Abstract
The impairment in diabetic wound healing represents a significant clinical problem. Chronic inflammation is thought to play a central role in the pathogenesis of this impairment. We have previously shown that treatment of diabetic murine wounds with mesenchymal stem cells (MSCs) can improve healing, but the mechanisms are not completely defined. MicroRNA-146a (miR-146a) has been implicated in regulation of the immune and inflammatory responses. We hypothesized that abnormal miRNA-146a expression may contribute to the chronic inflammation. To test this hypothesis, we examined the expression of miRNA-146a and its target genes in diabetic and nondiabetic mice at baseline and after injury. MiR-146a expression was significantly downregulated in diabetic mouse wounds. Decreased miR-146a levels also closely correlated with increased gene expression of its proinflammatory target genes. Furthermore, the correction of the diabetic wound-healing impairment with MSC treatment was associated with a significant increase in the miR-146a expression level and decreased gene expression of its proinflammatory target genes. These results provide the first evidence that decreased expression of miR-146a in diabetic wounds in response to injury may, in part, be responsible for the abnormal inflammatory response seen in diabetic wounds and may contribute to wound-healing impairment.
Chronic wounds are a common and severe complication of diabetes and are associated with significant morbidity and mortality (1). The impaired healing of diabetic wounds has been characterized by decreased production of chemokines (2), decreased angiogenesis (3), and an abnormal inflammatory response (4). Increasing evidence suggests that the persistent upregulation of inflammatory gene expression may contribute to the pathogenesis of the chronic diabetic wound through activation of inflammatory pathways (5–7). However, the molecular pathophysiology that underlies the abnormal inflammatory response in diabetic wound-healing impairment remains poorly defined.
Mesenchymal stem cells (MSCs) are multipotent cells derived from the stroma of bone marrow and other tissues and can give rise to multiple tissues in vivo and in vitro (8). MSCs have been demonstrated as active participators in tissue-repair by promoting production of multiple growth factors, cytokines, collagens, and matrix metalloproteinases (9–11). The ability of MSCs to regenerate tissues (12,13) and differentiate into multiple cell lineages, including keratinocytes, endothelial cells, and pericytes (14,15), highlights the potential of MSCs in wound-healing applications. MSCs have been shown to correct diabetic wound healing in mice by accelerating epithelialization and increasing granulation tissue and angiogenesis (16,17); however, the exact molecular mechanisms of this correction have not been well characterized.
MicroRNAs (miRNAs) are 19 to 22 nucleotides long and negatively regulate gene expression at the post-transcriptional level by binding to the 3′ untranslated region (UTR) of specific mRNAs (18). Several miRNAs have been found in skin tissue and are thought to play a crucial role in a wide range of biologic processes, including the regulation of innate and adaptive immune responses (19–21).
MiR-146a has been described as one of the key regulatory molecules in the inflammatory response. It can be induced by different proinflammatory stimuli, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α) (22,23). MiRNA-146a has also been reported to negatively regulate the innate immune response by targeting and repressing IL-1 receptor-associated kinase 1 (IRAK1) and TNF receptor-associated factor 6 (TRAF6) (23). As two key adapter molecules of the nuclear factor (NF)-κB pathway, IRAK1 and TRAF6 increase NF-κB activity, resulting in increased expression of the genes expressing IL-6 and IL-8 (24), which are key mediators of inflammation.
Dysregulation of miR-146a has been demonstrated in several chronic inflammatory diseases, such as psoriasis (21), rheumatoid arthritis (25), and systemic lupus erythematosus (26). Furthermore, miR-146a modulates innate immunity through regulation of Toll-like receptor signaling and cytokine responses (22,23). Targeted deletion of miR-146a in mice results in inflammatory disorders, demonstrating that miR-146a plays a key role as a molecular brake on inflammation (27). The role of miRNA-146a in the abnormal inflammatory response observed in diabetic wound-healing impairment, or the correction of this impairment, has not been examined.
We hypothesized that decreased miR-146a may, in part, be responsible for the abnormal inflammation observed in diabetic wounds and subsequent impairment in wound healing and that correction of the diabetic wound-healing impairment with MSC treatment is mediated by correction of abnormal miRNA-146a expression. To test these hypotheses, we examined, over time, the expression profile of miRNA-146a and its target genes during the wound-healing response in diabetic (db/db) and nondiabetic (db/+) mice, as well as after treatment with MSCs.
