Abstract
Glutamatergic neurotransmission mediated by N-methyl-d-aspartate (NMDA) receptors is vital for the cortical computations underlying cognition and might be disrupted in severe neuropsychiatric illnesses such as schizophrenia. Studies on this topic have been limited to processes in local circuits; however, cognition involves large-scale brain systems with multiple interacting regions. A prominent feature of the human brain’s global architecture is the anticorrelation of default-mode vs. task-positive systems. Here, we show that administration of an NMDA glutamate receptor antagonist, ketamine, disrupted the reciprocal relationship between these systems in terms of task-dependent activation and connectivity during performance of delayed working memory. Furthermore, the degree of this disruption predicted task performance and transiently evoked symptoms characteristic of schizophrenia. We offer a parsimonious hypothesis for this disruption via biophysically realistic computational modeling, namely cortical disinhibition. Together, the present findings establish links between glutamate’s role in the organization of large-scale anticorrelated neural systems, cognition, and symptoms associated with schizophrenia in humans.
Keywords: default-mode network, task-based activation, task-based deactivation, pharmacological manipulation, fMRI
Drug treatments for serious mental illnesses and investigations of the neurochemical bases of healthy cognition have, for the most part, targeted the slow neuromodulatory neurotransmitters, dopamine and serotonin (1). However, rapid excitatory glutamatergic and inhibitory γ-aminobutyric acid (GABA) signals mediate local and long-range cortical computations (2) and play a critical role in cognition and severe psychiatric illnesses such as schizophrenia (3–5). We investigated how disrupting the N-methyl-d-aspartate (NMDA) receptor component of fast glutamatergic neurotransmission via the administration of the NMDA receptor antagonist ketamine altered cognitive performance and systems-level neural activity and connectivity in healthy volunteers. Furthermore, we related these system-level neural changes to behavior and transiently evoked psychotic symptoms associated with schizophrenia.
Studies investigating glutamate’s role in cognition have largely focused on local circuits (5–7); however, cognition involves large-scale brain systems with multiple interacting regions. Recent neuroimaging work highlights the competitive relationships between two large-scale neural systems: a set of brain regions preferentially engaged during tasks that require goal-directed cognition and attention (task-positive) and the regions associated with resting conditions [default-mode network (DMN)] (8–10). The neurotransmitter mechanisms behind this inverse relationship remain unexplored, as does the role of this phenomenon in serious mental illness (11). Thus far, functional neuroimaging (fMRI) investigations of these large-scale neural systems have mostly been correlational (12), and the synaptic mechanisms for these effects have yet to be fully characterized. However, through the use of pharmacological challenges that provide the means for controlled neurochemical perturbations, casual experimental studies become possible (13). These perturbations are achieved via agents with well-characterized neuropharmacology (3) that can, through controlled manipulations of neurotransmission, transiently and reversibly induce symptoms that have face-validity with respect to psychiatric illness (14).
Here, we administered ketamine to healthy volunteers (Methods), which safely, transiently, and reversibly perturbs regional brain responses in a number of nodes integral to large-scale brain systems (15). Ketamine also alters synaptic function in the long-range connections that provide the connective basis of distributed brain systems (16). Infusing ketamine induces a transient state resembling schizophrenia in healthy volunteers (14), effects that have been related to individual differences in regional functional brain responses to cognitive tasks (13, 15, 17, 18). Here we investigated ketamine’s effects while subjects performed a demanding working memory (WM) task, a cognitive process involving short-term encoding and maintenance of information (19) that is profoundly impaired in schizophrenia (20) and that robustly modulates task-positive and DMN networks (10). Using blood oxygen-level–dependent (BOLD) imaging, we examined how modulating glutamatergic neurotransmission alters system-level activation and deactivation and the interplay between large-scale neural systems during WM, as well as the relevance of these changes for the psychosis-like phenomenology induced by ketamine. We hypothesized that reduction in signaling via NMDA receptors would attenuate task-related activation but also reduce the degree of DMN suppression: a phenomenon critical for cognitive performance (21, 22) but disrupted in neuropsychiatric illness (11, 23).
Lastly, we related observed experimental effects to a well-validated biophysically realistic computational model of WM (24) to study a leading hypothesized synaptic mechanism of NMDA blockade on neural activity, namely disrupted balance between cortical excitation/inhibition (5). With our computational modeling approach, we attempt to provide a framework for relating synaptic-level hypotheses to observed neural activity and cognition to better understand the complex and puzzling symptoms of serious mental illness (25). Together, the present findings highlight the functional utility of large-scale brain systems and the role of intact NMDA receptor operation in their interrelationship.
Results
Behavioral Effects.
