Abstract
Appropriate expression of IL-2 plays a central role during the priming and differentiation of T cells. A tight balance between IL-2 and the effector cytokine IL-17A is essential for immune homeostasis. Epigenetic mechanisms have been documented as a key component of cytokine regulation during lineage commitment. The molecular mechanisms that induce chromatin remodeling are less well understood. We investigated epigenetic regulators that mediate the diametric expression of IL-2 and IL-17A in naive, central memory, and effector memory CD4+ T cells. We demonstrate that cAMP response modulator (CREM)α contributes to epigenetic remodeling of IL2 in effector memory T cells through the recruitment of DNMT3a. CREMα also reduces CpG-DNA methylation of the IL17A promoter. CREMα expression is regulated at the epigenetic level by CpG-DNA methylation, which allows increased CREMα expression in effector memory CD4+ T cells. T cells from patients with systemic lupus erythematosus (SLE) express increased levels of CREMα and exhibit a phenotype that is similar to effector memory CD4+ T cells with epigenetically predetermined expression patterns of IL-2 and IL-17A. We conclude that CREMα mediates epigenetic remodeling of the IL2 and IL17A gene during T-cell differentiation in favor of effector memory T cells in health and disease.
Keywords: inflammation, gene regulation
Adaptive immune responses largely depend on T cells. Various T helper cell subsets are involved in host defense, and a tight balance between these populations and the subsequently produced pro- and anti-inflammatory cytokines is essential for immune homeostasis (1, 2). A growing body of literature documents that a disruption of this balance can result in chronic inflammation and autoimmunity or increased susceptibility to infections.
Our current knowledge of T-lymphocytic lineage commitment is limited and somewhat simplified. During T-cell priming, naive CD4+ T cells are exposed to antigens through their interaction with antigen-presenting cells in secondary lymphoid organs. In response to activation, T cells proliferate and differentiate into “effector” or “central” memory T cells. Primed CD4+ T cells either migrate to sites of inflammation or persist as circulating effector memory CD4+ T cells. Central memory CD4+ T cells migrate to secondary lymphatic tissues, where they wait for a secondary challenge to exert enhanced immune responses (2, 3). Sallusto et al. (1999) and others demonstrated that naive, central, and effector memory CD4+ T-cell subsets are defined by the absence or presence of surface markers, including the chemokine receptor CCR7 that reflects tissue-homing capacities to lymph nodes (3). Furthermore, each of the CD4+ T-cell subsets is characterized by specific cytokine expression patterns (4). Unprimed and not previously specialized naive CD4+ T cells are capable of expressing a wide range of cytokines, including IL-2. Effector memory subsets fail to express IL-2 but express effector proinflammatory cytokines, including IL-17A (1, 3, 4). However, the molecular mechanisms that underlie cytokine expression patterns and subsequently determine lineage fate are less well understood.
Epigenetic mechanisms represent a group of regulatory events that influence gene expression without altering the underlying genomic sequence. Specific epigenetic patterns have been documented as being involved during lineage commitment of immune cells and are largely responsible for the expression and silencing of various subset-defining cytokine genes, including the Th1 cytokine IFN-γ, the Th2 cytokine IL-4, and IL-10 and related cytokines in T cells and macrophages (4, 5). The epigenetic regulation of genes is accomplished by the reorganization of nucleosomes, resulting in decompaction/compaction of chromatin fibers and subsequent variable transcription factor and RNA polymerase recruitment to DNA. The main epigenetic modifications are cytidine-phosphate-guanosine (CpG-)DNA methylation and histone modifications which usually follow the same patterns. The mediators that connect these epigenetic modifications are less well understood (5, 6). Recent evidence indicates that CpG-DNA and histone methylation can be “translated” into one another through the recruitment of DNA methyltransferases (DNMTs) and/or histone methyltransferases with the help of adaptor molecules, such as the hetero chromatin protein 1, which can recruit DNMTs to sites with high levels of histone 3 methylation at lysine 9. DNMTs are historically classified as either “maintenance DNMTs,” including DNMT1, or DNMTs that are responsible for de novo CpG-DNA methylation, such as DNMT3a (6, 7).
