Abstract
Phagocytosis is a primary defense program orchestrated by monocytes/macrophages. Unregulated phagocytosis can lead to pathological conditions. In the current study we have demonstrated that Wnt5a stimulates phagocytosis through PI3 kinase–Rac1 and lipid-raft-dependent processes. Wnt5a-mediated augmentation in phagocytosis is suppressed by blocking expression of the putative Wnt5a receptor Frizzled 5. Enhanced phagocytosis of bacteria by Wnt5a–Fz5 signaling increases the secretion of proinflammatory cytokines, but not the bacterial killing rate. Furthermore, a small molecule inhibitor of Wnt production, IWP-2, which reduces secretion of functionally active Wnt5a, not only suppresses both phagocytosis and the secretion of proinflammatory cytokines but also accelerates the bacterial killing rate.
Keywords: inflammation, E. coli, sepsis
The complexities accompanying phagocytosis by macrophages under the influence of different intercellular milieu remain unresolved. Although crucial for innate immune functions, phagocytosis turned awry may enhance inflammation, thus aggravating pathological conditions (1–3). The molecular mechanisms underlying such phenomena are unclear. Because phagocytosis involves cytoskeletal alterations, we wondered if embryonic growth modulators such as Wnts, which influence cell polarity through cytoskeletal modulations, could also engender phagocytosis and thus foster inflammation (4–6). Accordingly, we addressed if Wnt5a, an embryonic growth modulator and a potential mediator of inflammation (7–10), stimulates phagocytosis of exogenous antigens by macrophages, and we analyzed its effects.
Wnt5a is a member of the Wnt family of secreted glycoprotein signal transducers. There are about 20 Wnts, which act as ligands to the Frizzled (Fz) family of heterotrimeric G-protein-coupled cell surface receptors. The Fz family receptors comprise about 10 members (11–16). Examples of some putative Fz receptors for Wnt5a are Fz5, Fz2, and Fz4 (17–19).
Wnt–Fz signaling plays a significant role in cell differentiation during mammalian development and has been broadly categorized into two types—canonical (β catenin mediated) and noncanonical (not β catenin mediated). During canonical Wnt signaling, the Wnt1/Wnt3a class of canonical Wnts facilitates the transcriptional activity of β catenin along with specific transcription factors of the Lef/Tcf family (5, 11–15). Several reports suggest that unlike the canonical Wnts, Wnt5a mediates β-catenin–independent signaling and promotes Rho/Rac GTPase activity in specific cell types (15–22). Nevertheless, a crosstalk between noncanonical Wnt5a signaling intermediates and canonical signaling intermediates is not unusual (18).
Both canonical and noncanonical Wnt signaling pathways play important roles in health and disease (7, 11). Wnt5a-mediated signaling is associated with inflammation in several disorders, such as rheumatoid arthritis, psoriasis, metabolic dysfunction in obesity, and sepsis (7–10, 23–25). In vitro studies have documented that Wnt5a not only promotes the expression of inflammatory cytokines such as IL-6, IL-8, and IL-12 but also provides cues for cytoskeletal modulations in several cell types (7, 8, 26–28).
Here we demonstrate that Wnt5a–Fz5 signaling in macrophages promotes phagocytosis through lipid raft clustering concomitant with Rac1–PI3 kinase–IκB kinase activation. Augmented phagocytosis with no enhancement in bacterial killing rate results in bacterial overload and elevated levels of proinflammatory cytokines. Interestingly, a small molecule inhibitor of Wnt production, IWP-2 (29), blocks both phagocytosis and the secretion of proinflammatory cytokines but facilitates bacterial killing.
Results
Wnt5a Signaling Stimulates the Internalization of Latex Beads by Macrophages.
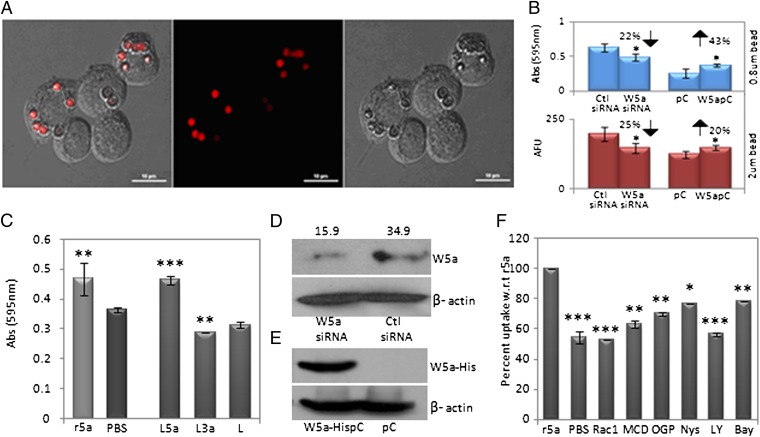
Activated Rho/Rac GTPases accompany phagocytic events in macrophages. Interestingly, Wnt5a signaling is associated with Rac GTPase activation (22, 30). We thus investigated if Wnt5a signaling contributes to phagocytosis using both 0.8 μm blue-dye-filled latex beads and 2 μm red fluorescent beads and estimated phagocytosis by absorbance and fluorescence measurements, respectively. RAW 264.7 macrophages expressing Wnt5a were either transfected with a Wnt5a expression vector (to up-regulate Wnt5a expression) or Wnt5a-specific siRNA (to inhibit Wnt5a expression), and the extent of phagocytosis by Wnt5a expression vector/siRNA transfected cells was compared with that of the appropriate controls. Fig. 1A depicts uptake of red fluorescent latex beads by RAW cells, by confocal microscopy. As demonstrated in Fig. 1B, although Wnt5a expression vector transfected cells phagocytosed latex beads with increased efficiency, inhibition of Wnt5a expression resulted in a considerable decrease in phagocytosis. Additionally, both recombinant Wnt5a and Wnt5a conditioned medium also stimulated latex bead internalization. Notably, Wnt3a, which belongs to the “canonical” class of Wnts, did not display any significant effect on phagocytosis of beads (Fig. 1C). Fig. S1 shows the presence of Wnt5a/Wnt3a protein in the appropriate conditioned media prepared from l-Wnt5a and l-Wnt3a cells. Fig. 1 D and E describes the transfection efficiency.