RESEARCH DESIGN AND METHODS
Animal model.
All experiments were approved and performed under the guidelines of the University of Mississippi Medical Center Animal Care and Use Committee. Twelve-week-old female mice homozygous for the Leprdb mutation (db/db) and age-matched female nondiabetic heterozygous littermates (db/+) were used in these experiments (BKS.Cg-Dock7m+/+Leprdb/J, strain No. 000642, Jackson Laboratory). At the time of experiments, db/db mice weighed greater than 45 g, with blood glucose levels in excess of 400 mg/dL, and db/+ heterozygote mice weighed less than 25 g, with blood glucose levels less than 250 mg/dL. Animals were given standard rodent chow and water ad libitum.
Mouse wound-healing model.
Mice were anesthetized with inhaled isoflurane. Each mouse was shaved and depilated before wounding. The dorsal skin was swabbed with alcohol and Betadine (Purdue Pharma, Stamford, CT). Each mouse underwent a single, dorsal, full-thickness wound (including panniculus carnosum) with an 8-mm punch biopsy (Miltex Inc., York, PA). All wounds were dressed with Tegaderm (3M, St. Paul, MN), which was subsequently removed on postoperative day 2. Postoperatively, the mice received a subcutaneous injection of an analgesic, Banamine (Schering-Plough Animal Health Corp., Union, NJ).
A full-thickness skin area, including the wounded area, was harvested on days 1, 3, 7, 14, and 21 after surgery. Dorsal skin of unwounded db/db and db/+ mice was also harvested for day 0 time point. Photographs were obtained with a Nikon camera using a ruler for each image. ImageJ software (National Institutes of Health, Bethesda, MD; http://rsbweb.nih.gov/ij/) was used to calculate the wound area of each mouse every other day. The same observer measured the size of each wound. Wound area was plotted as a function of time.
MSC isolation and treatment.
MSCs were isolated from bone marrow of transgenic adult mice expressing green fluorescent protein (GFP), as previously described (28). MSC identities were confirmed by cell surface markers. Full-thickness 8-mm wounds were created as described above. Diabetic (db/db) or nondiabetic (db/+) wounds were treated with 1 × 106 MSCs or PBS vehicle alone. Wound treatment (MSC or vehicle) was administered immediately after wounding by three intradermal injections spaced in a radial pattern from the wound edge in a total volume of 40 μL (10 μL per injection) using a Hamilton syringe.
Extraction of cytokines from Schirmer strips and enzyme-linked immunosorbent assay (ELISA) analysis.
Immediately after wound and MSC treatment, a strip was placed on the wound under the Tegaderm. The extraction of cytokines from the Schirmer strips was performed according to previously published methods (29). Briefly, 200 μL extraction buffer (0.5 mol/L NaCL with 0.5% Tween 20) was added to individual tubes containing a Schirmer tear strip and incubated for 3 h at room temperature on a rocker. Total protein concentration was quantified using a bicinchoninic acid protein assay (Thermo Scientific, Rockford, IL) that uses a standard curve generated from known concentrations of BSA (Thermo Scientific). All samples were run in duplicate. ELISA was then used to quantify the concentration of macrophage inflammatory protein 2 (MIP-2) protein levels (R&D Systems, Minneapolis, MN). Values were normalized by the respective protein concentration of the samples.
Histology and immunohistochemistry.
Wound tissues were processed as previously described (16,30). Immunohistochemical staining was performed on paraffin sections as described below. Antigen retrieval was performed using 1× Antigen Retrieval Citra (BioGenex HK086-9k, Fremont, CA) in a decloaking chamber using the factory default settings (Biocare Medical, Concard, CA). Slides were rinsed three times in 0.1% PBST (0.1% Triton100 in PBS) and blocked in 20% goat serum in 0.4% PBST for 60 min at room temperature. This was followed by overnight incubation at room temperature with primary antibodies against cluster of differentiation (CD) 45 (rabbit polyclonal ab10558, 1:100; Abcam, Cambridge, MA) in 5% goat serum and 0.1% PBST. The following day, slides were washed three times in 0.1% PBST and incubated with the appropriate anti-rabbit biotinylated secondary antibodies for 1 h at room temperature. Slides were washed three times in 0.1% PBST and mounted in Vectashield (Vector Laboratories, Burlingame, CA). The slides were then incubated with avidin-biotin-peroxidase complex (Vector Laboratory) and developed, as described by the manufacturer.
