Abstract
Direct evidence for a role of endogenous retinoic acid (RA), the active metabolite of vitamin A in the initial differentiation and meiotic entry of spermatogonia, and thus in the initiation of spermatogenesis is still lacking. RA is synthesized by dedicated enzymes, the retinaldehyde dehydrogenases (RALDH), and binds to and activates nuclear RA receptors (RARA, RARB, and RARG) either within the RA-synthesizing cells or in the neighboring cells. In the present study, we have used a combination of somatic genetic ablations and pharmacological approaches in vivo to show that during the first, prepubertal, spermatogenic cycle (i) RALDH-dependent synthesis of RA by Sertoli cells (SC), the supporting cells of the germ cell (GC) lineage, is indispensable to initiate differentiation of A aligned into A1 spermatogonia; (ii) RARA in SC mediates the effects of RA, possibly through activating Mafb expression, a gene whose Drosophila homolog is mandatory to GC differentiation; (iii) RA synthesized by premeiotic spermatocytes cell autonomously induces meiotic initiation through controlling the RAR-dependent expression of Stra8. Furthermore, we show that RA of SC origin is no longer necessary for the subsequent spermatogenic cycles but essential to spermiation. Altogether, our data establish that the effects of RA in vivo on spermatogonia differentiation are indirect, via SC, but direct on meiotic initiation in spermatocytes, supporting thereby the notion that, contrary to the situation in the female, RA is necessary to induce meiosis in the male.
Keywords: mouse, mutagenesis, retinoid antagonist, RAR/RXR heterodimer
Spermatogenesis is a complex and tightly regulated cell differentiation process, yielding mature spermatozoa from spermatogonia stem cells. Spermatogonia in the single-cell state, known as A single (As) spermatogonia, have traditionally been considered as spermatogonia stem cells in the mouse. Upon division, As spermatogonia give rise to two paired A (Ap) spermatogonia, then to a chain of 4–32 aligned (Aal) spermatogonia. As, Ap, and Aal are referred to as “undifferentiated spermatogonia” because they all retain stem cell properties (1). Subsequently Aal cells differentiate into A1 spermatogonia, which are irreversibly committed toward gamete production. They undergo a series of divisions generating B spermatogonia, which divide once more to yield premeiotic (i.e., preleptotene) spermatocytes (2). The entire process of spermatogenesis relies on functional interactions between germ cells (GC) and somatic, supporting cells, called Sertoli cells (SC), involving a complex assortment of hormones and cytokines, among which retinoic acid (RA), the biologically active form of vitamin A (retinol). In fact, vitamin A-deficient (VAD) mice are infertile because of an arrest of spermatogonia differentiation at the Aal–A1 transition, and treating them with either retinol or RA results in the complete recovery of spermatogenesis (3). As RA can trigger meiosis in female GC through initiating Stra8 expression (4, 5) and can additionally induce Stra8 expression in male GC (3), the paradigm has become that RA is required for meiotic initiation. However, a recent study showing that female GC can enter meiosis in a fetal ovary devoid of RA has challenged this model (6).
During embryonic development RA usually acts in a paracrine manner, one cell type controlling its synthesis, whereas a neighbor cell type responds to the signal (7). In cells synthesizing RA, conversion of retinol to its active metabolite depends upon retinaldehyde dehydrogenases (RALDH1 to RALDH3 encoded by Aldh1a1 to Aldh1a3 genes). In responding cells, RA binds to and activates nuclear RA receptors (RARA, RARB, and RARG isotypes), which are ligand-dependent transcriptional regulators. They usually function in the form of heterodimers with rexinoid receptors (RXRA, RXRB, and RXRG isotypes) and control expression of target genes through binding to RA response elements (RARE) located in the vicinity of the promoter (8). At the onset of spermatogenesis in the developing testis, Aldh1a1 and Aldh1a2 are expressed in SC, whereas Rara and Rarg are expressed in SC and Aal spermatogonia, respectively (9, 10). These expression patterns raise the possibility that RA triggering the differentiation of spermatogonia could be synthesized by SC and acts either in an autocrine manner through cell autonomously activating RARA-dependent events in SC or in a paracrine manner through activating RARG in Aal spermatogonia, which then become committed toward meiosis.
Thus, although RA seems to play a central role in the differentiation of spermatogonia, the mechanisms driving RA availability in these cells remain largely unknown. Moreover, it has not yet been established whether the effects of RA on spermatogonia differentiation are cell autonomous or mediated by SC, and the RA-controlled genetic cascades need to be clarified. In addition, direct evidence supporting a role for RA in meiotic entry of male GC during puberty is still lacking. In the present study, we provide answers to these questions through the phenotypic analysis of mice lacking all RALDH activities specifically in SC.
Results and Discussion
Ablation of RALDH in SC Impairs Differentiation of A1 Spermatogonia.
