Abstract
Purpose
To determine the safety of sentinel lymph node biopsy as a replacement for inguinal femoral lymphadenectomy in selected women with vulvar cancer.
Patients and Methods
Eligible women had squamous cell carcinoma, at least 1-mm invasion, and tumor size ≥ 2 cm and ≤ 6 cm. The primary tumor was limited to the vulva, and there were no groin lymph nodes that were clinically suggestive of cancer. All women underwent intraoperative lymphatic mapping, sentinel lymph node biopsy, and inguinal femoral lymphadenectomy. Histologic ultra staging of the sentinel lymph node was prescribed.
Results
In all, 452 women underwent the planned procedures, and 418 had at least one sentinel lymph node identified. There were 132 node-positive women, including 11 (8.3%) with false-negative nodes. Twenty-three percent of the true-positive patients were detected by immunohistochemical analysis of the sentinel lymph node. The sensitivity was 91.7% (90% lower confidence bound, 86.7%) and the false-negative predictive value (1-negative predictive value) was 3.7% (90% upper confidence bound, 6.1%). In women with tumor less than 4 cm, the false-negative predictive value was 2.0% (90% upper confidence bound, 4.5%).
Conclusion
Sentinel lymph node biopsy is a reasonable alternative to inguinal femoral lymphadenectomy in selected women with squamous cell carcinoma of the vulva.
INTRODUCTION
The modern sentinel lymph node biopsy (SLNB) concept was proposed by Morton et al1 as an alternative to observation or elective regional lymphadenectomy in women with early cutaneous melanoma. It hypothesizes that early lymphatic drainage from a primary tumor progresses to a preferred nodal drainage basin in a nonrandom fashion. The principle lymph node, or sentinel lymph node (SLN), is identified by the combination of preoperative lymphoscintigraphy and intraoperative localization by using blue dye and radiocolloid. This technique provides the opportunity for early intervention in women with SLN metastases and spares SLN-negative women the morbidity of unnecessary regional lymphadenectomy. Clinical trials have established the procedure as an integral component in the management of women with melanoma and breast cancer.2,3
Vulvar cancer is a relatively uncommon cancer affecting fewer than 4,000 women in the United States each year.4 Standard treatment consists of wide radical excision of the primary tumor and inguinal femoral lymphadenectomy. Even with the use of contemporary surgical techniques, half the women who undergo treatment of vulvar cancer suffer a wound complication.5 While wound disruption and acute infections are common and predominately short-lived complications, lymphedema and cellulitis can be lifelong issues. Unfortunately, in the last century, there has been little progress in the treatment of lymphedema beyond prevention through removal of fewer lymph nodes in fewer women.
Vulvar cancer is an excellent target for the SLN concept because the tumor is easy to inject with blue dye and radiocolloid and because lymph drainage is predictably to one or both of the groins. Multiple single-institution series6–10 established the feasibility of SLNB in women with vulvar cancer. The Gynecologic Oncology Group (GOG) has studied various surgical and radiotherapy techniques for women with vulvar cancer. Two previous attempts by the GOG to replace full inguinal femoral lymphadenectomy with either a less radical surgical procedure or radiotherapy were unsuccessful.11,12 Here, we report the results of the GOG-173 protocol [Phase III Study of Intraoperative Lymphatic Mapping in Patients With Invasive Squamous Cell Carcinoma of the Vulva], a prospective multi-institution validation trial to determine whether SLNB could replace inguinal femoral lymphadenectomy and become the standard for future treatment studies in women with vulvar cancer.
PATIENTS AND METHODS
Patients
The protocol was open to women at the 47 GOG member institutions and their affiliates. The institutional review board at each member institution (Appendix, online only) approved the protocol. Women with invasive squamous cell carcinoma of the vulva were eligible if the depth of invasion was at least 1 mm, the tumor was limited to the vulva, the primary tumor size was at least 2 cm and not larger than 6 cm, and there was no suggestion of inguinal lymph node metastases on physical examination. Women with a previous wide local excision were eligible if the medical record documented the size of the primary tumor. The women had to have adequate performance status to undergo surgery. All women gave written informed consent. Women who had prior groin irradiation, prior groin dissection, multifocal disease, recurrent vulvar cancer, or grossly inflamed tumor were excluded.
