest that the hospital-based EDC study cohort is representative of the local type 1 diabetes population in mortality in addition to sharing similar epidemiologic characteristics, as previously described (23). We thus believe the dramatic improvement in life expectancy is likely true for the general population with childhood-onset type 1 diabetes and not due to a preferential participation of healthier individuals in the EDC in later years. Furthermore, the improvement in life expectancy is far greater than that seen in the general population.
There are several potential explanations for the substantial increase in life expectancy between the two subcohorts. First, no early childhood deaths were observed in the more recent subcohort (1965–1980), with the first death occurring at age 12 years, compared with the first death occurring at age 6 months in the earlier cohort (1950–1964). The lack of early deaths in the 1965–1980 subcohort is likely related to the earlier recognition and improved treatment of type 1 diabetes in young children after the 1950s. Indeed, it has been reported that a large proportion of childhood deaths in type 1 diabetes were attributed to diabetic ketoacidosis or hypoglycemia (24,25).
A second potential explanation for the increase in life expectancy is that there was a general decline in the acute and long-term complications of type 1 diabetes in individuals diagnosed after 1965, because a greater proportion of their diabetes duration occurred during an era of better glucose monitoring and insulin administration (6,26).
The greater life expectancy may also be due specifically to the reduction of renal disease resulting from improved diabetes care. Several reports have demonstrated a decline in renal disease in type 1 diabetes (6,27,28). In addition, an increase in ACE inhibitor use within the Pittsburgh EDC cohort was associated with a decrease in death (29). In fact, the Finnish Diabetic Nephropathy (FinnDiane) study (30) and the Pittsburgh EDC study (31) have both recently shown that in the absence of renal disease and microalbuminuria, the long-term mortality risk in type 1 diabetes is not increased compared with the general population.
An increase in statin use is another possible contributor to increasing life expectancy in type 1 diabetes. Although historically, low rates of statin use in the EDC cohort have prevented detailed analysis of the effect of statins on mortality rates, the 2008 Cholesterol Treatment Trialists’ Collaborators’ meta-analysis reported a 9% reduction in mortality for each millimole per liter decrease in LDL cholesterol in people with diabetes (type 1 and type 2 combined) (32).
This report has several noted strengths. The Pittsburgh EDC study includes participants who were diagnosed during a 30-year period (1950–1980), allowing the study cohort to be divided into two subcohorts that likely experienced different natural histories of type 1 diabetes due to improvements in treatment. The EDC study has also obtained death certificates for all individuals, including those who were eligible to participate but died before the baseline examination, thus minimizing potential survival bias. Similarly, 130 individuals who were eligible, but declined examination, have provided survey and mortality follow-up. In addition, we were able to validate the life-expectancy estimates for the 1965–1980 subcohort by using the population-based ACR data collected during the same time period, which also had a very high 95% vital status ascertainment. It could be argued that it is not appropriate to include the 271 participants who are common to both the EDC 1965–1980 diagnosis subcohort and the ACR in this validation. However, if the rates of ACR are the gold standard, and they are as a true population-based cohort, then the similarity with EDC is an important validation regardless of the amount of overlap. This validation is not of methodology, but rather of whether the estimated rates seen in the hospital-based EDC are representative of the local type 1 diabetes population. Because the overlapping segment of EDC is itself part of the population, a bias would be created if these individuals were excluded from the ACR for this comparison.
A key limitation to these analyses is that complete lifetime follow-up is not possible for currently surviving participants because the EDC study is ongoing. Although we have attempted to correct for this incomplete follow-up by using Chiang’s maximum likelihood adjustment in our calculations of life expectancies, we note that these results are intended as a description of the particular cohort studied and may not be applicable to type 1 diabetes in general, particularly those diagnosed after adolescence. In addition, these findings may not be fully reflective of the life expectancy of a child diagnosed in 2012. Although a period or “current” life-table approach theoretically would address this issue, this is debatable because the “current” age-specific mortality rates that would be used would reflect, at older ages, a survival cohort of those diagnosed before improved care could contribute much to their prognosis.
We thus intend for these estimates to be used as relative comparisons of life expectancy between the two subcohorts being examined and to describe improvements in mortality and life expectancy over time. The EDC is a study of a hospital-based cohort and may not reflect the overall type 1 diabetes population; however, the ACR data presented clearly show that life expectancy is similar in the two cohorts, so these data likely present a reasonable estimate of the life expectancy of childhood-onset type 1 diabetes in this area.