Fig. 1.
Wnt5a-mediated stimulation of latex bead uptake. (A) Demonstration of 2 μm fluorescent red bead uptake by RAW 264.7 macrophages. (B) Differential uptake of blue beads and red beads by Wnt5a expression-vector-transfected and Wnt5a siRNA-transfected cells with respect to the empty vector and control siRNA-transfected cells. (C) Effect of Wnt5a-L cell–conditioned medium (L5a), Wnt3a-L cell–conditioned medium (L3a), and recombinant Wnt5a (r5a) on blue bead uptake with respect to the appropriate controls L-cell-conditioned medium (L) and PBS. (D) Quantitation of reduction in Wnt5a protein (∼43 kD) expression by siRNA transfection normalized to β -actin (∼42 kD) is shown above each corresponding band (J). (E) Immunoblot depicting expression-vector-specific Wnt5a expression in Wnt5a expression vector (Wnt5aHis-pc)–transfected cells but not in the empty vector (pc) transfected cells. (F) Inhibitors to Rac1 (5 µM), PI3 kinase (LY: 5 µM), lipid raft (Nys: 5 µM; MCD: 10 mM; OGP: 2.5 mM), and IκBkinase (Bay: 5 µM) reduce uptake of blue latex beads by 25%–50% with respect to r5a. A 46% reduction in uptake by PBS (control) with respect to r5a is shown as a reference. *P < 0.05; **P < 0.01;***P < 0.001; n = 3. (Scale bar: 10 µm.)
Importantly, there was considerable inhibition in recombinant Wnt5a-mediated phagocytosis by the lipid raft disrupting agents methyl-β–cyclodextrin (MCD); n-octyl-β–d-glucopyranoside (OGP); nystatin (nys), a Rac1-specific inhibitor; the PI3 kinase inhibitor LY294002, and the IκBkinase inhibitor BAY11-7082 (Fig. 1F). These results suggest that Wnt5a-induced enhancement in phagocytosis occurs to a fair extent via lipid rafts through PI3 kinase–Rac1–IκB-kinase–dependent processes. Confocal microscopy of Wnt5a pretreated cells stained with the cholera toxin B subunit (CTB), which binds to lipid rafts, was suggestive of Wnt5a-mediated clustering of lipid rafts at the cell surface (Fig. S2) (31–34). Unlike phagocytosis, Wnt5a did not stimulate pinocytosis of FITC dextran significantly (Fig. S3).
Wnt5a Signaling Promotes Internalization of Bacteria but Not Bacterial Killing.
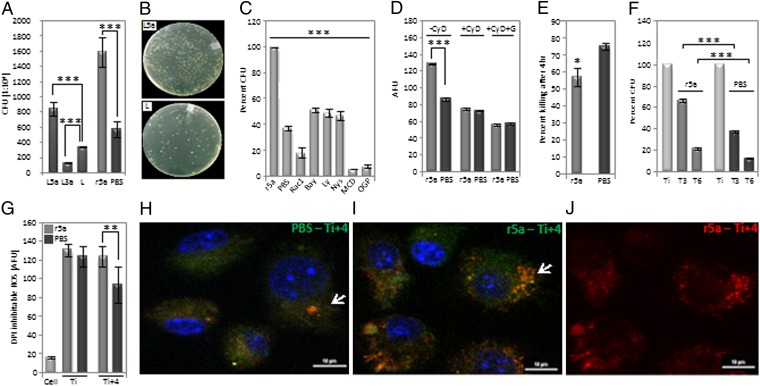
With Escherichia coli being a causative agent for many common diseases including urinary tract infections and sepsis and Wnt5a having been indicated as a stimulator of phagocytosis, it was important to investigate if Wnt5a signaling in macrophages influenced E. coli uptake and clearance. Pretreatment of the RAW 264.7 macrophage cell line with either Wnt5a-conditioned medium or recombinant Wnt5a resulted in about 2.5-fold higher E. coli uptake than the corresponding controls in 2 h as represented by the colony-forming units (CFUs) from internalized bacteria (Fig. 2 A and B). Wnt3a-conditioned medium actually decreased uptake compared with the control (Fig. 2A). Fig. S4 depicts the time-dependent increase in bacterial uptake by Wnt5a. Wnt5a-stimulated bacterial uptake was significantly inhibited by inhibitors to lipid rafts, Rac1, PI3 kinase, and IκB kinase (Fig. 2C). The observed internalization thus operates through a Rac1–PI3 kinase–IκB kinase–mediated pathway that is associated with lipid raft organization at the cell surface, similar to latex bead uptake (31–34).
Fig. 2.