Protocol and Procedures
There was no requirement for surgeon skill verification since vulvar cancer is so infrequent in any individual gynecologic oncologist's practice. There is no validated number for skill verification in vulvar cancer. At the time the study was written, the commonly referenced number of patients for a breast surgeon was 10 to 20. This was impractical for gynecologic oncologists. Women underwent a complete history and physical examination. A drawing of the primary tumor was obtained along with demographic and tumor information at enrollment. No preoperative cross-sectional imaging was required.
All women underwent intraoperative lymphatic mapping and SLNB with isosulfan blue dye. At the outset of the study, preoperative lymphoscintigraphy and intraoperative radiolocalization were optional. Beginning 2 years after study activation, preoperative lymphoscintigraphy and intraoperative radiolocalization were required (see the Amendments section for more information).
The decision to perform unilateral or bilateral SLNB and lymphadenectomy was determined by the location of the primary tumor. If the tumor came within 2 cm of the midline, bilateral SLNB and lymphadenectomies were performed regardless of lymphoscintigraphy findings.
Intraoperative intradermal injection of blue dye was performed following induction of anesthesia and sterile preparation and draping. Five minutes after dye injection, an incision was made in the groin, and blue efferent channels and blue lymph nodes were identified. A lymph node was considered sentinel if a blue channel led to it, even if the node was not blue. If radiocolloid was used intraoperatively, a handheld gamma counter was passed over the nodal tissue to identify lymph nodes emitting the tracer. A radioactive lymph node was considered sentinel if the activity was at least 10 times greater than background radioactivity. SLNs were labeled as blue, radioactive (hot), or both blue and hot. Following SLN identification, a completion lymphadenectomy was performed for all patients as described in the GOG surgical manual.
Histopathology
The method of labeling and processing SLNs was specified in the protocol. SLNs were subjected to ultra staging by serially sectioning them into 3-mm blocks. Frozen section evaluation was discouraged. At least two sections 40 microns apart were taken from each block and examined to determine whether they contained tumor cells. If routine hematoxylin and eosin staining was negative for metastatic disease on the first slide, immunohistochemical cytokeratin staining was performed on the second slide. Slides of the primary tumor, including slides demonstrating depth of invasion, were confirmed on GOG central pathology review; however, slides of lymph node metastases were not sent for central pathology review.
Statistical Analysis
The primary objective of this study was to estimate the negative predictive value of a sentinel groin lymph node (ie, the probability of all unilateral groin lymph nodes from a dissection being negative when the unilateral sentinel node is negative). By definition, the sentinel diagnostic procedure is 100% specific and, therefore, the predictive value of the procedure is a function of two variables: sensitivity (ie, the probability of the SLN being determined as positive for a patient with at least one positive groin lymph node from all nodes removed) and the frequency of occult positive groin nodes. Thus, the statistical design of this study was based on formulating a lower confidence boundary for the sensitivity (90% confidence or α = .10). We assumed that the sensitivity of the procedure would be constant over the entire population being studied.
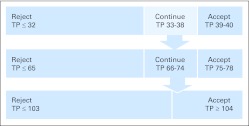
A three-phase sampling scheme was designed in the hope of reducing the required sample size. The decision to stop or continue was based on the conditional lower confidence boundary (LCB) estimate and its relationship to the minimally effective sensitivity values of 0.82 and 0.88 for stage I and stage II disease, respectively. Appendix Table A1 (online only) and Appendix Figure A1 (online only) give conditional LCBs for various scenarios and stopping rules, respectively, for the three-phase sampling scheme design. In particular, the study would close if the sensitivity was less than 0.82 (the minimally effective level for stage I disease) or if the conditional 90% LCB was greater than 0.88 (the minimally effective level for stage II disease). At the third and final accrual phase, the decision to consider the procedure effective was based on observing 104 or more true-positive nodes to detect sensitivity of at least 90% and false-negative predictive value of no more than 5% with at least 89% power. A total of 120 lymph node–positive women would be needed to determine the statistical end points if the study continued through all three phases. Expected accrual was 20 lymph node–positive women per year in light of accrual to previous GOG studies. Therefore, it was anticipated that the study would take a minimum of 2 years and a maximum of 6 years to complete.
Amendments
At the outset, the protocol did not require lymphoscintigraphy, in part out of concern that the addition of this imaging study would inhibit accrual because of the cost. Two years after study activation, mounting published evidence indicated that SLN detection was improved by the addition of preoperative lymphoscintigraphy and intraoperative radiolocalization. Therefore, preoperative lymphoscintigraphy and intraoperative radiolocalization were made mandatory. In 2007, when a nationwide shortage of isosulfan blue occurred, the study was amended to permit the use of other vital blue dyes such as methylene blue.