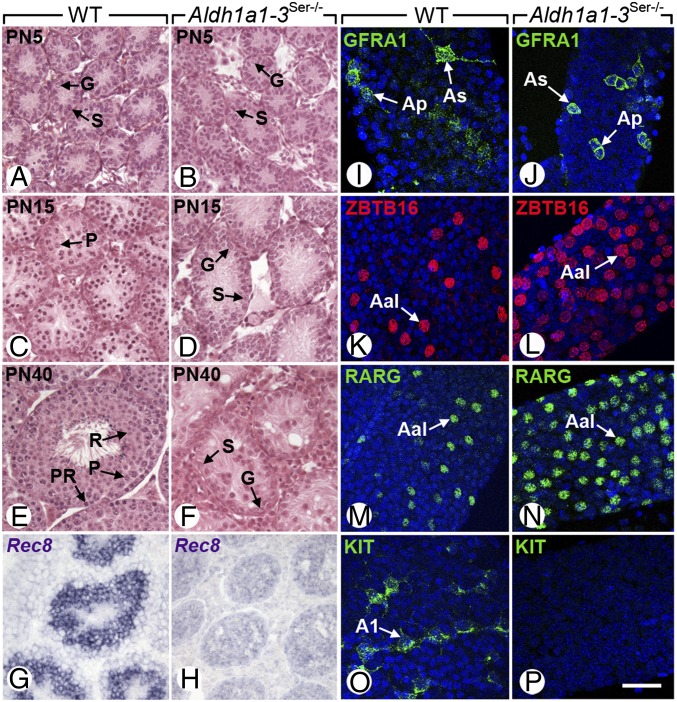
To generate Aldh1a1-3Ser−/− mutants, in which the RALDH genes were excised only in SC, mice carrying loxP-flanked alleles of the Aldh1a1, Aldh1a2, and Aldh1a3 genes (11–13) were crossed with mice bearing the Amh-Cre transgene, which is expressed from embryonic day 15 onward (14) and therefore excises RALDH coding sequence before the onset of spermatogenesis (2). At postnatal day 5 (PN5), no histological defects were detected in Aldh1a1-3Ser−/− mutant testes (n = 3) that contained spermatogonia and SC as their wild-type (WT) counterparts (Fig. 1 A and B). However, the expression of Sohlh1, Sohlh2, and Kit genes normally present in differentiated spermatogonia (15, 16) was significantly decreased in Aldh1a1-3Ser−/− testes, whereas expression of Ngn3, Nanos3, Ret, Zbtb16, and Rarg normally present in undifferentiated spermatogonia (17–20) was normal (Fig. S1A), indicating a delay in the process of spermatogonia differentiation in the absence of RALDH in SC. At PN15 and PN40 (n = 3 testes for each age and genotype), the seminiferous tubules of Aldh1a1-3Ser−/− mutants still contained only spermatogonia and SC (Fig. 1 D and F), whereas those of WT controls displayed normal spermatogenesis (Fig. 1 C and E). The absence of meiotic cells in Aldh1a1-3Ser−/− mutant testes was further assessed by the lack of Rec8 expression (Fig. 1 G and H). To characterize the spermatogonia in Aldh1a1-3Ser−/− testes at PN40 (n = 3 for each genotype), we analyzed the expression pattern of genes that are characteristic of given stages of differentiation, by in situ hybridization (ISH) analyses (Fig. S1) and whole-mount immunohistochemistry (IHC) (Fig. 1 I–P). Both WT and Aldh1a1-3Ser−/− seminiferous tubules contained Ret- (Fig. S1) and GFRA1-positive (Fig. 1 I and J) spermatogonia, which are characteristic of As and Ap stages (19). A large number of spermatogonia in Aldh1a1-3Ser−/− seminiferous tubules expressed ZBTB16 and RARG (Fig. 1 K–N; Fig. S1), which are markers of Aal spermatogonia (10, 21). In contrast, KIT, which is expressed in A1 to B spermatogonia, was never detected in the seminiferous tubules of Aldh1a1-3Ser−/− mutants (Fig. 1 O and P; Fig. S1). Thus, similarly to the situation found in VAD (3), spermatogonia in Aldh1a1-3Ser−/− mutants do not progress beyond the Aal to A1 transition, and the apparent high number of Aal spermatogonia (Fig. 1 L and N) likely reflects inhibition of their differentiation into A1 spermatogonia. These results indicate that ablation of RALDH in SC blocks spermatogonia differentiation at the Aal stage, thereby preventing the initiation of the first wave of spermatogenesis. We conclude that, if RA is actually the product generated by RALDH and is required to initiate spermatogonia differentiation, it must be synthesized by SC.
Fig. 1.
Spermatogenesis is impaired in Aldh1a1-3Ser−/− mutants because of an arrest of spermatogonia differentiation at the Aal stage. (A–F) Histological sections through testes of PN5, PN15, and PN40 WT and mutants. (G and H) ISH with antisense probes for Rec8 (purple signal) on testis sections of PN40 WT and mutant. (I–P) Whole-mount immunodetection of GFRA1 (green), ZBTB16 (red), and RARG and KIT (green) on seminiferous tubules of WT and mutants; the signals are superimposed with the DAPI nuclear counterstain. As, A single spermatogonia; Ap, A paired spermatogonia; Aal, A aligned spermatogonia; A1, A1 spermatogonia; G, spermatogonia; P, pachytene spermatocyte; PR, preleptotene spermatocyte; R, round spermatid; S, Sertoli cell. [Scale bar (in P for reference): G–P, 40 μm; in A–F, 60 μm.]
RA-Activated RARA in SC Drives the Initial Transition from Aal to A1 Spermatogonia, Possibly Through Cell Autonomously Inducing Expression of Mafb.
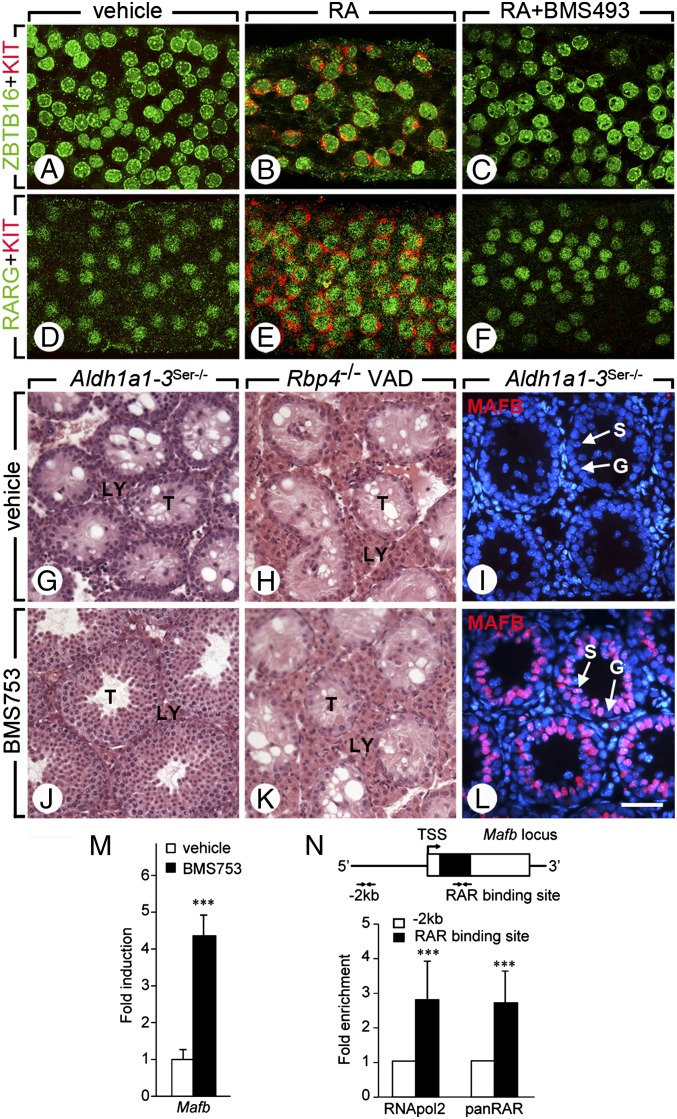
To show that impairment of spermatogonia differentiation was caused by a lack of RA in Aldh1a1-3Ser−/− testes, retinoids were administered to PN40 mutants (n = 3 for each condition). Twenty-four hours after a single injection of RA, a majority of spermatogonia in Aldh1a1-3Ser−/− seminiferous tubules expressed KIT (compare Fig. 2 A and D with Fig. 2 B and E). As expected, administration of retinol to Aldh1a1-3Ser−/− mutants had no effect on spermatogonia differentiation. Importantly, administration of BMS493 (a pan-RAR inverse agonist) together with RA inhibited the RA-induced differentiation of KIT-positive spermatogonia in Aldh1a1-3Ser−/− mutants (Fig. 2 C and F). These data support the view that Aldh1a1-3Ser−/− testes contained Aal spermatogonia, which are poised to resume differentiation into A1 spermatogonia upon RA administration. We conclude that RA produced by RALDH in SC promotes the initial transition from Aal to A1 spermatogonia, thereby driving the initiation of the first (i.e., pubertal) wave of spermatogenesis in mice. To determine whether RA