Abstract
Children with dyslexia often exhibit increased variability in sensory and cognitive aspects of hearing relative to typically developing peers. Assistive listening devices (classroom FM systems) may reduce auditory processing variability by enhancing acoustic clarity and attention. We assessed the impact of classroom FM system use for 1 year on auditory neurophysiology and reading skills in children with dyslexia. FM system use reduced the variability of subcortical responses to sound, and this improvement was linked to concomitant increases in reading and phonological awareness. Moreover, response consistency before FM system use predicted gains in phonological awareness. A matched control group of children with dyslexia attending the same schools who did not use the FM system did not show these effects. Assistive listening devices can improve the neural representation of speech and impact reading-related skills by enhancing acoustic clarity and attention, reducing variability in auditory processing.
Children with dyslexia, reading impairment not caused by deficits in ability or opportunity (1), often have difficulties with orienting and maintaining attention (2, 3). Although the ability to direct attention is still developing during the elementary school years (4), dyslexics have poorer task-dependent attentional shifting in both auditory and visual modalities than their typically developing peers even into adulthood (2, 3). These deficits may impact and be impacted by heightened variability in sensory processes, such as inconsistent representations of speech by the auditory nervous system, and could contribute to documented impairments in auditory processing (5–7) and difficulty with meaningfully disambiguating speech sounds (8). Children with dyslexia can exhibit abnormal subcortical processing of speech, particularly in response to acoustic elements crucial for differentiating speech sounds (9–11). Deficient auditory sensory representation and unsuccessful disambiguation of speech likely contribute to the well-documented impairments in phonological awareness and phonological memory seen in children with dyslexia (12–14), with auditory processing skills in prereaders predicting later language skill (15, 16). Because the auditory system integrates both sensory and cognitive facets of hearing, we suggest that through repeated, impaired interaction with sound, children with dyslexia can develop abnormal sensory representations of speech as well as abnormal cognitive skills for the interpretation of speech. For example, a child who repeatedly misperceives the sounds “cat” as “bat” or “pat” fails to make a robust sound-to-meaning connection between those sounds and their referent. However, because of this same integrative nature of the auditory system, deficient function can be improved with auditory training.
Auditory perception and neurophysiology can be altered with auditory training (17–23). These changes can be traced directly to cross-cortical and descending cortical influence on neural receptivity in animal models and are driven by the behavioral importance of sounds (18, 24). In humans, attention and working memory are important components of training-related changes (25) and may serve to direct descending cortical influence on auditory sensory function. Computer-based perceptual games, musical training, and language learning can provide effective training for children with developmental learning disorders, such as dyslexia, because they actively engage attention to sound. Classroom assistive listening devices, which can be worn throughout the school day, can also improve auditory processing by engendering enhancements in attention, as reported by both teachers and students (26–28). Assistive listening devices (i.e., classroom FM systems) also result in neurophysiologic enhancements in response to attended vs. ignored sounds (29). Such systems increase the signal-to-noise ratio of the speaker of interest (e.g., the teacher) (30) and create a more stable acoustic input by reducing the impact of background noise on the most vulnerable portion of speech sounds (31). These acoustic enhancements, along with accompanying improvements in auditory attention, lead to boosts in academic achievement, literacy, and phonological awareness, with the greatest benefits seen for children with learning impairments (32–34).
What are the biological mechanisms by which classroom FM system use improves auditory attention and phonological awareness in children with dyslexia? How might these benefits translate to the neural representation of speech? Here, we investigated the impact of classroom FM system use on auditory brainstem encoding of stop consonants, which can be deficient in children with dyslexia. Auditory brainstem function is stable from test to retest in the absence of intervention (35, 36), but can be altered by short-term auditory training (19, 20, 22), lifelong experience such as musical training and language experience (37, 38), and directed attention (39). Here we assessed auditory brainstem responses and reading performance in children with dyslexia before and after classroom FM system use for one academic year. We hypothesized that enhanced neural consistency would accompany improvement in reading skills in children wearing the FM systems but not in a control group of dyslexic children in the same classrooms who did not wear assistive listening devices. We further hypothesized that neural consistency would improve pervasively throughout the recording session and not simply offset neural fatigue.
Results
FM System Use Benefits Reading and Related Skills.
We assessed reading ability, phonological awareness, and auditory brainstem function in response to speech in 38 normal hearing children with dyslexia (ages 8–14 y, 16 girls). The majority of children (n = 31) attended private schools for children with severe reading and learning impairments that provide rich academic environments through the use of technology and individualized instruction. Nineteen children wore a personal FM system (similar to a Bluetooth receiver) throughout the school day for the duration of one academic year (Phonak EduLink). Similar to previous findings of improved literacy and related skills with FM system use (32, 33), children who used the FM system improved on phonological awareness and reading [Comprehensive Test of Phonological Processing (CTOPP) phonological awareness, t18 = −5.255, P < 0.001; Woodcock–Johnson (WJ)-III basic reading, t17 = −3.118, P = 0.006]. There were no significant correlations between pretest reading scores and change in reading scores for the dyslexic FM users (CTOPP phonological awareness, ρ = −0.323, P = 0.178; WJ-III basic reading, ρ = −0.399, P = 0.178), supporting that changes in reading ability were due to active intervention with FM system use and not regression to the mean. The matched control group of 19 children with dyslexia attending the same schools who did not use the FM systems did not improve on either of these measures (Table S1). These results are consistent with those of Flexer and colleagues (32) who reported that children using an FM system showed greater gains in reading-related skills than children in the same academic environment.
