INTRODUCTION
Modern multiagent chemotherapy regimens have increased the cure rate in acute lymphoblastic leukemia (ALL). In pediatric ALL, refinements of doses and schedules of chemotherapy combinations have increased the cure rate from less than 40% in the 1960s to 80% in 2000 without the addition of any novel chemotherapy drugs or targeted therapies.1–3 The pediatric regimens have included induction, consolidation, maintenance, and CNS treatment phases. Using similar strategies in adult ALL has improved the cure rates from less than 20% to 35% to 50%.4–6 The addition of asparaginase to pediatric chemotherapy regimens further improved the cure rates. Using similar regimens in adult ALL seems to improve the cure rates but at the expense of significant toxicity, particularly in patients age 45 years or older.7 Further intensification of existing chemotherapy regimens is unlikely to increase the cure rate and may significantly increase toxicities. Survivors of childhood ALL are at risk of multiple late effects related in part to the intensity of their therapy.8 Therefore, novel anti-ALL agents are needed to overcome chemotherapy resistance and reduce nonspecific toxicities.
Targeted therapy in ALL has shown promise in both children and adults. In Philadelphia chromosome–positive ALL, the discovery of the activity of BCR-ABL tyrosine kinase inhibitors and their addition to intensive chemotherapy has increased survival rates from less than 10% to approximately 50% in adults and from approximately 35% to 80% in children.9–12 Targeted therapy using monoclonal antibodies against cell surface markers of ALL cells has shown promising results.13–15 Herein, we will review the results and status of investigational monoclonal antibody–based therapies in ALL.
SURFACE ANTIGEN EXPRESSION ON LYMPHOBLASTS AND POTENTIAL TARGETED MONOCLONAL ANTIBODIES
Several differentiation antigens expressed on the surface of lymphoblasts are targetable with existing monoclonal antibody–based reagents. One such antigen is CD20, which functions as a calcium channel that ultimately influences cell-cycle progression and differentiation via downstream signaling pathways. Expression of CD20 is noted in approximately 25% to 50% of patients with precursor B-cell (pre-B) ALL and almost all cases of mature or Burkitt-type ALL (B-ALL).16–18 Recent studies reported that CD20 expression was upregulated in children with pre-B ALL following exposure to corticosteroids.18–21 Initiation of induction therapy was associated with an increase in the proportion of patients with CD20 expression from 45% to 81% and with increases in the intensity of CD20 expression and the percentage of blasts that express CD20.19 Lymphoblasts with CD20 upregulation were sensitive to rituximab when exposed in vitro. These observations could broaden the application of rituximab therapy to patients with low or absent CD20 expression through a sequential therapeutic approach such as using corticosteroids before delivering monoclonal antibody–based therapies.
CD22 is a member of the sialoglycoprotein family of adhesion molecules that regulates B-cell activation and interaction of B cells with T cells and antigen-presenting cells. Expression of CD22 has been demonstrated in more than 90% of patients with pre-B ALL and mature B-ALL.17,22 CD19 is a type I transmembrane glycoprotein of the immunoglobulin (Ig) superfamily, with expression restricted to B cells. CD19 is involved in B-cell fate and differentiation through the modulation of B-cell receptor signaling at multiple stages of B-cell development. CD19 is expressed in nearly all patients who have pre-B ALL and mature B-ALL.18 The CD52 antigen is a member of the glycosylphosphatidylinositol-anchored membrane glycoproteins, which appears to function in normal T-cell activation, release of cytokines, and signal transduction. Expression of CD52 is reported in 70% to 80% of patients with T-cell ALL; its expression in pre-B ALL has been reported in 70% of patients, but the true incidence is likely to be lower because of differing cut points for definitions of CD52 positivity.16
The frequency and intensity of antigen expression varies with biologic subtype and patient age. Variations in the reports of expression of different surface antigens may be related to techniques and to what is considered as positive on the basis of density and intensity of expression. Traditionally, significant expression referred to the presence of a surface antigen on at least 20% of ALL blasts. However, it is critical to the efficacy of monoclonal antibody–based therapeutic approaches that all blasts in a given patient express the antigen target. CD20 expression is often variably expressed across blasts, whereas CD19 and CD22 expressions are usually uniform. The degree of antigen expression and internalization rates are additional factors that might influence response to therapy. For example, in pre-B ALL, the average density of CD22 is about 4,000 sites per cell,22 but the surface density of CD19 may be 5- to 10-fold higher. Notably, CD19 internalization rates seem to be slower in comparison to those of CD22.23
MONOCLONAL ANTIBODY–BASED REAGENTS AND MECHANISM OF ACTION
Several monoclonal antibody–based reagents have potential application in the treatment of ALL. These include unconjugated monoclonal antibodies; monoclonal antibodies (or fragments) conjugated to cytotoxic agents (antibody drug conjugates); monoclonal antibodies conjugated to toxins (immunotoxins); and bispecific single-chain antibodies that redirect cytotoxic T lymphocytes (via CD3 expression) to preselected surface antigens on ALL cells (eg, CD19), bispecific T-cell engagers, and radionuclide conjugates.24,25
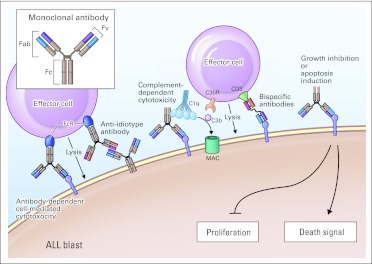
Monoclonal antibody–based reagents can kill leukemia blasts by direct and/or indirect mechanisms that vary with the antigen and the reagent (Figs 1 and 2). Binding by unconjugated monoclonal antibodies can, in some cases, directly induce cytotoxicity through inhibition of proliferation or triggering of cell death pathways. Indirect killing may occur via antibody-dependent cell-mediated cytotoxicity and/or complement-dependent cytotoxicity. Importantly, such immune-mediated cytotoxicity requires functional effector mechanisms, which may be deficient in patients with ALL.26 The cytotoxicity of monoclonal antibodies can increase by linkage to toxic moieties, including chemotherapy agents, toxins, and radioisotopes. Notably, such reagents armed with potent cytotoxic compounds do not require active immune response mechanisms for activity and can be effective even in profoundly immunocompromised patients.