Real-time quantitative PCR.
Wound samples from days 0, 1, 3, 7, 14, and 21 after surgery were homogenized in TRIzol (Life Technologies, Invitrogen), and total cellular RNA was isolated and purified following the manufacturer’s instructions. For mRNA analysis, mRNA was converted into cDNA using the SuperScript First-Strand Synthesis System (Invitrogen). Real-time quantitative PCR was performed with the CFX96 real-time PCR thermal cycler (Bio-Rad, Hercules, CA) to amplify samples in triplicate. Relative gene product amounts were reported for each gene compared with 18S ribosomal RNA. For miRNA analysis, miR-146a expression was examined with the TaqMan MicroRNA Assay Kit. MultiScribe Reverse Transcriptase was used for RT-PCR, and TaqMan primers for has-miR-146a (assay ID 000468) were used to monitor miRNA expression. U6 (assay ID 001973) was used as an internal miRNA control. Results were reported as means ± SEM. A Student t test was performed using 2-ΔΔCT values of each sample. A value of P < 0.05 was considered significant.
RESULTS
Delayed diabetic wound closure.
Diabetic wounds demonstrated a significant delay in wound closure. At 9 days after wounding, the wound surface area of diabetic wounds was 40.8 ± 8.4 mm2, and was significantly larger compared with nondiabetic wounds (23.7 ± 7.3 mm2, P < 0.04). In addition, the time to wound closure was also significantly delayed in mice with diabetes (19 days) compared with nondiabetic mice (14 days). These results are consistent with our previous reports of the diabetic wound-healing impairment (16,31,32).
Decreased miR-146a expression in diabetic wounds.
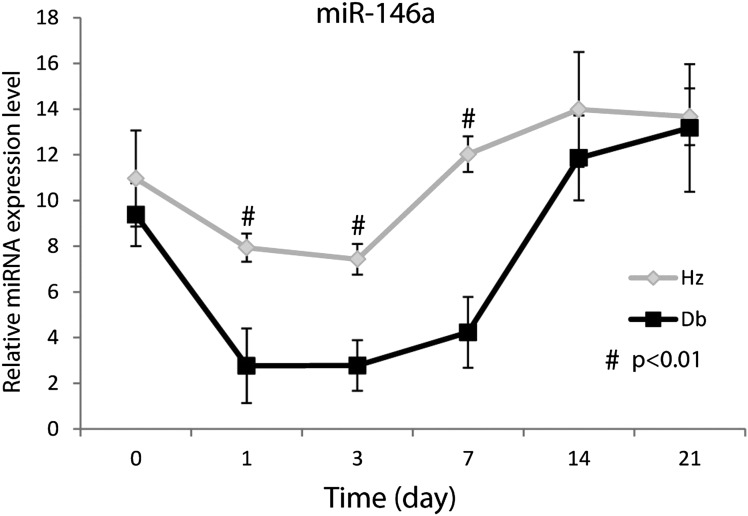
At baseline, diabetic and nondiabetic skin (day 0) demonstrated similar levels of miRNA-146a expression (Fig. 1). In response to injury, miR-146a expression level was significantly reduced in diabetic wounds compared with nondiabetic wounds at 1, 3, and 7 days after wounding. By day 14 after wounding, there was no statistical difference in miRNA-146a expression between diabetic and nondiabetic wounds.
FIG. 1.
Dynamic expression level changes of miR-146a during the wound-healing process. Real-time quantitative RT-PCR analysis of miR-146a levels in db/+ (n = 5) and db/db (n = 5) wounds at 0, 1, 3, 7, 14, and 21 days after dermal injury. MiR-146a gene expression was calculated after normalizing with U6. #P < 0.01 comparing db/db wounds with db/+ wounds.
Increased expression of miRNA-146a target genes in diabetic wounds.