acts in an autocrine manner through activating RARA in SC or in a paracrine manner through activating RARG in Aal spermatogonia, we performed organotypic cultures of PN12 Aldh1a1-3Ser−/− testes (n = 3–5) in the presence of BMS753 or BMS961, which are RARA- and RARG-selective agonists, respectively (12). Within 6 h, both BMS753 and BMS961 induced a robust expression of Cyp26a1 mRNA, a well characterized RA-target gene, indicating that both retinoids were functional in this assay. Importantly, Stra6, expressed by SC, and Crabp1, expressed in spermatogonia (9, 22), were induced only by BMS753 and BMS961, respectively (Fig. S2). These results, together with the fact that RARA is expressed in SC and RARG in spermatogonia (23), indicate that BMS753 and BMS961 activated selectively RARA and RARG, respectively. Most interestingly, in organotypic cultures, Kit expression was significantly increased only in the presence of BMS753 (Fig. S2). Moreover, in vivo, spermatocytes and spermatids were observed in the seminiferous tubules of Aldh1a1-3Ser−/− mutants 3 wk after administration of a single dose of BMS753 (Fig. 2 G and J). Therefore, activating RARA can resume spermatogonia differentiation in Aldh1a1-3Ser−/− mutants. We conclude that RA produced by SC drives the transition from Aal to A1 spermatogonia required for the first wave of spermatogenesis through activating RARA in an autocrine manner.
Fig. 2.
Initiation of the first wave of spermatogenesis requires RARA, which controls Mafb expression in SCs. (A–F) Whole-mount immunodetection of ZBTB16 or RARG (green) and KIT (red) on seminiferous tubules of Aldh1a1-3Ser−/− mutants 24 h after treatment with vehicle, RA, or RA+BMS493. (G and H; J and K) Histological sections through testes of Aldh1a1-3Ser−/− and Rbp4−/− mutants 3 wk after treatment with vehicle or BMS753. (I and L) Immunodetection of MAFB on testes sections from Aldh1a1-3Ser−/− mutants 8 h after treatment with vehicle or BMS753. (M) Relative expression of Mafb mRNA quantified by quantitative RT-PCR in testes of Aldh1a1-3Ser−/− mutants cultured in the presence of cycloheximide and treated for 2 h with vehicle (white bars) and BMS753 (black bars). (N) Schematic representation of Mafb locus and quantitative analysis by qPCR of DNA recovered from MSC-1 cell chromatin immunoprecipitated using antibodies directed against RNApol2 or all RAR isotypes (pan-RAR) at Mafb locus. It is a single exon-containing gene. The untranslated and translated regions are depicted by open and closed boxes, respectively, and the transcription start site (TSS) by a broken arrow. The locations of primers used for qPCR are indicated at −2 kb and in Mafb. Mean fold enrichment of three experiments at RAR binding site (black bars) is relative to the amount of DNA recovered at −2 kb (set at 1, white bars). Error bars represent SEM (n = 4); ***P < 0.001. G, spermatogonia; LY, Leydig cell; S, Sertoli cell; T, seminiferous tubule. [Scale bar (in L for reference): A–F, 15 μm; G–K, 50 μm; I–L, 20 μm.]
To investigate whether similar RARA-dependent events in SC also operate in the reinitiation of spermatogenesis in a VAD testis, Rbp4-null adult males fed a VAD diet for 14 wk (a reliable model of postnatal vitamin A deficiency; ref. 24) were treated with BMS753 or with its vehicle only (n = 7 for each treatment). Interestingly, spermatogenesis did not initiate upon administration of BMS753 to VAD Rbp4-null mutants; only SC and spermatogonia were present in their seminiferous epithelium, as in the vehicle-treated situation (Fig. 2 H and K). It must be stressed that spermatogenesis can resume in Rbp4-null males if vitamin A is restored in the diet or if RA is administered (24). These results indicate that RA-activated RARA in SC is able to drive the initial transition from Aal to A1 spermatogonia, but not the following ones and suggest that RA needs to act elsewhere in the seminiferous epithelium to induce subsequent cycles of spermatogenesis.
The neonatal testis at PN6 contains two distinct populations of undifferentiated GC: gonocyte-derived undifferentiated spermatogonia and gonocytes that are able to differentiate directly into A1 spermatogonia to initiate the first prepubertal wave of spermatogenesis (2, 25). The signals underlying the fate of these two subpopulations were thought to be under the control of somatic cells but yet unidentified (25). According to our study, it is now conceivable that the signal committing gonocytes to differentiation as A1 spermatogonia relies on RA-activated RARA in SC. However, undifferentiated spermatogonia also rely on RA as attested by the requirement of retinol (or RA) to resume spermatogenesis in VAD adult males (3, 24). The Aal-to-A1 transitions during all but the first wave of spermatogenesis may depend on RAR-dependent signals distinct from those controlled by RARA in SC for commitment of gonocytes.
