Abstract
Proteinase-Activated rreceptor-2 (PAR2), a G-protein–coupled Receptor, activated by serine proteinases, is reported to have both protective and proinflammatory effects in the airway. Given these opposing actions, both inhibitors and activators of PAR2 have been proposed for treating asthma. PAR2 can signal through two independent pathways: a β-arrestin–dependent one that promotes leukocyte migration, and a G-protein/Ca2+ one that is required for prostaglandin E2 (PGE2) production and bronchiolar smooth muscle relaxation. We hypothesized that the proinflammatory responses to PAR2 activation are mediated by β-arrestins, whereas the protective effects are not. Using a mouse ovalbumin model for PAR2-modulated airway inflammation, we observed decreased leukocyte recruitment, cytokine production, and mucin production in β-arrestin-2−/− mice. In contrast, PAR2-mediated PGE2 production, smooth muscle relaxation, and decreased baseline airway resistance (measures of putative PAR2 “protective” effects) were independent of β-arrestin-2. Flow cytometry and cytospins reveal that lung eosinophil and CD4 T-cell infiltration, and production of IL-4, IL-6, IL-13, and TNFα, were enhanced in wild-type but not β-arrestin-2−/− mice. Using the forced oscillation technique to measure airway resistance reveals that PAR2 activation protects against airway hyperresponsiveness by an unknown mechanism, possibly involving smooth muscle relaxation. Our data suggest that the PAR2-enhanced inflammatory process is β-arrestin-2 dependent, whereas the protective anticonstrictor effect of bronchial epithelial PAR2 may be β-arrestin independent.
Currently, 300 million people suffer from asthma resulting in nearly 250,000 asthma-related deaths reported annually, ∼80% occurring in low- and lower-middle–income regions. The development of new medications that inhibit cellular inflammation may reduce morbidity rates, and attempts to manage this disease have identified proteinase-activated receptor-2 (PAR2) as an attractive new target (1). PAR2 is a G-protein–coupled receptor (GPCR) that is widely expressed in bronchial epithelial cells, leukocytes, and airway smooth muscle, where it may be activated by proteinases secreted from invading pathogens, inhaled proteinases, or by locally released proteinases such as tissue kallikreins or tryptase (2–5). Serine proteinases activate PAR2 by cleaving its N terminus, revealing a tethered ligand (SLIGRL/SLIGKV, human/mouse) that self-activates the receptor, leading to G-protein coupling and β-arrestin recruitment. Peptides corresponding to the tethered ligand and peptidomimetics such as 2-furoyl-LIGRL-ornithine-NH2 (2fAP) are commonly used to activate PAR2, both in cultured cells and in vivo (6, 7). We previously reported that PAR2 can activate two independent signaling pathways, one transduced by “classical” G-protein–coupled signaling and the other by a G-protein–independent, β-arrestin–mediated signaling pathway (8–12). Although the two signaling pathways can target common downstream effectors, the outcomes can be distinct and even opposing. For example, β-arrestins can scaffold the actin-severing protein, cofilin, with its upstream activator (Chronophin) while inhibiting its negative regulator (LIMK). This scaffold has been identified in fibroblasts, tumor cells, and primary mouse leukocytes, and is crucial for PAR2-stimulated chemotaxis. Downstream of the G-protein–coupled pathway, this same process is inhibited (11, 12).
In keeping with its opposing signals in vitro, studies done in vivo suggest that PAR2 activation can play diametrically opposed roles in allergic asthma. In favor of a proinflammatory role for PAR2, the recruitment of leukocytes to the lungs in a murine ovalbumin (OVA) model of allergic inflammatory airway disease was reduced in PAR2−/− mice and increased in PAR2-overexpressing mice (13, 14). The inflammatory response to OVA is also enhanced by the intranasal administration of PAR2 peptide agonists in wild-type (WT) mice (15). These inflammatory responses involve cell migration, leading to the hypothesis that they are β-arrestin dependent. β-Arrestins are also required for leukocyte chemotaxis downstream of a number of other GPCRs, including several chemokine receptors known to be involved in allergic asthma (11, 12, 16). In favor of a protective role, administration of PAR2 agonists promotes prostanoid-induced cytoprotection in rodent and human airways, and bronchoconstriction is elevated in PAR2−/− mice (17). Prostaglandin E2 (PGE2) production also inhibits eosinophil migration and degranulation (17, 18). PAR2-induced PGE2 production uses a Gαq-Ca2+–coupled mechanism that we hypothesize is independent of β-arrestins (19, 20). Depending on the balance of PAR2 signals between G-protein– and β-arrestin–dependent pathways, PAR2 agonists may be capable of either compounding or curbing allergic asthma. This study examines the potential role of the β-arrestin signaling pathway in the proinflammatory and protective actions of PAR2 in the airway.
Results
PAR2 Induces Cellular Airway Inflammation in WT but Not β-Arrestin-2−/− Mice.