Fig 1.
Mechanisms of action of monoclonal antibodies. Monoclonal antibodies consist of two heavy chains and two light chains. The amino acid sequence of the variable (v) ends of each of the four chains determines the specificity of antigen binding. The other end of the molecule (Fc; crystallizable or complement fixing fragment) activates complement and engages immune effector cells through Fc-receptor (FcR) binding. Antibody-dependent cell-mediated cytotoxicity (ADCC): Immune effector cells lyse target cells coated with antibodies. Binding of the Fc portion of immunoglobulin (Ig) molecules by FcRs on effector cells leads to activation of effector cell functions and cytotoxicity. Anti-idiotype antibodies directed against unique regions of the Ig variable domains expressed on blasts can also activate ADCC. Complement-dependent cytotoxicity (CDC): IgM and IgG antibodies bind the first component of complement (C1q) initiating the complement cascade, which terminates in the generation of the membrane attack complex (MAC) and cell lysis. Intermediate components of the complement cascade function as opsonins facilitating CDC and ADCC. The third component of complement (C3b) binds directly to C3b receptors (C3bR) on effector cells, which leads to complement-dependent cell-mediated cytoxicity. Bispecific antibodies: Monoclonal antibody constructs with dual specificity can facilitate direct cell-mediated cytotoxicity through linkage of immune effector cells and leukemic blasts. Direct effects of unconjgated monoclonal antibodies: Monoclonal antibodies that bind surface receptors can inhibit cell proliferation or induce apoptosis through effects on intracellular signaling pathways. ALL, acute lymphoblastic leukemia; CD3, cluster designation 3; Fab, antigen binding fragment; Fv, variable fragment.
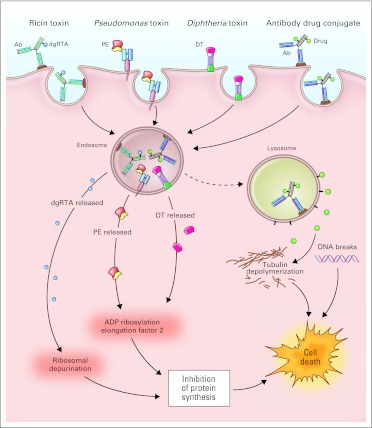
Fig 2.
Mechanisms of action of monoclonal antibody (Ab) conjugates. Monoclonal antibodies and their fragments can be conjugated or linked to cytotoxic agents. Chemotherapy and toxin conjugates must be internalized via receptor-mediated endocytosis, whereas internalization is not required for radioisotope conjugates. After internalization, the active cytotoxic component is released and mediates cell death. Ricin-based immunotoxins depurinate ribosomal RNA and inhibit protein synthesis. Pseudomonas (PE)- and diphtheria (DT) -derived immunotoxins ADP ribosylate elongation factor-2 and inhibit protein synthesis. Antibody drug conjugates mediate cytotoxicity by drug-specific actions (eg, targeting tubulin by maytansin and auristatin, and induction of DNA breaks by calicheamicin). dgRTA, deglycosylated ricin A chain.
Unconjugated monoclonal antibodies include CD20 monoclonal antibodies such as rituximab, and the presumed more potent antibodies ofatumumab and GA-101. Monoclonal antibodies conjugated to cytotoxic agents include inotuzumab ozogamicin (CD22 monoclonal antibody conjugated to calicheamcin), SAR3419 (CD19 monoclonal antibody conjugated to maytansin, a potent antimitotic cytotoxic drug), as well as several other CD19 and CD22 monoclonal antibodies conjugated with auristatin (also an inhibitor of mitosis). Monoclonal antibodies have also been conjugated with toxins such as Pseudomonas and diphtheria toxins. Finally, blinatumomab is an example of a bispecific monoclonal antibody directing cytotoxic T cells to surface antigens on ALL cells. The reasons behind the efficacy of particular monoclonal antibodies may be related to density of expression of surface antigens and their internalization, efficacy of particular toxins, achievable dose levels and pharmacokinetics, and frequency of administration. For example, CD20 and CD52 are not internalized after antibody binding, whereas CD19 is, albeit relatively slowly in comparison to CD22.23
Experience With Rituximab (unconjugated CD20 monoclonal antibody)
The original experience with rituximab (Rituxan; Genentech, South San Francisco, CA) in lymphoproliferative disorders demonstrated encouraging activity, particularly in indolent lymphomas. Subsequent studies that combined chemotherapy with rituximab showed significant improvements in survival in aggressive lymphomas by using chemoimmunotherapy with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) and rituximab.27 Studies in chronic lymphocytic leukemia (CLL) combining fludarabine with or without cyclophosphamide and rituximab demonstrated survival benefit with the addition of rituximab.28,29 The experience was particularly impressive in CLL since single-agent rituximab has minimal anti-CLL activity.