RESULTS
Patients
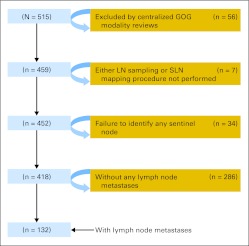
From December 1999 through January 2009, 515 women were accrued from 47 member institutions. After disqualifications shown in the CONSORT diagram (Fig 1), 459 women were eligible and 452 underwent lymphatic mapping, SLNB, and inguinal femoral lymphadenectomy. Clinical characteristics of eligible women are provided in Table 1. In all, 772 groin dissections were performed (bilateral in 320 women and unilateral in 132 women). Table 2 provides details on 418 women who had at least one SLN identified and thus were evaluable for the sensitivity analysis. No significant allergic reactions to blue dye were reported.
Fig 1.
CONSORT diagram. GOG, Gynecologic Oncology Group; LN, lymph node; SLN, sentinel lymph node.
Table 1.
Patient Characteristics (N = 459)*
| Characteristic | No. of Patients | % |
|---|---|---|
| Age, years | ||
| ≤ 40 | 25 | 5.4 |
| 41-50 | 65 | 14.2 |
| 51-60 | 101 | 22.0 |
| 61-70 | 77 | 16.8 |
| 71-80 | 118 | 25.7 |
| ≥ 81 | 73 | 15.9 |
| Ethnicity | ||
| Hispanic | 9 | 2.0 |
| Non-Hispanic | 393 | 85.6 |
| Unknown/not specified | 57 | 12.4 |
| Race | ||
| Black | 22 | 4.8 |
| White | 423 | 92.2 |
| Unknown/not specified | 14 | 3.1 |
| Performance status | ||
| 0 | 336 | 73.2 |
| 1 | 107 | 23.3 |
| 2 | 14 | 3.1 |
| 3 | 2 | 0.4 |
| Tumor size, cm | ||
| 2.0-2.9 | 174 | 37.9 |
| 3.0-3.9 | 123 | 26.8 |
| 4.0-4.9 | 80 | 17.4 |
| ≥ 5.0 | 82 | 17.9 |
| Tumor grade | ||
| 1 | 141 | 30.7 |
| 2 | 265 | 57.7 |
| 3 | 53 | 11.6 |
Clinical characteristics of all 459 patients who remained in the study after centralized Gynecologic Oncology Group (GOG) review, including seven patients who did not undergo either lymphatic mapping or sentinel lymph node biopsy.
Table 2.
SLNB Sensitivity Analysis
| Analysis | SLNB Result | Lymph Node Metastasis |
Total | Statistics |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Present | Absent | Sensitivity | 90% CI | NPV (%) | 90% CI | FNPV (%) | 90% CI | |||
| By patients | Positive | 121 | 0 | 121 | ||||||
| Negative | 11 | 286 | 297 | |||||||
| Total | 132 | 286 | 418 | 91.7 | 86.7 to 95.3 | 96.3 | 93.9 to 97.9 | 3.7 | 2.1 to 6.1 | |
| By groin | Positive | 140 | 0 | 140 | ||||||
| Negative | 12 | 441 | 453 | |||||||
| Total | 152 | 441 | 593 | 92.1 | 87.5 to 95.4 | 97.4 | 95.7 to 98.5 | 2.7 | 1.5 to 4.3 | |
| In tumors < 4.0 cm | Positive | 67 | 0 | 67 | ||||||
| Negative | 4 | 198 | 202 | |||||||
| Total | 71 | 198 | 269 | 2.0 | 0.7 to 4.5 | |||||
| In tumors ≥ 4.0 cm | Positive | 54 | 0 | 54 | ||||||
| Negative | 7 | 88 | 95 | |||||||
| Total | 61 | 88 | 149 | 7.4 | 3.5 to 13.4 | |||||
Abbreviations: FNPV, false-negative predictive value; NPV, negative predictive value; SLNB, sentinel lymph node biopsy.
Incidence of Lymph Node Metastases in Women With SLNs Identified
The incidence of lymph node metastases among women with at least one SLN identified was 31.6% (132 of 418). The incidence of lymph node metastases was significantly higher in women with larger tumors. Specifically, the rate of lymph node metastases was 26.4% (71 of 269) in women with primary tumors 2.0 to 3.9 cm and 40.9% (61 of 149) in women with primary tumors 4.0 to 6.0 cm (P = .0029). An analysis of the reliability of the SLN procedure relative to the primary tumor location is the subject of a secondary pending analysis.