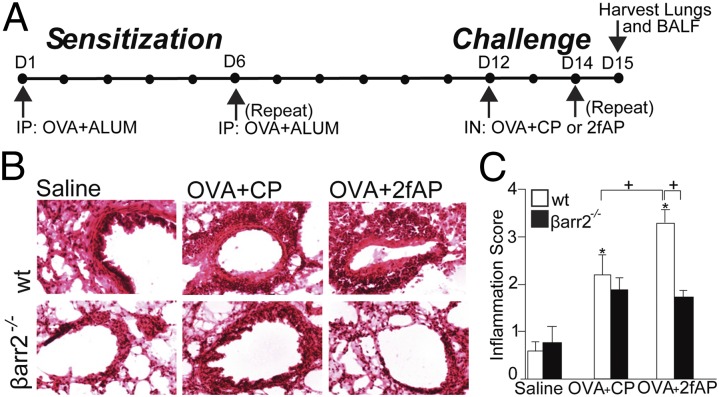
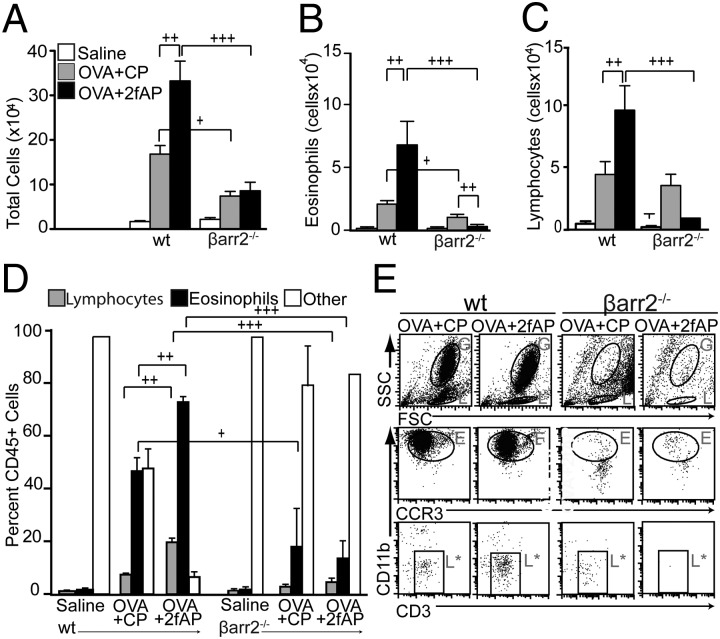
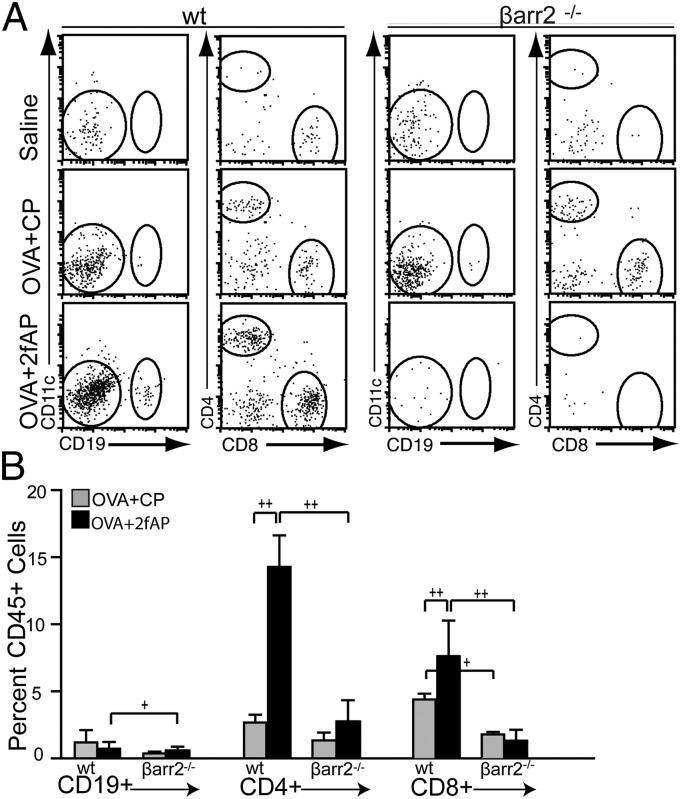
To assess a role for β-arrestin-2 in PAR2-induced cellular airway inflammation, we used a modification of a previously described OVA-induced murine model of allergic asthma (15). Mice were sensitized with an i.p. injection of saline (as a negative control) or OVA/alum, on days 1 and 6. This was followed on days 12 and 14 by an intranasal (i.n.) challenge of saline or OVA plus either a PAR2 agonist (2fAP) or a scrambled negative control peptide [2-furoyl-OLRIGL-NH2 (CP)]. Optimal 2fAP concentrations were determined by conducting a dose–response curve (Fig. S1). The mice were killed on day 15, bronchoalveolar lavage fluid (BALF) and lungs were collected, and cellular inflammation was assessed (Fig. 1A). In this short-term model, the response to OVA alone is less pronounced, and the additive effect of PAR2 on OVA-induced inflammation is more evident. Analysis of H&E-stained lung tissue sections reveals that influx of inflammatory cells into the perivascular and peribronchial regions from all WT mice treated with OVA (receiving either CP or 2fAP with the i.n. challenges) was increased compared with saline controls (Fig. 1B). Lung sections were scored for characteristics associated with acute and chronic inflammation as described in Materials and Methods revealing that the inflammation index was 1.5-fold greater for OVA+2fAP-treated WT compared with the OVA+CP-treated mice (Fig.1C). In contrast, OVA+2fAP treatment was unable to exacerbate inflammation in β-arrestin-2−/− mice. Although OVA+CP treatment elevated total BALF cells for both WT and β-arrestin-2−/− mice relative to their respective saline controls, the effect was significantly less in β-arrestin-2−/− mice, consistent with previous reports (21). Challenge with OVA+2fAP in WT animals augmented the influx of inflammatory cells (16.6 ± 3.5-fold increase over saline), but in β-arrestin-2−/− mice, no augmentation of total cells was observed with 2fAP (Fig. 2A). Differential counts, performed from cytospin preparations (Fig. S2), revealed challenge of WT mice with OVA+CP alone increased lung recruitment of both eosinophils (Fig. 2B) and lymphocytes (Fig. 2C) compared with saline controls. Challenge with OVA+2fAP further increased the numbers of both cell types in the BALF of WT mice by 3- and 2.5-fold, respectively, over those observed with OVA+CP. In contrast, the numbers of eosinophils and lymphocytes recovered from the BALF of β-arrestin-2−/− mice receiving OVA+CP was significantly lower than those in similarly treated WT mice, and the proinflammatory effect of 2fAP was not observed in β-arrestin-2−/− mice. In β-arrestin-1−/− mice, the inflammation induced by OVA alone and the PAR2-stimulated augmentation of inflammation were not significantly different from that of similarly treated WTs, suggesting the requirement is specific to β-arrestin-2 (Fig. S3). Flow-cytometric analysis, using forward and side scatter (FSC/SSC) and expression of cell surface markers CCR3 and CD3 to calculate the percentage of CD45+ BALF cells that were either eosinophils or lymphocytes, respectively (Fig. 2 D and E), revealed that BALF from OVA+CP-treated WT mice contained ∼50% eosinophils and 9% lymphocytes. In contrast, the inflammatory response to OVA+CP in β-arrestin-2−/− mice was significantly muted (19% CCR3+; <5% CD3+). Whereas PAR2 activation by 2fAP elevated BALF levels of both eosinophils (72%) and lymphocytes (22%) in WT mice, no such effect was observed in similarly treated β-arrestin-2−/− mice. Similar results were observed in digested lung tissue (Fig. S4). Lymphocytes, identified by SSC, were further analyzed for expression of lineage-specific markers (Fig. 3A). When 2fAP treatment replaces that of CP in the context of OVA, a significant elevation in BALF CD4+ and CD8+ cells occurs, but only in WT mice. Consistent with the other BALF data, OVA+CP treatment has a significantly reduced inflammatory effect on β-arrestin-2−/− mice relative to WTs (Fig. 3B). Both WT and β-arrestin-2−/− mice, challenged with either OVA+CP or OVA+2fAP, showed a similar increase in production of OVA-IgE, compared with saline-treated controls (Table 1), suggesting that the differences in cellular inflammation observed in β-arrestin-2−/− mice were not due to their inability to become sensitized to ovalbumin. Measurement of surface PAR2 levels in the airways of WT and β-arrestin-1 and -2−/− mice demonstrated that there was no significant difference in surface receptor levels, suggesting the effect was not due to decreased PAR2 expression (Fig. S5).