Encouraged by these findings, investigators combined rituximab with chemotherapy in ALL subsets that exhibited CD20 cell surface expression. Mature B-ALL and Burkitt leukemia exhibit strong CD20 expression on the leukemic cells. Hyper-CVAD [fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone] chemotherapy was combined with rituximab and was administered as two doses with each of the first four cycles of hyper-CVAD, for a total of eight doses. In an update of 51 patients treated with the combination, the complete remission (CR) rate was 95%. Compared with prior experience with hyper-CVAD without rituximab (n = 44), the addition of rituximab resulted in a 4-year survival rate of 77% versus 50% (P = .03). The 4-year survival was slightly but not significantly improved in patients younger than age 60 years (78% v 70%) but was significantly improved among older patients (75% v 19%; P < .01).13,15 In a study of mature B-ALL, Burkitt lymphoma, and diffuse large B-cell lymphoma (Multicentre Study to Optimize Therapy of B-ALL and High-grade Non-Hodgkin's Lymphoma in Adults [GMALL-B-ALL/NHL 2002], 18,30 the German study group also combined chemotherapy with rituximab (total of eight doses). Among 146 patients treated, the CR rate was 90%; the 3-year survival rate among patients younger than age 55 years was 91%, and among patients age 55 years or older, it was 84%. In the subset of patients with mature B-ALL (n = 84), the CR rate was 83%; the 3-year survival rate was 79% among younger patients and 39% among older patients. Rizzieri et al31 updated the Cancer and Leukemia Group B (CALGB) group experience with chemoimmunotherapy in Burkitt leukemia, comparing the addition of four doses of rituximab (Rituximab, Chemotherapy, and Filgrastim in Treating Patients With Burkitt's Lymphoma or Burkitt's Leukemia [CALGB 10002]) to their historical experience with chemotherapy alone (Combination Chemotherapy in Treating Patients With Non-Hodgkin's Lymphoma or Acute Lymphocytic Leukemia [CALGB 9251]). In the historical experience, the cure rate was approximately 50%. With the addition of rituximab, the 5-year survival rate was 90% in the low-risk group (n = 56), 70% in the intermediate-risk group (n = 29), and 50% in the high-risk group (n = 20).31 These experiences suggest that the combination of chemotherapy and rituximab is the new standard of care in mature B-ALL and Burkitt lymphoma.
The addition of rituximab to chemotherapy was then investigated in patients with CD20+ pre-B ALL (non-Burkitt). Thomas et al32 investigated chemoimmunotherapy with hyper-CVAD and rituximab in 173 patients, 97 with CD20+ ALL. They compared that experience to the use of hyper-CVAD chemotherapy alone in 109 patients, 53 with CD20+ ALL. The overall CR rate was 95% with chemoimmunotherapy, and the 3-year rates of CR duration and survival were 60% and 50%, respectively. Among younger patients (age < 60 years) with CD20+ ALL, chemoimmunotherapy was associated with significantly higher rates of CR duration (70% v 38%; P < .01) and survival (75% v 47%; P = .003).32 No benefit was noted among patients age 60 years or older with CD20+ ALL, in part because of deaths in CR from infectious complications in the setting of a high rate of negativity in this group for minimal residual disease (MRD) by multiparameter flow cytometry. The German study group also reported similar experiences in the German Multicenter Trial for Treatment of Newly Diagnosed Acute Lymphoblastic Leukemia in Adults (GMALL 07/2003).33 In their study, rituximab was administered for a total of eight doses with chemotherapy in standard-risk ALL and for a total of three doses before allogeneic stem-cell transplantation in high-risk ALL. Among patients younger than age 55 years, the addition of rituximab was associated with a 5-year remission duration rate of 80% versus 47% without rituximab, and with a 5-year survival rate of 71% versus 51% without rituximab.33
These studies suggest that the addition of rituximab to chemotherapy has improved outcome in CD20+ pre-B ALL, particularly among younger adults. Future studies should address the role of extended rituximab therapy (beyond eight doses) and rituximab with attenuated chemotherapy (to reduce risk of infectious complications) in older patients. Whether it is useful to give rituximab to patients with low expression of CD20+ (less than 20%) or following upregulation of CD20 expression (eg, with corticosteroid pretreatment) needs to be further explored.
Data are limited on the use of rituximab in pediatric ALL. Clinical trials completed to date have used rituximab in combination with chemotherapy in the setting of mature B-ALL and Burkitt lymphoma.34,35 Anecdotal reports of single-agent activity include a CR in a child with mature B-ALL and clearance of MRD in a child with pre-B ALL with rituximab alone.36,37
Ofatumumab (HuMax-CD20; GlaxoSmithKline, Collegeville, PA and Genmab, Copenhagen, Denmark) is a second-generation fully human anti-CD20 IgG1 monoclonal antibody. This is directed at a unique small-loop epitope of CD20 (different binding site than that for rituximab) with a longer release time from the target site and a superior complement-dependent cellular cytotoxicity effect compared with rituximab. Ofatumumab has shown activity in patients with fludarabine-refractory and rituximab-refractory CLL.38,39 Studies combining anti-ALL chemotherapy regimens with ofatumumab or with other more potent CD20 monoclonal antibodies such as GA-101 are warranted.
Experience With Alemtuzumab (unconjugated CD52 monoclonal antibody)
Alemtuzumab, a CD52 monoclonal antibody, has demonstrated efficacy in a range of benign and malignant tumors, in particular CLL, resulting in approval by the US Food and Drug Administration of alemtuzumab as salvage and first-line therapy for CLL.40,41 In a pilot study, Stock et al42 treated 24 patients with newly diagnosed CD52+ ALL who were in CR following intensive chemotherapy with alemtuzumab administered at 30 mg subcutaneously three times a week for 4 weeks (total 12 doses). Cytomegalovirus infection was noted in two patients. There was a median 1-log decrease of MRD during alemtuzumab therapy. After a median follow-up of 51 months, the median disease-free survival was 53 months and the median survival was 55 months. Previously, pilot experience with single-agent alemtuzumab in six adults with refractory CD52+ ALL was negative.43 A phase II study of alemtuzumab in children with relapsed T-cell ALL and pre-B ALL was closed prematurely because of poor accrual.44 One (8%) of 13 patients treated with single-agent alemtuzumab on that trial achieved a CR. Additional rare CRs with CD52+ pre-B ALL have been reported with single-agent alemtuzumab.45
Alemtuzumab has been reported to target the CD52 upregulation induced as a mechanism of resistance to rituximab. Studies of chemotherapy regimens incorporating alemtuzumab with or without rituximab will clarify the potential benefit and toxicity profiles of this chemoimmunotherapy approach.