Wnt5a stimulated bacterial internalization and restricted killing. (A) Depiction of ∼twofold difference in bacterial internalization by (i) L5a and L3a vs. L and (ii) r5a vs. PBS as assessed by CFUs from internalized bacteria. (B) Separate demonstration of CFU, L5a vs. L. (C) A 50%–90% decrease in bacterial internalization by use of the designated inhibitors along with r5a as judged by CFU, with PBS being used as a reference. (D) GFP–E. coli internalization by RAW cells pretreated separately with r5a and PBS in the absence or presence of cytochalasin D (CyD) and gentamycin (G), as determined by fluorescence measurement, with AFU depicting arbitrary fluorescence units. (E) Differential killing in r5a vs. PBS pretreated RAW cells infected with bacteria as studied by CFU. (F) About 10%–29% more retention of bacteria 3 h (T3) and 6 h (T6) after treatment of 2-h-infected RAW cells with r5a vs. PBS. (G) Depiction of DPI-inhibitable ROS of RAW 264.7 cells pretreated with r5a or PBS 2 h after bacterial uptake (Ti) and 4 h postuptake (Ti+4) as measured by DCFDH-DA fluorescence, with only cells being used as a reference; *P < 0.05, **P < 0.01, ***P < 0.001, n = 3. (H and I) Endosomal/lysosomal trafficking of GFP–E. coli depicting orange-yellow hue (green of GFP–E. coli and red of LysoTracker) in r5a or PBS pretreated RAW cells 4 h after bacterial internalization. (J) Depiction of endosomal/lysosomal stain with LysoTracker red. (Scale bar: 10 µm.)
To differentiate between E. coli binding and internalization by Wnt5a signaling, GFP–E. coli uptake by RAW cells was assessed both in the presence and absence of cytochalasin-D, an inhibitor of actin polymerization and uptake. RAW cells pretreated separately with recombinant Wnt5a and PBS were incubated with cytochalasin-D before addition of GFP–E. coli. Although the fluorescence associated with Wnt5a pretreated cells was higher than that of the control as anticipated in the absence of cytochalasin-D, in the presence of cytochalasin-D it was the same. Additionally, upon a brief treatment with gentamycin, which is expected to kill cell-bound bacteria, the GFP fluorescence of cytochalasin-D-treated cells diminished (Fig. 2D). Taken together, these results suggest that Wnt5a signaling enhances bacterial uptake primarily by facilitating internalization, without affecting binding significantly.
The absolute numbers of bacteria killed within a certain period were much higher in Wnt5a pretreated cells than in the corresponding controls on account of increased rates of internalization. The bacterial killing rate, however, always decreased slightly in Wnt5a pretreated cells compared with the corresponding controls (Fig. 2E). To separately validate the negative effect of Wnt5a on bacterial killing, recombinant Wnt5a protein or PBS (control) was directly added to RAW cells that had internalized bacteria for 2 h, and bacterial killing was assessed after 3 and 6 h separately. Killing was distinctly reduced in the presence of Wnt5a compared with the corresponding control (Fig. 2F), indicating that Wnt5a signaling not only does not support but may in fact hinder killing of internalized bacteria. The difference in cell numbers upon Wnt5a or PBS treatment was not significant (Table S1).
Interestingly, although cell-associated DPI-inhibitable Reactive Oxygen Species (ROS) increased with phagocytosis of bacteria as estimated by 2′,7′-dichlorfluorescein-diacetate (DCFH-DA) fluorescence, there was no significant change in ROS level upon Wnt5a-stimulated enhancement in bacterial uptake for 2 h compared with the control (Ti; Fig. 2G). Bacterial killing as documented in the estimated 4 h after bacterial internalization (Fig. 2E) correlated with increased persistence in DPI-inhibitable ROS in Wnt5a pretreated cells compared with the corresponding controls, implying prolonged activation of the macrophage phagosome oxidase (Ti+4; Fig. 2G). The endosomal/lysosomal trafficking of GFP–E. coli in the infected macrophages as evident from the orange-yellow hue (green of GFP–E. coli + red of lysotracker dye) (Fig. 2H) also correlated with bacterial killing in the estimated 4 h after internalization.
Wnt5a-Mediated Phagocytosis Is Dependent on Fz5.
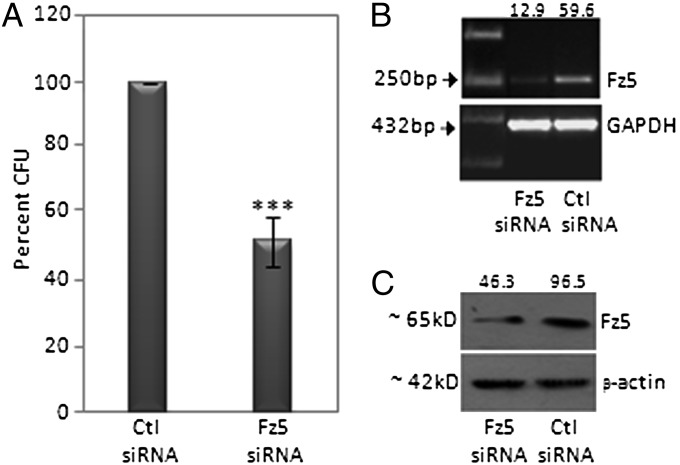
Fz5, which has homology with the heterotrimeric G protein–coupled receptors, is a putative receptor for Wnt5a (17). It was thus important to evaluate the involvement of Fz5 in Wnt5a-stimulated phagocytosis. As depicted in Fig. 3A, Wnt5a incubation of RAW cells having partial knockdown of Fz5 with Fz5 siRNA exhibited lesser uptake of E. coli than the appropriate control. Fig. 3 B and C demonstrate the reduction in Fz5 mRNA and protein expression, respectively, upon Fz5 siRNA transfection.
Fig. 3.
Inhibited bacterial internalization by siRNA-mediated reduction in Fz5 expression. (A) A 51% reduction in bacterial internalization in RAW cells (Fz5 siRNA transfected vs. control siRNA transfected) after infection with bacteria for 2 h. (B and C) Quantitation of decrease in Fz5 mRNA (B: RT-PCR) and protein (C: immunoblot) by siRNA transfection normalized to GAPDH and β-actin, respectively. ***P < 0.001; n = 3.