To gain insights into the genetic cascade controlled by RARA in SC, we treated organotypic cultures (n = 4) of PN12 Aldh1a1-3Ser−/− testes with BMS753 for 2 h in the presence of the translation inhibitor cycloheximide, thereby allowing changes in the expression of only direct target genes. Microarray expression profiling identified only a few genes that were deregulated twofold or more upon BMS753 treatment, among which was Mafb (formerly Kreisler). We confirmed by reverse transcription–quantitative PCR (RT-qPCR) that expression of Mafb mRNA was strongly increased upon short-term activation of RARA (Fig. 2M). In addition, MAFB was detected by IHC in the SC nuclei of Aldh1a1-3Ser−/− mutants treated with BMS753 for 8 h (n = 5), but not in nuclei from testes exposed to vehicle (Fig. 2 I and L). Importantly, chromatin immunoprecipitation assays with a pan-RAR antibody performed on cultured MSC-1 SC (26) treated for 2 h with RA revealed a robust RAR-binding site at the end of the Mafb coding region, indicating that this gene is a direct target of RA-activated RARA in SC (Fig. 2N). Because no consensus RARE was found in the sequence, the motif on which RARA binds remains unknown. This motif is unlikely to recruit RARA/RXR heterodimers because RARA acts in SC through a noncanonical pathway, independently of RXR (9). Control of Mafb expression by RA-activated RARA in SC seems particularly relevant to spermatogonia differentiation because the MAFB transcription factor often instructs cell lineage commitment (27) and because its Drosophila ortholog Traffic Jam is required, in somatic cells of the fly gonad for GC differentiation (28). The involvement of MAFB in the RA-dependent differentiation of spermatogonia is, however, not testable through a genetic approach in the mouse as null mutants are not viable (29, 30), conditional alleles of Mafb are not available, and mice bearing the chemically induced Kr mutation (31) are deficient in MAFB only in the hindbrain region (32).
RA Is Necessary to Initiate Meiosis in Spermatocytes, Through Controlling Stra8 Expression in a Cell-Autonomous Manner.
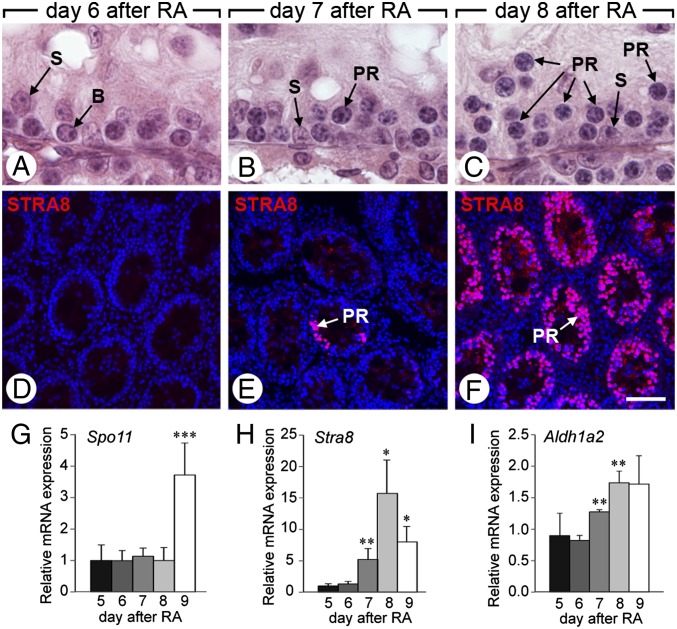
The kinetics of the first spermatogenesis was followed in Aldh1a1-3Ser−/− mutants (n = 3–5) that were “rescued” by a single injection of RA to resume the Aal–A1 transition. Six days after RA injection, only B spermatogonia were present in the seminiferous tubules (Fig. 3A), and neither STRA8 protein (Fig. 3D) nor Rec8 mRNA (Fig. S3), which are markers of meiosis (33), was detected. At day 7, few preleptotene spermatocytes were observed in the seminiferous tubules (Fig. 3B), and some STRA8-positive cells were detected in 30 ± 12% of the tubules (Fig. 3E). At day 8, a large number of preleptotene spermatocytes were observed in all tubule sections (Fig. 3C), whereas STRA8 (Fig. 3F) and Rec8 (Fig. S3) expressions extended to virtually all tubules. The mRNA levels of Spo11, which is normally expressed from the leptotene stage of meiosis, and of Stra8 were quantified from days 5 to 9 after RA injection. Stra8 expression significantly increased at day 8 and then dropped at day 9 (Fig. 3G), whereas Spo11 peaked at day 9 (Fig. 3H), indicating that differentiation of preleptotene spermatocytes started 7 d after RA-induced Aal–A1 transition, and that their further differentiation into leptotene spermatocytes occurred at day 9.
Fig. 3.
Meiosis initiates 7 d after the Aal-A1 transition in Aldh1a1-3Ser−/− mutants rescued by a single injection of RA. (A–C) Histological sections through testes of Aldh1a1-3Ser−/− mutants 6, 7, and 8 d after treatment with RA. (D–F) Immunodetection of STRA8 (red) superimposed to the DAPI nuclear counterstain (blue) in the same samples. (G–I) Relative expression of Spo11, Stra8, and Aldh1a2 mRNA quantified by RT-qPCR in testes of Aldh1a1-3Ser−/− mutants between 5 and 9 d after treatment with RA. Error bars represent SEM (n = 4); *P < 0.05, **P < 0.01, ***P < 0.001. B, B spermatogonia; PR, preleptotene spermatocyte; S, Sertoli cell. [Scale bar (in F for reference): A–C, 10 μm; D–F, 70 μm.]