Experience With Epratuzumab (unconjugated CD22 monoclonal antibody)
Epratuzumab, a humanized monoclonal antibody targeting CD22, has recently been studied in pediatric patients with first relapse of pre-B ALL. Therapy consisted of single-agent epratuzumab 360 mg/m2 intravenously (IV) for four doses in an initial cohort, followed by weekly doses of epratuzumab for 4 weeks in combination with standard four-drug re-induction therapy. Among the initial cohort of 15 patients treated, one patient achieved a partial response with single-agent therapy.46 Dose-limiting toxicities were seizures in one patient and liver function test abnormalities in one patient. The addition of epratuzumab to re-induction chemotherapy was well tolerated, with no apparent significant increase in toxicity. Although epratuzumab did not improve the second remission rates, among patients who attained CR, postinduction MRD-negative rates were higher in comparison with those of historical controls treated with chemotherapy alone (42% v 25%).47
Experience With Blinatumomab (CD3+CD19+ Bispecific Monoclonal Antibody)
Blinatumomab belongs to a novel class of bispecific single-chain antibodies that engages T cells with its anti-CD3 arm and redirects them via its anti-CD19 arm to CD19-expressing lymphoblasts. Within minutes after these cells make contact, the T cells become activated and induce perforin-mediated death of the targeted ALL cells. Past experiences indicate poor prognosis for patients with ALL in first CR with persistent MRD unless they promptly undergo allogeneic stem-cell transplantation.48 Topp et al49 treated 21 adults with ALL in first CR but with persistent MRD (> 10−4 by polymerase chain reaction at 16 weeks from the start of induction-consolidation chemotherapy). Blinatumomab was administered at a dose of 15 μg/m2 by continuous infusion over 24 hours daily for 4 weeks with that regimen being potentially repeated after a 2-week hiatus. Sixteen of 21 patients became MRD negative, 12 of whom were molecularly refractory to prior chemotherapy. With a median follow-up of 15 months, the 1-year probability of relapse-free survival was 78% overall and 60% in patients who did not undergo allogeneic stem-cell transplantation.49 More recently, blinatumomab was investigated in adults with active refractory/relapsed ALL. An early analysis reported marrow CRs in 12 of 18 treated patients, with all of them achieving MRD negativity.50 CRs have also been reported in three children with relapsed pre-B ALL after stem-cell transplantation.51 Blinatumomab is currently undergoing pivotal trials in ALL in first CR and in refractory/relapsed ALL with the aim of potential approval of this treatment as single-agent therapy for these conditions.
Experience With Inotuzumab (CD22 monoclonal antibody conjugated to calicheamcin)
Inotuzumab ozogamicin is a CD22 monoclonal antibody bound to calicheamcin. Calicheamcin is a natural product of Micromonospora echinospora and is significantly more toxic than chemotherapy.52,53 It binds to the minor DNA groove and causes double-strand DNA breaks resulting in cell apoptosis. Inotuzumab binds CD22 with subnanomolar affinity, is rapidly internalized, and delivers the conjugated calicheamcin intracellularly. Phase I/II studies showed encouraging activity in lymphomas.54,55 Response rates ranged from 60% to 80% and were durable. The phase II proposed dose schedule was 1.8 mg/m2 IV once every 3 to 4 weeks. Dose-limiting toxicities were myelosuppression and reversible liver function abnormalities. In a phase II study of inotuzumab 1.3 to 1.8 mg/m2 IV once every 3 to 4 weeks in 49 patients with refractory/relapsed ALL, 65% were experiencing their second or beyond salvage treatment regimen, and 30% had Philadelphia chromosome–positive ALL or translocation (4;11). Overall, nine (18%) achieved CR, 14 (29%) had marrow CR without recovery of platelet counts, and five (10%) had marrow CR without recovery of neutrophil or platelet counts.56 The overall response rate was 57%. The treatment was well tolerated, with only two patients (4%) dying within 4 weeks of start of therapy from non–drug-related complications. Among the 28 patients achieving response, 18 had chromosomal abnormalities at the start of therapy and 16 (89%; 43% of all patients) achieved a complete cytogenetic response. Multiparameter flow cytometry for MRD was performed in 27 patients achieving marrow CR; reversal to MRD-negative status was observed in 17 patients (63%). The median survival of all patients was 4.5 months. Among the nine patients achieving CR, the estimated 9-month survival was 78%; among the other 19 patients achieving marrow CR, the median survival was 6.7 months. Twenty-two of the 49 patients were able to proceed to subsequent allogeneic stem-cell transplantation. Adverse effects included liver function abnormalities that were severe in 31% of the patients but were reversible. Five patients undergoing allogeneic stem-cell transplantation developed veno-occlusive disease; this was thought to be possibly related to the preparative regimens, which included N,N′N′-triethylenethiophosphoramide (thioTEPA) and clofarabine. Changing the preparative regimens in subsequent patients resulted in no further instances of veno-occlusive disease. This experience indicated that inotuzumab is a highly active single agent for the treatment of refractory/relapsed ALL. Although responses were short-lasting, this might be because of the refractory nature of the study group. Subsequent studies will evaluate inotuzumab in earlier salvage therapy, in combination with chemotherapy for first-line ALL therapy, for treatment for MRD, and as part of combined monoclonal antibody therapy regimens.
Other Monoclonal Antibodies Conjugated to Cytotoxic Agents
SAR3419 is a humanized IgG1 CD19 monoclonal antibody conjugated to maytansin, a highly potent tubulin inhibitor.57 Studies of SAR3419 in lymphoma showed a response rate of 30% to 40%.58–60 Corneal toxicity was the dose-limiting toxicity but was reversible. A phase II study of SAR3419 administered at a dose of 55 to 70 mg/m2 IV once per week for 4 weeks and then once every other week over a period of 8 weeks is ongoing in adults with refractory/relapsed ALL. CD19 and CD22 monoclonal antibodies conjugated with auristatin, another cytotoxic spindle tubulin inhibitor, are available and would be of interest.