Fig. 1.
PAR2-induced lung inflammation is reduced in β-arrestin-2−/− mice. (A) Timeline of OVA-induced sensitization and PAR2 challenge in WT and β-arrestin-2−/− mice (24 per OVA treatment group, 18 per saline treatment group, 128 total mice). (B) Representative images of H&E-stained frozen lung sections from mice treated as described in Fig. 1A. (C) Histological inflammation scoring for peribronchial thickness and leukocyte invasion on a scale from 0 to 4 (see SI Materials and Methods for details; 20 sections from each mouse, four separate experiments, scored double-blinded). +Significant difference between groups (P < 0.01). *All groups differed significantly from saline-treated mice (P < 0.05, n = 15 for saline, n = 20 for OVA+CP and OVA+2fAP). Statistics in this and all subsequent figures were determined by ANOVA with Tukey HSD posttests.
Fig. 2.
PAR2-induced recruitment of leukocytes to the lungs requires β-arrestin-2. (A–C) Bar graphs depict cell numbers in BALF: total cells (A), eosinophils (B), and lymphocytes (C). Numbers from all OVA-treated mice were statistically different from saline controls except eosinophils in β-arrestin-2−/− treated with OVA+2fAP. (D) Flow-cytometric determination of the percentage of CD45+ cells that were lymphocytes, eosinophils, or other cells. Significant differences between bracketed groups are indicated as follows: +P < 0.05, ++P < 0.01, and +++P > 0.001. (E) Representative scatter plots of flow-cytometric analysis of BALF cells. High FSC/SSC, CD45+ granulocyte populations (G) were analyzed for the presence of CD11b and CCR3 [eos (E)]. Low SSC, CD45+ lymphocyte (L) populations were further analyzed for the presence of the T-lymphocyte marker, CD3 (L*) (n = 18 for saline, n = 24 each for OVA+CP and OVA+2fAP).
Fig. 3.
PAR2 promotes β-arrestin–dependent recruitment of CD4+ T cells. (A) Representative scatter plots of flow-cytometric analysis. CD45+/low SSC cells were analyzed for immunoreactivity to CD19+/CD11c− (CD19+ B cells), CD19−/CD4+/CD8− (CD4 T cells) and CD19−/CD4−/CD8+ (CD8 T cells). (B) Graph showing the %CD45+ cells that were CD19+, CD4+, or CD8+. +P < 0.05 and ++P < 0.01 show significant difference between bracketed groups. n = 15 for saline, n = 20 for OVA+CP and OVA+2fAP.
Table 1.
Ovalbumin IgE levels in serum after OVA+2fAP-induced airway inflammation model
| Mouse strain | Sensitization | Challenge | OVA-specific IgE (OD450) |
| WT | None | Saline | 0.15 ± 0.02 |
| WT | OVA+Al(OH3) | OVA+CP | *0.68 ± 0.13 |
| WT | OVA+Al(OH3) | OVA+2fAP | *0.60 ± 0.23 |
| βarr2−/− | None | Saline | 0.15 ± 0.03 |
| βarr2−/− | OVA+Al(OH3) | OVA+CP | *0.70 ± 0.14 |
| βarr2−/− | OVA+Al(OH3) | OVA+2fAP | *0.65 ± 0.16 |
Serum samples from mice treated as described in Fig. 1A were assayed for OVA-IgE levels by ELISA. Values are expressed as mean ± SEM optical density (OD) (n = 8 for each treatment group). *Statistically significant differences from saline control group, as determined by ANOVA, P < 0.001 (n = 15 for saline, n = 20 for OVA+CP and OVA+2fAP).
To determine the extent to which 2fAP is able to access cells expressing PAR2 within the pleural cavity, we examined the labeling of airway cells with rhodamine-conjugated 2fAP (Rh-2fAP) 24 h after i.n. administration. We observed Rh-2fAP-labeled cells distributed along both the basement membrane and apical surface of airway epithelia in WT mice, but not PAR2−/−, mice (Fig. S6). These data raise the possibility that PAR2 expressed on leukocytes might mediate their chemotaxis into the lungs. This hypothesis was further supported by the observation that the cellular inflammation observed in response to OVA+2fAP is partially mediated by hematopoietic PAR2. We observed that OVA+2fAP-induced airway inflammation was reduced after adoptive transfer of PAR2−/− bone marrow cells into WT mice (Fig. S7). Conversely, when WT bone marrow was transplanted into PAR2−/− mice and these mice were challenged with OVA+2fAP, recruitment of immune cells to the lungs was partially restored.
PAR2-Induced Cytokine Production Is Partially Dependent on β-Arrestin-2.