In conclusion, life expectancy improved from the 1950–1964 to 1965–1980 type 1 diabetes diagnosis subcohorts of the Pittsburgh EDC study, a hospital-based cohort of childhood-onset type 1 diabetes. A similar improvement between diagnosis subcohorts was observed regardless of sex or pubertal status at diagnosis. Further investigation shows this life expectancy is similar to community-based life expectancy, suggesting childhood-onset type 1 diabetes diagnosed in the late 1960s and 1970s is associated with only a 4- to 6-year loss-of-life expectancy compared with >17 years for those diagnosed in the 1950s and early 1960s. These results support the need for insurance companies to update their analysis of the life expectancy of those with childhood-onset type 1 diabetes, because the current weighting of insurance premiums is based on earlier, outdated estimates.
ACKNOWLEDGMENTS
The Pittsburgh EDC study was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (R01-DK034818), which had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of data; or the preparation, review, or approval of the manuscript.
No potential conflicts of interest relevant to this article were reported.
R.G.M. contributed to the study concept and design, to acquisition, analysis, and interpretation of the data, to drafting the manuscript and critical review of the manuscript for important intellectual content, to statistical analysis, and to administrative, technical, or material support. A.M.S. contributed to the study concept and design, to acquisition, analysis, and interpretation of the data, to critical review of the manuscript for important intellectual content, and to statistical analysis. R.K.S. contributed to the study concept and design, analysis and interpretation of the data, critical review of the manuscript for important intellectual content, and statistical analysis. T.J.S. contributed to the study concept and design, analysis and interpretation of the data, critical review of the manuscript for important intellectual content, statistical analysis, and to administrative, technical, or material support. T.J.O. supervised the study and contributed to study concept and design, analysis and interpretation of the data, critical review of the manuscript for important intellectual content, obtaining funding, and to administrative, technical, or material support. T.J.O. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
These results were presented at the 71st Scientific Sessions of the American Diabetes Association, San Diego, California, 24–28 June 2011.
The authors acknowledge the long-term help of the EDC participants.
REFERENCES
- 1.Borch-Johnsen K, Kreiner S, Deckert T. Mortality of type 1 (insulin-dependent) diabetes mellitus in Denmark: a study of relative mortality in 2930 Danish type 1 diabetic patients diagnosed from 1933 to 1972. Diabetologia 1986;29:767–772 [DOI] [PubMed] [Google Scholar]
- 2.Sartor G, Nyström L, Dahlquist G. The Swedish Childhood Diabetes Study: a seven-fold decrease in short-term mortality? Diabet Med 1991;8:18–21 [DOI] [PubMed] [Google Scholar]
- 3.Borch-Johnsen K. Improving prognosis of type 1 diabetes. Mortality, accidents, and impact on insurance. Diabetes Care 1999;22(Suppl. 2):B1–B3 [PubMed] [Google Scholar]
- 4.Podar T, Solntsev A, Reunanen A, et al. Mortality in patients with childhood-onset type 1 diabetes in Finland, Estonia, and Lithuania: follow-up of nationwide cohorts. Diabetes Care 2000;23:290–294 [DOI] [PubMed] [Google Scholar]
- 5.Asao K, Sarti C, Forsen T, et al. Diabetes Epidemiology Research International Mortality Study Group Long-term mortality in nationwide cohorts of childhood-onset type 1 diabetes in Japan and Finland. Diabetes Care 2003;26:2037–2042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pambianco G, Costacou T, Ellis D, Becker DJ, Klein R, Orchard TJ. The 30-year natural history of type 1 diabetes complications: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes 2006;55:1463–1469 [DOI] [PubMed] [Google Scholar]
- 7.Skrivarhaug T, Bangstad HJ, Stene LC, Sandvik L, Hanssen KF, Joner G. Long-term mortality in a nationwide cohort of childhood-onset type 1 diabetic patients in Norway. Diabetologia 2006;49:298–305 [DOI] [PubMed] [Google Scholar]
- 8.Secrest AM, Becker DJ, Kelsey SF, LaPorte RE, Orchard TJ. All-cause mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes: the Allegheny County type 1 diabetes registry. Diabetes Care 2010;33:2573–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNally PG, Raymond NT, Burden ML, et al. Trends in mortality of childhood-onset insulin-dependent diabetes mellitus in Leicestershire: 1940-1991. Diabet Med 1995;12:961–966 [DOI] [PubMed] [Google Scholar]
- 10.Brown LJ, Scott RS, Moir CL. All-cause mortality in the Canterbury (New Zealand) insulin-treated Diabetic Registry population. Diabetes Care 2001;24:56–63 [DOI] [PubMed] [Google Scholar]
- 11.Goodkin G. Mortality factors in diabetes. A 20 year mortality study. J Occup Med 1975;17:716–721 [PubMed] [Google Scholar]
- 12.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA 2003;290:1884–1890 [DOI] [PubMed] [Google Scholar]
- 13.Manuel DG, Schultz SE. Diabetes health status and risk factors. In Diabetes in Ontario. An Institute for Clinical Evaluative Sciences Practice Atlas. Hux J, Booth G, Slaughter PM, Laupacis A, Eds. Toronto, Ontario, Canada, Canadian Diabetes Association, 2003, p. 77–94 [Google Scholar]
- 14.Chiang CL. The life table and its applications. Malabar, FL, Krieger Publishing Co, 1984 [Google Scholar]
- 15.Smith DP. Formal demography. New York, Plenum Press, 1992 [Google Scholar]
- 16.Kostraba JN, Dorman JS, LaPorte RE, et al. The investigation of age at onset as a risk factor for mortality in persons with insulin-dependent diabetes mellitus using Cox proportional hazards models. Am J Epidemiol 1991;133:67–72 [DOI] [PubMed] [Google Scholar]
- 17.Survival. A program for life tables and related measures [computer program]. Version 10.0. Houston, University of Texas School of Public Health, 2008.