FM System Use Improves the Consistency of Neural Responses to Sound.
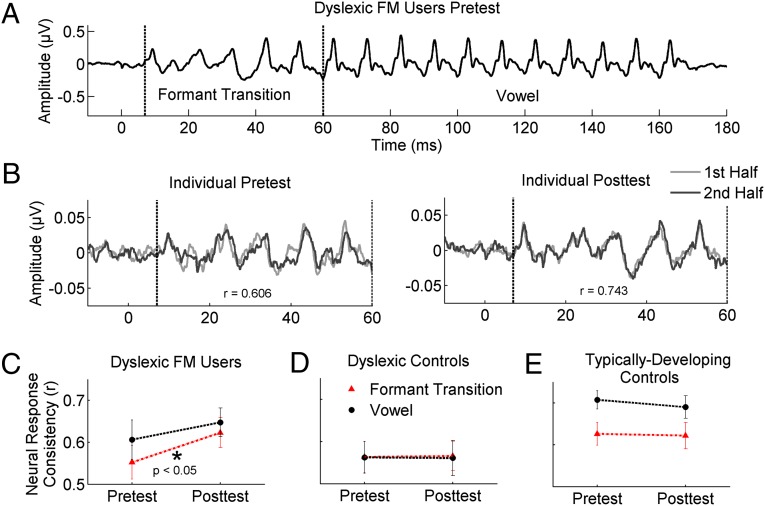
After children used the FM system for 1 y, their auditory brainstem responses to speech became more consistent, as evidenced by a larger correlation between the first and second halves of the recording (Fig. 1 A and B). More consistent responses are reflected by r-values closer to 1. Improvement in neural response consistency was observed for the response to the formant transition of the speech syllables (7–60 ms; t18 = −2.260, P = 0.036) but not to the steady-state vowel (60–180 ms; t18 = −1.173, P = 0.256; Fig. 1C). No changes were seen for the matched control group who did not use FM systems (7–60 ms, t18 = −0.170, P = 0.867; 60–180 ms, t18 = −0.180, P = 0.859; Fig. 1D). The formant transition, which uniquely distinguishes the three stop-consonant syllables, is composed of rapid spectrotemporal changes and is crucial for the discrimination and identification of stop consonants (11). Additionally, it is within the response to the formant transition period and not the steady-state vowel that neural encoding deficits are seen in children with dyslexia (9, 10). Thus, classroom FM system use yielded improvements in the consistency of the neural representation of dynamic components of speech important for distinguishing consonants.
Fig. 1.
FM system use increases the consistency of auditory brainstem representation of speech. (A) Responses from dyslexic children before FM system use with the formant transition (7–60 ms) and vowel (60–180 ms) portions of the speech syllables marked. (B) Response consistency, quantified as the correlation between responses collected during the first half of the recording session (light gray) and those collected in the second half of the recording session (dark gray), improved with FM system use during the formant transition for this representative individual. The more consistent response at posttest is reflected by a higher r-value. (C) After FM system use for one school year, children with dyslexia had more consistent speech-evoked brainstem responses, particularly for the response to the formant transition (red triangles; trending at P = 0.036). Significant changes were not seen in response to the vowel portion (black circles). (D) Control children with dyslexia who did not use the FM systems show no change in response consistency in either the formant transition (red triangles) or the vowel (black circles) portions of the response. (E) Response consistency also does not change for typically developing controls in either the formant transition (red triangles) or the vowel (black circles) portions.
Benefits with FM system use were not driven by changes in ongoing neural activity, as neither prestimulus amplitude at pretest nor change in prestimulus amplitude predicted change in phonological awareness (ρ = 0.119, P = 0.626 and ρ = −0.187, P = 0.443, respectively) or response consistency (ρ = 0.018, P = 0.943 and ρ = −0.121, P = 0.622, respectively). Therefore, the benefits of FM system use are particular to the consistency of the response to sound and not a reduction in general neurophysiologic noise.
Improvement in Neural Consistency Is Greatest for “Learners.”