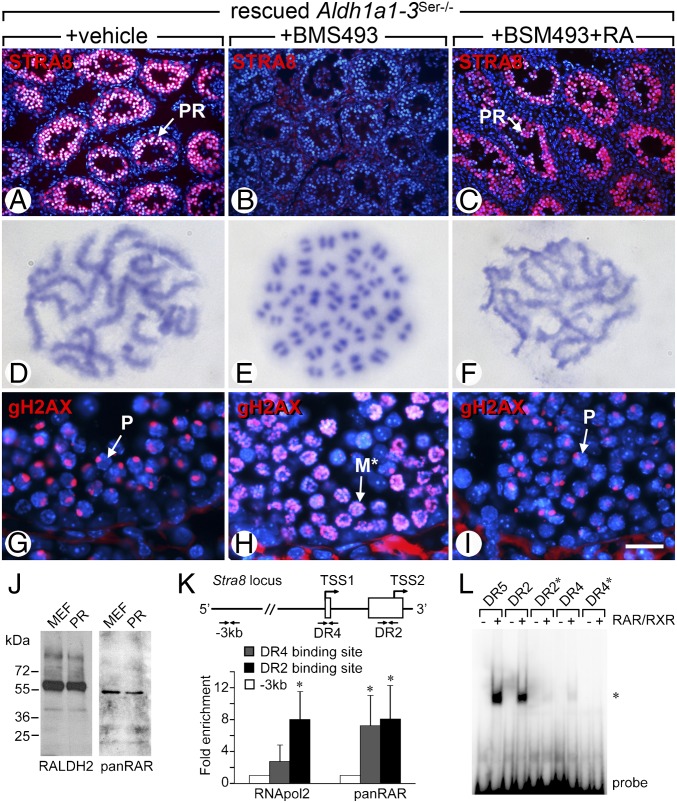
Because RA half-life is <1 h in the mouse (34), the amount of residual RA present at day 7 in rescued Aldh1a1-3Ser−/− mutants is virtually null [i.e., ∼10−40 pg/kg body weight (bw)]. This observation casts doubts on the fact that RA drives meiosis if, at the onset of meiosis, SC still represent the sole source of RA in the seminiferous epithelium. To test whether some RA was actually necessary for meiosis, we treated rescued Aldh1a1-3Ser−/− mutants (n = 3 for each condition) with the pan-RAR inverse agonist BMS493 either alone or in combination with RA at 6, 7, and 8 d after the Aal–A1 transition, which correspond to the period of meiotic initiation. BMS493 administration impaired the formation of preleptotene spermatocyte (Fig. S4) and abrogated STRA8 expression (Fig. 4 A and B), whereas coadministration of RA prevented the effects of BMS493 (Fig. 4C). Most importantly, GC in BMS493-treated rescued Aldh1a1-3Ser−/− males displayed abnormalities which are identical to those of Stra8-null spermatocytes (33), namely (i) hypercondensed chromatin structures resembling mitosis metaphase chromosomes and reacting positively with an antibody against the phosphorylated form of histone H3 (Fig. S4); (ii) 40 unpaired univalent chromosomes instead of 19 bivalents plus the sex chromosomes (Fig. 4 D and E); (iii) distribution of phosphorylated H2AX (gH2AX) on all chromosomes instead of restriction to the XY body at the pachytene stage (Fig. 4 G and H). Because all these defects were prevented when RA was administered together with BMS493 (Fig. 4 F and I; Fig. S4), we conclude that BMS493 actually impaired the RA-induced signal responsible for STRA8 expression. Moreover, because SC devoid of all RALDH cannot produce RA, another source of RA necessarily exists for meiotic initiation in the rescued Aldh1a1-3Ser−/− mutants.
Fig. 4.
Meiotic initiation requires RA-activated RAR in preleptotene spermatocytes to induce Stra8 expression. (A–C) Immunodetection of STRA8 (red) and DAPI nuclear counterstain (blue) at the onset of meiosis (day 8 after rescue) on histological sections from testes of Aldh1a1-3Ser−/− mutants rescued by RA (day 0), and then treated with vehicle, BMS493, or BMS493+RA from days 6 to 8. (D–F) Giemsa-stained metaphase spreads at the pachytene stage (day 12 after rescue). (G–I) Immunodetection of gH2AX (red) and DAPI nuclear counterstain (blue) on histological sections at the pachytene stage (day 12 after rescue). (J) Western blot analysis of protein extracts from FACS-purified preleptotene spermatocytes from rescued Aldh1a1-3Ser−/− mutants (PR), using antibodies directed against RALDH2 or all RAR isotypes (pan-RAR). Protein extracts from mouse embryonic fibroblasts (MEF) transfected with RALDH2- and RAR-coding plasmids were used as positive controls. (K) Schematic representation of Stra8 locus and quantitative analysis by qPCR of DNA recovered from testis chromatin (prepared using RA-rescued Aldh1a1-3Ser−/− mutants at day 8) immunoprecipitated using antibodies directed against RNApol2 or all RAR isotypes (pan-RAR) at Stra8 locus. The untranslated exons and the two transcription start sites (TSS1 and TSS2) are depicted by open boxes and broken arrows, respectively. The locations of primers used for qPCR are indicated at −3 kb and in Stra8. Mean fold enrichment of three experiments at DR4 (gray bars) and DR2 (black bars) binding sites is relative to the amount of DNA recovered at −3 kb (set at 1, white bars). (L) EMSA showing that RAR/RXR heterodimers bind strongly to the DR2 and weakly to the DR4 of Stra8 (asterisk). Binding is abolished when DR2 and DR4 sequences are changed (DR2* and DR4*, respectively). Binding to canonical RARE of Rarb2 gene was used as a positive control (DR5). Error bars represent SEM (n = 5); *P < 0.05. M*, metaphase-like cells; P, pachytene spermatocytes; PR, preleptotene spermatocyte. [Scale bar (in I for reference): A–C, 100 μm; D–F, 1.5 μm; G–I, 10 μm.]
Interestingly, Aldh1a2 mRNA expression significantly increased 7 and 8 d after the Aal–A1 transition in rescued Aldh1a1-3Ser−/− mutants (Fig. 3I; Fig. S3). In agreement with this finding, RALDH2 protein was detected by Western blot (Fig. 4J) in FACS-purified preleptotene spermatocytes from rescued Aldh1a1-3Ser−/− mutants (Fig. S5), indicating that the alternate source of RA in Aldh1a1-3Ser−/− mutants at the time of meiotic initiation is represented by preleptotene spermatocytes. In addition to RALDH2, these cells also contained at least one RAR isotype, as assessed by Western blot using a pan-RAR antibody (Fig. 4J), making possible that RA-activated RAR cell autonomously controlled meiosis initiation through turning on Stra8. Accordingly, ChIP assays performed on rescued Aldh1a1-3Ser−/− testes at day 8 revealed two binding sites for RAR in Stra8 gene in the promoter region (Fig. 4K). The first site located within the most upstream transcription start site (TSS1) contained a consensus RARE with half-sites separated by 4 bp, and the second site located close to the downstream TSS (TSS2) contained a consensus RARE with half-sites separated by 2 bp (hereafter referred to as DR4 and DR2, respectively). Electrophoretic mobility shift assay (EMSA) further revealed that RAR/RXR heterodimers bound weakly to DR4, but strongly to DR2, i.e., almost as efficiently as to the canonical DR5 RARE located in Rarb2 gene (Fig. 4L), and binding was abolished when the sequence of DR4 and DR2 were modified (DR4* and DR2* in Fig. 4L). Furthermore, DR2 but not DR4 was able to compete for binding of RAR/RXR to the canonical DR5 (Fig. S6).