The anti-CD33 conjugate gemtuzumab ozogamicin (Mylotarg; Pfizer) has been used occasionally to treat patients who have ALL with CD33 expression, with anecdotal reports of successful remission induction in that setting.61
Experience With Monoclonal Antibodies Conjugated to Immunotoxins
Immunotoxins are proteins that consist of two primary components: a targeting moiety for cell binding and a bacterial or plant toxin that is internalized and causes cell death. BL22 (CAT-3888) is an anti-CD22 immuotoxin composed of a variable fragment (Fv) derived from a monoclonal antibody directed toward CD22 and fused to a 38-kDa fragment of Pseudomonas aeruginosa exotoxin A (RFB4 [dsFv]-PE38).62 Following preclinical studies demonstrating the cytotoxic effect of BL22 against CD22+ cell lines and leukemic cells from patient samples, BL22 was also found to be highly active in phase I/II human studies in hairy cell leukemia.63 Wayne et al22 evaluated BL22 in a phase I study in childhood ALL. BL22 was administered at doses of 10 to 40 μg/kg every other day for three or six doses every 3 to 4 weeks. No dose-limiting toxicities were noted. Among 23 patients treated, 16 (70%) showed reductions of leukemic blasts; four patients had more than 2-log reductions of circulating blasts, and four patients had normalizations of peripheral blast counts. No objective CRs or partial responses were noted.22
Optimization of the monoclonal antibody structure and more continuous exposure schedules are ongoing efforts and may improve the efficacy of this approach. BL22 has moderate affinity for CD22 (kDa 85 nmol/L). Mutagenesis studies selected an Fv with higher binding affinity (kDa 6 nmol/L) and improved in vitro cytotoxicity against CD22-expressing hematologic malignancies, including ALL.64,65 The new higher-affinity version of BL22 was named HA22 (high-affinity BL22) and later CAT-8015 or moxetumomab pasudotox. Studies with moxetumomab in hairy cell leukemia showed an objective response rate of 84% with 44% CRs.24 Significantly, among 19 evaluable patients with heavily pretreated childhood ALL treated with HA22 5 to 40 μg/kg IV once every other day over 12 days, four patients achieved CRs, one had a partial response, and eight patients had hematologic improvement, for an objective response rate of 26% and an overall clinical activity rate of 68%.66
Other additional monoclonal antibodies directed toward CD19 and CD22 and bound to diphtheria immunotoxin are under investigation in early phase I/II studies. Combotox, which is a combination of anti-CD19 and anti-CD22 deglycosylated ricin A chain immunotoxins, was tested in children with pre-B ALL. Seventeen patients were treated, and a maximum-tolerated dose of 5 mg/m2 was established. Three CRs (18%) were seen, and hematologic activity was noted in other patients.67
Experience With Monoclonal Antibodies Conjugated to Radionuclides
In ALL, radioimmunoconjugates are concentrated in the bone marrow and consequently are associated with severe myelosuppression. Thus, the application of radioimmunotherapy has been limited to myeloablative conditioning before allogeneic stem-cell transplantation.68
FUTURE PROSPECTS
Future studies will establish the role of these monoclonal antibody–based therapies and their toxicity profile not only in the salvage setting but also in first-line therapy for high-risk adult ALL. In standard-risk ALL, the persistence of MRD at 16 weeks from the start of first-line therapy has been associated with a poor outcome with a median time to subsequent relapse of 2 to 6 months and estimated 3-year survival rates of only 10% to 20%.69 Although allogeneic stem-cell transplantation may overcome this adverse outcome in select patients, monoclonal antibody therapies, as has been observed with blinatumomab, offer a less toxic and highly effective alternative approach to eradicate the MRD and prolong relapse-free survival. Older patients with de novo ALL have poor tolerance to current first-line chemotherapy regimens. Despite respectable CR rates of 60% to 80% with modern ALL regimens, the failure rate in these patients is high, with up to 30% of patients dying in CR from complications related to intensive chemotherapy. Investigating low-intensity chemotherapy concurrently with monoclonal antibodies or combinations of monoclonal antibodies with minimal or no chemotherapy in such patients is worthwhile. Similarly, it is a desirable goal to reduce chemotherapy-related toxicities in the pediatric setting. Adding monoclonal antibodies to chemotherapy in first-line treatment of ALL may increase cure rates and obviate the need to further intensify cytotoxic regimens. In this context, measuring response to combinations of monoclonal antibodies with or without chemotherapy in ALL (as was the case with tyrosine kinase inhibitors in chronic myeloid leukemia) may require the assessment of deeper levels of response than are measured by simple morphology and assessment of percentage of blasts. Response evaluation may rely on more precise and quantitative estimations of persistent MRD as measured by multiparameter flow cytometry studies and/or quantification of molecular residual disease. Finally, it is hoped that combinations of monoclonal antibody–based therapies with or without chemotherapy could be a potential future strategy that is curative in ALL, similar to the strategy of nonchemotherapy approaches with the use of all transretinoic acid and arsenic trioxide in acute promyelocytic leukemia.
Footnotes
Supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research, and by National Institutes of Health Grant No. CA016672.
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
A.S.W. is a co-inventor on patents assigned to the National Institutes of Health.