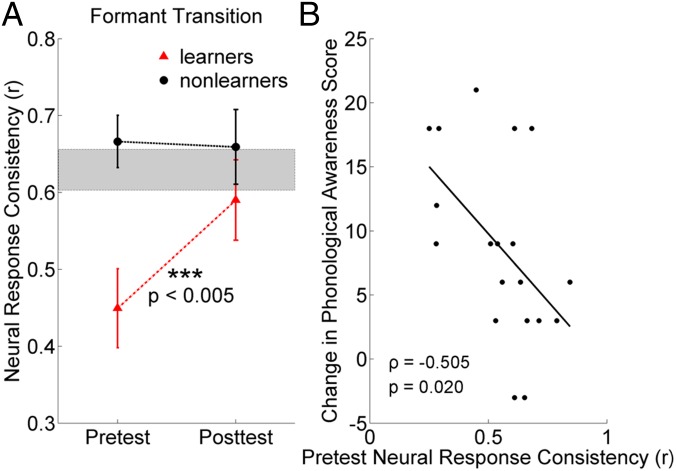
Although training-related neural changes were evident at the group level, the effects were driven by a subset of participants who showed the greatest improvements in phonological awareness. Participants were classified as learners if their improvement on CTOPP phonological awareness was greater than eight standard score points (n = 10, M = 14.10, SD = 4.9); “nonlearners” did not meet this criterion (n = 9, M = 2.67, SD = 3.5). Learners had significantly poorer subcortical response consistency in the formant transition relative to an age- and sex-matched group of typically developing children (n = 26; SI Materials and Methods) at pretest (t35 = −3.207, P = 0.003); however, they did not differ from typically developing peers at posttest (t35 = 0.589, P = 0.559; Fig. 2A). Nonlearners did not differ from typically developing peers in response consistency at either pre- or posttest (pretest, t34 = 0.630, P = 0.533; posttest, t34 = 0.581, P = 0.565; Fig. 2A). Responses of learners became more consistent (t9 = −4.033, P = 0.003), whereas the responses of nonlearners did not (t8 = 0.047, P = 0.964; Fig. 2A). Although causality cannot be determined, this outcome suggests that changes in response consistency and changes in phonological awareness skills are linked, supported by a marginally significant correlation between changes in the two variables across the whole group of FM users (ρ = 0.430, P = 0.066). It appears that as the auditory brainstem becomes better able to consistently represent speech syllables, phonological awareness skills likewise improve.
Fig. 2.
Changes in neural consistency are greatest for children with the largest improvements in phonological awareness. (A) When children in the FM group were divided into “learners” and “nonlearners” on the basis of their improvement in phonological awareness, learners (more than eight points improvement) showed significant gains in neural response consistency during the formant transition. Initially poorer in response consistency than typically developing peers (gray block, mean ± 1 SEM) at pretest, learners (red triangles) were not different from typically developing peers at posttest. Nonlearners (black circles) showed no change in neural response consistency during the formant transition with FM system use and were not different from typically developing children at pretest or posttest. (B) Across the whole group of FM system users, poorer response consistency during the formant transition at pretest predicted the greatest improvements in phonological awareness with FM system use. Note that the change in phonological awareness score was calculated as posttest minus pretest so a larger number represents improvement.
Neural Metrics Predict Benefit from FM System Use.
In FM system users, there was a trend that pretest response consistency predicted the amount of improvement in phonological awareness, with worse response consistency at pretest predicting the greatest gains in phonological awareness (ρ = −0.530, P = 0.020; Fig. 2B). Given that children with the poorest response consistency at pretest had the greatest to gain with FM system use, assistive listening devices may be particularly effective for children with the greatest variability in auditory processing.
Mechanisms of Neural Response Consistency.
The consistency, or lack thereof, of the neural response may be due to at least two mechanisms. Inconsistent responses may be due to neural fatigue over the course of the experiment, reflected by weaker responses during the second half of the recording session relative to the first. Alternatively and/or additionally, responses may be variable trial by trial, irrespective of the time of assessment during the recording. Our initial results showed improvements in response consistency from the first half of the recording to the second half with FM system use. We see nearly identical improvement in neural consistency when we calculate response consistency over the even-numbered events throughout the recording relative to the odd-numbered events (FM group, t18 = −1.895, P = 0.074; learners, t9 = −5.645, P < 0.001). Because the consistency of the response improves when sampling throughout the recording session, this result suggests that the observed enhancements of response consistency with classroom FM system use did not reflect a decrease in neural fatigue, but rather an improvement of trial-by-trial consistency.
Neural Effects Are Not Seen for the Academic Environment or Maturation Alone.
There were no neural changes seen for the matched group of children with dyslexia who primarily attended the same schools or for an age- and sex-matched group of 26 typically developing children. The absence of changes in the control groups suggests that, without intervention, neural response consistency is not expected to change in either typically developing or dyslexic children. Thus, the benefits seen here were specific to classroom FM system use and are greater than may be expected from an enhanced academic environment or maturation alone. See SI Materials and Methods for additional participant information and SI Results and Table S1 for statistical comparisons.
Discussion
Children with dyslexia who used classroom assistive listening devices (FM systems) had more consistent auditory brainstem responses to speech after 1 year, a change not seen for children in the same classrooms who did not use the assistive listening devices. The consistency of the neural representation of speech before FM use was predictive of the improvement in phonological awareness after FM use, and children with the greatest improvement in phonological awareness showed the greatest improvement in neural response consistency. It appears that neural response variability constituted an obstacle in the effective processing of sound in the development of reading skills for these children, an obstacle that was alleviated by enhanced signal quality and greater interactions with the meaningful speech of teachers.
We suggest that inconsistent neural processing of sound underlies and reflects the variability in auditory processing that is common in children with dyslexia. Children with the poorest performance on auditory discrimination tasks are often those with the greatest variability in performance (40). Neural correlates of this variability were seen previously for auditory cortical responses to trains of speech syllables presented in noise, where children with learning impairments had reduced consistency of responses over the course of the syllable train relative to typically developing children (41). Evidence from the auditory brainstem also suggests more variable auditory processing in children with dyslexia who, unlike typically developing children, profit less from repeating elements in the acoustic environment (42). Reduced response consistency may reflect less synchronous neural activity (43) and contribute to excessively delayed auditory brainstem responses to speech sounds in children with reading and learning impairments (10, 44, 45).