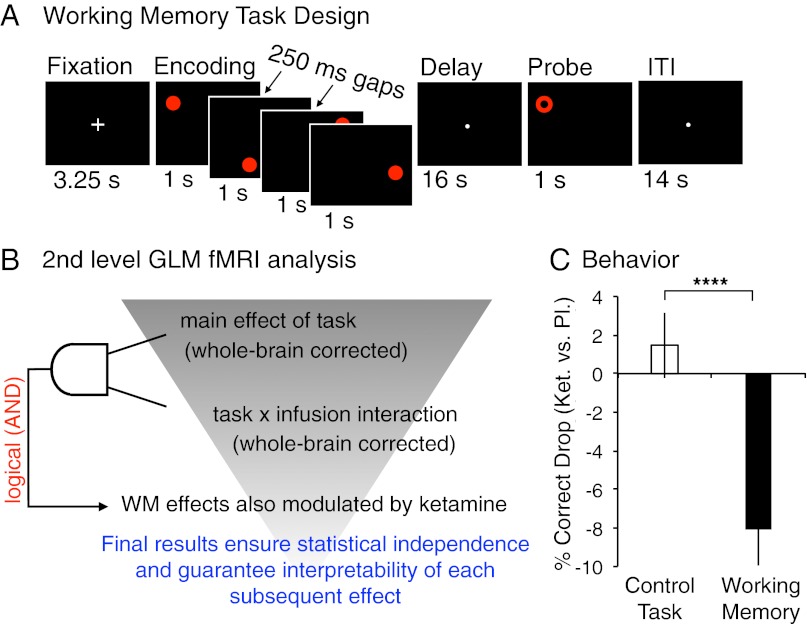
We first assessed the impact of ketamine on performance of the spatial WM task (Fig. 1A). Confirming hypothesized effects, results revealed a significant accuracy reduction for WM vs. control trials following ketamine administration [t(18) = 5.65; P < 0.0001] (Fig. 1C; for details, see SI Text and Fig. S1), which was in line with well-established deleterious effects of ketamine on WM across species (26, 27).
Fig. 1.
(A) Subjects encoded four or two (not shown; see Methods) circle locations and, after a delay, indicated whether the circle was presented at that location or not (probe). Subjects also completed a control task where four gray circles appeared but were explicitly asked not to encode the circles. During the probe phase, another gray circle was shown, requiring a motor response but no recall. (B) Second-level conjunction fMRI analysis strategy: (i) we computed a main effect of task (i.e., WM vs. control condition) type I error corrected at the whole-brain level (Fig. 2); and (ii) we computed a task × infusion interaction, revealing regions differentially modulated by ketamine across task conditions. Regions identified this way are not guaranteed to be involved in WM. That is, regions showing a task × infusion interaction may not show engagement during WM (i.e., main effect of task). Thus, we computed a conjunction (logical AND) between these effects. The surviving regions were ensured to show both a task main effect and modulation of this effect by ketamine (Fig. 3A and Fig. S2). (C) Percentage drop in accuracy (% correct) is shown for the control (white bar) and WM (black bar) tasks following ketamine vs. placebo infusion (difference plotted). ****P < 0.0001 (see SI Text, Behavioral Results for complete behavioral analysis). Error bars reflect ±1 SEM.
Task-Based Activation and Deactivation.
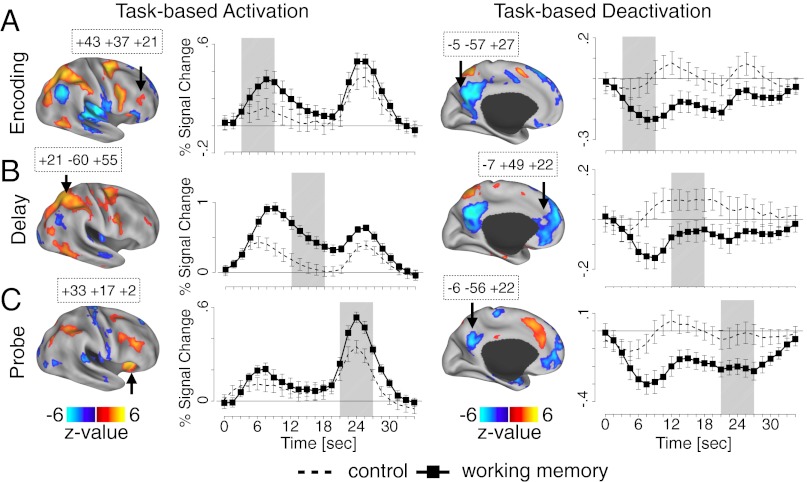
Performing WM tasks robustly modulates task-positive regions (28) but also involves deactivation of the DMN (10). We first verified that the cognitive task engaged regions previously shown to be active during WM (28) by computing a main effect of task condition (WM vs. control trials) during encoding, delay, and probe phases of the trial (Fig. 2 A–C and SI Text, fMRI Analyses). Analyses confirmed robust WM effects across the three WM phases, in terms of both task-based activation (Fig. 2, Left) and deactivation (Fig. 2, Right), in correspondence with the DMN (8) (for all WM-modulated regions, see Tables S1–S3).
Fig. 2.
WM effects for encoding (A), delay (B), and probe (C) phases are shown. Maps illustrate task-based WM activations (orange-yellow) and deactivations (blue) (Left and Right, respectively). All displayed foci met a whole-brain correction (SI Text, fMRI Analyses). Time courses are shown for exemplar regions identified using an assumed hemodynamic response function (SI Text, fMRI Analyses), exhibiting significant WM (black squares) vs. control task (dashed lines) effects. Approximate trial epochs (encoding, delay, probe) are marked with gray vertical bars. Canonical WM responses are evident across all epochs. Region coordinates are marked in boxes. For complete list of task-modulated foci, see Tables S1–S3.
Ketamine Modulation of Task-Based Activation and Deactivation.