Wnt5a-Stimulated Phagocytosis of E. coli Is Associated with a Marked Increase in Proinflammatory Cytokines.
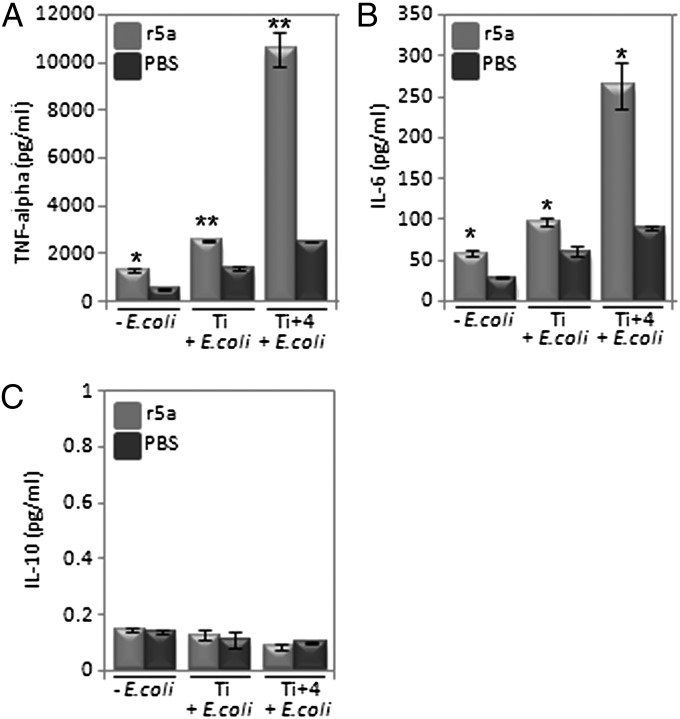
As demonstrated in Fig. 4 A and B, secretion of the proinflammatory cytokines TNF-α and IL-6 increased with the internalization and killing of E. coli. Secretion of TNF-α was heightened by about twofold upon Wnt5a-stimulated increase in bacterial uptake (Ti) and increased to greater than fourfold 4 h after internalization (Ti+4). In the case of IL-6, the corresponding fold differences with controls were about 1.5-fold and threefold, respectively. Similar to previously published reports (8, 35), secreted levels of TNF-α and IL-6 were higher in Wnt5a-treated cells, although to a lesser degree, even in the absence of E. coli. Changes in the levels of the anti-inflammatory cytokine IL-10 were negligible (Fig. 4C).
Fig. 4.
Cytokine profile accompanying phagocytosis. (A) Depiction of 1.8–4.2-fold increase (r5a vs. PBS pretreatment) in TNF-α produced by RAW cells after E. coli internalization for 2 h (Ti) and 4 h thereafter (Ti+4) as determined by ELISA. Increased level of TNF-α was noted in r5a pretreated cells also in the absence of E. coli. (B) A 1.5–3-fold increase in IL6 produced by RAW cells under similar conditions. (C) Depiction of no significant change in IL10 after similar treatment *P < 0.05; **P < 0.01; n = 3.
IWP-2 Inhibits Internalization, Facilitates Bacterial Killing, and Regulates Proinflammatory Cytokine Secretion.
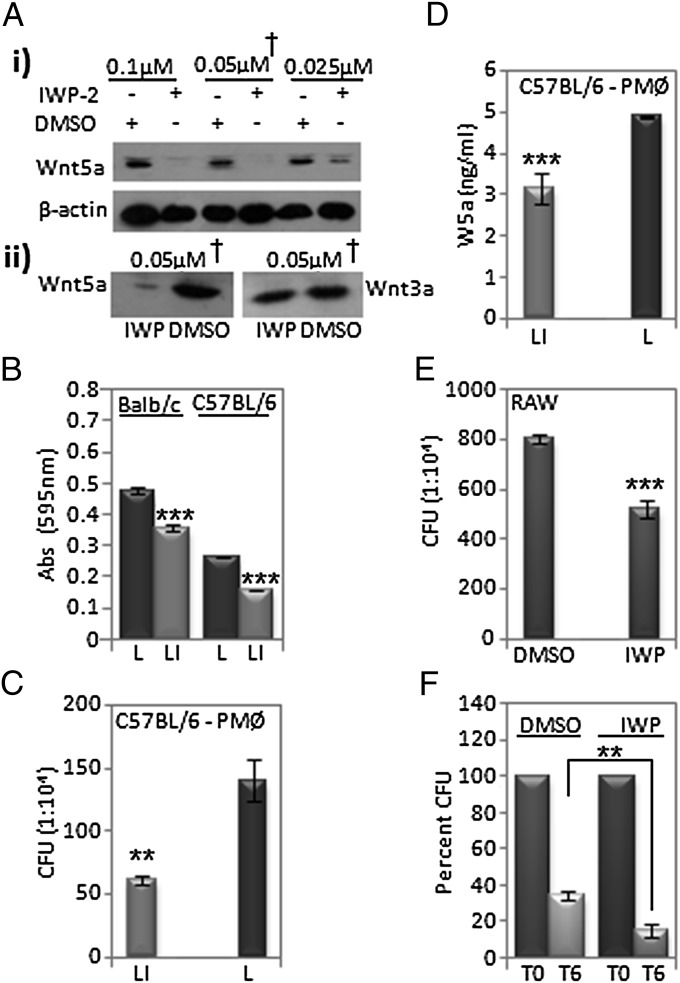
The small molecule inhibitor of Wnt production, IWP-2, was discovered as an inhibitor of O-acylation, a posttranslational modification that is required for Wnt secretion (29). We confirmed that IWP-2 when added to mouse l-Wnt5a cells decreased secretion of Wnt5a in a dose-dependent manner (Fig. 5 Ai). Furthermore, upon pretreatment of both l-Wnt5a and l-Wnt3a cells with 0.05 μM IWP-2 for 2 d, we found that IWP-2 had a much more significant effect on Wnt5a secretion than on Wnt3a secretion (Fig. 5Aii). IWP-2 was used accordingly both as a free drug and as liposome formulation on RAW cells and mouse peritoneal phagocytes to test its efficacy as a regulator of phagocytosis, bacterial killing, and cytokine secretion.