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Employment or Leadership Position: None Consultant or Advisory Role: Deborah Thomas, Pfizer (C), Talon Therapeutics (C) Stock Ownership: None Honoraria: Deborah Thomas, Pfizer Research Funding: Hagop Kantarjian, Micromet, Pfizer, sanofi-aventis; Alan S. Wayne, Cooperative research and development agreement between the National Cancer Institute and MedImmune Expert Testimony: None Other Remuneration: None
AUTHOR CONTRIBUTIONS
Conception and design: Hagop Kantarjian, Deborah Thomas, Alan S. Wayne, Susan O'Brien
Manuscript writing: All authors
Final approval of manuscript: All authors
REFERENCES
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. doi: 10.1056/NEJMra052603. [DOI] [PubMed] [Google Scholar]
- 2.Pui CH, Carroll WL, Meshinchi S, et al. Biology, risk stratification, and therapy of pediatric acute leukemias: An update. J Clin Oncol. 2011;29:551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaynon PS, Trigg ME, Heerema NA, et al. Children's Cancer Group trials in childhood acute lymphoblastic leukemia: 1983-1995. Leukemia. 2000;14:2223–2233. doi: 10.1038/sj.leu.2401939. [DOI] [PubMed] [Google Scholar]
- 4.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 5.Faderl S, O'Brien S, Pui CH, et al. Adult acute lymphoblastic leukemia: Concepts and strategies. Cancer. 2010;116:1165–1176. doi: 10.1002/cncr.24862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gökbuget N, Hoelzer D, Arnold R, et al. Treatment of adult ALL according to protocols of the German multicenter study group for adult ALL (GMALL) Hematol Oncol Clin North Am. 2000;14:1307–1325. doi: 10.1016/s0889-8588(05)70188-x. [DOI] [PubMed] [Google Scholar]
- 7.Huguet F, Leguay T, Raffoux E, et al. Pediatric-inspired therapy in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia: The GRAALL-2003 study. J Clin Oncol. 2009;27:911–918. doi: 10.1200/JCO.2008.18.6916. [DOI] [PubMed] [Google Scholar]
- 8.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 9.Thomas DA, Faderl S, Cortes J, et al. Treatment of Philadelphia chromosome-positive acute lymphocytic leukemia with hyper-CVAD and imatinib mesylate. Blood. 2004;103:4396–4407. doi: 10.1182/blood-2003-08-2958. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi F, O'Brien S, Thomas D, et al. First report of phase 2 study of dasatinib with hyper-CVAD for the frontline treatment of patients with Philadelphia chromosome-positive (Ph+) acute lympoblastic leukemia. Blood. 2010;116:2070–2077. doi: 10.1182/blood-2009-12-261586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bassan R, Rossi G, Pogliani EM, et al. Chemotherapy-phased imatinib pulses improve long-term outcome of adult patients with Philadelphia chromosome-positive acute lymphoblastic leukemia: Northern Italy Leukemia Group protocol 09/00. J Clin Oncol. 2010;28:3644–3652. doi: 10.1200/JCO.2010.28.1287. [DOI] [PubMed] [Google Scholar]
- 12.Schultz KR, Bowman WP, Aledo A, et al. Improved early event-free survival with imatinib in Philadelphia chromosome-positive acute lymphoblastic leukemia: A Children's Oncology Group study. J Clin Oncol. 2009;27:5175–5181. doi: 10.1200/JCO.2008.21.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas DA, Faderl S, O'Brien S, et al. Chemoimmunotherapy with hyper-CVAD plus rituximab for the treatment of adult Burkitt and Burkitt-type lymphoma or acute lymphoblastic leukemia. Cancer. 2006;106:1569–1580. doi: 10.1002/cncr.21776. [DOI] [PubMed] [Google Scholar]
- 14.Gokbuget N, Hoelzer D. Rituximab in the treatment of adult ALL. Ann Hematol. 2006;85:117–119. [Google Scholar]
- 15.Thomas D, O'Brien S, Kantarjian HM. Monoclonal antibody therapy with rituximab for acute lymphoblastic leukemia. Hematol Oncol Clin North Am. 2009;23:949–971. doi: 10.1016/j.hoc.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Piccaluga PP, Arpinati M, Candoni A, et al. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52:325–327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 17.Raponi S, De Propris MS, Intoppa S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: Analysis of 52 cases. Leuk Lymphoma. 2011;52:1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 18.Hoelzer D, Gökbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26:25–32. doi: 10.1016/j.blre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Dworzak MN, Schumich A, Printz D, et al. CD20 up-regulation in pediatric B-cell precursor acute lymphoblastic leukemia during induction treatment: Setting the stage for anti-CD20 directed immunotherapy. Blood. 2008;112:3982–3988. doi: 10.1182/blood-2008-06-164129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watt T, Park S, Cooper T. CD20 up-regulation in induction therapy for childhood B lymphoblastic leukemia. Blood. 2010;116 (abstr 2124) [Google Scholar]
- 21.Dworzak MN, Gaipa G, Schumich A, et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: Evidence for a drug-induced regulatory phenomenon—Results of the AIEOP-BFM-ALL-FLOW-MRD-Study Group. Cytometry B Clin Cytom. 2010;78:147–153. doi: 10.1002/cyto.b.20516. [DOI] [PubMed] [Google Scholar]