Increases in neural consistency were seen solely in response to the formant transition of the speech stimuli, the most acoustically dynamic portion of the syllables. This finding is not unexpected for a host of reasons. First, the spectrotemporal changes present in the formant transition aid in the meaningful disambiguation of speech sounds (11). Impaired speech sound identification may contribute to impaired phonological awareness and memory skills, thought to be strong contributing factors to the poor literacy of children with dyslexia (12–14). Second, FM system use may preferentially impact the formant transition portions of speech, which are particularly vulnerable to the degrading effects of background noise (31). Third, it is in response to this complex portion of a syllable that children with dyslexia show deficient neural encoding of timing and harmonic aspects of speech relative to their typically developing peers (9, 10). Therefore, the benefits seen with FM system use for consistency of the response to the formant transition may stem from its enhancement of the signal-to-noise ratio of this region, which is notoriously difficult for poor readers to represent. We hypothesize that linguistic analysis is more successful when the auditory brainstem responds more stably to this spectrotemporally complex portion. In support of this, we found that increased consistency of the neural response was linked to improvement in phonological awareness, suggesting that, although causality cannot be determined, more faithful representations of the linguistically meaningful stimulus acoustics were used by higher-order systems. As a result, phonological representations and/or phonological memory skills benefited. Because changes were also seen for reading ability, it would appear that changes to phonological representations and/or memory were pervasive and yielded benefits for literacy. As children with dyslexia and other communication impairments are known to have deficient auditory function linked to impaired phonological processing and reading skills (5, 6, 12–16), it is encouraging that enhanced auditory stability of vulnerable speech components contributes to improvements in reading and related skills.
We conjecture that assistive listening devices resulted in neural and behavioral changes through improved clarity of the acoustic signal and a subsequent enhancement of auditory attention. FM systems enhance the signal-to-noise ratio of the teacher’s voice, which may contribute to the arousal, orientation, and selection of attention by the students. We speculate that in this way a portion of the cognitive burden of attending is lessened. Reducing the cognitive load of attending by providing an improved auditory signal may allow for greater resources to be allocated to the meaningful speech of the teacher and the academic curriculum, increasing motivation for classroom-related activities and enhancing students’ opportunities for reading improvements (26). Among children in the current study who used FM systems, there was a trend that greater improvement in brainstem response consistency after FM system use was linked to reduced inattentiveness via a parent-rating scale [Attention Deficit Hyperactivity Disorder (ADHD) Rating Scale IV: ρ = −0.403, P = 0.087]. Because this correlation exists only within the group of children who used FM systems, any biases about their children using the devices were likely the same across parents. Additionally, parents did not interact with their children while the children used the devices in class; instead, their assessment is based on their experience with their child’s everyday attention skills. There is previous evidence of changes in physiological responses reflecting attention for children using FM systems (29); however, the present study included only parental assessments of attention behavior and not neurophysiologic or behavioral reaction-time data to support changes in attention. Future studies should include these measures in conjunction with subcortical and behavioral assessments.
The enhanced signal-to-noise ratio provided by the FM system may improve auditory brainstem function by providing the nervous system with a clearer acoustic signal. Making the signal more robust (particularly the acoustically complex formant transition) facilitates the listener’s awareness of these sounds and decreases the impact of background noise on perception. A clearer acoustic signal and greater awareness of sound would foster the development of robust sound-to-meaning relationships and could, via corticofugal mechanisms, lead to more precise temporal encoding of sound. Importantly, the FM systems were not used during testing; instead they engendered a lasting change in brainstem function by enhancing signal-to-noise ratio over the course of the school year. Auditory brainstem responses become less synchronous in the presence of background noise (46), particularly for children with dyslexia who are more adversely impacted by background noise than their typically developing peers (47–49). Decreasing the impact of background noise on the acoustic signal would relieve responses from degradation and could serve to enhance response stability even in the absence of noise, particularly for reading-impaired children who likely suffer even when small amounts of background noise are present. We suggest the interaction of attention and enhanced signal quality led to improved experience with meaningful speech that likely contributed to the increase in neural consistency seen here and impacted performance in phonological awareness and reading.
As the auditory nervous system reflects both the sensory and the cognitive contributions to hearing, such as auditory attention, it is likely that repeated interaction with inconsistent representations of sounds reinforces variable auditory perceptual skills in children with dyslexia. Increased neural variability in response to speech, on a trial-by-trial basis throughout the recording session, appears to provide a biological foundation for dyslexia in some children. The present results suggest that active engagement with sound, specifically the meaningful speech of a teacher, and increased participation in an academic curriculum can reduce auditory processing variability in children with dyslexia. Decreases in auditory variability were seen electrophysiologically, suggesting a fundamental change in how the auditory system represents and accesses speech. Improvements in the consistency of neural responses to speech were linked to improvements in reading-related skills and may indicate that enhanced sensory representation of the contrastive elements of speech contribute to improved reading ability, even for children with pervasive reading impairments. Increases in the consistency of neural responses to the most acoustically complex and dynamic portion of speech sounds were seen for an intervention program that additionally increases student motivation and attention in class, has documented benefits for literacy, can be worn throughout the school day without interruption to the academic plan, and can be easily and immediately implemented. Through enhanced signal perception, active engagement with sound, and reduced cognitive burden of attending, children with dyslexia achieved gains in reading-related skills and exhibited auditory neuroplasticity with classroom FM system use.