Characteristics of SLNs
Among the 418 women (92.5%) who had at least one SLN identified at surgery, the SLNs were both blue and hot in 254 women (61%), were blue only in 100 women (24%), and were hot only in 64 women (15%). The mean number of SLNs per groin was 1.54. The mean number of all lymph nodes per groin was 8.94 (range, one to 32 lymph nodes; interquartile range, six to 11 lymph nodes).
Rates of SLN identification were similar in the 48 women who had a prior wide local excision and had blue dye with or without radiocolloid injected around the scar (87.5%; 42 of 48) and the 376 women who had blue dye with or without radiocolloid injected around the tumor (93.1%; 376 of 404; P = .15). Twenty-one women had SLNs that appeared grossly involved with tumor at surgery. Of note, the SLN was the only positive node in 73 (55.3%) of the 132 node-positive women.
Sensitivity Analysis
The sensitivity analysis is summarized in Table 2. Of the 132 node-positive women, 11 had false-negative findings on SLNB (8.3%; 90% CI, 4.7% to 13.4%). The sensitivity was 91.7%, and the false-negative predictive value [1-negative predictive value] was 3.7%. When the data were analyzed by groin rather than by patient, the false-negative predictive value was 2.7%. For women with tumors smaller than 4 cm, the false-negative predictive value was 2.0%, and for women with tumors 4 to 6 cm, the false-negative predictive value was 7.4%.
False-Negative Nodes
The false-negative rates for SLNs identified by dye and radiocolloid, dye alone, and radiocolloid alone were 1.6%, 2.0%, and 7.8%, respectively. Of the 11 false-negative patients, six were the first, second, or third accession from an institution. Only one institution had two false-negative patients. Appendix Table A2 (online only) provides a detailed listing of tumor characteristics, surgery type, and the SLN identification method for the 11 patients with false-negative results. Table 3 lists the cumulative number of false-negatives after each accrual phase.
Table 3.
Cumulative Counts of Eligible Patients, Incidence of Lymph Node Metastasis, and False Negatives by Accrual Phase (step)
| Variable | Accrual Phase |
|||
|---|---|---|---|---|
| One | Two | Three | Final* | |
| Sentinel lymph node identified | 112 | 273 | 390 | 418 |
| Lymph node metastasis | 40 | 80 | 120 | 132 |
| False negatives | 3 | 6 | 10 | 11 |
Accrual extended beyond the planned sample size to ensure at least 120 lymph node–positive patients.
Histopathology
Immunohistochemical analysis of SLNs was performed in 200 (71%) of the 285 women with negative SLNs on routine hematoxylin and eosin analysis. Twenty-three percent of true-positive cases were detected by immunohistochemistry when routine hemotoxylin and eosin staining did not reveal metastatic disease.
DISCUSSION
The study reported here was the latest effort by the GOG to improve outcomes for women with vulvar cancer. The 1976 GOG-37 protocol report demonstrated the superiority of groin and pelvic radiotherapy compared with pelvic lymphadenectomy in women undergoing radical vulvectomy who had groin metastases.13,14 The GOG has collected extensive surgical pathologic data confirming the relationship of depth of invasion to risk of nodal metastases, most notably the insignificant risk of lymph node spread for any depth of invasion less than 1 mm.15 In 1979, DiSaia et al16 proposed that the superficial inguinal lymph nodes served as SLNs and that if these nodes were negative, deeper femoral nodes were also negative. They confirmed their observation in a larger single-institution trial.17 The GOG 74 protocol [Carcinoma of the Vulva Treated with Ipsilateral Superficial Inguinal Lymphadenectomy and Modified Radical Hemivulvectomy] was designed to test the utility of superficial inguinal lymphadenectomy in the group setting; however, the results were disappointing. There was an unexpectedly high number of groin relapses, and this approach was abandoned.12 The GOG conducted protocol 88 [RT vs bilateral groin dissection] to compare lymphadenectomy with groin irradiation; again, results were disappointing, with an unexpectedly high rate of groin recurrence in those receiving primary groin irradiation.11
The results of this trial closely replicate the results of the GROINS V (Gronigen International Sentinel Nodes Vulva) study.18 This multi-institutional trial conducted primarily in the Netherlands, used an observational study design and included women with vulvar cancer who had primary tumor size less than 4 cm and who had negative findings on SLNB. Assuming that the eight women with groin relapse of the 403 women enrolled onto the study represented women with false-negative findings on SLNB, the false-negative rate in that study was 5.9%, and the false-negative predictive value was 2.9% (Table 4). These values are remarkably similar to our false-negative rate and false-negative predictive value for women with tumors less than 4 cm.