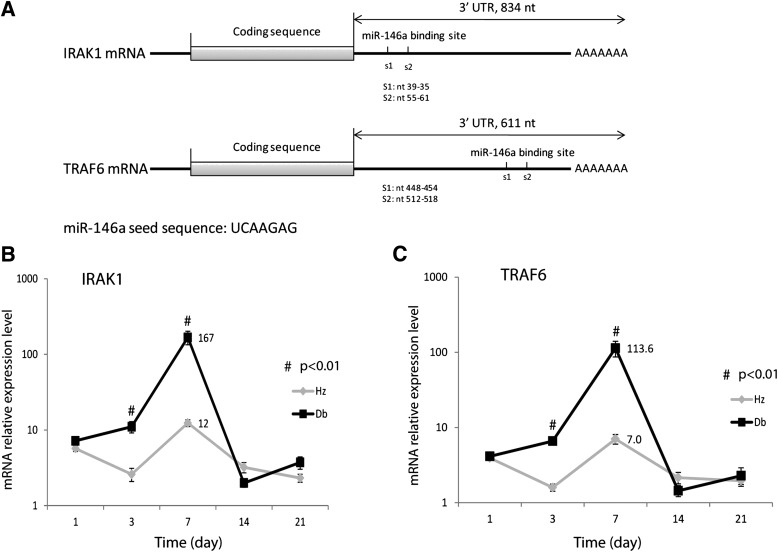
Two predicted miRNA-146a binding sites were found in the gene sequence for IRAK1 (nt 39-35, nt 55-61) and TRAF6 (nt 448-454, nt 512-518) 3′ UTR (Fig. 2A). Real-time PCR analysis of IRAK1 and TRAF6 gene expression demonstrated that diabetic wounds had significantly increased expression of IRAK1 (Fig. 2B) and TRAF6 (Fig. 2C) at 3 and 7 days after wounding compared with nondiabetic wounds. This increased expression correlated with the time course of decreased miRNA-146a expression in diabetic wounds.
FIG. 2.
Dynamic changes of miR-146a direct targets IRAK1 and TRAF6 during the wound-healing process. A: IRAK1 and TRAF6 mRNA structure and predicted targets of miR-146a by the TargetScan Web site (http://www.targetscan.org/). Real-time quantitative RT-PCR analysis of mRNA for IRAK1 (B) and TRAF6 (C) in db/+ (Hz; n = 5) and db/db (n = 5) wounds at 1, 3, 7, 14, and 21 days after dermal injury. #P < 0.01 comparing db/db wounds with db/+ wounds.
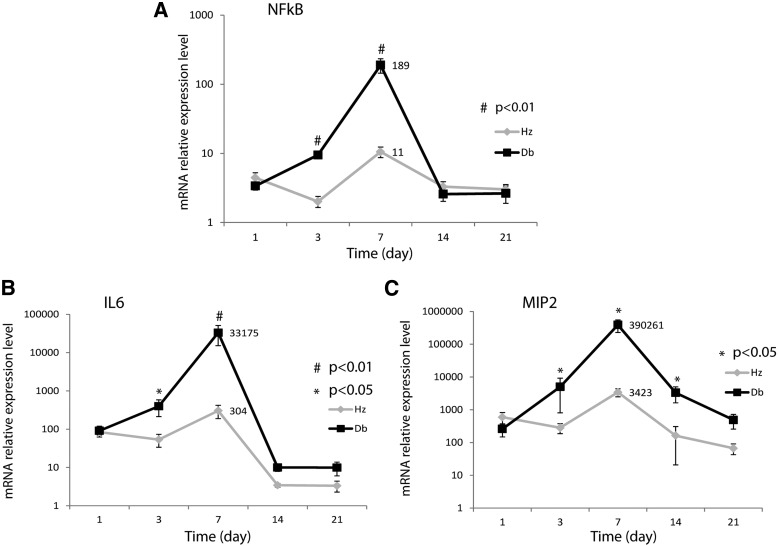
IRAK1 and TRAF6 are two key adapter molecules of the NF-κB pathway and regulate NF-κB activity, so we also examined the time course of NF-κB expression in diabetic and nondiabetic wounds. At 3 and 7 days after wounding, diabetic wounds demonstrated significantly increased NF-κB expression (Fig. 3A) compared with nondiabetic wounds, reaching more than an 18-fold difference at day 7 after wounding.
FIG. 3.
Dynamic changes of proinflammatory factors during the wound-healing process. Real-time quantitative RT-PCR analysis of mRNA for NF-κB (A), IL-6 (B), and MIP-2 (C) in db/+ (Hz; n = 5) and db/db (n = 5) wounds at 1, 3, 7, 14, and 21 days after dermal injury. #P < 0.01, *P < 0.05 comparing db/db wounds with db/+ wounds.