Materials and Methods
Participants.
A total of 38 dyslexic children ages 8–14 y participated in this study, divided into an FM-using group and a control group. All participants had normal hearing, defined as air-conduction thresholds <20 dB hearing level (HL) for pure tones at octave intervals from 250 to 8,000 Hz with air-bone threshold gaps <10 dB for pure tones from 500 to 4,000 Hz, click-evoked brainstem responses [100-μs stimulus presented at 31.3 Hz and 80 dB sound pressure level (SPL)] within laboratory-internal norms for 8- to 12-y olds, 95% confidence intervals for full-scale intelligence quotients (IQs) including scores >80 on the Wechsler Abbreviated Scale of Intelligence (50), and no current or prior neurological disorders. Children were classified as dyslexic by fulfilling the following two criteria: They must score below 100 or more than 15 points lower than their full-scale IQ on the Test of Silent Word Reading Fluency (51) or the Sight subtest of the Test of Oral Word Reading Efficiency (52) and they must be diagnosed with a learning, reading, and/or attention disorder by a professional. Thirty-two children met the criteria for both behavioral tests. Additionally, 21 children had at least one parent or sibling who was diagnosed with a learning, reading, and/or attention disorder. Eighteen children had diagnosed attention disorders, but only 2 did not have diagnosed reading or learning impairments. All but 7 children attended the Hyde Park Day Schools in Chicago, IL, a system of prestigious, private institutions for children with severe reading impairments that were not adequately remediated by the students’ home schools. The goal of the school system is to return children to their home school within approximately 2 y of enrollment and thus the academic environment is enriched and has very individual-focused instructional techniques. The children not attending the Hyde Park Day Schools had recently transitioned out of the schools (n = 2) or were siblings of participants in the study who did (n = 5) and were part of both the control (n = 5) and the FM-using groups (n = 2).
Children were quasi-randomly assigned to FM system use or control groups on the basis of the date they entered the study, with an attempt to control the proportion of males and females and average age in each group. The FM group was slightly older than the control group of children with dyslexia (mean difference, 13 mo; t36 = −2.061, P = 0.047) and had a slightly longer test–retest interval (mean difference, 1.6 mo; t36 = −2.985, P = 0.005). The two groups did not differ on IQ (t36 = 1.118, P = 0.271). Although the control group had more boys than girls (13 boys, 6 girls), the difference in numbers was not statistically significant (χ2 = 2.579, P = 0.108). There were equal numbers of boys and girls in the FM group (10 boys, 9 girls; χ2 = 0.053, P = 0.819). The 18 children with comorbid attention disorders were divided equally among the two groups. All children participated in the testing battery during the summer (pretest), progressed through one academic year, and returned for the same testing battery the following summer (posttest). During the intervening school year, control children with dyslexia had no additional intervention beyond their academic environment whereas children in the FM group wore Phonak EduLinks bilaterally for the entire school year. The Phonak EduLinks were fitted by the first author along with oversight from a Phonak employee and were worn bilaterally at full volume by all students. Teachers wore the Campus STM transmitter and FM systems were used in classes that were primarily lecture based, on average half of the school day (∼4 h). Children were not required to wear the FM system during silent study periods, during classes involving physical activity such as physical education and occupational therapy, or during standardized assessment periods unless requested by the teacher or student. The FM systems were not used during the testing battery in the laboratory. The first author conducted training sessions for both the students and the teachers at the start of the academic year and assessed compliance and technical upkeep through weekly visits. Compliance was reported to be generally good and over the 8 mo of the academic year, each child used the device for an estimated 420 h.
Behavioral Assessments.
The Wechsler Abbreviated Scale of Intelligence (50), the Sight Word subtest of the Test of Oral Word Reading Efficiency (52), and the Test of Silent Word Reading Fluency (51) were used as inclusion measures (as described above). The first two subtests of the CTOPP (53) were administered, yielding the phonological awareness cluster score. The Word Attack and Letter/Word Identification subtests of the Woodcock–Johnson III Tests of Achievement Battery (54) were administered to yield the basic reading composite score. Attention deficits were assessed via parent report with the ADHD Rating Scale IV (55).
Electrophysiological Recordings.
Stimuli were 170-ms /ba/, /da/, and /ga/ syllables synthesized using a Klatt (56) synthesizer with a 50-ms formant transition in which the first, second, and third formants were dynamic. The fundamental frequency, fourth formant, and fifth formant were stable throughout. Please see ref. 9 for additional stimulus details. Stimuli were presented at 80 dB SPL monaurally to the right ear through insert earphones (ER-3; Etymotic Research) by NeuroScan Stim 2 presentation software (Compumedics). Stimuli were presented in alternating polarities (180° out of phase), intermixed with each other and five other speech stimuli, at a rate of 4.35 Hz.