Table 4.
SLNB Sensitivity Analysis in the GROINS V Study
| SLNB Result | Lymph Node Metastasis |
Total | Statistics |
|||
|---|---|---|---|---|---|---|
| Present | Absent | Sensitivity (%) | NPV (%) | FNPV (%) | ||
| Positive | 127 | 0 | 127 | |||
| Negative | 8 | 268 | 276 | |||
| Total | 135 | 268 | 403 | 94.1 | 97.1 | 2.9 |
Abbreviations: FNPV, false-negative predictive value; GROINS V, Gronigen International Sentinel Nodes Vulva study; NPV, negative predictive value; SLNB, sentinel lymph node biopsy.
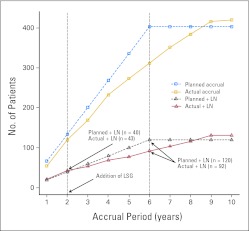
There are several weaknesses of our study that require mention. First, the study took longer to complete than anticipated. The false-negative rate might have been lower if surgeon skill verification had been required. We anticipate that a learning curve would result in a decreasing rate of false-negative nodes as investigators learned the procedure. Performance did not improve during the study, presumably because new centers and surgeons were constantly being added. The amendment to require lymphoscintigraphy coincided with a decline in accrual (Fig 2), suggesting how sensitive GOG member institutions are to cost. In addition, some individual investigators might have adopted the procedure as their standard and stopped offering the study to women since women in the study would require full lymphadenectomy. Second, the rate of performance of immunohistochemical analysis was much lower than required by the protocol. There was some resistance to conducting the costly and time-consuming immunohistochemistry studies, which suggests poor communication among gynecologic oncologists, pathologists, and the GOG statistical office. Since immunohistochemistry detects more true-positive nodes and reduces the number of false-negative nodes, omission of immunohistochemical analysis might have lengthened the study and led to an overestimation of the false-negative predictive value. Central review of immunohistochemistry slides was not required, which limits our analysis regarding the role of the size of the metastases on outcomes. Third, we were unable to evaluate the impact of the procedure on groin recurrence risk or on quality of life (because of reduced surgical evaluation) since all women underwent full lymphadenectomy following the sentinel node procedure. Both outcomes are the subject of ongoing investigation. Finally, we did not capture complications that may be unique to the procedure as well as we had planned to. As experience has grown with mapping studies in other solid tumors, procedure-specific complications such as hypersensitivity reactions, pseudohypoxia, paresthesias, seroma, and infection have been attributed to the procedure. These effects and their impact on survivorship are important parameters.
Fig 2.
Projected versus actual accrual and incidence of lymph node metastasis. LN, lymph node; LSG, lymphoscintigraphy.
On the basis of our experience, we believe that the safety of future studies that include SLNB will be enhanced if confirmation of institutional competence with the procedure, including pathologic ultra staging, is obtained. Preoperative imaging provides an opportunity to exclude women with grossly involved but not palpable SLNs who might benefit by going directly to lymphadenectomy. Finally, future studies should define single grossly involved lymph nodes that are not hot or blue as sentinel, since they are clearly the first site of metastases.
In conclusion, this study met its predetermined statistical goals for the incorporation of SLNB into future GOG treatment studies of women with vulvar cancer. Women with primary tumors smaller than 4 cm who met the eligibility criteria for this trial can be counseled preoperatively that if the SLN is negative, they have a less than 3% risk of a groin relapse due to a false-negative SLN. With the addition of preparative imaging, growing surgical experience, and improved histopathologic techniques, it is hoped that this rate can be reduced even further. We believe that the results of this trial coupled with the results of the GROINS V study provide adequate evidence that SLNB should be offered to well-selected patients by well-trained and informed gynecologic oncologists. In clinical settings where vulvar cancer is rare and surgeons' experience is limited, referral to a high-volume center or surgeon is appropriate.