Allergic asthma involves a Th2 response orchestrated by cytokines and chemokines secreted by epithelial cells, T cells, and other invading leukocytes. Using a cytometric bead array to assay cytokine levels, we observed increased IL-4, IL-6, IL-13, TNFα, IL-10, and IL-12p70 levels in OVA+2fAP-challenged compared with OVA+CP-treated WT mice (Fig. 4). In β-arrestin-2−/− mice, no PAR2-mediated increase in IL-4, IL-6, IL-13, and TNFα levels was observed, but levels of IL-10 and IL-12p70 were still increased with 2fAP treatment (Fig. 4 E and F). Similar to what was reported previously (15), in this short-term model of airway inflammation, no significant changes in cytokine levels other than IL-6 were seen between animals challenged with OVA+CP and saline-treated controls (Fig. 4B).
Fig. 4.
PAR2-induced cytokine production in WT and β-arrestin-2−/− mice. BALF supernatants from mice, treated as described in Fig. 1A, were analyzed by cytometric bead array for the presence of IL-4 (A), IL-6 (B), IL-13 (C), TNFα (D), IL-10 (E), and IL-12p70 (F). *,**Statistically significant differences from saline controls (*P < 0.01 and **P < 0.001); +statistically significant differences between bracketed groups (P < 0.01) (n = 15 for saline, n = 20 each for OVA+CP and OVA+2fAP).
One of the up-regulated cytokines, IL-13, is known to play a key role in goblet cell hyperplasia and increased mucin production during asthma (22). To determine whether PAR2 increased mucin production via a β-arrestin–dependent mechanism, lung sections were stained with Alcian blue to identify goblet cells and goblet cell numbers were quantified. OVA+2fAP significantly increased mucin production above that observed in saline-treated and OVA+CP-treated animals. Consistent with the dependence of IL-13–mediated mucin production on β-arrestin-2, PAR2-stimulated mucin production was also abolished in the β-arrestin-2−/− animals (Fig. 5) but not in β-arrestin-1−/− animals (Fig. S8).
Fig. 5.
Goblet cell hyperplasia and mucin production in response to PAR2 is abolished in β-arrestin-2−/− mice. (A) Lung tissue from each of the treatment groups shown in Fig. 1A was stained with Alcian blue to reveal acidic mucins and costained with neutral red. The arrows indicate mucin-producing goblet cells. (B) Double-blind quantification of the number of mucin-producing cells per millimeter of basement membrane. (C) Quantification of intensity of Alcian blue stain. Significant difference from saline treated mice (*P < 0.05; **P < 0.005). Significant differences between bracketed groups are indicated as follows: +P < 0.05. Twenty images of at least two mice from three independent experiments (120 total) were analyzed for B and C.
PAR2-Mediated Bronchiolar Relaxation Is Independent of β-Arrestin-2.
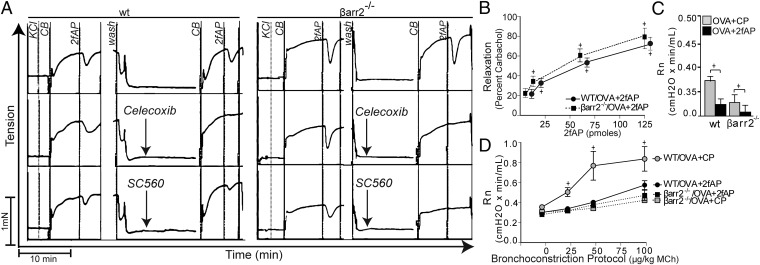
The major protective effect reported for PAR2 is smooth muscle relaxation, which is mediated by prostaglandins (e.g.PGE2) released from airway epithelial cells. PGE2 levels were significantly increased in the BALF of both WT and β-arrestin-2−/− mice receiving OVA+2fAP (Table 2), suggesting β-arrestin-2 is not required for this PAR2-mediated response. We next investigated 2fAP-induced smooth muscle relaxation from WT and β-arrestin-2−/− mice. Treatment of first-order bronchiolar rings with 2fAP caused a rapid relaxation response, the magnitude and duration of which was similar in WT and β-arrestin-2−/− bronchioles (Fig. 6 A and B). In keeping with previous reports (23), relaxation in both animal groups was abolished by selective inhibitors of either COX1 or COX2 (Fig. 6A). We conclude that PAR2-induced smooth muscle relaxation is independent of β-arrestin-2.
Table 2.
PGE2 levels in BALF after OVA+2fAP-induced airway inflammation model
| Mouse strain | Sensitization | Challenge | PGE2 levels, pg/mL |
| WT | None | Saline | 1.4 ± 0.23 |
| WT | OVA+Al(OH3) | OVA+CP | 2.2 ± 0.18 |
| WT | OVA+Al(OH3) | OVA+2fAP | *3.4 ± 0.15 |
| βarr2−/− | None | Saline | 1.8 ± 0.31 |
| βarr2−/− | OVA+Al(OH3) | OVA+CP | 2.2 ± 0.29 |
| βarr2−/− | OVA+Al(OH3) | OVA+2fAP | **3.2 ± 0.06 |
BALF supernatants were assayed for prostaglandin (PGE2) levels using PGE2 Express AChE tracer ELISA. Values are mean ± SEM (n = 8). Statistically significant differences from control group are indicated as follows: *P = 0.0003, **P = 0.0001 (n = 15 for saline, n = 20 for OVA+CP and OVA+2fAP).
Fig. 6.
PAR2-induced smooth muscle relaxation is maintained in β-arrestin-2−/− mice. (A) Representative myograph traces of tension in bronchiole smooth muscle from WT (Left) and β-arrestin-2−/− mice (Right) after pretreatment with vehicle (Top), COX-2 inhibitor (Celecoxib, 0.2 µM) (Middle), or COX1 inhibitor (SC-560, 0.1 µM) (Bottom). Treatments with KCl (to assess viability), carbachol (CB), and 2fAP are indicated. (B) Average relaxation (percentage of the CB-induced tension) from three independent experiments is graphed as a function of [2fAP]. +Significant differences in tension with 2fAP (P < 0.01). (C and D) Newtonian resistance (Rn) was assessed in mice, treated as described in Fig. 1A. (C) Graph of baseline resistance values in WT and β-arrestin-2−/− mice that received OVA+CP or OVA+2fAP. (D) Resistance after bronchoconstriction with indicated doses of methacholine (MCh). +Significant difference between bracketed groups (P < 0.01, n = 6 per treatment group). +Significant differences in MCh responsiveness compared with baseline (P < 0.05, n = 6 per treatment group).