Responses were collected from a vertical Ag-AgCl electrode montage (Cz active, forehead ground, ipsilateral earlobe reference) by NeuroScan Acquire (Compumedics) and digitized at 20,000 Hz by a Synamp2 system (Compumedics). Data were offline bandpass filtered from 70 to 2,000 Hz (12 dB/octave roll off) and epoched into 230-ms windows (40 ms of prestimulus activity), and responses ± 35 μV were rejected as artifact. A total of 6,000 artifact-free responses (3,000 for each polarity) were obtained for each of the three sounds.
To assess response consistency, two different methods were used to generate 3,000 sweep averages, each representing half of the recording events, which were then compared with each other. In one method, the average of responses collected during the first half of the recording was compared with the average from the second half. For the other method, responses from even-numbered events across the recording were averaged together and compared with responses from odd-numbered events.
For both methods, response consistency was assessed by calculating the correlation between the two 3,000-sweep averages. Correlations were conducted separately for the segments of the response arising from the formant transition (7–60 ms) and the steady-state vowel (60–180 ms). Response consistencies were averaged across the /ba/, /da/, and /ga/ stimuli to form one metric. All data were Fisher transformed before statistical analyses; however, values reported in Figs. 1 and 2 were converted back to r-values for visual purposes.
Statistical Analyses.
Multiple comparisons were controlled for, using a Holm–Bonferroni correction (an iterative Bonferroni correction of α/n, α/n − 1, etc., for each comparison) with specific application to the test–retest comparisons in the dyslexic FM group. On the basis of previous evidence, we predicted a priori that FM system use would yield benefits in neural and behavioral function. As such, one-tailed, paired t tests were used to assess training effects in the dyslexic FM group. Relationships between neural function and behavior, evaluated with Spearman’s correlations due to the small sample size, were evaluated with two-tailed tests. Because the typically developing (see SI Materials and Methods for participant details) and dyslexic control groups were predicted to show no change from test to retest, the most conservative approach is not to correct for multiple comparisons and allow any significant changes to be observed (contrary to our hypothesis); thus one-tailed, paired t tests were used (α = 0.10). For similar reasons, when comparing the age, the test–retest interval, etc., of the groups using two-tailed, independent t tests, α was set to 0.05. Data from all groups were confirmed to be normally distributed, using the Kolmogorov–Smirnov test.
Supplementary Material
Acknowledgments
We thank the families and children for participating and the administrators and staff at the Hyde Park Day Schools for their enthusiastic collaboration. The authors also thank the members of the Auditory Neuroscience Laboratory for their assistance with data collection and Erika Skoe, Trent Nicol, Samira Anderson, and Dana Strait for their careful review of the manuscript. This work was supported by National Institutes of Health Grant R01DC01510, Phonak AG Grant CNV0055193, the Hugh Knowles Center at Northwestern University, and a Northwestern University Cognitive Science Interdisciplinary Research Fellowship (awarded to J.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 16406.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206628109/-/DCSupplemental.
References
- 1.Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biol Psychiatry. 2005;57:1301–1309. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 2.Facoetti A, et al. Multisensory spatial attention deficits are predictive of phonological decoding skills in developmental dyslexia. J Cogn Neurosci. 2010;22:1011–1025. doi: 10.1162/jocn.2009.21232. [DOI] [PubMed] [Google Scholar]
- 3.Lallier M, et al. Behavioral and ERP evidence for amodal sluggish attentional shifting in developmental dyslexia. Neuropsychologia. 2010;48:4125–4135. doi: 10.1016/j.neuropsychologia.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 4.Pearson DA, Lane DM. Auditory attention switching: A developmental study. J Exp Child Psychol. 1991;51:320–334. doi: 10.1016/0022-0965(91)90039-u. [DOI] [PubMed] [Google Scholar]
- 5.Tallal P, Miller SL, Jenkins WM, Merzenich MM. The role of temporal processing in developmental language-based learning disorders: Research and clinical implications. In: Blachman B, editor. Foundations of Reading Acquisition and Dyslexia: Implications for Early Intervention. Mahwah, NJ: Lawrence Erlbaum; 1997. pp. 49–66. [Google Scholar]
- 6.Goswami U, et al. Language-universal sensory deficits in developmental dyslexia: English, Spanish, and Chinese. J Cogn Neurosci. 2011;23:325–337. doi: 10.1162/jocn.2010.21453. [DOI] [PubMed] [Google Scholar]
- 7.Hari R, Renvall H. Impaired processing of rapid stimulus sequences in dyslexia. Trends Cogn Sci. 2001;5:525–532. doi: 10.1016/s1364-6613(00)01801-5. [DOI] [PubMed] [Google Scholar]
- 8.Serniclaes W, Sprenger-Charolles L. Categorical perception of speech sounds and dyslexia. Curr Psychol Lett Behav Brain Cogn 1(10) 2003. Available at http://cpl.revues.org/index379.html.