Although the requirement of RA for meiosis in male GC was suggested for some time, no straightforward evidence for this proposal was yet provided. This paradigm emerged from the ability of exogenous RA to induce premature meiotic initiation in fetal male GC (reviewed in ref. 5) and from VAD models, which display a meiotic failure simply because spermatogonia are blocked at the undifferentiated stage (e.g., ref. 35). In this context, the present genetic study identifies endogenous RA in male GC as the driving force responsible for meiotic initiation in vivo. We propose that RA-activated RAR/RXR heterodimers cell autonomously control STRA8 production in preleptotene spermatocytes, essentially through their binding to a DR2 located close to Stra8 gene TSS2, and thereby stably commit male GC to the meiotic cell cycle (33). Although our findings conclusively show that endogenous RA can be the long-searched meiosis-inducing factor in postnatal male GC, it is possible that signals other than RA play this role in fetal female GC (36–38). In agreement with this proposal, both Stra8 expression and meiosis initiate normally in fetal ovaries devoid of RA (6). In addition, the DR2 element in Stra8 is not, or very poorly, bound by RAR in WT female GC, casting doubts on the physiological induction of Stra8 by RA-activated RAR/RXR heterodimers (6). Accordingly, aside of RA, several factors such as DMRT1 (39), MSX1/MSX2 (40), and RSPO1 (41) are required to promote Stra8 expression and meiotic initiation in the fetal ovary.
RALDH in SC Are Required for Spermiation but Dispensable for both the Oscillating Activity of SC and Perpetuation of Spermatogonia Differentiation.
Interestingly, rescued Aldh1a1-3Ser−/− testes analyzed between 3 and 20 wk following induction of the initial Aal to A1 transition by a single injection of RA displayed a complete spermatogenesis with mature spermatozoa (Fig. 2K; Fig. S7). However, a large number of mature spermatids (i) failed to align at the luminal side of the seminiferous epithelium and (ii) were retained beyond the normal time of spermiation (Fig. S7). In addition, the epididymides of these mutants contained only abnormal spermatozoa displaying signs of necrosis (Fig. S7), such as separation of the acrosome from the nucleus (42). Thus, RALDH activity in SC is required for spermiation. Because this process is also impaired in mice lacking either RARA or RXRB specifically in SC (23), we conclude that RA originating from SC is required for the proper release of elongated spermatids into the lumen of the seminiferous tubules, through activating in an autocrine manner RARA/RXRB heterodimers.
That spermatogenesis lasts for months after a single injection of retinoid in Aldh1a1-3Ser−/− mutants implies that, once the very first wave of spermatogenesis has occurred, RALDH activity in SC becomes fully dispensable to spermatogonia differentiation. Along the same lines, analysis by IHC or ISH at adulthood in rescued Aldh1a1-3Ser−/− mutants (n = 3) showed that Gata1, Ar, Stra6, and Lgals1 gene expressions were oscillating (Fig. S8) as it is the case in the normal mouse testis. Thus, although the oscillating activity of SC requires both RARA in SC (23, 43, 44) and RA (44), RALDH activity in SC is dispensable to the control of the cyclic expression of genes by RA-activated RARA. In this context, RA is necessarily produced somewhere else than in SC. Aside from preleptotene spermatocytes (see above), pachytene spermatocytes represent an alternative source of RA because these cells robustly express Aldh1a2 mRNA at stages VII–VIII of the seminiferous epithelium cycle (23), i.e., at the time when Aal spermatogonia transform into A1 (2). We propose that RA generated through the activity of RALDH2 in pachytene spermatocytes acts in a paracrine fashion to stimulate spermatogonia differentiation from the second cycle onward. Thus, through cyclically modulating the RA environment in the seminiferous epithelium from the second wave of spermatogenesis onward, spermatogonia progeny (i.e., spermatocytes) may provide a sophisticated system controlling both the oscillating activity of SC, and the synchrony between the differentiation of Aal spermatogonia and meiotic initiation to allow perpetuation of spermatogenesis and therefore constant production of gametes.
Materials and Methods
Detailed procedures and antibodies are indicated in SI Text.
Mice and Treatment.
Mice on a mixed C57BL/6–129/Sv (50–50%) genetic background were housed in an animal facility licensed by the French Ministry of Agriculture (Agreement B67-218-5), and animal experiments were supervised by M.M. (Agreement 67-62) who is qualified in mouse experimentations, in compliance with the European legislation on care and use of laboratory animals and with the Ethics Committee of Institut de Génétique et de Biologie Moléculaire et Cellulaire (IGBMC). For VAD studies, mice were fed a VAD diet (TD86143; Harlan Laboratories) as described (24). All-trans-RA (5 or 25 mg/kg bw; MP Biomedicals), BMS493 (50 mg/kg bw), and BMS753 (25 mg/kg bw, Tocris Bioscience) were administered by i.p. injections. Organotypic cultures were performed on testes from PN12 Aldh1a1-3Ser−/− mice.
Flow Cytometry.
Preleptotene spermatocytes were purified as described (45). Dispersed cells were sequentially stained with Hoechst 33342 and propidium iodide, and sorting was performed using Aria II cell sorter (Becton Dickinson).
Histology, ISHs, IHC, and Preparation of Germ Cell Nuclear Spreads.