Consistent with a prior report (21), an increase in airway hyperresponsiveness (AHR) was observed in WT animals receiving i.n. challenges of OVA+CP, but this effect was absent in β-arrestin-2−/− mice, (Fig. 6D). Interestingly, both baseline airway resistance and methacholine-induced AHR were significantly reduced in WT mice treated with OVA+2fAP (Fig. 6 C and D), consistent with the protective effects of PAR2 reported by others (17). The already low level of baseline airway resistance in OVA-treated β-arrestin-2−/− mice was decreased with the addition of 2fAP, suggesting that the protective effect of PAR2 on airway smooth muscle relaxation is independent of β-arrestin-2 (Fig. 6C). However, 2fAP was not able to lower the airway response to methacholine in β-arrestin-2−/− mice, perhaps owing to the already reduced magnitude of the response in these mice (Fig. 6D). The abrogation of OVA-induced AHR in β-arrestin2−/− animals has been reported previously and studies with chimeric mice suggest that this effect is independent of the cellular inflammation (24).
Discussion
Efforts directed at exploiting the potential “protective” bronchodilator effects of PAR2 agonists for the treatment of asthma have been hindered by studies demonstrating that PAR2 agonists can cause inflammatory responses in various tissues (13, 17, 25). The debate as to whether PAR2 is a protective or proinflammatory receptor in allergic inflammatory airway disease resembles the continued controversy surrounding the function of the β2-adrenoceptor (β2-AR), the prototypical GPCR, in asthma. Although β-agonists are bronchodilators of choice to alleviate acute bronchospasm, their chronic use is associated with loss of bronchoprotection, worsening of asthma control, and asthma-related death (26). In murine models, the asthma phenotype is significantly promoted by chronic β-agonist treatment (27, 28) or impaired in mice having genetic or pharmacologic ablation of β2-ARs (29, 30). Similarly, mice lacking β-arrestin-2 do not develop the asthma phenotype (21), placing β-arrestin-2 downstream of GPCRs in the proasthmatic signaling pathway. Thus, the seemingly paradoxical effects of PAR2 and β2-AR agonists on the asthma phenotype can likely be explained by the activation of dual signaling pathways, a proinflammatory β-arrestin–dependent signaling pathway in addition to the classical G-protein–mediated bronchorelaxation pathway.
Our studies provide insight to explain the apparently opposing responses of PAR2 activation. We, and others, have previously shown that, in a 25-d murine multiple-OVA challenge model, development of the asthma phenotype is significantly impaired in mice lacking either β-arrestin-2 or PAR2 (13, 14, 21). To differentiate between PAR2-specific effects that are mediated by β-arrestin-2, and the general β-arrestin-2-OVA–induced allergic responses also we used a 15-d OVA model in the current study. At this time point, the magnitude of the asthma phenotype induced by OVA is low, whereas the PAR2 agonist exacerbating effects are high (15), allowing the asthma phenotype-inducing effects of PAR2 to be highlighted. We show that the “inflammatory” leukocyte infiltration response in our asthma model depends on β-arrestin-2–mediated signaling, whereas many of the protective bronchodilator effects of PAR2 activation (such as PGE2 production and subsequent bronchiolar smooth muscle relaxation) do not. The results shown here are consistent with previous studies demonstrating two independent signaling pathways downstream of PAR2: a β-arrestin-2–dependent pathway leading to actin cytoskeletal changes and cell migration, and a G-protein–dependent one that promotes Ca2+ mobilization and diacylglycerol formation (10, 11). We have previously shown formation of a β-arrestin scaffold containing the actin filament-severing protein cofilin and its upstream activator in primary bone marrow leukocytes, and have demonstrated that this signaling pathway is crucial for PAR2-stimulated cell migration (11, 12). PAR2 has also been reported to increase PGE2 levels by multiple mechanisms, some resulting in rapid, and others delayed, PGE2 release, all of which appear to occur through Gαq-dependent, β-arrestin–independent signaling (8–11, 18, 20). Our data suggest that both COX1 and COX2 appear to be interlinked to generate the relaxant response, because a selective inhibition of either enzyme blocked the relaxant response. Taken together, these results indicate that this protective signaling axis is independent of β-arrestin-2, despite reports that IL-1 and angiotensin II can increase PGD2 and PGE2 levels via a β-arrestin–dependent pathway in vitro (31, 32). The signaling pathway activated by a PAR2 mechanism that does not involve β-arrestin-2 merits further investigation. Taken together, these studies support the hypothesis that these two independent PAR2 signaling pathways may direct opposing responses in vivo.
Another important hallmark of cellular airway inflammation is the production of cytokines. Both the resident airway cells and invading eosinophils and CD4+ T cells can secrete these factors. Because the lung level of both cell types was reduced in β-arrestin-2−/− mice, it is not surprising that the 2fAP-induced increases in cytokines such as IL-6, IL-13, and TNFα were also reduced. IL-13 is important for goblet cell hyperplasia and mucin production such as was observed in the WT mice receiving OVA+2fAP. Consistent with β-arrestin dependence of IL-13 production, mucin production and goblet cell number were significantly reduced in OVA-treated β-arrestin-2−/− mice. Increased acidic mucin is clinically relevant, as mucus plugging is often associated with severity of disease in humans.
Like human asthma, one of the defining features of allergic inflammatory airways disease in mice is AHR, a measure of the sensitivity and reactivity of airway narrowing to a bronchoconstrictor. We assessed airway reactivity, the more clinically relevant component of AHR (33), by measuring the resistance response to multiple increasing concentrations of the bronchoconstrictor, methacholine. WT mice treated with 2fAP in the context of OVA displayed a statistically significant and physiologically relevant decrease in airway reactivity relative to those treated with CP. Because the effect of OVA treatment on AHR in β-arrestin-2−/− mice is so muted, it is difficult to assess whether this protective effect of PAR2 in vivo requires β-arrestins; however, a significant reduction in baseline resistance was observed in mice receiving OVA+2fAP compared with OVA+CP in both WT and β-arrestin-2−/− animals, suggesting that PAR2-mediated airway smooth muscle relaxation is independent of β-arrestin-2. The protective effect of 2fAP on AHR in mice appears to be dominant over the proinflammatory effect because AHR decreased despite the concomitant enhanced airways inflammation and mucin phenotypes, which are typically associated with increased AHR. Our data support the notion that the paradoxical effects of PAR2 activation are mediated by dual signaling pathways. Separation of these dual pathways in vivo can be challenging, especially if the receptor activates opposing outcomes. For example, in guinea pig bronchial preparations the prostanoid-mediated epithelium-dependent bronchodilator action of PAR2 is masked by the concurrent PAR2-mediated triggering of airway hyperresponsiveness and only revealed in the presence of indomethacin (34). PAR2-mediated production of airway prostaglandins likely underlies the protective effect of 2fAP on AHR observed here as well. To definitively determine whether the protective effects of PAR2 on AHR are independent of β-arrestins in vivo will likely require using a model that results in a more robust AHR phenotype in β-arrestin-2−/− mice.