- 18.Diabetes Epidemiology Research International Mortality Study Group Major cross-country differences in risk of dying for people with IDDM. Diabetes Care 1991;14:49–54 [DOI] [PubMed] [Google Scholar]
- 19.LaPorte RE, Fishbein HA, Drash AL, et al. The Pittsburgh insulin-dependent diabetes mellitus (IDDM) registry. The incidence of insulin-dependent diabetes mellitus in Allegheny County, Pennsylvania (1965-1976). Diabetes 1981;30:279–284 [DOI] [PubMed] [Google Scholar]
- 20.Secrest AM, Becker DJ, Kelsey SF, Laporte RE, Orchard TJ. Cause-specific mortality trends in a large population-based cohort with long-standing childhood-onset type 1 diabetes. Diabetes 2010;59:3216–3222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arias E. United States Life Tables, 2006. National Vital Statistics Reports, Vol 58, No 21. Hyattsville, MD, National Center for Health Statistics, 2010 [PubMed] [Google Scholar]
- 22.Ioacara S, Lichiardopol R, Ionescu-Tirgoviste C, et al. Improvements in life expectancy in type 1 diabetes patients in the last six decades. Diabetes Res Clin Pract 2009;86:146–151 [DOI] [PubMed] [Google Scholar]
- 23.Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes 1982;31:136–144 [DOI] [PubMed] [Google Scholar]
- 24.Scibilia J, Finegold D, Dorman J, Becker D, Drash A. Why do children with diabetes die? Acta Endocrinol Suppl (Copenh) 1986;279:326–333 [DOI] [PubMed] [Google Scholar]
- 25.Diabetes Epidemiology Research International Mortality Study Group International evaluation of cause-specific mortality and IDDM. Diabetes Care 1991;14:55–60 [DOI] [PubMed] [Google Scholar]
- 26.Nathan DM, Zinman B, Cleary PA, et al. Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Research Group Modern-day clinical course of type 1 diabetes mellitus after 30 years’ duration: the diabetes control and complications trial/epidemiology of diabetes interventions and complications and Pittsburgh epidemiology of diabetes complications experience (1983-2005). Arch Intern Med 2009;169:1307–1316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura R, Dorman JS, Bosnyak Z, Tajima N, Becker DJ, Orchard TJ, Diabetes Epidemiology Research International Mortality Study. Allegheny County Registry Incidence of ESRD and survival after renal replacement therapy in patients with type 1 diabetes: a report from the Allegheny County Registry. Am J Kidney Dis 2003;42:117–124 [DOI] [PubMed] [Google Scholar]
- 28.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 [DOI] [PubMed] [Google Scholar]
- 29.Sobolewski BA, Zgibor JC, Orchard TJ. ACE inhibitors and calcium channel blockers: patterns of use and associations with mortality in type 1 diabetes. Diabetes Res Clin Pract 2004;65:37–43 [DOI] [PubMed] [Google Scholar]
- 30.Groop PH, Thomas MC, Moran JL, et al. FinnDiane Study Group The presence and severity of chronic kidney disease predicts all-cause mortality in type 1 diabetes. Diabetes 2009;58:1651–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orchard TJ, Secrest AM, Miller RG, Costacou T. In the absence of renal disease, 20 year mortality risk in type 1 diabetes is comparable to that of the general population: a report from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetologia 2010;53:2312–2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kearney PM, Blackwell L, Collins R, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet 2008;371:117–125 [DOI] [PubMed] [Google Scholar]