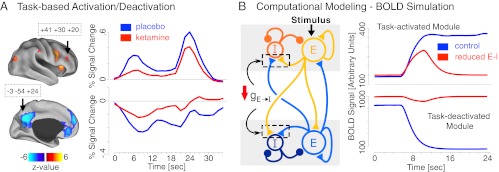
Next, using a stringent conjunction masking technique (29) (Fig. 1B), we independently tested for effects of ketamine only in areas that were explicitly engaged during WM (Fig. 2), ensuring a principled method for isolating ketamine modulation of task-related regions. We computed a whole-brain–corrected task condition × infusion interaction map and then computed a conjunction (logical AND) between surviving results and the map showing main effects of task condition. We repeated this procedure across task phases. This approach ensures that observed BOLD modulations by ketamine are present in regions explicitly modulated by the WM task (SI Text, fMRI Analyses). Results revealed a number of WM-related regions modulated by ketamine across encoding and delay (Fig. S2 and Table S4). We highlight two exemplar regions exhibiting identified effects: the dorso-lateral prefrontal cortex [Brodmann area (BA) 46; Fig. 3A, Upper] and the precuneus (BA 31; Fig. 3A, Lower) (see Fig. S3 for all regions). These effects illustrate that ketamine attenuated signal in task-activated regions, as well as deactivation of regions overlapping the DMN (8). No regions exhibited a ketamine modulation of WM signals at the probe phase. Consistent with hypotheses, these results indicate that ketamine not only attenuates encoding and early delay signals during WM but also disrupts suppression of regions typically deactivated during demanding cognitive operations (10). To further verify preferential effects of ketamine in regions involved in WM (and to rule out potential vascular confounds), we examined a motor cortex region that showed a strong response to the button press during the probe phase. There was no BOLD signal attenuation by ketamine in this motor region; in fact, the response for ketamine was numerically higher (Fig. S3).
Fig. 3.
(A) Regions exhibiting WM effects and modulation of this effect by ketamine for task-based activation (Upper) and task-based deactivation (Lower). WM time courses are shown for dorso-lateral prefrontal cortex (Upper) and precuneus (Lower) for ketamine (red) and placebo (blue) (coordinates are shown in boxes). Complete list of foci exhibiting significant effects across encoding and delay phases is presented in Table S4 and Fig. S2. The probe phase analysis did not reveal significant modulation by ketamine. Note: less negative value for DMN under ketamine reflects less deactivation relative to baseline compared with the placebo condition. (B) Computational model scheme, comprised of task-activated (Upper) and task-deactivated (Lower) modules followed by modeling results. We modeled the effects of ketamine as a reduction of NMDA conductance onto inhibitory interneurons (gE-I). We examined whether “disinhibition” via reduced NMDA conductance onto GABA cells (E-I) would result in effects similar to BOLD findings under ketamine. Here, we present predicted BOLD signal derived from the simulated local field potential (LFP) on the time scale comparable to a single WM trial in the experiment to appropriately juxtapose model simulations to BOLD empirical observations. For complete modeling implementation, BOLD simulation details, and comparison with firing-rate results, see SI Text, Computational Modeling and Figs. S4–S6.
Examining Hypothesized Synaptic Effects of Ketamine via Computational Modeling.
The above analysis suggests a breakdown in WM-related BOLD signal in both task-activated and task-deactivated regions. As noted, a leading hypothesis for ketamine’s effects at the synaptic level, derived from both animal and human work, postulates disinhibition via preferential antagonism of NMDA receptors on inhibitory interneurons (3, 30). How can we reconcile the observed reduction in BOLD signal and WM performance with disinhibition? To investigate hypothesized effects of ketamine on the cortical microcircuit level, which may shed light on observed BOLD effects, we adapted a well-validated biophysically plausible computational model of WM (24). Our model is comprised of two modules: a task-activated module, which is a recurrent microcircuit capable of WM computations at the time scale relevant to a single WM trial; and a task-deactivated module representing the DMN characterized by high baseline firing rate and deactivation at task onset (31). The microcircuit modules interact through long-range, net inhibitory projections (SI Text, Computational Modeling). The biophysical realism in the model allows for direct implementation of pharmacological manipulations at the synaptic level (32) (Methods and SI Text, Computational Modeling for model operation and pharmacological implementation). Here, we related hypothesized ketamine effects, highlighted by preclinical findings (3, 30) to observed BOLD effects: we preferentially disrupted NMDA conductance onto GABAergic interneurons (i.e., E-I conductance). We contrasted E-I reduction with a preferential reduction in recurrent excitation on pyramidal cells (i.e., E-E reduction; see Figs. S5 and S6). To ensure model simulations describe processes qualitatively similar to empirical observations, we computed BOLD signal based on model-generated activity, which reproduced described effects (Fig. 3B; see SI Text, Computational Modeling for details). Although at achieved concentrations ketamine likely also disrupts recurrent excitation (i.e., E-E conductance), E-I reduction alone was sufficient to account for both the attenuation in task-related activation and the lack of DMN suppression (see Figs. S5 and S6). Furthermore, there are two mechanisms by which reduced E-I strength could potentially disrupt the proper pattern of activation and deactivation: (i) long-range (net inhibitory) connections between modules are weakened, impairing the ability of the task-activated module to shut down the task-negative module; and (ii) local disinhibition renders a hyperactive microcircuit less sensitive to the long-range input, so that the already high-firing task-deactivated module cannot be shut down even with an equal-strength, long-range suppressive input. We found that local microcircuit E-I reduction, as opposed to long-range reduction, plays the dominant role in disrupting model function (Fig. S4).