PAR2 is up-regulated in the airways of patients with chronic asthma (35), which along with the reported proinflammatory effects of PAR2, has led to an interest in inhibitors of PAR2-activating proteinases or of the receptor itself as therapeutic agents for treating asthma (36). In contrast, the protective effects of PAR2 have also generated interest in PAR2 agonists as therapeutic agents for asthma, with the idea being that agonists of PAR2 might promote bronchodilatation (2, 17, 37, 38). Although these same agonists can exacerbate other aspects of lung inflammation (13–15, 39), our work reveals the potential of developing “biased” PAR2 agonists or antagonists that can selectively attenuate the β-arrestin-2–dependent signal pathway and optimize the protective PAR2 G-protein–mediated signaling responses. Studies identifying naturally occurring biased PAR2 signaling by cryptic proteolytic cleavage and mutant tethered ligands point to the feasibility of developing such agonists (1, 40, 41). Furthermore, the recent identification of pepducins that specifically inhibit PAR2 raises the possibility that development of small molecule β-arrestin–specific PAR2 antagonists might be possible (42). Indeed, pharmacologic manipulation of biased signaling may underlie the mortality advantage observed in congestive heart failure patients treated with carvedilol versus metoprolol (43, 44). Our findings identify PAR2, like the β1-AR, as a GPCR capable of eliciting G-protein– and β-arrestin–dependent signals that regulate organ system function and disease features in disparate ways. However, in this instance, we have PAR2-mediated β-arrestin signaling driving airway pathology and G-protein signaling combating it, whereas in heart failure β1-AR–mediated G-protein signaling is pathogenic and β-arrestin signaling is cardioprotective. Taken together, direct targeting of the β-arrestin-2–dependent signaling pathway may hold tremendous therapeutic promise in the treatment of asthma. The potential to selectively modulate PAR2-specific signaling pathways that mediate protective or proinflammatory effects sets the stage for the development of pathway-specific therapeutic agents for the treatment of asthma and other inflammatory diseases.
Materials and Methods
Animals.
All animal procedures were in accordance with the guidelines on the use and care of laboratory animals set by the National Institutes of Health and approved by the Institutional Animal Care and Use Committee at University of California, Riverside, and Duke University. β-Arrestin2−/− mice in a C57BL/6 background were provided by Robert Lefkowitz (Duke University Medical Center). PAR2−/− mice were provided by Robin Plevin (University of Strathclyde, Glasgow, Scotland) and were developed by KOWA Pharmaceuticals. WT C57BL/6 (WT) mice were from The Jackson Laboratory. All animals are bred in-house.
Sensitization and Challenge with Ovalbumin.
Age-matched male C57BL/6 β-arrestin-1−/− and β-arrestin-2−/− mice (2–4 mo old) were sensitized on days 1 and 6 with an i.p. injection of OVA/alum (Sigma) [10 μg of OVA and 2 mg of Al(OH)3 in 0.5 mL of saline] or saline alone. On days 12 and 14, they were given an i.n. challenge of 25 μL of OVA [0.2% (wt/vol) in saline] containing either PAR2 activating peptide (2fAP at 2.5 nmol) or PAR2 control peptide (CP; 2.5 nmol), or given saline alone. Peptides were synthesized by Genemed. Control WT and β-arrestin2−/− mice received saline for i.p. injections and i.n. challenges. On day 15, mice were euthanized and BALF and lungs were collected. Lung digests and histology were performed as previously described (15) or analyzed for AHR as described below.
ELISA Immunoassays and Cytokine Bead Arrays.
OVA-IgE ELISA immunoassays and Prostaglandin AChR capture assays (MD BioProducts) were performed according to the manufacturer’s instructions. Experimental details are provided in SI Materials and Methods.
BALF Analysis.
Cells from BALF samples were pelleted, washed, and resuspended in FACS buffer for flow cytometry. Total cell numbers were determined by hemocytometer counts. One hundred microliters of lavage fluid were spun onto slides using Shandon Cytospin 3 as previously described (12). Cell differentials were determined by classifying 200 cells using standard morphological criteria. A minimum of 10 images was analyzed for each mouse; n = 16 mice per treatment (4 mice per treatment group repeated 4 times). For flow cytometry, BALF cells were divided in two antibody-staining sets and analyzed as described in SI Materials and Methods.
Lung Histology.
Extracted lungs were either flash frozen in OCT or fixed and embedded in paraffin. Five-micrometer sections were stained using standard H&E protocol and imaged with an upright Nikon Eclipse E600 using PAXIT! software. Sections were scored for epithelial and perivascular inflammation using a 0–4 point scale, as described in SI Materials and Methods. For analysis of mucin, formalin-fixed lung tissue was paraffin embedded and 5-µm sections were cleared, hydrated, and stained with Alcian blue for 30 min (to identify mucin proteins) and neutral red for 5 min (to identify cell nuclei). Sections were then dehydrated and mounted with resinous mounting media, and slides were imaged as described above.
Smooth Muscle Relaxation and AHR.
These methods have been described previously (20, 27, 45, 46), and experimental details are provided in SI Materials and Methods. Briefly, airway responsiveness in mice was measured using the forced oscillation technique, an invasive method that measures lung impedance. From this measure, Newtonian resistance, an indicator of airway luminal diameter, was calculated according to the constant-phase model.
Data and Statistical Analysis.