NF-κB has been shown to directly regulate the expression of IL-6 and IL-8 (23,24). In the mouse, MIP-2 is the equivalent of IL-8 (33). We also examined the expression of IL-6 and MIP-2 in diabetic and nondiabetic wounds by real-time PCR. Diabetic wounds demonstrated significantly increased expression of IL-6 (Fig. 3B) and MIP-2 (Fig. 3C) at 3 and 7 days after wounding, with 109- and 114-fold differences for IL-6 and MIP-2, respectively, at 7 days. These results are consistent with the expression patterns of IRAK1, TRAF6, and NF-κB, and inversely related to miRNA-146a expression in diabetic wounds.
MSC treatment increases miR-146a expression, attenuates inflammatory gene expression, and improves diabetic wound healing.
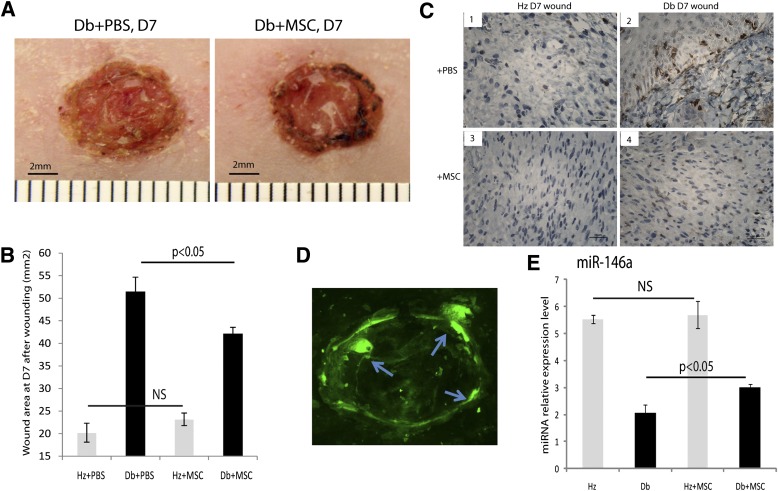
Treatment of diabetic wounds with MSCs resulted in a significant improvement in wound closure (Fig. 4A), consistent with previous descriptions (16,28). At 7 days after wounding, fluorescent stereomicroscopy demonstrated numerous GFP+ MSCs in the wound base and the wound margin (Fig. 4D). Wound size measurement at day 7 using Image J (Fig. 4B) indicated wound size was significantly reduced in MSC-treated diabetic wounds (n = 10) compared with vehicle-treated diabetic wounds (n = 10; 42.1 ± 1.4 vs. 51.5 ± 3.2 mm2, P < 0.03). At day 7, there were numerous CD45+ inflammatory cells in the diabetic wounds (Fig. 4C2) compared with relatively few inflammatory cells in the nondiabetic wounds (Fig. 4C1). Treatment of diabetic wounds with MSCs resulted in a significant reduction in the number of CD45+ inflammatory cells in the diabetic wounds at 7 days (Fig. 4C4), similar to the that seen in nondiabetic wounds (Fig. 4C3).
FIG. 4.
MSC treatment accelerates wound closure and upregulates miR-146a level in diabetic wounds. A: Diabetic wounds at day (D) 7 treated with vehicle (left) and MSCs (right). B: Quantitative analysis of wound closure at day 7 in diabetic wounds treated with vehicle and MSCs (n = 10). C: CD45 immunostaining of sections from nondiabetic (C1, 3) and diabetic wounds (C2, 4) treated with vehicle (top) and MSCs (bottom). D: Fluorescent image demonstrates GFP signal at the wound edge (arrows indicate the injection sites). E: Real-time PCR analysis of miR-146a expression level in db/+ (Hz; n = 5) and db/db (n = 5) wounds at day 7 with MSC or vehicle treatment. *P < 0.05 comparing db/db wounds treated with MSCs with db/db wounds treated with vehicle. (A high-quality digital representation of this figure is available in the online issue.)