- 22.Wayne AS, Kreitman RJ, Findley HW, et al. Anti-CD22 immunotoxin RFB4 (dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: Preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16:1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Du X, Beers R, Fitzgerald DJ, et al. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68:6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.FitzGerald DJ, Wayne AS, Kreitman RJ, et al. Treatment of hematologic malignancies with immunotoxins and antibody-drug conjugates. Cancer Res. 2011;71:6300–6309. doi: 10.1158/0008-5472.CAN-11-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palanca-Wessels MC, Press OW. Improving the efficacy of radioimmunotherapy for non-Hodgkin lymphomas. Cancer. 2010;116:1126–1133. doi: 10.1002/cncr.24801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haining WN, Cardoso AA, Keczkemethy HL, et al. Failure to define window of time for autologous tumor vaccination in patients with newly diagnosed or relapsed acute lymphoblastic leukemia. Exp Hematol. 2005;33:286–294. doi: 10.1016/j.exphem.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 27.Coiffier B, Thieblemont C, Van Den Neste E, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: A study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116:2040–2045. doi: 10.1182/blood-2010-03-276246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–1764. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 29.Elter T, Gercheva-Kyuchukova L, Pylylpenko H, et al. Fludarabine plus alemtuzumab versus fludarabine alone in patients with previously treated chronic lymphocytic leukemia: A randomised phase 3 trial. Lancet Oncol. 2011;12:1204–1213. doi: 10.1016/S1470-2045(11)70242-X. [DOI] [PubMed] [Google Scholar]
- 30.Hoelzer D, Gökbuget N, Beck J, et al. Subtype adjusted therapy improves outcome of elderly patients with acute lymphoblastic leukemia. Blood. 2004;104 (suppl 1; abstr 2732) [Google Scholar]
- 31.Rizzieri DA, Johnson JL, Byrd JC, et al. Efficacy and toxicity of rituximab and brief duration, high intensity chemotherapy with filgrastim support for Burkitt or Burkitt-like leukemia/lymphoma: Cancer and Leukemia Group B (CALGB) study 10002. Blood. 2010;116 doi: 10.1111/bjh.12736. (abstr 858) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas DA, O'Brien S, Faderl S, et al. Chemoimmunotherapy with a modified hyper-CVAD and rituximab regimen improves outcome in de novo Philadelphia chromosome-negative precursor B-lineage acute lymphoblastic leukemia. J Clin Oncol. 2010;28:3880–3889. doi: 10.1200/JCO.2009.26.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoelzer D, Huettmann A, Kaul F, et al. Immunochemotherapy with rituximab improves molecular CR rate and outcome in CD20+ B-lineage standard and high risk patients: Results of 263 CD20+ patients studied prospectively in GMALL study 07/2003. Blood. 2010;116 (abstr 170) [Google Scholar]
- 34.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: A report from the Children's Oncology Group. Pediatr Blood Cancer. 2009;52:177–181. doi: 10.1002/pbc.21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiramizu B, Goldman S, Kusao I, et al. Minimal disease assessment in the treatment of children and adolescents with intermediate-risk (Stage III/IV) B-cell non-Hodgkin lymphoma: A Children's Oncology Group report. Br J Haematol. 2011;153:758–763. doi: 10.1111/j.1365-2141.2011.08681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Corbacioglu S, Eber S, Gungor T, et al. Induction of long-term remission of a relapsed childhood B-acute lymphoblastic leukemia with rituximab chimeric anti-CD20 monoclonal antibody and autologous stem cell transplantation. J Pediatr Hematol Oncol. 2003;25:327–329. doi: 10.1097/00043426-200304000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Claviez A, Eckert C, Seeger K, et al. Rituximab plus chemotherapy in children with relapsed or refractory CD20-positive B-cell precursor acute lymphoblastic leukemia. Haematologica. 2006;91:272–273. [PubMed] [Google Scholar]
- 38.Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol. 2010;28:3525–3530. doi: 10.1200/JCO.2010.27.9836. [DOI] [PubMed] [Google Scholar]
- 39.Wierda W, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1749–1755. doi: 10.1200/JCO.2009.25.3187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keating MJ, Flinn I, Jain V, et al. Therapeutic role of alemtuzumab (Campath-1H) in patients who have failed fludarabine: Results of a large international study. Blood. 2002;99:3554–3561. doi: 10.1182/blood.v99.10.3554. [DOI] [PubMed] [Google Scholar]
- 41.Hillmen P, Skotnicki AB, Robak T, et al. Alemtuzumab compared with chlorambucil as first-line therapy for chronic lymphocytic leukemia. J Clin Oncol. 2007;25:5616–5623. doi: 10.1200/JCO.2007.12.9098. [DOI] [PubMed] [Google Scholar]
- 42.Stock W, Sanford B, Lozanski G, et al. Alemtuzumab can be incorporated into front-line therapy of adult acute lymphoblastic leukemia (ALL): Final phase I results of a Cancer and Leukemia Group B study (CALGB 10102) Blood. 2009;114:22. (abstr 838) [Google Scholar]
- 43.Tibes R, Keating MJ, Ferrajoli A, et al. Activity of alemtuzumab in patients with CD52-positive acute leukemia. Cancer. 2006;106:2645–2651. doi: 10.1002/cncr.21901. [DOI] [PubMed] [Google Scholar]
- 44.Angiolillo AL, Yu AL, Reaman G, et al. A phase II study of Campath-1H in children with relapsed or refractory acute lymphoblastic leukemia: A Children's Oncology Group report. Pediatr Blood Cancer. 2009;53:978–983. doi: 10.1002/pbc.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Laporte JP, Isnard F, Garderet L, et al. Remission of adult acute lymphocytic leukemia with alemtuzumab. Leukemia. 2004;18:1557–1558. doi: 10.1038/sj.leu.2403422. [DOI] [PubMed] [Google Scholar]
- 46.Raetz EA, Cairo MS, Borowitz MJ, et al. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: A Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26:3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Raetz AE, Cairo MS, Borowitz MJ, et al. Reinduction chemoimmunotherapy with epratuzumab in relapsed acute lymphoblastic leukemia (ALL) in children, adolescents and young adults: Results from Children's Oncology Group (COG) study ADVL04P2. Blood. 2011;118 doi: 10.1002/pbc.25454. (abstr 573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brüggemann M, Raff T, Flohr T, et al. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107:1116–1123. doi: 10.1182/blood-2005-07-2708. [DOI] [PubMed] [Google Scholar]