Testis samples were fixed in Bouin's fluid and embedded in paraffin. Histological sections were stained with hematoxylin and eosin. ISH using digoxigenin-labeled probes for detection of Aldh1a2, Kit, Lgals1, Rec8, Ret, and Zbtb16 expression were performed as described (23, 24). Freshly prepared seminiferous tubules fixed in PBS containing 4% (wt/vol) paraformaldehyde (PFA) and 10% (vol/vol) methanol were incubated with one of the primary antibodies. The IHC process was repeated in the case of a double staining using a distinct primary antibody followed by incubation with the appropriate secondary antibody. Freshly frozen sections were postfixed in acetone then in 4% (wt/vol) PFA in PBS. Immunodetections were performed as described (23, 33). The samples were mounted on glass slides in Vectashield (Vector Laboratories) containing DAPI and observed with confocal laser or fluorescence microscopes. Metaphase spreads of spermatocytes were prepared as described (33).
Quantitative Analysis of Gene Expression by RT-PCR.
Total RNA was prepared using TRIzol reagent (Life Technologies). Reverse transcription of total RNA followed by PCR amplification of cDNA was performed using QuantiTect Reverse Transcription (Qiagen) and LightCycler 480 SYBR Green I Master (Roche Diagnostics) kits, respectively. Primers were as indicated in Table S1. Triplicates of each sample were used in each experimental condition. The transcript levels were normalized relative to that of Actb transcripts, whose expression is not changed by the mutation or by retinoid administration. Data were expressed as fold induction relative to vehicle condition. Statistical significance was assessed by Student t test.
Chromatin Immunoprecipitation (ChIP).
To prepare chromatin, cells or dissected testes were fixed with 0.4% PFA (wt/vol), before being sonicated to shear DNA to an average size of 500 bp. For each reaction, 100 μg of chromatin was first incubated with 6 μg of ChIP-grade anti–pan-RAR or anti-RNApol2 antibody and then with protein G-Sepharose. The purified DNA was analyzed by qPCR and was compared with input DNA. Quantitation was determined by the enrichment of the binding site compared with a site located upstream the TSS. The sequences of the oligonucleotides used are described in Table S1.
EMSAs.
They were performed as previously described (46). The sequences of the oligonucleotides used are described in Table S1.
Supplementary Material
Acknowledgments
We thank Muriel Klopfenstein for help as well as Amin Choukrallah, Emmanuel Moutier, and Abdelali Lehraiki for sharing with us their expertise in ChIP, EMSA, and organotypic culture, respectively. We also thank Florian Guillou and Pascal Dollé for providing us the Amh-Cre and the loxP-flanked Aldh1a2 transgenic lines, respectively, as well as all of the common services and platforms of IGBMC who significantly contributed to this work, notably persons from FACS (Claudine Ebel), electron microscopy (Nadia Messaddeq), and confocal imaging (Pascal Kessler and Marc Koch) facilities. This work was supported by grants from Centre National de la Recherche Scientifique, Institut National de la Santé et de la Recherche Médicale, Hôpital Universitaire de Strasbourg; Fondation pour la Recherche Médicale (FRM) Grant DEQ20071210544; and Agence Nationale de la Recherche Contracts ANR-09-BLAN-0286, ANR-10-BLAN-1239-04, and ANR-09-BLAN-0282. M.R. was supported by French Ministry of Research and Technology grants and FRM Grant FDT20110922849.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1214936109/-/DCSupplemental.
References
- 1.Nakagawa T, Sharma M, Nabeshima Y, Braun RE, Yoshida S. Functional hierarchy and reversibility within the murine spermatogenic stem cell compartment. Science. 2010;328:62–67. doi: 10.1126/science.1182868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- 3.Hogarth CA, Griswold MD. The key role of vitamin A in spermatogenesis. J Clin Invest. 2010;120:956–962. doi: 10.1172/JCI41303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowles J, Koopman P. Retinoic acid, meiosis and germ cell fate in mammals. Development. 2007;134:3401–3411. doi: 10.1242/dev.001107. [DOI] [PubMed] [Google Scholar]
- 5.Griswold MD, Hogarth CA, Bowles J, Koopman P. Initiating meiosis: The case for retinoic acid. Biol Reprod. 2012;86:35. doi: 10.1095/biolreprod.111.096610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar S, et al. Sex-specific timing of meiotic induction is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun. 2011;2:151. doi: 10.1038/ncomms1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambon P. The nuclear receptor superfamily: A personal retrospect on the first two decades. Mol Endocrinol. 2005;19:1418–1428. doi: 10.1210/me.2005-0125. [DOI] [PubMed] [Google Scholar]
- 9.Vernet N, et al. Retinoic acid metabolism and signaling pathways in the adult and developing mouse testis. Endocrinology. 2006;147:96–110. doi: 10.1210/en.2005-0953. [DOI] [PubMed] [Google Scholar]
- 10.Gely-Pernot A, et al. Spermatogonia differentiation requires retinoic acid receptor γ. Endocrinology. 2012;153:438–449. doi: 10.1210/en.2011-1102. [DOI] [PubMed] [Google Scholar]
- 11.Dupé V, et al. A newborn lethal defect due to inactivation of retinaldehyde dehydrogenase type 3 is prevented by maternal retinoic acid treatment. Proc Natl Acad Sci USA. 2003;100:14036–14041. doi: 10.1073/pnas.2336223100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matt N, et al. Retinoic acid-dependent eye morphogenesis is orchestrated by neural crest cells. Development. 2005;132:4789–4800. doi: 10.1242/dev.02031. [DOI] [PubMed] [Google Scholar]
- 13.Vermot J, et al. Conditional (loxP-flanked) allele for the gene encoding the retinoic acid-synthesizing enzyme retinaldehyde dehydrogenase 2 (RALDH2) Genesis. 2006;44:155–158. doi: 10.1002/gene.20195. [DOI] [PubMed] [Google Scholar]
- 14.Lécureuil C, Fontaine I, Crepieux P, Guillou F. Sertoli and granulosa cell-specific Cre recombinase activity in transgenic mice. Genesis. 2002;33:114–118. doi: 10.1002/gene.10100. [DOI] [PubMed] [Google Scholar]
- 15.Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- 16.Barrios F, et al. SOHLH1 and SOHLH2 control Kit expression during postnatal male germ cell development. J Cell Sci. 2012;125:1455–1464. doi: 10.1242/jcs.092593. [DOI] [PubMed] [Google Scholar]