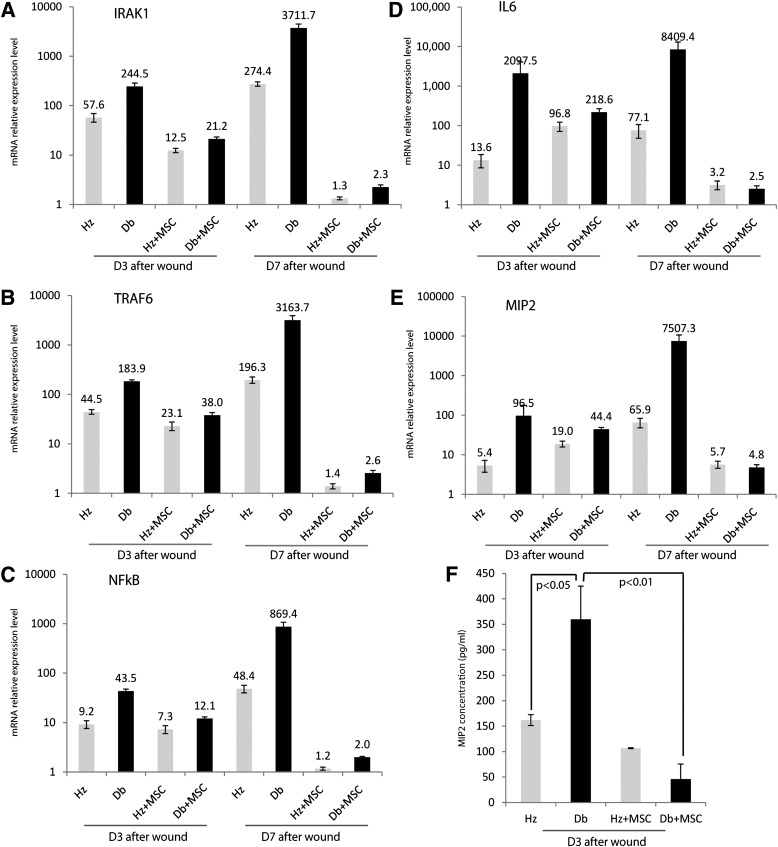
The effect of MSC-mediated correction of the diabetic wound-healing impairment on miRNA-146a and its target genes was assessed using real-time PCR. MSC-treated diabetic wounds exhibited significantly increased miR-146a expression compared with vehicle-treated controls (n = 5 per group, P < 0.05; Fig. 4E). Increased miR-146a gene expression in MSC-treated diabetic wounds was also associated with a significant downregulation of IRAK1, TRAF6, NF-κB, IL-6, and MIP-2 gene expression in wounds at days 3 and 7 (Fig. 5A–E). MIP-2 protein level was also significantly attenuated with MSC treatment (Fig. 5F).
FIG. 5.
MSC treatment downregulates levels of NF-κB, IL-6, and MIP-2 in diabetic wounds. Real-time quantitative RT-PCR analysis of mRNA, IRAK1 (A), TRAF6 (B), NF-κB (C), IL-6 (D), and MIP-2 (E) in db/+ (Hz; n = 5) and db/db (n = 5) wounds at days (D) 3 and 7 after dermal injury with MSCs or vehicle treatment. F: ELISA analysis of wound MIP-2 protein concentration.
DISCUSSION
In this study we demonstrate that miR-146a expression is significantly reduced during diabetic wound healing and is associated with a dramatic increase in the proinflammatory target genes IRAK1, TRAF6, and NF-κB, resulting in increased IL-6 and MIP-2 gene expression. We also show that MSC correction of the diabetic wound-healing impairment is associated with increased miR-146a expression and attenuated expression of proinflammatory and inflammatory genes. To our best knowledge, this is the first evidence that downregulated expression of miR-146a in response to injury in diabetic wounds may be partly responsible for the abnormal inflammatory response seen in diabetic wounds and may contribute to wound-healing impairment.
Wound healing is a synchronized process that progresses through the molecular events of cell recruitment, cell proliferation, extracellular matrix deposition, and remodeling. The precise and optimum response of various inflammatory elements and growth factors is essentially required during the normal wound-healing process (34). In the case of diabetes, many factors can impair wound healing, including extrinsic factors (wound infection, high glucose level, oxidative stress) and intrinsic factors (neuropathy, vascular problems, and other associated effects due to diabetes) (35).
Wound inflammation plays a central role in tissue regeneration, and timely initiation and resolution of inflammation are equally important for functional tissue repair. Strongly induced or unresolved inflammation can be harmful, causing pathologic manifestations and leading to chronic inflammatory disorders, as we observed in the diabetic wounds. Indeed, prolonged persistence of polymorphonuclear neutrophils and macrophages at the wound sites partly contribute to impaired wound healing in the diabetic mouse model (6). Because inflammation is crucial to wound repair, it is no surprise that nature applies multiple layers of regulation to keep inflammation in check. In this study, we provide evidence that miRNAs are involved in the pathogenesis of diabetic wound healing and demonstrate a link between miR-146a and the extensive and prolonged inflammation response in diabetic wounds.