Ketamine Modulation of Task-Based Connectivity.
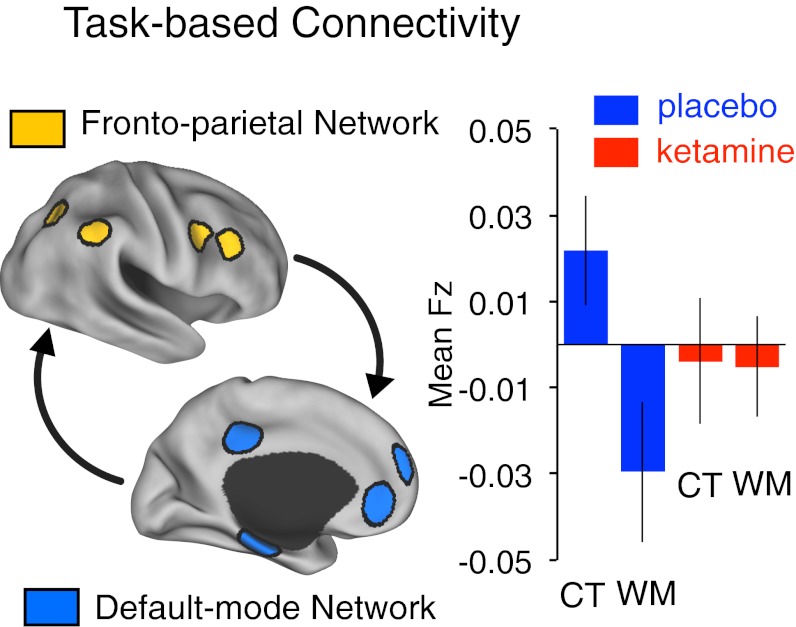
Given that glutamate plays a role in both local and long-range cortical computations, we also examined the possibility that NMDA receptor blockade may induce a reduction in task-based functional connectivity (tb-fcMRI) between large-scale anticorrelated systems. This second-level hypothesis was compelling because ketamine modulated task-related activation and deactivation in these systems. To this end, we selected a set of independent seeds previously identified as part of the fronto-parietal (FP) and DMN (8, 33). We focused on the delay phase of the trial using a previously validated technique developed to circumvent the possibility that overall task structure drives observed relationships (21) (for details see SI Text, tb-fcMRI). As hypothesized, there was a significant modulation of tb-fMRI between the FP-DMN networks during the delay, confirmed statistically by a task × infusion interaction [F(1,18) = 11.09; P < 0.004] (Fig. 4). This effect was preferential for the FP-DMN tb-fcMRI (when examining the cingulo-opercular system there was no task × infusion interaction, although there was a main effect of infusion, Fig. S7). Given emerging concerns that spurious head movement can confound connectivity results (34, 35), we implemented an additional volume censoring (“scrubbing”) movement correction (36, 37). The tb-fcMRI results remained significant after movement scrubbing [F(1,18) = 6.1; P < 0.025]. For completeness, we present both sets of results in Fig. S7 and additional movement analysis details (SI Text, tb-fcMRI).
Fig. 4.
tb-fcMRI during the delay phase between independently selected FP and DMN regions following ketamine (red) and placebo (blue) infusion, shown for the control (CT) and WM task conditions (all seed coordinates are listed in Table S5; for tb-fcMRI details, see SI Text, tb-fcMRI). Error bars reflect ±1 SEM.
Ketamine Disrupts Performance-Related Deactivation.