Fig. 5.
Inhibition of phagocytosis by inhibitor of Wnt production, IWP-2, in vitro. (A) Dose-dependent decrease in Wnt5a and Wnt3a levels in supernatant of l-Wnt5a and l-Wnt3a cells treated with IWP-2, showing β-actin levels in corresponding cell lysates. (B) Decrease in uptake of blue dye latex beads by mouse peritoneal macrophages (liposome-IWP i.e., LI vs. liposome i.e., L pretreatment for about 48 h) as determined by A595. (C) Decrease in E. coli uptake by peritoneal macrophages (LI vs. L pretreatment) of C57BL/6 mice as determined by CFU upon infection with bacteria for 2 h. (D) Reduction in Wnt5a secretion upon treatment of C57BL/6 mouse peritoneal macrophages with LI vs. L (control). (E) Decrease in bacterial uptake (IWP vs. DMSO) by RAW cells after similar bacterial infection. (F) Decrease in retention of internalized bacteria by RAW cells upon treatment of E. coli–infected RAW cells with IWP-2 vs. DMSO for 6 h, as determined by CFU. **P < 0.01; ***P ≤ 0.001; n = 3.
Peritoneal macrophages, harvested from the peritoneal lavage fluid of both Balb/C and C57BL/6 mice and pretreated with IWP-2-liposome, internalized 25% and 40% less blue-dye-filled latex beads, respectively, than the free liposome-treated controls as measured by A595 (Fig. 5B). Likewise, incubation of similarly prepared cultures of peritoneal macropahges with E. coli resulted in remarkably lesser uptake in the IWP-liposome pretreated cells than in the corresponding controls, as judged by counting CFUs from internalized bacteria (Fig. 5C). Secretion of Wnt5a in IWP-liposome pretreated macrophages was also clearly reduced, compared with the corresponding control (Fig. 5D). Additionally, RAW cells pretreated with IWP-2 internalized considerably lesser number of E. coli than just DMSO (control) pretreated cells (Fig. 5E). Furthermore, in accordance with the apparently negative influence of Wnt5a on bacterial killing rate, incubation of E. coli–infected RAW macrophages with either IWP-2 or DMSO resulted in a greater bacterial killing rate following IWP-2 treatment compared with control (Fig. 5F). As analyzed by flow cytometry, IWP-2 treatment did not cause any cell toxicity (Fig. S5).
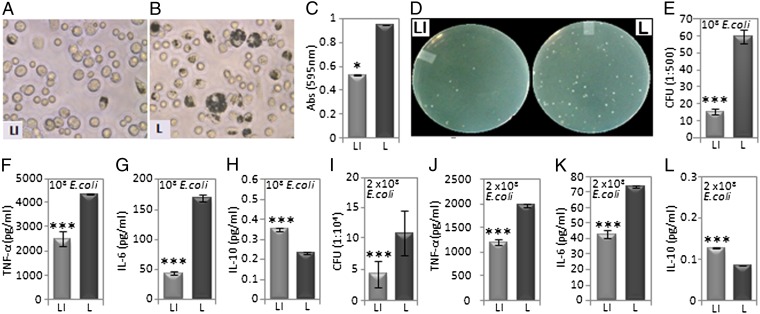
To evaluate the efficacy of IWP-2 in vivo, 200 μL each of IWP-2-liposome or free liposome was separately injected into C57BL/6 mice intraperitoneally about 2 h before injection of a similar volume of either blue-dye-filled latex beads or E. coli DH5α. IWP-2 caused significant reduction in the uptake of blue beads (Fig. 6 A–C) as well as E. coli as assessed by CFUs (Fig. 6 D and E) in peritoneal lavage cells within 2 h. In addition, the levels of TNF-α and IL-6 in the lavage fluid of the corresponding mice were reduced by 2–4-fold compared with control values (Fig. 6 F and G). Interestingly, IWP-2 even induced a considerable increase in secretion of the anti-inflammatory cytokine IL-10 (Fig. 6H). IWP-2 induced reduction in E. coli CFUs (Fig. 6I) and TNF-α/IL-6 level (Fig. 6 J and K), and a slight elevation in IL-10 level (Fig. 6L) persisted 24 h postinjection. Equal numbers of E. coli–infected RAW cells and peritoneal macrophages that were pretreated with IWP yielded a similar cytokine profile (Fig. S6). To further explore the physiological relevance of the immune regulatory activity of IWP-2, 3-mo-old C57BL/6 mice were injected i.p. first with either IWP-2 or DMSO and then with E. coli for 3 consecutive days based on a previously published model of peritonitis (36). A considerable decrease in both intracellular bacteria and secreted levels of TNF-α and IL-6 was noticed in IWP-2-treated mice compared with controls after 3 d, with IL-10 levels remaining almost the same. Importantly, the secreted level of Wnt5a in the peritoneal lavage was also almost twofold lower in the IWP-2-treated mice compared with controls. Bacterial CFU obtained from the cardiac blood of IWP-2-treated mice was also significantly lower than controls, implying that blockade of Wnt5a-mediated phagocytosis may restrict bacterial dissemination (Fig. S7).