Recently, we identified the transcription factor cAMP response element modulator (CREM)α as a link between CpG-DNA methylation, histone H3K18 deacetylation, and H3K27 trimethylation (8). We aimed to investigate a potential role of CREMα during lineage commitment of CD4+ T cells by determining cytokine expression patterns. Together with the cAMP response element binding protein (CREB), CREM is a member of the ATF superfamily of transcription factors. This family is involved in numerous physiological processes, including immune homeostasis, steroid metabolism, and spermatogenesis (9, 10). As a result of its specific exon composition, CREM exerts both trans-activating and trans-repressing effects on target genes. These effects are mediated through CREM recruitment to CRE binding sites (TGACGTCA) or CRE half-sites in cis-regulatory elements. CREMα is overexpressed in T cells from patients with systemic lupus erythematosus (SLE) (11–13). SLE is a chronic autoimmune disorder that can affect every organ, including the kidneys, lungs, central nervous system, intestine, joints, and skin. T cells from SLE patients are characterized by severe signaling anomalies resulting in altered cytokine production (14). CREMα has been linked to reduced IL-2 and increased IL-17A expression through diametric trans-regulatory effects on the IL2 and IL17A genes (8, 15–17). CREMα expression is controlled by trans-activation and trans-repression of the CREM promoter P1 (9, 10). In SLE patients, dephosphorylated Sp-1 trans-activates CREM P1 in a disease activity–dependent manner (10, 12).
Here, we demonstrate the involvement of CREMα in the lineage determination of effector memory CD4+ T cells, where CREMα mediates epigenetic remodeling of the IL2 gene through the recruitment of DNMT3a. CREMα also mediates reduced CpG-DNA methylation of IL17A in IL-17A expressing effector memory CD4+ T cells. We demonstrate that CREM promoter activity in T cells is controlled by CpG-DNA methylation. Effector memory CD4+ T cells and total T cells from SLE patients exhibit low levels of CpG-DNA methylation of the CREM promoter P1, allowing increased CREMα expression and contributing to epigenetic remodeling of IL2 and IL17A.
Experimental Procedures
Study Subjects and T-Cell Culture.
T cells from SLE patients, healthy controls, and individuals with rheumatoid arthritis (RA) were purified from whole blood as reported previously (15) (SI Experimental Procedures). Epidemiologic and clinical information is displayed in Table S1 (SLE) and Table S2 (RA).
Flow Cytometry and T-Cell Sorting.
T cells from SLE patients and controls were stained with fluorochrome-labeled antibodies (BioLegend) against CD3, CD4, CD45RA, and CCR7. Labeled T cells were then subjected to either analysis on a 5 laser LSR II (BD Sciences) flow cytometer or sorting on a FacsAria II cell sorter (BD Biosciences). Naive, central memory, and effector memory CD4+ T cells were defined as reported previously (3).
mRNA Analysis.
Total RNA from control and SLE T cells was isolated and used via standard procedures (SI Experimental Procedures).
Methylated CpG-DNA Immunoprecipitation.
The methylated CpG-DNA immunoprecipitation assay (Zymo Research) was used according to the manufacturer’s instructions. Methylated DNA was recovered and subjected to PCR analysis on an ABI OneStepPlus real-time PCR system (SI Experimental Procedures).
Coimmunoprecipitation of DNMT3a with CREMα.
Jurkat T cells or HEK293T cells were transfected with pcDNA3.1 and expression plasmids for CREMα, pcDNA3.1 and DNMT3a, or CREMα and DNMT3a using Lipofectamine 2000 (Invitrogen). Lysates were subjected to coimmunoprecipitation (SI Experimental Procedures).
Cotransfection of Jurkat T cells with CREMα Expression Plasmids and Control or DNMT3a siRNA.
Jurkat T cells were transfected with expression plasmids and 10 nM control siRNA or DNMT3a-specific siRNA (OriGene) (SI Experimental Procedures).
Luciferase Reporter Constructs.
Reporter constructs spanning the proximal 500 bp of the human CREM promoter P1 have been described previously (10) (SI Experimental Procedures).