Studies have shown that MSCs can accelerate healing in nondiabetic and diabetic mouse models (16,17,28) and can improve healing in human wounds (17). This correction with MSC therapy involves multiple aspects of the wound-healing response, including re-epithelialization, granulation tissue formation, and neovascularization (16,28). Many of the effects of MSC treatment are thought to be due to the release of soluble factors that regulate the local cellular response to injury (36–38), affecting multiple signaling pathways. One of the novel and most striking findings of this study is that wounds treated with MSCs significantly upregulate miR-146a expression. This upregulation of miR-146a is closely associated with a dramatic downregulation of its target genes, IRAK1 and TRAF6, and their associated pathway components NF-κB, IL-6, and MIP-2. These data provide evidence that MSCs enhance wound repair by altering miR-146a expression levels and attenuating the inflammatory response.
Our results demonstrate that miR-146a plays an important role in diabetic wound healing by regulating proinflammatory genes expression and that modulation of miR-146a level by MSC treatment can decrease wound inflammation and enhance wound closure. In addition, our results suggest that miR-146a may also represent a potential useful marker of inflammation as well as a potential therapeutic target for modification of the diabetic wound-healing response.
ACKNOWLEDGMENTS
The research presented in this article was supported by National Institutes of Health Diabetes Pathfinder Grant 7DP2 DK-083085-01 to K.W.L.
No potential conflicts of interest relevant to this article were reported.
J.X. researched the data and wrote the manuscript. W.W., L.Z., and W.D.-M. researched the data. M.W.M. and M.E.M. reviewed and edited the manuscript. K.W.L. contributed to discussion and wrote the manuscript. K.W.L. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Parts of this study were reported at the Wound Healing Society Annual Meeting, Dallas, Texas, 14–17 April 2011.
REFERENCES
- 1.Jeffcoate WJ, Harding KG. Diabetic foot ulcers. Lancet 2003;361:1545–1551 [DOI] [PubMed] [Google Scholar]
- 2.Jude EB, Blakytny R, Bulmer J, Boulton AJ, Ferguson MW. Transforming growth factor-beta 1, 2, 3 and receptor type I and II in diabetic foot ulcers. Diabet Med 2002;19:440–447 [DOI] [PubMed] [Google Scholar]
- 3.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev 2003;23:117–145 [DOI] [PubMed] [Google Scholar]
- 4.Fahey TJ, 3rd, Sadaty A, Jones WG, 2nd, Barber A, Smoller B, Shires GT. Diabetes impairs the late inflammatory response to wound healing. J Surg Res 1991;50:308–313 [DOI] [PubMed] [Google Scholar]
- 5.Seitz O, Schürmann C, Hermes N, et al. Wound healing in mice with high-fat diet- or ob gene-induced diabetes-obesity syndromes: a comparative study. Exp Diabetes Res 2010;2010:476969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetzler C, Kämpfer H, Stallmeyer B, Pfeilschifter J, Frank S. Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000;115:245–253 [DOI] [PubMed] [Google Scholar]
- 7.Grice EA, Snitkin ES, Yockey LJ, Bermudez DM, Liechty KW, Segre JA, NISC Comparative Sequencing Program Longitudinal shift in diabetic wound microbiota correlates with prolonged skin defense response. Proc Natl Acad Sci U S A 2010;107:14799–14804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci 2006;119:2204–2213 [DOI] [PubMed] [Google Scholar]
- 9.Fathke C, Wilson L, Hutter J, et al. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells 2004;22:812–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim DH, Yoo KH, Choi KS, et al. Gene expression profile of cytokine and growth factor during differentiation of bone marrow-derived mesenchymal stem cell. Cytokine 2005;31:119–126 [DOI] [PubMed] [Google Scholar]
- 11.Parikka V, Väänänen A, Risteli J, et al. Human mesenchymal stem cell derived osteoblasts degrade organic bone matrix in vitro by matrix metalloproteinases. Matrix Biol 2005;24:438–447 [DOI] [PubMed] [Google Scholar]
- 12.Barry FP. Biology and clinical applications of mesenchymal stem cells. Birth Defects Res C Embryo Today 2003;69:250–256 [DOI] [PubMed] [Google Scholar]
- 13.Crevensten G, Walsh AJ, Ananthakrishnan D, et al. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng 2004;32:430–434 [DOI] [PubMed] [Google Scholar]
- 14.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol 2008;180:2581–2587 [DOI] [PubMed] [Google Scholar]