Statistical significance was determined using one-way ANOVA and Tukey honestly significant difference (HSD) posttests to compare treatment groups. Data, graphs, and statistical analyses were performed using FACS DIVA, FlowJo, Microsoft Excel 2003, or GraphPad Prism 5.0.
Supplementary Material
Acknowledgments
We thank Dr. Robert Lefkowitz for β-arrestin-2−/− mice and Dr. Robin Plevin for PAR2−/− mice. This work was funded by National Institutes of Health Grants 1R21HL092388 (to K.A.D.), R01HL084123 and R01HL093103 (to J.K.L.W.), and R01NS072298 (to E.H.W.), and the Canadian Institutes of Health Research (M.D.H.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1208881109/-/DCSupplemental.
References
- 1.Ramachandran R, Noorbakhsh F, Defea K, Hollenberg MD. Targeting proteinase-activated receptors: Therapeutic potential and challenges. Nat Rev Drug Discov. 2012;11:69–86. doi: 10.1038/nrd3615. [DOI] [PubMed] [Google Scholar]
- 2.Cocks TM, Moffatt JD. Protease-activated receptor-2 (PAR2) in the airways. Pulm Pharmacol Ther. 2001;14:183–191. doi: 10.1006/pupt.2001.0285. [DOI] [PubMed] [Google Scholar]
- 3.Oikonomopoulou K, et al. Proteinase-mediated cell signalling: Targeting proteinase-activated receptors (PARs) by kallikreins and more. Biol Chem. 2006;387:677–685. doi: 10.1515/BC.2006.086. [DOI] [PubMed] [Google Scholar]
- 4.Arizmendi NG, et al. Mucosal allergic sensitization to cockroach allergens is dependent on proteinase activity and proteinase-activated receptor-2 activation. J Immunol. 2011;186:3164–3172. doi: 10.4049/jimmunol.0903812. [DOI] [PubMed] [Google Scholar]
- 5.Boitano S, et al. Potent agonists of the protease activated receptor 2 (PAR2) J Med Chem. 2011;54:1308–1313. doi: 10.1021/jm1013049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hollenberg MD, et al. Derivatized 2-furoyl-LIGRLO-amide, a versatile and selective probe for proteinase-activated receptor 2: Binding and visualization. J Pharmacol Exp Ther. 2008;326:453–462. doi: 10.1124/jpet.108.136432. [DOI] [PubMed] [Google Scholar]
- 7.McGuire JJ, Saifeddine M, Triggle CR, Sun K, Hollenberg MD. 2-Furoyl-LIGRLO-amide: A potent and selective proteinase-activated receptor 2 agonist. J Pharmacol Exp Ther. 2004;309:1124–1131. doi: 10.1124/jpet.103.064584. [DOI] [PubMed] [Google Scholar]
- 8.DeFea KA, et al. beta-Arrestin-dependent endocytosis of proteinase-activated receptor 2 is required for intracellular targeting of activated ERK1/2. J Cell Biol. 2000;148:1267–1281. doi: 10.1083/jcb.148.6.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge L, Ly Y, Hollenberg M, DeFea K. A beta-arrestin-dependent scaffold is associated with prolonged MAPK activation in pseudopodia during protease-activated receptor-2-induced chemotaxis. J Biol Chem. 2003;278:34418–34426. doi: 10.1074/jbc.M300573200. [DOI] [PubMed] [Google Scholar]
- 10.Wang P, DeFea KA. Protease-activated receptor-2 simultaneously directs beta-arrestin-1-dependent inhibition and Galphaq-dependent activation of phosphatidylinositol 3-kinase. Biochemistry. 2006;45:9374–9385. doi: 10.1021/bi0602617. [DOI] [PubMed] [Google Scholar]
- 11.Zoudilova M, et al. Beta-arrestin-dependent regulation of the cofilin pathway downstream of protease-activated receptor-2. J Biol Chem. 2007;282:20634–20646. doi: 10.1074/jbc.M701391200. [DOI] [PubMed] [Google Scholar]
- 12.Zoudilova M, et al. beta-Arrestins scaffold cofilin with chronophin to direct localized actin filament severing and membrane protrusions downstream of protease-activated receptor-2. J Biol Chem. 2010;285:14318–14329. doi: 10.1074/jbc.M109.055806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmidlin F, et al. Protease-activated receptor 2 mediates eosinophil infiltration and hyperreactivity in allergic inflammation of the airway. J Immunol. 2002;169:5315–5321. doi: 10.4049/jimmunol.169.9.5315. [DOI] [PubMed] [Google Scholar]
- 14.Takizawa T, et al. Abrogation of bronchial eosinophilic inflammation and attenuated eotaxin content in protease-activated receptor 2-deficient mice. J Pharmacol Sci. 2005;98(1):99–102. doi: 10.1254/jphs.scz050138. [DOI] [PubMed] [Google Scholar]
- 15.Ebeling C, et al. Proteinase-activated receptor 2 activation in the airways enhances antigen-mediated airway inflammation and airway hyperresponsiveness through different pathways. J Allergy Clin Immunol. 2005;115:623–630. doi: 10.1016/j.jaci.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 16.DeFea KA. Stop that cell! Beta-arrestin-dependent chemotaxis: A tale of localized actin assembly and receptor desensitization. Annu Rev Physiol. 2007;69:535–560. doi: 10.1146/annurev.physiol.69.022405.154804. [DOI] [PubMed] [Google Scholar]
- 17.Cocks TM, et al. A protective role for protease-activated receptors in the airways. Nature. 1999;398:156–160. doi: 10.1038/18223. [DOI] [PubMed] [Google Scholar]
- 18.Henry PJ. The protease-activated receptor2 (PAR2)-prostaglandin E2-prostanoid EP receptor axis: A potential bronchoprotective unit in the respiratory tract? Eur J Pharmacol. 2006;533:156–170. doi: 10.1016/j.ejphar.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 19.Kawao N, et al. Signal transduction for proteinase-activated receptor-2-triggered prostaglandin E2 formation in human lung epithelial cells. J Pharmacol Exp Ther. 2005;315:576–589. doi: 10.1124/jpet.105.089490. [DOI] [PubMed] [Google Scholar]
- 20.Nagataki M, Moriyuki K, Sekiguchi F, Kawabata A. Evidence that PAR2-triggered prostaglandin E2 (PGE2) formation involves the ERK-cytosolic phospholipase A2-COX-1-microsomal PGE synthase-1 cascade in human lung epithelial cells. Cell Biochem Funct. 2008;26:279–282. doi: 10.1002/cbf.1434. [DOI] [PubMed] [Google Scholar]