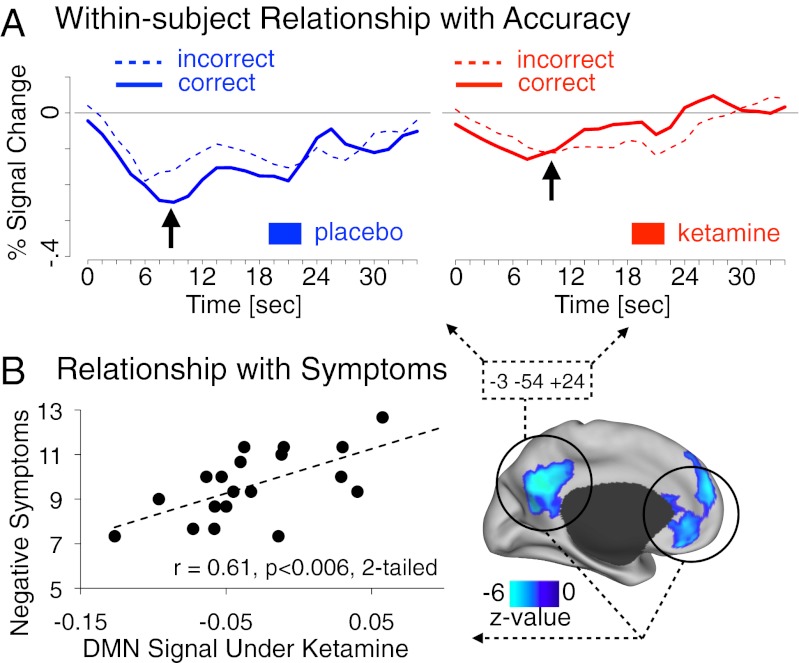
Next, we examined whether ketamine effects on WM in the task-based analysis relate to behavioral performance. We computed an additional model with WM accuracy as a covariate to enable examination of the within-subject, trial-by-trial relationship between behavioral performance and brain activity (38) (SI Text, fMRI Analyses). We tested whether foci showing a ketamine modulation of WM-related signals also exhibit a differential pattern of responses related to performance accuracy. To test this hypothesis, we computed an accuracy (correct vs. incorrect WM trials) × infusion (ketamine vs. placebo) interaction specifically for WM trials. We did so across task phases, but only delay-related results revealed significant findings. Among the foci identified in Fig. 3A, three DMN regions showed a significant accuracy × infusion interaction: middle temporal gyrus [F(1,18) = 7.93; P < 0.015]; precuneus [F(1,18) = 5.29; P < 0.035], and superior frontal gyrus [F(1,18) = 5.05; P < 0.04]. We highlight effects for the precuneus (Fig. 5A) given the consistent pattern of results across all three regions: during placebo infusion there was a greater reduction of task-based deactivation for correct vs. incorrect trials. However, following ketamine administration there was less task-based suppression on accurate trials. These results replicate prior findings showing that DMN suppression is vital for WM performance (21, 22) and suggest that NMDA blockade attenuates the ability for such performance-relevant suppression to take place. Notably, these patterns were evident in task-deactivated regions, but we did not observe an accuracy × infusion modulation of regions exhibiting WM-related activation.
Fig. 5.
(A) Correct vs. incorrect WM time courses are shown across placebo (blue) and ketamine (red) for the precuneus region that exhibited effects of ketamine on WM (defined via the conjunction approach, box shows region coordinates). (B) Relationship between the magnitude of DMN deactivation during WM following ketamine administration and severity of negative symptoms associated with schizophrenia. Subjects showing least DMN suppression during WM were most symptomatic. This effect was specific to WM trials; during control trials under ketamine, this relationship was not evident (r = 0.11).
DMN Signal and Ketamine-Induced Psychiatric Symptoms.
We also examined the degree to which ketamine-induced modulations of brain responses related to symptom severity: establishing links between cognition, brain function, and psychiatric symptoms. To that end, we computed a correlation between positive, negative, and dissociative symptoms measures (for clinical measure details, see SI Text, Clinical Measures) and the degree of DMN deactivation during WM (we averaged across all DMN regions modulated by ketamine; see SI Text, fMRI Analyses). Only the relationship with negative symptoms reached significance [r = 0.61; P < 0.006; two-tailed, Bonferroni-corrected] (Fig. 5B), indicating that the most symptomatic subjects had the least ability to suppress DMN during WM.
Discussion
Present results suggest that intact NMDA receptor function is critical for optimal cognitive performance, at least in the context of WM, as well as seamless engagement and deactivation of distributed anticorrelated systems in the human brain. Here, we focused on pharmacologically disrupting fast glutamatergic neurotransmission. Prior work attempting to improve cognition found that enhancing slow neuromodulation via modafinil resulted in more potent deactivation of the DMN (39). Minzenberg et al. (39) hypothesized that disrupted reciprocal inhibition, mediated via GABA interneurons, might be responsible for the breakdown of the “mirror” anticorrelated structure of these large-scale systems. Indeed, one leading hypothesis of ketamine’s effects is preferential antagonism of NMDA conductance on GABA cells, which would lead to disinhibition (3, 30, 40). Our experimental results implicate NMDA receptor activity in optimal local and long-range circuit function. When induced via ketamine, NMDA receptor hypofunction may result in cognitive deficits and negative symptoms associated with schizophrenia. This may be driven by disrupting the balance of excitation/inhibition in the cortical microcircuit, as recently suggested by preclinical work (5). Our modeling simulations offer a framework for relating this hypothesis, at the synaptic level, to experimental results, suggesting that disrupted NMDA conductance on GABA cells could contribute to the observed effects.
The model is a proof-of-principle instantiation of one leading hypothesis of what may be ketamine’s effects on task-based BOLD response in certain regions and its modulation of cellular-level phenomena (30). The reciprocal inhibition between microcircuit modules is an important assumption based on leading hypotheses of the intrinsic configuration of these large-scale systems (8). We explicitly used this assumed antagonistic configuration to relate synaptic-level hypotheses to systems-level observations. Using this framework, we found that local E/I balance is the crucial property of the model, suggesting a perspective on the importance of local circuit properties in controlling the nature of large-scale interactions between brain areas during cognitive tasks. That is, the observed relationship between modules was very sensitive to NMDA receptor manipulation within each module. The modeling results also highlight that a subtle E-I perturbation resulted in disinhibition and was robust across a range of E-I reductions (Fig. S6). In contrast, E-E perturbations result in a set of regimes that were not observed experimentally, namely reduced firing across both task-activated and task-deactivated modules. This pattern might be observed under a higher level of ketamine that may result in elevated level of NMDA blockade that also strongly affects E-E conductance (e.g., anesthesia).