- 15.Wu Y, Chen L, Scott PG, Tredget EE. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007;25:2648–2659 [DOI] [PubMed] [Google Scholar]
- 16.Badillo AT, Redden RA, Zhang L, Doolin EJ, Liechty KW. Treatment of diabetic wounds with fetal murine mesenchymal stromal cells enhances wound closure. Cell Tissue Res 2007;329:301–311 [DOI] [PubMed] [Google Scholar]
- 17.Falanga V, Iwamoto S, Chartier M, et al. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng 2007;13:1299–1312 [DOI] [PubMed] [Google Scholar]
- 18.Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of post-transcriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet 2008;9:102–114 [DOI] [PubMed] [Google Scholar]
- 19.Yi R, O’Carroll D, Pasolli HA, et al. Morphogenesis in skin is governed by discrete sets of differentially expressed microRNAs. Nat Genet 2006;38:356–362 [DOI] [PubMed] [Google Scholar]
- 20.Yi R, Poy MN, Stoffel M, Fuchs E. A skin microRNA promotes differentiation by repressing ‘stemness’. Nature 2008;452:225–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonkoly E, Wei T, Janson PC, et al. MicroRNAs: novel regulators involved in the pathogenesis of psoriasis? PLoS ONE 2007;2:e610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheedy FJ, O’Neill LA. Adding fuel to fire: microRNAs as a new class of mediators of inflammation. Ann Rheum Dis 2008;67(Suppl. 3):iii50–iii55 [DOI] [PubMed] [Google Scholar]
- 23.Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-kappaB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A 2006;103:12481–12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhaumik D, Scott GK, Schokrpur S, et al. MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8. Aging (Albany NY) 2009;1:402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakasa T, Miyaki S, Okubo A, et al. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum 2008;58:1284–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang Y, Luo X, Cui H, et al. MicroRNA-146A contributes to abnormal activation of the type I interferon pathway in human lupus by targeting the key signaling proteins. Arthritis Rheum 2009;60:1065–1075 [DOI] [PubMed] [Google Scholar]
- 27.Boldin MP, Taganov KD, Rao DS, et al. miR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med 2011;208:1189–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Javazon EH, Keswani SG, Badillo AT, et al. Enhanced epithelial gap closure and increased angiogenesis in wounds of diabetic mice treated with adult murine bone marrow stromal progenitor cells. Wound Repair Regen 2007;15:350–359 [DOI] [PubMed] [Google Scholar]
- 29.VanDerMeid KR, Su SP, Ward KW, Zhang JZ. Correlation of tear inflammatory cytokines and matrix metalloproteinases with four dry eye diagnostic tests. Invest Ophthalmol Vis Sci 2012;53:1512–1518 [DOI] [PubMed] [Google Scholar]
- 30.Herdrich BJ, Danzer E, Davey MG, et al. Regenerative healing following foetal myocardial infarction. Eur J Cardiothorac Surg 2010;38:691–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Badillo AT, Chung S, Zhang L, Zoltick P, Liechty KW. Lentiviral gene transfer of SDF-1alpha to wounds improves diabetic wound healing. J Surg Res 2007;143:35–42 [DOI] [PubMed] [Google Scholar]
- 32.Bermudez DM, Xu J, Herdrich BJ, Radu A, Mitchell ME, Liechty KW. Inhibition of stromal cell-derived factor-1α further impairs diabetic wound healing. J Vasc Surg 2011;53:774–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tekamp-Olson P, Gallegos C, Bauer D, et al. Cloning and characterization of cDNAs for murine macrophage inflammatory protein 2 and its human homologues. J Exp Med 1990;172:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med 1999;341:738–746 [DOI] [PubMed] [Google Scholar]
- 35.Blakytny R, Jude E. The molecular biology of chronic wounds and delayed healing in diabetes. Diabet Med 2006;23:594–608 [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Tredget EE, Wu PY, Wu Y. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One 2008;3:e1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim WS, Park BS, Sung JH, et al. Wound healing effect of adipose-derived stem cells: a critical role of secretory factors on human dermal fibroblasts. J Dermatol Sci 2007;48:15–24 [DOI] [PubMed] [Google Scholar]
- 38.Lee EY, Xia Y, Kim WS, et al. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen 2009;17:540–547 [DOI] [PubMed] [Google Scholar]