- 21.Walker JKL, et al. β-Arrestin-2 regulates the development of allergic asthma. J Clin Invest. 2003;112:566–574. doi: 10.1172/JCI17265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izuhara K, et al. The mechanism of mucus production in bronchial asthma. Curr Med Chem. 2009;16:2867–2875. doi: 10.2174/092986709788803196. [DOI] [PubMed] [Google Scholar]
- 23.McGuire JJ, Hollenberg MD, Andrade-Gordon P, Triggle CR. Multiple mechanisms of vascular smooth muscle relaxation by the activation of proteinase-activated receptor 2 in mouse mesenteric arterioles. Br J Pharmacol. 2002;135:155–169. doi: 10.1038/sj.bjp.0704469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hollingsworth JW, et al. Both hematopoietic-derived and non-hematopoietic-derived beta-arrestin-2 regulates murine allergic airway disease. Am J Respir Cell Mol Biol. 2010;43:269–275. doi: 10.1165/rcmb.2009-0198OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergnolle N, Hollenberg MD, Sharkey KA, Wallace JL. Characterization of the inflammatory response to proteinase-activated receptor-2 (PAR2)-activating peptides in the rat paw. Br J Pharmacol. 1999;127:1083–1090. doi: 10.1038/sj.bjp.0702634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker JKL, Penn RB, Hanania NA, Dickey BF, Bond RA. New perspectives regarding β2-adrenoceptor ligands in the treatment of asthma. Br J Pharmacol. 2011;163(1):18–28. doi: 10.1111/j.1476-5381.2010.01178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin R, et al. Chronic treatment in vivo with β-adrenoceptor agonists induces dysfunction of airway β2-adrenoceptors and exacerbates lung inflammation in mice. Br J Pharmacol. 2012;165:2365–2377. doi: 10.1111/j.1476-5381.2011.01725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen LP, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen LP, et al. Beta2-adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Callaerts-Vegh Z, et al. Effects of acute and chronic administration of beta-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathurin K, et al. An interaction between L-prostaglandin D synthase and arrestin increases PGD2 production. J Biol Chem. 2011;286:2696–2706. doi: 10.1074/jbc.M110.178277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kendall RT, et al. The beta-arrestin pathway-selective type 1A angiotensin receptor (AT1A) agonist [Sar1,Ile4,Ile8]angiotensin II regulates a robust G protein-independent signaling network. J Biol Chem. 2011;286:19880–19891. doi: 10.1074/jbc.M111.233080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Busse WW. The relationship of airway hyperresponsiveness and airway inflammation: Airway hyperresponsiveness in asthma: Its measurement and clinical significance. Chest. 2010;138(2 Suppl):4S–10S. doi: 10.1378/chest.10-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barrios VE, Jarosinski MA, Wright CD. Proteinase-activated receptor-2 mediates hyperresponsiveness in isolated guinea pig bronchi. Biochem Pharmacol. 2003;66:519–525. doi: 10.1016/s0006-2952(03)00292-2. [DOI] [PubMed] [Google Scholar]
- 35.Knight DA, et al. Protease-activated receptors in human airways: Upregulation of PAR-2 in respiratory epithelium from patients with asthma. J Allergy Clin Immunol. 2001;108:797–803. doi: 10.1067/mai.2001.119025. [DOI] [PubMed] [Google Scholar]
- 36.Kanke T, et al. Novel antagonists for proteinase-activated receptor 2: Inhibition of cellular and vascular responses in vitro and in vivo. Br J Pharmacol. 2009;158(1):361–371. doi: 10.1111/j.1476-5381.2009.00342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.De Campo BA, Henry PJ. Stimulation of protease-activated receptor-2 inhibits airway eosinophilia, hyperresponsiveness and bronchoconstriction in a murine model of allergic inflammation. Br J Pharmacol. 2005;144:1100–1108. doi: 10.1038/sj.bjp.0706150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Agostino B, et al. Activation of protease-activated receptor-2 reduces airways inflammation in experimental allergic asthma. Clin Exp Allergy. 2007;37:1436–1443. doi: 10.1111/j.1365-2222.2007.02793.x. [DOI] [PubMed] [Google Scholar]
- 39.Alshurafa HN, et al. A protease activated receptor-2 (PAR-2) activating peptide, tc-LIGRLO-NH2, induces protease release from mast cells: Role in TNF degradation. BMC Pharmacol. 2004;4:12. doi: 10.1186/1471-2210-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramachandran R, et al. Agonist-biased signaling via proteinase activated receptor-2: Differential activation of calcium and mitogen-activated protein kinase pathways. Mol Pharmacol. 2009;76:791–801. doi: 10.1124/mol.109.055509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramachandran R, et al. Neutrophil elastase acts as a biased agonist for proteinase-activated receptor-2 (PAR2) J Biol Chem. 2011;286:24638–24648. doi: 10.1074/jbc.M110.201988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sevigny LM, et al. Interdicting protease-activated receptor-2-driven inflammation with cell-penetrating pepducins. Proc Natl Acad Sci USA. 2011;108:8491–8496. doi: 10.1073/pnas.1017091108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Remme WJ, et al. Effect of carvedilol and metoprolol on the mode of death in patients with heart failure. Eur J Heart Fail. 2007;9:1128–1135. doi: 10.1016/j.ejheart.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 44.Wisler JW, et al. A unique mechanism of beta-blocker action: Carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schuessler TF, Bates JH. A computer-controlled research ventilator for small animals: Design and evaluation. IEEE Trans Biomed Eng. 1995;42:860–866. doi: 10.1109/10.412653. [DOI] [PubMed] [Google Scholar]
- 46.Proskocil BJ, Fryer AD. 2005. Beta2-agonist and anticholinergic drugs in the treatment of lung disease. Proc Am Thorac Soc 2(4):305–310; discussion 311–312.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.