The management of women with positive findings on SLNB was not addressed in this study. There is extensive literature from other disease sites that indicate the size of the metastasis is a critical element in determining treatment. There may even be false-positive patients in whom the identification of micrometastases or isolated tumor cells is clinically insignificant. Categorizing immunohistochemistry-only patients as node positive can lead to overtreatment. Nevertheless, treatment guidelines from breast cancer should not be adapted to vulvar cancer without further investigation in spite of parallels in the development of surgery for both diseases. Squamous cell carcinoma of the vulva is not hormonally active or chemosensitive. Patients with vulvar cancer who relapse frequently do so locally and die from locally advanced disease. This is in contrast to many breast cancers that are considered sensitive to chemotherapy and hormonal therapy.
The GOG (GOG 270 study) has joined the Gronigen International Study on Sentinel Nodes in Vulvar Cancer (GROINSS VII), which addresses the management of SLN-positive women. The protocol requires surgeon skill verification, preoperative imaging, and central pathology review. We hope that international collaboration will help shorten the duration of studies that improve outcomes for women with vulvar cancer.
Appendix
The following institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group Professional Corporation, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, The Cleveland Clinic Foundation, Washington University School of Medicine, Memorial Sloan-Kettering Cancer Center, Columbus Cancer Council, MD Anderson Cancer Center, Fox Chase Cancer Center, Women's Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Fletcher Allen Health Care, University of Wisconsin Hospital, The Hospital of Central Connecticut, and Georgia Center for Oncology Research & Education Consortium, and Community Clinical Oncology Program.
Fig A1.
Stopping rules associated with the three-phase sampling scheme. TP, true positive.
Table A1.
Three-Phase Sampling Scheme
| Phase | Action | No. of True Positives | Sensitivity Estimate | Conditional Lower Confidence Boundary | Unconditional Lower Confidence Boundary |
|---|---|---|---|---|---|
| 1 | S | 0-26 | — | — | — |
| S | 27 | 0.6750 | — | 0.5624 | |
| S | 28 | 0.7000 | — | 0.5884 | |
| S | 29 | 0.7250 | — | 0.6147 | |
| S | 30 | 0.7500 | — | 0.6412 | |
| S | 31 | 0.7750 | — | 0.6682 | |
| S | 32 | 0.8000 | — | 0.6955 | |
| C | 33 | 0.8250 | — | 0.7233 | |
| C | 34 | 0.8500 | — | 0.7515 | |
| C | 35 | 0.8750 | — | 0.7804 | |
| C | 36 | 0.9000 | — | 0.8100 | |
| C | 37 | 0.9250 | — | 0.8406 | |
| C | 38 | 0.9500 | — | 0.8724 | |
| S | 39 | 0.9750 | — | 0.9062 | |
| S | 40 | 1.0000 | — | 0.9441 | |
| 2 | S | 33-60 | — | — | — |
| S | 61 | 0.7625 | 0.6735 | 0.6906 | |
| S | 62 | 0.7750 | 0.6750 | 0.7040 | |
| S | 63 | 0.7875 | 0.7103 | 0.7174 | |
| S | 64 | 0.8000 | 0.7144 | 0.7309 | |
| S | 65 | 0.8125 | 0.7577 | 0.7445 | |
| C | 66 | 0.8250 | 0.7670 | 0.7582 | |
| C | 67 | 0.8375 | 0.7774 | 0.7720 | |
| C | 68 | 0.8500 | 0.7889 | 0.7858 | |
| C | 69 | 0.8625 | 0.8013 | 0.7998 | |
| C | 70 | 0.8750 | 0.8145 | 0.8140 | |
| C | 71 | 0.8875 | 0.8281 | 0.8282 | |
| C | 72 | 0.9000 | 0.8421 | 0.8426 | |
| C | 73 | 0.9125 | 0.8560 | 0.8572 | |
| C | 74 | 0.9250 | 0.8696 | 0.8721 | |
| S | 75 | 0.9375 | 0.8823 | 0.8872 | |
| S | 76 | 0.9500 | 0.8933 | 0.9026 | |
| S | 77 | 0.9625 | 0.9014 | 0.9184 | |
| S | 78 | 0.9750 | 0.9053 | 0.9348 | |
| 3 | S | 66-98 | — | — | — |
| S | 99 | 0.8250 | 0.7839 | 0.7723 | |
| S | 100 | 0.8333 | 0.7896 | 0.7814 | |
| S | 101 | 0.8417 | 0.7961 | 0.7905 | |
| S | 102 | 0.8500 | 0.8033 | 0.7996 | |
| S | 103 | 0.8583 | 0.8111 | 0.8088 | |
| S | 104 | 0.8667 | 0.8193 | 0.8180 | |
| S | 105 | 0.8750 | 0.8278 | 0.8273 | |
| S | 106 | 0.8833 | 0.8365 | 0.8366 | |
| S | 107 | 0.8917 | 0.8452 | 0.8460 | |
| S | 108 | 0.9000 | 0.8537 | 0.8554 | |
| S | 109 | 0.9083 | 0.8618 | 0.8649 | |
| S | 110 | 0.9167 | 0.8692 | 0.8745 | |
| S | 111 | 0.9250 | 0.8752 | 0.8842 | |
| S | 112 | 0.9333 | 0.8794 | 0.8940 | |
| S | 113 | 0.9417 | 0.8917 | 0.9039 | |
| S | 114 | 0.9500 | 0.8931 | 0.9139 |
Abbreviations: C, continue; S, stop.