- 49.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 50.Topp MS, Goekbuget N, Zugmaier G, et al. Anti-CD19 BiTE blinatumomab induces high complete remission rate in adult patients with relapsed B-precursor ALL: Updated results of an ongoing phase II trial. Blood. 2011;118 (abstr 252) [Google Scholar]
- 51.Handgretinger R, Zugmaier G, Henze G, et al. Complete remission after blinatumomab-induced donor T-cell activation in three pediatric patients with post-transplant relapsed acute lymphoblastic leukemia. Leukemia. 2011;25:181–184. doi: 10.1038/leu.2010.239. [DOI] [PubMed] [Google Scholar]
- 52.Thorson JS, Sievers EL, Ahlert J, et al. Understanding and exploiting nature's chemical arsenal: The past, present and future of calicheamicin research. Cur Pharm Des. 2000;6:1841–1879. doi: 10.2174/1381612003398564. [DOI] [PubMed] [Google Scholar]
- 53.Ricart AD. Antibody-drug conjugates of calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab ozogamicin. Clin Cancer Res. 2011;17:6417–6427. doi: 10.1158/1078-0432.CCR-11-0486. [DOI] [PubMed] [Google Scholar]
- 54.Advani A, Coiffier B, Czuczman MS, et al. Safety, pharmacokinetics, and preliminary clinical activity of inotuzumab ozogamicin, a novel immunoconjugate for the treatment of B-cell non-Hodgkin's lymphoma: Results of a phase I study. J Clin Oncol. 2010;28:2085–2093. doi: 10.1200/JCO.2009.25.1900. [DOI] [PubMed] [Google Scholar]
- 55.Di Joseph JF, Dougher MM, Kalyandrug LB, et al. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin's B-cell lymphoma. Clin Cancer Res. 2006;12:242–249. doi: 10.1158/1078-0432.CCR-05-1905. [DOI] [PubMed] [Google Scholar]
- 56.Kantarjian H, Thomas D, Jorgensen J, et al. Inotuzumab ozogamicin, an anti-CD22 calecheamicin conjugate, for refractory and relapsed acute lymphocytic leukaemia: A phase 2 study. Lancet Oncol. 2012;13:403–411. doi: 10.1016/S1470-2045(11)70386-2. [DOI] [PubMed] [Google Scholar]
- 57.Blanc V, Bousseau A, Caron A, et al. SAR3419: An anti-CD19-Maytansinoid Immunoconjugate for the treatment of B-cell malignancies. Clin Cancer Res. 2011;17:6448–6458. doi: 10.1158/1078-0432.CCR-11-0485. [DOI] [PubMed] [Google Scholar]
- 58.Coiffier B, Ribrag V, Dupuis J, et al. Phase I/II study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered weekly to patients (pts) with relapsed/refractory B-cell non-Hodgkin's lymphoma (NHL) J Clin Oncol. 2011;29:508s. (suppl; abstr 8017) [Google Scholar]
- 59.Polson AG, Ho WY, Ramakrishnan V. Investigational antibody-drug conjugates for hematological malignancies. Expert Opin Investig Drugs. 2011;20:75–85. doi: 10.1517/13543784.2011.539557. [DOI] [PubMed] [Google Scholar]
- 60.Younes A, Gordon L, Kim S, et al. Phase I multi-dose escalation study of the anti-CD19 maytansinoid immunoconjugate SAR3419 administered by intravenous (IV) infusion every 3 weeks to patients with relapsed/refractory B-cell non-Hodgkin's lymphoma (NHL) Blood. 2009;114 doi: 10.1200/JCO.2011.39.4403. (abstr 585) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zwaan CM, Reinhardt D, Jürgens H. Gemtuzumab ozogamicin in pediatric CD33-positive acute lymphoblastic leukemia: First clinical experiences and relation with cellular sensitivity to single agent calicheamicin. Leukemia. 2003;17:468–470. doi: 10.1038/sj.leu.2402749. [DOI] [PubMed] [Google Scholar]
- 62.Kreitman RJ, Pastan I. Antibody fusion proteins: Anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res. 2011;17:6398–6405. doi: 10.1158/1078-0432.CCR-11-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kreitman RJ, Stetler-Stevenson M, Margulies I, et al. Phase II trial of recombinant immunotoxin RFB4 (dsFv)-PE38 (BL22) in patients with hairy cell leukemia. J Clin Oncol. 2009;27:983–990. doi: 10.1200/JCO.2008.20.2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Salvatore G, Beers R, Margulies I, et al. Improved cytotoxic activity toward cell lines and fresh leukemia cells of a mutant anti-CD22 immunotoxin obtained by antibody phage display. Clin Cancer Res. 2002;8:995–1002. [PubMed] [Google Scholar]
- 65.Mussai F, Campana D, Bhojwani D, et al. Cytotoxicity of the anti-CD22 immunotoxin HA22 (CAT-8015) against paediatric acute lymphoblastic leukaemia. Br J Haematol. 2010;150:352–358. doi: 10.1111/j.1365-2141.2010.08251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wayne AS, Bhojwani D, Silverman LB, et al. A novel anti-CD22 immunotoxin, moxetumomab pasudotox: Phase I study in pediatric acute lymphoblastic leukemia (ALL) Blood. 2011;118 doi: 10.1182/blood-2017-02-749101. (abstr 248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrera L, Bostrom B, Gore L, et al. A phase I study of Combotox in pediatric patients with refractory B-lineage acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2009;31:936–941. doi: 10.1097/MPH.0b013e3181bdf211. [DOI] [PubMed] [Google Scholar]
- 68.Matthews DC, Appelbaum FR, Eary JF, et al. Development of a marrow transplant regimen for acute leukemia using targeted hematopoietic irradiation delivered by 131I-labeled anti-CD45 antibody, combined with cyclophosphamide and total body irradiation. Blood. 1995;85:1122–1131. [PubMed] [Google Scholar]
- 69.Gökbuget N, Kneba M, Raff T, et al. Adults with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. doi: 10.1182/blood-2011-09-377713. doi: 10.1182/blood-2011-09-377713 [epub ahead of print on March 22, 2012] [DOI] [PubMed] [Google Scholar]