- 17.Buaas FW, et al. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- 18.Yoshida S, et al. Neurogenin3 delineates the earliest stages of spermatogenesis in the mouse testis. Dev Biol. 2004;269:447–458. doi: 10.1016/j.ydbio.2004.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Tokuda M, Kadokawa Y, Kurahashi H, Marunouchi T. CDH1 is a specific marker for undifferentiated spermatogonia in mouse testes. Biol Reprod. 2007;76:130–141. doi: 10.1095/biolreprod.106.053181. [DOI] [PubMed] [Google Scholar]
- 20.Lolicato F, et al. Potential role of Nanos3 in maintaining the undifferentiated spermatogonia population. Dev Biol. 2008;313:725–738. doi: 10.1016/j.ydbio.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 21.Filipponi D, et al. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bouillet P, et al. Developmental expression pattern of Stra6, a retinoic acid-responsive gene encoding a new type of membrane protein. Mech Dev. 1997;63:173–186. doi: 10.1016/s0925-4773(97)00039-7. [DOI] [PubMed] [Google Scholar]
- 23.Vernet N, et al. Prepubertal testis development relies on retinoic acid but not rexinoid receptors in Sertoli cells. EMBO J. 2006;25:5816–5825. doi: 10.1038/sj.emboj.7601447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghyselinck NB, et al. Retinoids and spermatogenesis: Lessons from mutant mice lacking the plasma retinol binding protein. Dev Dyn. 2006;235:1608–1622. doi: 10.1002/dvdy.20795. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida S, et al. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- 26.Peschon JJ, et al. Directed expression of an oncogene to Sertoli cells in transgenic mice using mullerian inhibiting substance regulatory sequences. Mol Endocrinol. 1992;6:1403–1411. doi: 10.1210/mend.6.9.1331774. [DOI] [PubMed] [Google Scholar]
- 27.Stanley ER. Lineage commitment: Cytokines instruct, at last! Cell Stem Cell. 2009;5:234–236. doi: 10.1016/j.stem.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li MA, Alls JD, Avancini RM, Koo K, Godt D. The large Maf factor Traffic Jam controls gonad morphogenesis in Drosophila. Nat Cell Biol. 2003;5:994–1000. doi: 10.1038/ncb1058. [DOI] [PubMed] [Google Scholar]
- 29.Blanchi B, et al. MafB deficiency causes defective respiratory rhythmogenesis and fatal central apnea at birth. Nat Neurosci. 2003;6:1091–1100. doi: 10.1038/nn1129. [DOI] [PubMed] [Google Scholar]
- 30.Moriguchi T, et al. MafB is essential for renal development and F4/80 expression in macrophages. Mol Cell Biol. 2006;26:5715–5727. doi: 10.1128/MCB.00001-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cordes SP, Barsh GS. The mouse segmentation gene kr encodes a novel basic domain-leucine zipper transcription factor. Cell. 1994;79:1025–1034. doi: 10.1016/0092-8674(94)90033-7. [DOI] [PubMed] [Google Scholar]
- 32.Eichmann A, et al. The expression pattern of the mafB/kr gene in birds and mice reveals that the kreisler phenotype does not represent a null mutant. Mech Dev. 1997;65:111–122. doi: 10.1016/s0925-4773(97)00063-4. [DOI] [PubMed] [Google Scholar]
- 33.Mark M, et al. STRA8-deficient spermatocytes initiate, but fail to complete, meiosis and undergo premature chromosome condensation. J Cell Sci. 2008;121:3233–3242. doi: 10.1242/jcs.035071. [DOI] [PubMed] [Google Scholar]
- 34.Nau H. Species differences in pharmacokinetics and drug teratogenesis. Environ Health Perspect. 1986;70:113–129. doi: 10.1289/ehp.8670113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li H, Palczewski K, Baehr W, Clagett-Dame M. Vitamin A deficiency results in meiotic failure and accumulation of undifferentiated spermatogonia in prepubertal mouse testis. Biol Reprod. 2011;84:336–341. doi: 10.1095/biolreprod.110.086157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Best D, Sahlender DA, Walther N, Peden AA, Adams IR. Sdmg1 is a conserved transmembrane protein associated with germ cell sex determination and germline-soma interactions in mice. Development. 2008;135:1415–1425. doi: 10.1242/dev.019497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kocer A, Reichmann J, Best D, Adams IR. Germ cell sex determination in mammals. Mol Hum Reprod. 2009;15:205–213. doi: 10.1093/molehr/gap008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Tilly JL. Epigenetic status determines germ cell meiotic commitment in embryonic and postnatal mammalian gonads. Cell Cycle. 2010;9:339–349. doi: 10.4161/cc.9.2.10447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Krentz AD, et al. DMRT1 promotes oogenesis by transcriptional activation of Stra8 in the mammalian fetal ovary. Dev Biol. 2011;356:63–70. doi: 10.1016/j.ydbio.2011.05.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Le Bouffant R, et al. Msx1 and Msx2 promote meiosis initiation. Development. 2011;138:5393–5402. doi: 10.1242/dev.068452. [DOI] [PubMed] [Google Scholar]
- 41.Chassot AA, et al. RSPO1/β-catenin signaling pathway regulates oogonia differentiation and entry into meiosis in the mouse fetal ovary. PLoS One. 2011;6:e25641. doi: 10.1371/journal.pone.0025641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holstein AF, Roosen-Runge EC, Schirren C. Illustrated Pathology of Human Spermatogenesis. Berlin: Grosse; 1988. p. 278. [Google Scholar]
- 43.Parvinen M. Cyclic functions of Sertoli cells. In: Russell LD, Griswold MD, editors. The Sertoli Cell. Clearwater, FL: Cache River Press; 1993. pp. 331–347. [Google Scholar]
- 44.Sugimoto R, Nabeshima Y, Yoshida S. Retinoic acid metabolism links the periodical differentiation of germ cells with the cycle of Sertoli cells in mouse seminiferous epithelium. Mech Dev. 2012;128:610–624. doi: 10.1016/j.mod.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 45.Getun IV, Torres B, Bois PR. Flow cytometry purification of mouse meiotic cells. J Vis Exp. 2011;50:e2602. doi: 10.3791/2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hwang JJ, Chambon P, Davidson I. Characterization of the transcription activation function and the DNA binding domain of transcriptional enhancer factor-1. EMBO J. 1993;12:2337–2348. doi: 10.1002/j.1460-2075.1993.tb05888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.