The model provides a biologically plausible framework for relating experimental findings following neuropharmacological manipulations to cellular-level hypotheses. As recently proposed by Montague et al. (25), this approach can help hone our understanding of synaptic disruptions, relate these hypotheses to system-level observations and ultimately to behavior observed in severe mental illness, for instance, the significant relationship between DMN signal and behavior in the present experiment.
Indeed, at the systems level, a growing literature associates DMN abnormalities with neuropsychiatric illness (23). A recent investigation examined the effect of transcranial magnetic stimulation (TMS) on modulating DMN function but did not report psychiatric symptomatology in healthy volunteers (41). Conversely, a recent study of schizophrenia patients linked DMN deactivation, cognition, and symptom severity (11). It may be possible that these two investigations capture the phenomenon on different time-scales: perturbing the DMN transiently via TMS may not be sufficiently potent or persistent to induce profound psychiatric phenomena, but more extended “tonic” manipulation via NMDA receptor blockade may begin to resemble what is observed in patients. Our experimental findings further highlight the functional relevance of suppressing the DMN during WM. As argued previously, DMN activity may reflect “passive” mental phenomena, such as self-referential, future-oriented thought (42). Whitfield-Gabrieli et al. suggest that “over-engagement” of the default network could lead to an exaggerated focus on one’s own thoughts and feelings, as well as an ambiguous integration between one’s internal and external environment (11). In that sense, tonic activation of the DMN in schizophrenia, if unregulated, may result in a state marked by exaggerated self-relevant processing, blurring the boundary between neural computations relevant for internal percepts and external reality. Furthermore, inadequately suppressed tonic activity in the DMN may obstruct goal-directed cognition and interfere with motivated pursuits, both characteristic of the negative syndrome (43). Indeed, failure to deactivate the DMN has been linked with poor cognitive performance in healthy populations (22) and lack of task-based DMN deactivation has been observed in schizophrenia, even before DMN literature evolved (44). Therefore, overactivity in the DMN, resulting from cortical disinhibition, may exacerbate cognitive dysfunction observed in neuropsychiatric conditions by reducing cortical efficiency and increasing noise during goal-directed cognition. The present findings also implicate the role of fast glutamatergic neurotransmission in the emergence of negative symptoms, which are suboptimally treated by medication targeting dopamine and serotonin (45).
Taken together, these results offer evidence that modulating glutamate neurotransmission in humans alters the relationship between large-scale, task-positive and task-negative neural systems in a performance and symptom-related manner. Our experimental findings and simulations are in line with one hypothesized pathophysiological mechanism for cognitive impairment and negative symptoms: that of reduced NMDA conductance on GABA interneurons, consistent with cortical disinhibition proposed previously (4, 5). These observations provide a possible framework for treatment development aimed at ameliorating these debilitating and inadequately treated symptoms associated with schizophrenia.
Methods
Subjects.
Nineteen healthy, neurologically and psychiatrically intact right-handed volunteers (10 male) with a mean ± SD age of 27.5 ± 6.3 y completed the study (see SI Text, Subjects for complete recruitment details).
Experimental Task Design and Infusion.
While in the scanner, subjects completed a well-validated delayed spatial WM task, described in our prior work (46), developed to mimic primate physiology experiments (47) (Fig. 1A). During scanning two phases of testing occurred. First, subjects were administered saline while they completed a series of scans. Second, subjects underwent the same series of scans during administration of ketamine. Subjects completed 32 WM trials and 16 control task trials (i.e., no WM encoding and maintenance requirement but requiring a probe response to control for motor effects) per infusion, resulting in 64 WM and 32 control trials per visit. Subjects completed three such visits (see SI Text, Overall Experimental Design and SI Text, Infusion Protocol for experimental design and infusion details). The WM task included trials with two and four encoding locations, the former with extended inter-trial interval (ITI) (2500 ms), to make both the same length. The control task always contained four locations. All reported analyses collapse across two and four locations given no load effects on reported behavioral (SI Text, Behavioral Results) and neuroimaging analyses (SI Text, fMRI Analyses). Following scanning, subjects underwent a battery of clinical measures to quantify the presence, nature, and severity of psychotic symptoms (SI Text, Clinical Measures).
fMRI Acquisition and Analysis.
All data were acquired using a 3T Tim Trio (Siemens) scanner at the Yale University School of Medicine (see SI Text, fMRI Acquisition for acquisition details). For the fMRI task-based analyses, we used FIDL software to preprocess and construct the general linear models (developed at NeuroImaging Laboratories, Washington University in St. Louis; version 2.64) (48). For functional connectivity, we implemented validated in-house MatLab software developed at Washington University in St. Louis that we used in our prior work (21, 49-51). For preprocessing and analysis details, see SI Text, fMRI Preprocessing, SI Text, fMRI Analyses, and SI Text, tb-fcMRI.
Computational Modeling.
Complete model implementation, interaction between modules, pharmacological implementation, and dependence on parameters are presented in SI Text, Computational Modeling and Figs. S4–S6.