Table A2.
SLN Identification and Tumor Characteristics Among the 11 Patients With False-Negative Results
| ID | Lymphadenectomy (surgery type) | Tumor |
SLN |
|||
|---|---|---|---|---|---|---|
| Sites | Location | Size (cm) | Grossly Involved | Identification* | ||
| FN01 | Bilateral | C | Midline | 4.0 | No | Hot only |
| FN02 | Bilateral | L | Ambiguous | 2.5 | No | Hot only |
| FN03 | Bilateral | F, P, L | Midline | 5.0 | No | Blue and hot |
| FN04 | Bilateral | C, L | Midline | 3.0 | Yes | Blue and hot |
| FN05 | Unilateral | L | Lateral | 4.0 | No | Blue and hot |
| FN06 | Bilateral | C, V, L | Midline | 5.0 | Yes | Hot only |
| FN07 | Unilateral | L | Lateral | 4.0 | No | Blue and hot |
| FN08 | Bilateral | L | Ambiguous | 5.0 | Yes | Hot only |
| FN09 | Unilateral | L | Lateral | 2.5 | Yes | Blue and hot |
| FN10 | Bilateral | C, L | Midline | 6.0 | No | Blue only |
| FN11 | Bilateral | C | Midline | 2.0 | No | Blue and hot |
Abbreviations: C, clitoris; F, posterior Fourchette; FN, false negative; ID, identification number; L, labium; P, perineum; SLN, sentinel lymph node; V, vestibule.
Blue, intraoperative intradermal injection of blue dye. Hot, use of radiocolloid intraoperatively with handheld gamma counter passed over the nodal tissue to identify lymph nodes emitting the tracer (radioactive).
Footnotes
Processed as a Rapid Communication manuscript. Listen to the podcast by Dr Schorge at www.jco.org/podcasts
Support information appears at the end of this article.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Clinical trial information can be found for the following: NCT00003325.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: Charles F. Levenback, Robert L. Coleman, Cornelia L. Trimble
Provision of study materials or patients: Michael A. Gold, Patricia L. Judson, Koen De Geest, Nick M. Spirtos, Ronald K. Potkul, Emma C. Rossi
Collection and assembly of data: Charles F. Levenback, Shamshad Ali, Robert L. Coleman, Michael A. Gold, Jeffrey M. Fowler, Patricia L. Judson, Maria C. Bell, Nick M. Spirtos, Ronald K. Potkul, Mario M. Leitao Jr, Jamie N. Bakkum-Gamez, Emma C. Rossi, Samuel S. Lentz, James J. Burke II, Linda Van Le
Data analysis and interpretation: Charles F. Levenback, Shamshad Ali, Robert L. Coleman
Manuscript writing: All authors
Final approval of manuscript: All authors
Support
Supported by National Cancer Institute Grants No. CA 27469 to the Gynecologic Oncology Group Administrative Office and No. CA 37517 to the Gynecologic Oncology Group Statistical and Data Center.