- 9.Hornickel J, Skoe E, Nicol T, Zecker SG, Kraus N. Subcortical differentiation of stop consonants relates to reading and speech-in-noise perception. Proc Natl Acad Sci USA. 2009;106:13022–13027. doi: 10.1073/pnas.0901123106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chandrasekaran B, Kraus N. 2012. Biological factors contributing to reading ability: Subcortical auditory function. Developmental Dyslexia: Early Precursors, Neurobehavioral Markers and Biological Substrates, The Dyslexia Foundation and the Extraordinary Brain Series, eds Benasich AA, Fitch RH (Paul H Brookes Publishing, Baltimore)
- 11.Miller GA, Nicely PE. An analysis of perceptual confusions among some English consonants. J Acoust Soc Am. 1955;27:338–352. [Google Scholar]
- 12.Richardson U, Thomson JM, Scott SK, Goswami U. Auditory processing skills and phonological representation in dyslexic children. Dyslexia. 2004;10:215–233. doi: 10.1002/dys.276. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs S. Phonological awareness: An investigation into the developmental role of vocabulary and short-term memory. Educ Psychol. 2004;24:13–25. [Google Scholar]
- 14.Gathercole SE, Alloway TP, Willis C, Adams A-M. Working memory in children with reading disabilities. J Exp Child Psychol. 2006;93:265–281. doi: 10.1016/j.jecp.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Benasich AA, Thomas JJ, Choudhury N, Leppänen PHT. The importance of rapid auditory processing abilities to early language development: Evidence from converging methodologies. Dev Psychobiol. 2002;40:278–292. doi: 10.1002/dev.10032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsao F-M, Liu H-M, Kuhl PK. Speech perception in infancy predicts language development in the second year of life: A longitudinal study. Child Dev. 2004;75:1067–1084. doi: 10.1111/j.1467-8624.2004.00726.x. [DOI] [PubMed] [Google Scholar]
- 17.David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proc Natl Acad Sci USA. 2012;109:2144–2149. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao E, Suga N. Experience-dependent plasticity in the auditory cortex and the inferior colliculus of bats: Role of the corticofugal system. Proc Natl Acad Sci USA. 2000;97:8081–8086. doi: 10.1073/pnas.97.14.8081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song JH, Skoe E, Banai K, Kraus N. Training to improve hearing speech in noise: Biological mechanisms. Cereb Cortex. 2011;122:1890–1898. doi: 10.1093/cercor/bhr196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Russo NM, Nicol TG, Zecker SG, Hayes EA, Kraus N. Auditory training improves neural timing in the human brainstem. Behav Brain Res. 2005;156:95–103. doi: 10.1016/j.bbr.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Merzenich MM, et al. Temporal processing deficits of language-learning impaired children ameliorated by training. Science. 1996;271:77–81. doi: 10.1126/science.271.5245.77. [DOI] [PubMed] [Google Scholar]
- 22.Carcagno S, Plack CJ. Subcortical plasticity following perceptual learning in a pitch discrimination task. J Assoc Res Otolaryngol. 2011;12:89–100. doi: 10.1007/s10162-010-0236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keuroghlian AS, Knudsen EI. Adaptive auditory plasticity in developing and adult animals. Prog Neurobiol. 2007;82:109–121. doi: 10.1016/j.pneurobio.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 24.Atiani S, Elhilali M, David SV, Fritz JB, Shamma SA. Task difficulty and performance induce diverse adaptive patterns in gain and shape of primary auditory cortical receptive fields. Neuron. 2009;61:467–480. doi: 10.1016/j.neuron.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seitz AR, Dinse HR. A common framework for perceptual learning. Curr Opin Neurobiol. 2007;17:148–153. doi: 10.1016/j.conb.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Blake R, Field B, Foster C, Platt F, Wertz P. Effect of FM auditory trainers on attending behaviors of learning-disabled children. Lang Speech Hear Serv Sch. 1991;22:111–114. [Google Scholar]
- 27.Purdy SC, Smart JL, Baily M, Sharma M. Do children with reading delay benefit from the use of personal FM systems in the classroom? Int J Audiol. 2009;48:843–852. doi: 10.3109/14992020903140910. [DOI] [PubMed] [Google Scholar]
- 28.Rosenberg GG, et al. Improving classroom acoustics (ICA): A three-year FM sound field classroom amplification study. J Educ Audiol. 1999;7:8–28. [Google Scholar]
- 29.Friederichs E, Friederichs P. Electrophysiologic and psycho-acoustic findings following one-year application of a personal ear-level FM device in children with attention deficit and suspected central auditory processing disorder. J Educ Audiol. 2005;12:31–36. [Google Scholar]
- 30. Crandell CC, Smaldino JJ, Flexer C, eds (2005) Sound Field Amplification: Applications to Speech Perception and Classroom Acoustics (Thomson Delmar Learning, Clifton Park, NY), 2nd Ed.
- 31.Nishi K, Lewis DE, Hoover BM, Choi S, Stelmachowicz PG. Children’s recognition of American English consonants in noise. J Acoust Soc Am. 2010;127:3177–3188. doi: 10.1121/1.3377080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Flexer C, Biley KK, Hinkley A, Harkema C, Holcomb J. Using sound-field systems to teach phonemic awareness to pre-schoolers. Hearing J. 2002;55(3):38–44. [Google Scholar]
- 33.Darai B. 2000. Using sound field FM systems to improve literacy scores. ADVANCE Speech-Language Pathol Audiol 10:5–7.