Supplementary Material
Acknowledgments
We thank Gretchen Hermes and David Matuskey for their skill and time running subject infusions; the nursing staff, Core Laboratory, and support staff of the Human Research Unit at Yale–New Haven Hospital; the MRI technicians at the Anlyan Center for all their support; the Clinical Neuroscience Research Unit staff at the Connecticut Mental Health Center as well as Department of Mental Health and Addiction Services for their support throughout the study; and Megan Ichinose and Taylor McGuinness for assistance with the final dataset. We thank Dr. Eve Marder and Gabrielle Gutierrez for providing a framework for how to visualize complex multidimensional parameter space data. We thank two anonymous reviewers for their excellent and constructive critique, comments, and suggestions on how to improve the manuscript. This research was supported by AstraZeneca Pharmaceuticals and by National Institutes of Health Grant R01 MH062349 (to X.-J.W.). The Janssen Research Foundation provided drug and some study support to the Department of Veterans Affairs (J.H.K.).
Footnotes
Conflict of interest statement: J.H.K. has been a paid consultant for AbbVie, Inc. (formerly Abbott Laboratories); Aisling Capital, LLC; AstraZeneca Pharmaceuticals; Bristol-Myers Squibb; Eisai, Inc.; Eli Lilly and Co.; Gilead Sciences, Inc.; Lundbeck Research USA; Medivation, Inc.; Otsuka Pharmaceutical Development & Commercialization, Inc.; Roche F. Hoffmann-La Roche Ltd; Sage Therapeutics, Inc.; Shire Pharmaceuticals; Takeda Industries; and Teva Pharmaceutical Industries, Ltd. J.H.K. serves as a member of the Scientific Advisory Boards for CHDI Foundation, Inc.; Lohocla Research Corporation; Mnemosyne Pharmaceuticals, Inc.; Naurex, Inc.; and Pfizer Pharmaceuticals. J.H.K. is a member of the Board of Directors for the Coalition for Translational Research in Alcohol and Substance Use Disorders, is the President Elect of the American College of Neuropsychopharmacology, and is the Editor of Biological Psychiatry. J.H.K. is listed as an inventor on the patent: Seibyl JP, Krystal JH, Charney DS (1995) US Patent 5,447,948. J.H.K. is listed as an inventor on a patent application by Yale University related to targeting the glutamatergic system for the treatment of neuropsychiatric disorders (application no. PCTWO06108055A1). J.H.K. is listed on a pending patent application related to intranasal administration of ketamine to treat depression. The other authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208494109/-/DCSupplemental.
References
- 1.Kapur S, Remington G. Serotonin-dopamine interaction and its relevance to schizophrenia. Am J Psychiatry. 1996;153:466–476. doi: 10.1176/ajp.153.4.466. [DOI] [PubMed] [Google Scholar]
- 2.Wang X-J. Decision making in recurrent neuronal circuits. Neuron. 2008;60:215–234. doi: 10.1016/j.neuron.2008.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krystal JH, et al. NMDA receptor antagonist effects, cortical glutamatergic function, and schizophrenia: Toward a paradigm shift in medication development. Psychopharmacology (Berl) 2003;169:215–233. doi: 10.1007/s00213-003-1582-z. [DOI] [PubMed] [Google Scholar]
- 4.Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- 5.Yizhar O, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: A novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. J Neurosci. 1997;17:2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moghaddam B, Adams BW. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281:1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 8.Fox MD, et al. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raichle ME, Snyder AZ. A default mode of brain function: A brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. [DOI] [PubMed] [Google Scholar]
- 10.Shulman GL, et al. Common blood flow changes across visual tasks: I. Increases in subcortical structures and cerebellum but not in nonvisual cortex. J Cogn Neurosci. 1997;9:624–647. doi: 10.1162/jocn.1997.9.5.624. [DOI] [PubMed] [Google Scholar]
- 11.Whitfield-Gabrieli S, et al. Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first-degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA. 2009;106:1279–1284. doi: 10.1073/pnas.0809141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biswal BB, et al. Toward discovery science of human brain function. Proc Natl Acad Sci USA. 2010;107:4734–4739. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Honey G, Bullmore E. Human pharmacological MRI. Trends Pharmacol Sci. 2004;25:366–374. doi: 10.1016/j.tips.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Krystal JH, et al. Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans. Psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Arch Gen Psychiatry. 1994;51:199–214. doi: 10.1001/archpsyc.1994.03950030035004. [DOI] [PubMed] [Google Scholar]
- 15.Honey GD, et al. Individual differences in psychotic effects of ketamine are predicted by brain function measured under placebo. J Neurosci. 2008;28:6295–6303. doi: 10.1523/JNEUROSCI.0910-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephan KE, Baldeweg T, Friston KJ. Synaptic plasticity and dysconnection in schizophrenia. Biol Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 17.Corlett PR, et al. Frontal responses during learning predict vulnerability to the psychotogenic effects of ketamine: Linking cognition, brain activity, and psychosis. Arch Gen Psychiatry. 2006;63:611–621. doi: 10.1001/archpsyc.63.6.611. [DOI] [PubMed] [Google Scholar]