Fig. 6.
Influence of IWP-2 on phagocytosis and cytokine profile in vivo. (A and B) Depiction of differential uptake of blue-dye-filled latex beads in 2 h by peritoneal phagocytes of C57BL/6 mice injected i.p. with LI or L before i.p. injection of latex beads. (C) Quantitation of the latex bead uptake, LI vs. L. (D) Representation of differential uptake of E. coli by peritoneal phagocytes of C57BL/6 mice injected i.p. with LI or L before i.p. injection of 108 CFU of E. coli. (E) Quantitation of the E. coli uptake, LI vs. L, by the peritoneal cells. (F–H) Differential levels of TNF-α, IL6, and IL10 in the peritoneal lavage fluid (LI vs. L) of the corresponding mice. (I) Quantitation of E. coli in peritoneal cells of similarly LI vs. L-injected C57BL/6 mice, 24 h after i.p. injection of 2 × 108 CFU of E. coli as determined by CFU. (J–L) Differential levels of TNF-α, IL6, and IL10 in the peritoneal lavage fluid (LI vs. L) of the corresponding mice. *P < 0.05; ***P < 0.001; n = 3 mice per group.
Discussion
The objective of our study was to ascertain how Wnt5a signaling influences phagocytosis and its consequences. We found that Wnt5a stimulates phagocytosis of both latex beads and E. coli through Rac1–PI3 kinase–IκB kinase–dependent processes involving lipid raft clustering. Wnt5a-mediated bacterial uptake is clearly associated with an increase in proinflammatory cytokine secretion but not with an increase in bacterial killing. Importantly, uptake is inhibited by a partial knockdown of the putative Wnt5a receptor Fz5 and preincubation of cells with a small molecule inhibitor of Wnt production— IWP-2 (29). Administration of IWP-2 furthermore leads to markedly reduced TNF-α/IL-6 secretion and accelerated killing of E. coli.
Wnt5a–Fz5-mediated activation of PI3 kinase and Rac1 perhaps stimulates the assembly of scavenger receptors through the clustering of lipid raft microdomains in macrophages, with the ensuing cytoskeletal rearrangements setting the stage for enhanced internalization of latex beads and E. coli (31–34, 37–39). Increased uptake of E. coli could also be contributed by the facilitated association of membrane lipid rafts with CD14, which promotes infection by Gram-negative bacteria (40). Moreover, recruitment of IκB kinase into lipid raft clusters could not only support internalization but also incite cytokine expression through activation of NFκB (41). Wnt5a–Fz5-mediated high internalization rates of E. coli/latex beads are likely to make room for additional binding of the same, thus causing amplification of signaling. Based on the classification of Wnt5a-induced activation of Rac1 as noncanonical (22), one may define Wnt5a-induced phagocytosis through lipid raft clustering as a noncanonical mode of Wnt signaling.
It is not clear why Wnt5a signaling restricts bacterial killing despite enhancing internalization. It is quite likely that the levels of phagosome oxidase activation and extent of phagosome–lysosome fusion upon Wnt5a-induced bacterial internalization are not sufficient to increase the bacterial killing rate. Perhaps lipid raft clustering facilitated by Wnt5a also partly inhibits phagosome–lysosome fusion (33). The reported involvement of TLR4 in suppression of bacterial clearance suggests that Rac–PI3 kinase–mediated cytoskeletal changes induced by Wnt5a–Fz5 may promote TLR4 signaling by bacterial components at the phagosome level (4, 42). The resultant surge in proinflammatory cytokines could in turn restrict the bacterial killing rate (42–45).
As a secreted protein, Wnt5a may exert its stimulatory effects on macrophages through autocrine and/or paracrine modes upon binding to the Fz5 receptor. The ability of Wnt5a–Fz5 signaling to stimulate phagocytosis of E. coli but not its killing makes it a likely mediator of uncontrolled inflammation or sepsis during infection (46). Such a possibility is supported by a recent report that relates improved sepsis survival in mice to inhibition of scavenger receptor function and thus phagocytosis (47). Moreover, the correlation of lower-than-control levels of inflammatory cytokines in E. coli–infected mice injected with IWP-2 with similarly lower levels of secreted Wnt5a (Fig. S6) are in accordance with the presence of Wnt5a in the sera of patients with sepsis (24).
In light of our understanding that Wnt5a may regulate immunostimulation by E. coli during sepsis, it is of interest to investigate the interactive potential of Wnt5a and lipopolysaccharide (LPS), a major immune regulatory component of E. coli. Although on one hand Wnt5a facilitates LPS-induced proinflammatory cytokine production in tissue culture cells (24), on the other hand it also promotes a tolerogenic effect of LPS by suppressing proinflammatory cytokine secretion (48). Our data suggest that Wnt5a-mediated immunostimulation is regulated by the dose of LPS. Although low doses (10 ng/mL) of LPS support a Wnt5a-induced proinflammatory cytokine profile similar to that observed by E. coli DH5α, high doses (100 ng/mL) promote a tolerogenic effect by Wnt5a through suppression of proinflammatory cytokine secretion (Fig. S8). The role of Wnt5a in the initiation and progression of sepsis, however, is unclear. Our study suggests that a preponderance of Wnt5a-Fz5-expressing macrophages at sites of infection could trigger the beginning of sepsis through increased phagocytosis and sustenance/dissemination of bacterial infection. The Wnt5a production inhibitor IWP-2 mediated a decrease in phagocytosis and proinflammatory cytokine secretion, but an increase in bacterial killing validates that endogenous Wnt5a has the potential to sustain bacterial infection and a proinflammatory (M1) state (49). It is thus important to evaluate how the influence of Wnt5a signaling on phagocytosis regulates the course of sepsis.