- 34.Johnston KN, John AB, Kreisman NV, Hall JW, 3rd, Crandell CC. Multiple benefits of personal FM system use by children with auditory processing disorder (APD) Int J Audiol. 2009;48:371–383. doi: 10.1080/14992020802687516. [DOI] [PubMed] [Google Scholar]
- 35.Hornickel J, Knowles E, Kraus N. Test-retest consistency of speech-evoked auditory brainstem responses in typically-developing children. Hear Res. 2012;284:52–58. doi: 10.1016/j.heares.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Song JH, Nicol T, Kraus N. Test-retest reliability of the speech-evoked auditory brainstem response. Clin Neurophysiol. 2011;122:346–355. doi: 10.1016/j.clinph.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kraus N, Chandrasekaran B. Music training for the development of auditory skills. Nat Rev Neurosci. 2010;11:599–605. doi: 10.1038/nrn2882. [DOI] [PubMed] [Google Scholar]
- 38.Krizman J, Marian V, Shook A, Skoe E, Kraus N. Subcortical encoding of sound is enhanced in bilinguals and relates to executive function advantages. Proc Natl Acad Sci USA. 2012;109:7877–7881. doi: 10.1073/pnas.1201575109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rinne T, et al. Auditory selective attention modulates activation of human inferior colliculus. J Neurophysiol. 2008;100:3323–3327. doi: 10.1152/jn.90607.2008. [DOI] [PubMed] [Google Scholar]
- 40.Moore DR, Ferguson MA, Edmondson-Jones AM, Ratib S, Riley A. Nature of auditory processing disorder in children. Pediatrics. 2010;126:e382–e390. doi: 10.1542/peds.2009-2826. [DOI] [PubMed] [Google Scholar]
- 41.Wible B, Nicol T, Kraus N. Abnormal neural encoding of repeated speech stimuli in noise in children with learning problems. Clin Neurophysiol. 2002;113:485–494. doi: 10.1016/s1388-2457(02)00017-2. [DOI] [PubMed] [Google Scholar]
- 42.Chandrasekaran B, Hornickel J, Skoe E, Nicol T, Kraus N. Context-dependent encoding in the human auditory brainstem relates to hearing speech in noise: Implications for developmental dyslexia. Neuron. 2009;64:311–319. doi: 10.1016/j.neuron.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Don M, Allen AR, Starr A. Effect of click rate on the latency of auditory brain stem responses in humans. Ann Otol Rhinol Laryngol. 1977;86:186–195. doi: 10.1177/000348947708600209. [DOI] [PubMed] [Google Scholar]
- 44.Johnson KL, Nicol TG, Zecker SG, Kraus N. Auditory brainstem correlates of perceptual timing deficits. J Cogn Neurosci. 2007;19:376–385. doi: 10.1162/jocn.2007.19.3.376. [DOI] [PubMed] [Google Scholar]
- 45.Banai K, et al. Reading and subcortical auditory function. Cereb Cortex. 2009;19:2699–2707. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burkard RF, Don M. The auditory brainstem response. In: Burkard RF, Don M, Eggermont JJ, editors. Auditory Evoked Potentials: Basic Principles and Clinical Applications. Baltimore: Lippincott Williams & Wilkins; 2007. pp. 229–253. [Google Scholar]
- 47.Anderson S, Skoe E, Chandrasekaran B, Kraus N. Neural timing is linked to speech perception in noise. J Neurosci. 2010;30:4922–4926. doi: 10.1523/JNEUROSCI.0107-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bradlow AR, Kraus N, Hayes E. Speaking clearly for children with learning disabilities: Sentence perception in noise. J Speech Lang Hear Res. 2003;46:80–97. doi: 10.1044/1092-4388(2003/007). [DOI] [PubMed] [Google Scholar]
- 49.Ziegler JC, Pech-Georgel C, George F, Lorenzi C. Speech-perception-in-noise deficits in dyslexia. Dev Sci. 2009;12:732–745. doi: 10.1111/j.1467-7687.2009.00817.x. [DOI] [PubMed] [Google Scholar]
- 50.Woerner C, Overstreet K. Wechsler Abbreviated Scale of Intelligence (WASI) San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 51.Mather N, Hammill DD, Allen EA, Roberts R. Test of Silent Word Reading Fluency (TOSWRF) Austin: Pro-Ed; 2004. [Google Scholar]
- 52.Torgesen JK, Wagner RK, Rashotte CA. Test of Word Reading Efficiency (TOWRE) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 53.Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing (CTOPP) Austin, TX: Pro-Ed; 1999. [Google Scholar]
- 54.Woodcock RW, McGrew KS, Mather N. Woodcock-Johnson III Tests of Achievement. Itasca, IL: Riverside Publishing; 2001. [Google Scholar]
- 55.DuPaul GJ, Power TJ, Anastopoulos AD, Reid R. ADHD Rating Scale IV. New York: Guilford; 1998. [Google Scholar]
- 56.Klatt DH. Software for a cascade/parallel formant synthesizer. J Acoust Soc Am. 1980;67:971–995. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.