REFERENCES
- 1.Morton DL, Wen DR, Wong JH, et al. Technical details of intraoperative lymphatic mapping for early stage melanoma. Arch Surg. 1992;127:392–399. doi: 10.1001/archsurg.1992.01420040034005. [DOI] [PubMed] [Google Scholar]
- 2.Balch CM, Cascinelli N. Sentinel-node biopsy in melanoma. N Engl J Med. 2006;355:1370–1371. doi: 10.1056/NEJMe068147. [DOI] [PubMed] [Google Scholar]
- 3.Singletary SE, Allred C, Ashley P, et al. Revision of the American Joint Committee on Cancer staging system for breast cancer. J Clin Oncol. 2002;20:3628–3636. doi: 10.1200/JCO.2002.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 5.Gaarenstroom KN, Kenter GG, Trimbos JB, et al. Postoperative complications after vulvectomy and inguinofemoral lymphadenectomy using separate groin incisions. Int J Gynecol Cancer. 2003;13:522–527. doi: 10.1046/j.1525-1438.2003.13304.x. [DOI] [PubMed] [Google Scholar]
- 6.Levenback C, Coleman RL, Burke TW, et al. Intraoperative lymphatic mapping and sentinel node identification with blue dye in patients with vulvar cancer. Gynecol Oncol. 2001;83:276–281. doi: 10.1006/gyno.2001.6374. [DOI] [PubMed] [Google Scholar]
- 7.de Hullu JA, Hollema H, Piers DA, et al. Sentinel lymph node procedure is highly accurate in squamous cell carcinoma of the vulva. J Clin Oncol. 2000;18:2811–2816. doi: 10.1200/JCO.2000.18.15.2811. [DOI] [PubMed] [Google Scholar]
- 8.Rob L, Robova H, Pluta M, et al. Further data on sentinel lymph node mapping in vulvar cancer by blue dye and radiocolloid Tc99. Int J Gynecol Cancer. 2007;17:147–153. doi: 10.1111/j.1525-1438.2007.00806.x. [DOI] [PubMed] [Google Scholar]
- 9.Sideri M, De Cicco C, Maggioni A, et al. Detection of sentinel nodes by lymphoscintigraphy and gamma probe guided surgery in vulvar neoplasia. Tumori. 2000;86:359–363. doi: 10.1177/030089160008600431. [DOI] [PubMed] [Google Scholar]
- 10.Ansink AC, Sie-Go DM, van der Velden J, et al. Identification of sentinel lymph nodes in vulvar carcinoma patients with the aid of a patent blue V injection: A multicenter study. Cancer. 1999;86:652–656. doi: 10.1002/(sici)1097-0142(19990815)86:4<652::aid-cncr14>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 11.Stehman FB, Bundy BN, Thomas G, et al. Groin dissection versus groin radiation in carcinoma of the vulva: A Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1992;24:389–396. doi: 10.1016/0360-3016(92)90699-i. [DOI] [PubMed] [Google Scholar]
- 12.Stehman FB, Bundy BN, Dvoretsky PM, et al. Early stage I carcinoma of the vulva treated with ipsilateral superficial inguinal lymphadenectomy and modified radical hemivulvectomy: A prospective study of the Gynecologic Oncology Group. Obstet Gynecol. 1992;79:490–497. [PubMed] [Google Scholar]
- 13.Homesley HD, Bundy BN, Sedlis A, et al. Radiation therapy versus pelvic node resection for carcinoma of the vulva with positive groin nodes. Obstet Gynecol. 1986;68:733–740. [PubMed] [Google Scholar]
- 14.Kunos C, Simpkins F, Gibbons H, et al. Radiation therapy compared with pelvic node resection for node-positive vulvar cancer: A randomized controlled trial. Obstet Gynecol. 2009;114:537–546. doi: 10.1097/AOG.0b013e3181b12f99. [DOI] [PubMed] [Google Scholar]
- 15.Sedlis A, Homesley H, Bundy BN, et al. Positive groin lymph nodes in superficial squamous cell vulvar cancer: A Gynecologic Oncology Group Study. Am J Obstet Gynecol. 1987;156:1159–1164. doi: 10.1016/0002-9378(87)90132-3. [DOI] [PubMed] [Google Scholar]
- 16.DiSaia PJ, Creasman WT, Rich WM. An alternate approach to early cancer of the vulva. Am J Obstet Gynecol. 1979;133:825–832. doi: 10.1016/0002-9378(79)90119-4. [DOI] [PubMed] [Google Scholar]
- 17.Berman ML, Soper JT, Creasman WT, et al. Conservative surgical management of superficially invasive stage I vulvar carcinoma. Gynecol Oncol. 1989;35:352–357. doi: 10.1016/0090-8258(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 18.Van der Zee AG, Oonk MH, De Hullu JA, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]