Materials and Methods
Cells and reagents were purchased from the USA, Germany, and Biobharati, Kolkata, India as specified in SI Materials and Methods. Procedures for mice infection, preparation of l-Wnt5a– and l-Wnt3a–conditioned medium, RNA isolation and RT-PCR, ELISA and immunoblotting, cell transfection, estimation of phagocytosis and bacterial killing, confocal microscopy, measurement of reactive oxygen species, and preparation of liposome-IWP2 are described in SI Materials and Methods. The Animal Ethics Committee of the Indian Institute of Chemical Biology approved all animal experiments.
Supplementary Material
Acknowledgments
This work was supported by a Department of Biotechnology, Government of India grant and institutional funding. We thank S. Mandal for some of the preliminary experiments, A. Chakravarty and S. Roy for help with animal experiments, A. Banerjee and D. Sarkar for confocal microscopy, and D. Carson for helpful discussions. D.N. and G.M. were supported by Council of Scientific and Industrial Research and Department of Biotechnology, Government of India, respectively.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1207789109/-/DCSupplemental.
References
- 1.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 2.Weissmann G. The pathogenesis of rheumatoid arthritis. Bull NYU Hosp Jt Dis. 2006;64:12–15. [PubMed] [Google Scholar]
- 3.Duffield JS. The inflammatory macrophage: A story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 4.Stuart LM, Ezekowitz RA. Phagocytosis and comparative innate immunity: Learning on the fly. Nat Rev Immunol. 2008;8:131–141. doi: 10.1038/nri2240. [DOI] [PubMed] [Google Scholar]
- 5.Cadigan KM, Nusse R. Wnt signaling: A common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- 6.Witze ES, Litman ES, Argast GM, Moon RT, Ahn NG. Wnt5a control of cell polarity and directional movement by polarized redistribution of adhesion receptors. Science. 2008;320:365–369. doi: 10.1126/science.1151250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sen M. Wnt signalling in rheumatoid arthritis. Rheumatology (Oxford) 2005;44:708–713. doi: 10.1093/rheumatology/keh553. [DOI] [PubMed] [Google Scholar]
- 8.Sen M, et al. Expression and function of wingless and frizzled homologs in rheumatoid arthritis. Proc Natl Acad Sci USA. 2000;97:2791–2796. doi: 10.1073/pnas.050574297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, et al. Wnt5a induces endothelial inflammation via β-catenin-independent signaling. J Immunol. 2010;185:1274–1282. doi: 10.4049/jimmunol.1000181. [DOI] [PubMed] [Google Scholar]
- 10.George SJ. Wnt pathway: A new role in regulation of inflammation. Arterioscler Thromb Vasc Biol. 2008;28:400–402. doi: 10.1161/ATVBAHA.107.160952. [DOI] [PubMed] [Google Scholar]
- 11.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 12.Moon RT, Brown JD, Torres M. WNTs modulate cell fate and behavior during vertebrate development. Trends Genet. 1997;13:157–162. doi: 10.1016/s0168-9525(97)01093-7. [DOI] [PubMed] [Google Scholar]
- 13.Willert K, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, et al. Activation of a frizzled-2/β-adrenergic receptor chimera promotes Wnt signaling and differentiation of mouse F9 teratocarcinoma cells via Galphao and Galphat. Proc Natl Acad Sci USA. 1999;96:14383–14388. doi: 10.1073/pnas.96.25.14383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulte G, Bryja V. The Frizzled family of unconventional G-protein-coupled receptors. Trends Pharmacol Sci. 2007;28:518–525. doi: 10.1016/j.tips.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Slusarski DC, Corces VG, Moon RT. Interaction of Wnt and a Frizzled homologue triggers G-protein-linked phosphatidylinositol signalling. Nature. 1997;390:410–413. doi: 10.1038/37138. [DOI] [PubMed] [Google Scholar]
- 17.He X, et al. A member of the Frizzled protein family mediating axis induction by Wnt-5A. Science. 1997;275:1652–1654. doi: 10.1126/science.275.5306.1652. [DOI] [PubMed] [Google Scholar]
- 18.Mikels AJ, Nusse R. Purified Wnt5a protein activates or inhibits β-catenin-TCF signaling depending on receptor context. PLoS Biol. 2006;4:e115. doi: 10.1371/journal.pbio.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen WE, et al. Dishevelled 2 recruits β-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- 20.Kühl M, Sheldahl LC, Park M, Miller JR, Moon RT. The Wnt/Ca2+ pathway: A new vertebrate Wnt signaling pathway takes shape. Trends Genet. 2000;16:279–283. doi: 10.1016/s0168-9525(00)02028-x. [DOI] [PubMed] [Google Scholar]
- 21.Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- 22.Sato A, Yamamoto H, Sakane H, Koyama H, Kikuchi A. Wnt5a regulates distinct signalling pathways by binding to Frizzled2. EMBO J. 2010;29:41–54. doi: 10.1038/emboj.2009.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ouchi N, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira C, Schaer DJ, Bachli EB, Kurrer MO, Schoedon G. Wnt5A/CaMKII signaling contributes to the inflammatory response of macrophages and is a target for the antiinflammatory action of activated protein C and interleukin-10. Arterioscler Thromb Vasc Biol. 2008;28:504–510. doi: 10.1161/ATVBAHA.107.157438. [DOI] [PubMed] [Google Scholar]