- 18.Honey RA, et al. Acute ketamine administration alters the brain responses to executive demands in a verbal working memory task: An FMRI study. Neuropsychopharmacology. 2004;29:1203–1214. doi: 10.1038/sj.npp.1300438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonides J, et al. The mind and brain of short-term memory. Annu Rev Psychol. 2008;59:193–224. doi: 10.1146/annurev.psych.59.103006.093615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Park S. Working memory impairments in schizophrenia: A meta-analysis. J Abnorm Psychol. 2005;114:599–611. doi: 10.1037/0021-843X.114.4.599. [DOI] [PubMed] [Google Scholar]
- 21.Anticevic A, Repovs G, Shulman GL, Barch DM. When less is more: TPJ and default network deactivation during encoding predicts working memory performance. Neuroimage. 2010;49:2638–2648. doi: 10.1016/j.neuroimage.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daselaar SM, Prince SE, Cabeza R. When less means more: Deactivations during encoding that predict subsequent memory. Neuroimage. 2004;23:921–927. doi: 10.1016/j.neuroimage.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 23.Fox MD, Greicius M. Clinical applications of resting state functional connectivity. Front Syst Neurosci. 2010;4:19. doi: 10.3389/fnsys.2010.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Compte A, Brunel N, Goldman-Rakic PS, Wang XJ. Synaptic mechanisms and network dynamics underlying spatial working memory in a cortical network model. Cereb Cortex. 2000;10:910–923. doi: 10.1093/cercor/10.9.910. [DOI] [PubMed] [Google Scholar]
- 25.Montague PR, Dolan RJ, Friston KJ, Dayan P. Computational psychiatry. Trends Cogn Sci. 2012;16:72–80. doi: 10.1016/j.tics.2011.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neill JC, et al. Animal models of cognitive dysfunction and negative symptoms of schizophrenia: Focus on NMDA receptor antagonism. Pharmacol Ther. 2010;128:419–432. doi: 10.1016/j.pharmthera.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Morgan CJ, Curran HV. Acute and chronic effects of ketamine upon human memory: A review. Psychopharmacology (Berl) 2006;188:408–424. doi: 10.1007/s00213-006-0572-3. [DOI] [PubMed] [Google Scholar]
- 28.Owen AM, McMillan KM, Laird AR, Bullmore E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum Brain Mapp. 2005;25:46–59. doi: 10.1002/hbm.20131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nichols TE, Brett M, Andersson J, Wager TD, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Kotermanski SE, Johnson JW. Mg2+ imparts NMDA receptor subtype selectivity to the Alzheimer’s drug memantine. J Neurosci. 2009;29:2774–2779. doi: 10.1523/JNEUROSCI.3703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci USA. 2009;106:5948–5953. doi: 10.1073/pnas.0812035106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunel N, Wang XJ. Effects of neuromodulation in a cortical network model of object working memory dominated by recurrent inhibition. J Comput Neurosci. 2001;11:63–85. doi: 10.1023/a:1011204814320. [DOI] [PubMed] [Google Scholar]
- 33.Dosenbach NU, et al. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Satterthwaite TD, et al. Impact of in-scanner head motion on multiple measures of functional connectivity: Relevance for studies of neurodevelopment in youth. Neuroimage. 2012;60:623–632. doi: 10.1016/j.neuroimage.2011.12.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Dijk KRA, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2012 doi: 10.1016/j.neuroimage.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pessoa L, Gutierrez E, Bandettini P, Ungerleider L. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35:975–987. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- 39.Minzenberg MJ, Yoon JH, Carter CS. Modafinil modulation of the default mode network. Psychopharmacology (Berl) 2011;215:23–31. doi: 10.1007/s00213-010-2111-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Greene R. Circuit analysis of NMDAR hypofunction in the hippocampus, in vitro, and psychosis of schizophrenia. Hippocampus. 2001;11:569–577. doi: 10.1002/hipo.1072. [DOI] [PubMed] [Google Scholar]
- 41.Eldaief MC, Halko MA, Buckner RL, Pascual-Leone A. Transcranial magnetic stimulation modulates the brain’s intrinsic activity in a frequency-dependent manner. Proc Natl Acad Sci USA. 2011;108:21229–21234. doi: 10.1073/pnas.1113103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buckner RL. The serendipitous discovery of the brain’s default network. Neuroimage. 2011;62:1137–1145. doi: 10.1016/j.neuroimage.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 43.Barch DM, Dowd EC. Goal representations and motivational drive in schizophrenia: The role of prefrontal-striatal interactions. Schizophr Bull. 2010;36:919–934. doi: 10.1093/schbul/sbq068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer-Lindenberg A, et al. Evidence for abnormal cortical functional connectivity during working memory in schizophrenia. Am J Psychiatry. 2001;158:1809–1817. doi: 10.1176/appi.ajp.158.11.1809. [DOI] [PubMed] [Google Scholar]
- 45.Buchanan RW, et al. The Cognitive and Negative Symptoms in Schizophrenia Trial (CONSIST): The efficacy of glutamatergic agents for negative symptoms and cognitive impairments. Am J Psychiatry. 2007;164:1593–1602. doi: 10.1176/appi.ajp.2007.06081358. [DOI] [PubMed] [Google Scholar]
- 46.Driesen NR, et al. Impairment of working memory maintenance and response in schizophrenia: Functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64:1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 48.Ollinger JM, Corbetta M, Shulman GL. Separating processes within a trial in event-related functional MRI. Neuroimage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- 49.Anticevic A, Repovs G, Barch DM. Resisting emotional interference: Brain regions facilitating working memory performance during negative distraction. Cogn Affect Behav Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2011 doi: 10.1093/schbul/sbq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973. doi: 10.1016/j.biopsych.2010.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.