- 25.Reischl J, et al. Increased expression of Wnt5a in psoriatic plaques. J Invest Dermatol. 2007;127:163–169. doi: 10.1038/sj.jid.5700488. [DOI] [PubMed] [Google Scholar]
- 26.Blumenthal A, et al. The Wingless homolog WNT5A and its receptor Frizzled-5 regulate inflammatory responses of human mononuclear cells induced by microbial stimulation. Blood. 2006;108:965–973. doi: 10.1182/blood-2005-12-5046. [DOI] [PubMed] [Google Scholar]
- 27.Weeraratna AT, et al. Wnt5a signaling directly affects cell motility and invasion of metastatic melanoma. Cancer Cell. 2002;1:279–288. doi: 10.1016/s1535-6108(02)00045-4. [DOI] [PubMed] [Google Scholar]
- 28.Wang Q, et al. A novel role for Wnt/Ca2+ signaling in actin cytoskeleton remodeling and cell motility in prostate cancer. PLoS ONE. 2010;5:e10456. doi: 10.1371/journal.pone.0010456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen B, et al. Small molecule-mediated disruption of Wnt-dependent signaling in tissue regeneration and cancer. Nat Chem Biol. 2009;5:100–107. doi: 10.1038/nchembio.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bokoch GM. Regulation of innate immunity by Rho GTPases. Trends Cell Biol. 2005;15:163–171. doi: 10.1016/j.tcb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Nagao G, Ishii K, Hirota K, Makino K, Terada H. Role of lipid rafts in innate immunity and phagocytosis of polystyrene latex microspheres. Colloids Surf B Biointerfaces. 2011;84:317–324. doi: 10.1016/j.colsurfb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 32.Lafont F, van der Goot FG. Bacterial invasion via lipid rafts. Cell Microbiol. 2005;7:613–620. doi: 10.1111/j.1462-5822.2005.00515.x. [DOI] [PubMed] [Google Scholar]
- 33.Naroeni A, Porte F. Role of cholesterol and the ganglioside GM(1) in entry and short-term survival of Brucella suis in murine macrophages. Infect Immun. 2002;70:1640–1644. doi: 10.1128/IAI.70.3.1640-1644.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seveau S, et al. A FRET analysis to unravel the role of cholesterol in Rac1 and PI 3-kinase activation in the InlB/Met signalling pathway. Cell Microbiol. 2007;9:790–803. doi: 10.1111/j.1462-5822.2006.00832.x. [DOI] [PubMed] [Google Scholar]
- 35.Li B, et al. WNT5A signaling contributes to Aβ-induced neuroinflammation and neurotoxicity. PLoS ONE. 2011;6:e22920. doi: 10.1371/journal.pone.0022920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anand RJ, et al. A role for connexin43 in macrophage phagocytosis and host survival after bacterial peritoneal infection. J Immunol. 2008;181:8534–8543. doi: 10.4049/jimmunol.181.12.8534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiyanagi T, et al. Involvement of cholesterol-enriched microdomains in class A scavenger receptor-mediated responses in human macrophages. Atherosclerosis. 2011;215:60–69. doi: 10.1016/j.atherosclerosis.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Yin HL, Janmey PA. Phosphoinositide regulation of the actin cytoskeleton. Annu Rev Physiol. 2003;65:761–789. doi: 10.1146/annurev.physiol.65.092101.142517. [DOI] [PubMed] [Google Scholar]
- 39.Li N, et al. Monocyte lipid rafts contain proteins implicated in vesicular trafficking and phagosome formation. Proteomics. 2003;3:536–548. doi: 10.1002/pmic.200390067. [DOI] [PubMed] [Google Scholar]
- 40.Haziot A, et al. Resistance to endotoxin shock and reduced dissemination of gram-negative bacteria in CD14-deficient mice. Immunity. 1996;4:407–414. doi: 10.1016/s1074-7613(00)80254-x. [DOI] [PubMed] [Google Scholar]
- 41.Sebald A, Mattioli I, Schmitz ML. T cell receptor-induced lipid raft recruitment of the I kappa B kinase complex is necessary and sufficient for NF-kappa B activation occurring in the cytosol. Eur J Immunol. 2005;35:318–325. doi: 10.1002/eji.200425024. [DOI] [PubMed] [Google Scholar]
- 42.Anand RJ, et al. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J Pediatr Surg. 2007;42:927–932, discussion 933. doi: 10.1016/j.jpedsurg.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 43.Rossol M, et al. LPS-induced cytokine production in human monocytes and macrophages. Crit Rev Immunol. 2011;31:379–446. doi: 10.1615/critrevimmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 44.Laroux FS, Romero X, Wetzler L, Engel P, Terhorst C. Cutting edge: MyD88 controls phagocyte NADPH oxidase function and killing of gram-negative bacteria. J Immunol. 2005;175:5596–5600. doi: 10.4049/jimmunol.175.9.5596. [DOI] [PubMed] [Google Scholar]
- 45.Lee JH, et al. Modulation of bacterial growth by tumor necrosis factor-α in vitro and in vivo. Am J Respir Crit Care Med. 2003;168:1462–1470. doi: 10.1164/rccm.200302-303OC. [DOI] [PubMed] [Google Scholar]
- 46.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 47.Leelahavanichkul A, et al. Class B scavenger receptor types I and II and CD36 targeting improves sepsis survival and acute outcomes in mice. J Immunol. 2012;188:2749–2758. doi: 10.4049/jimmunol.1003445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bergenfelz C, et al. Wnt5a induces a tolerogenic phenotype of macrophages in sepsis and breast cancer patients. J Immunol. 2012;188:5448–5458. doi: 10.4049/jimmunol.1103378. [DOI] [PubMed] [Google Scholar]
- 49.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.