Statistical Analysis.
Paired two-tailed Student t test and Pearson’s product moment was used for statistical analysis of transfection experiments. Relative mRNA expression levels and methylation indices were analyzed for statistical significance using nonparametric Mann–Whitney U test as the obtained data did not follow a Gaussian distribution (Kolmorov-Smirnov normality test).
Results
Bioinformatic Analysis of the IL2 and IL17A Genes.
To investigate CpG-DNA methylation patterns across the human IL2 and IL17A genes, we defined regions of interest as reported previously (8, 15). We aligned the mouse and human IL2 and IL17A genes (VISTA Genome Browser, http://pipeline.lbl.gov/cgi-bin/gateway2), and determined conserved noncoding sequences (CNS), exons, and UTRs. Three regions of interest (CNS1-3) were defined within the IL2 promoter and one was chosen in the IL17A promoter based on sequence conservation, the number of CpGs, and the presence of regulatory regions (Fig. S1 A and B). The IL2 promoter CNS3 covers the core 300 bp promoter, including the –180 CRE site that is responsible for CREMα effects on IL-2 expression in T cells (8, 12).
IL-2 Expressing CD4+ T Cells Exhibit Reduced CpG-DNA Methylation of the IL2 Promoter.
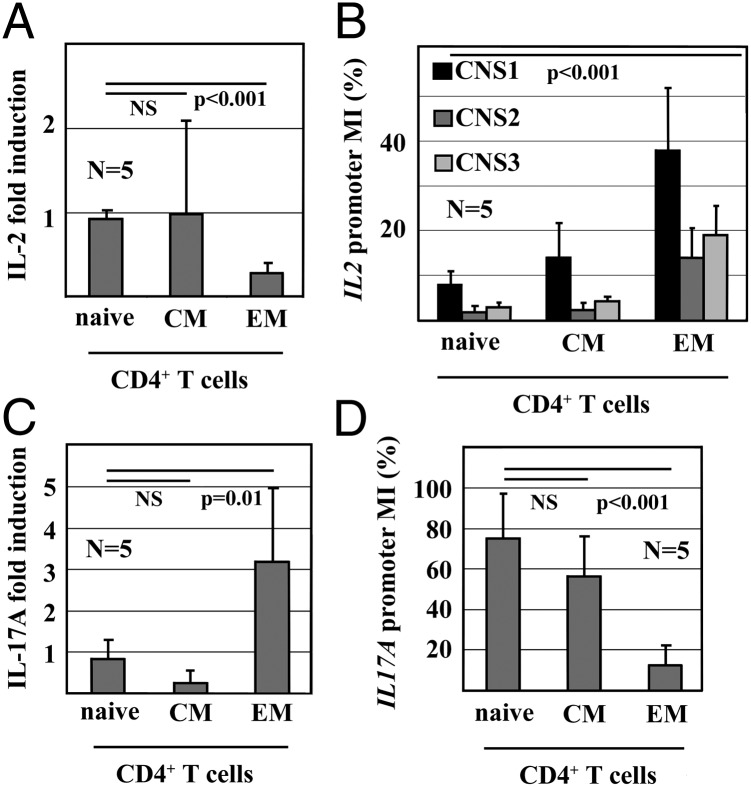
To better understand the regulation of IL-2 expression during T-cell lineage determination, we investigated dynamic epigenetic modifications in human naive (CD3+CD4+CD45RA+CCR7−), central memory (CD3+CD4+CD45RA−CCR7+), and effector memory (CD3+ CD4+CD45RA−CCR7−) CD4+ T cells. In response to T-cell activation with anti-CD3 and anti-CD28 antibodies for 12 h, naive and central memory CD4+ T cells express IL-2 mRNA, whereas effector memory CD4+ T cells fail to express IL-2 (Fig. 1A). IL-2 expression is reflected by CpG-DNA methylation of the IL2 promoter. Naive and central memory CD4+ T cells exhibit low degrees of CpG-DNA methylation in all investigated regions (methylation index [MI]: <15%), effector memory CD4+ T cells are methylated to significantly higher degrees (MI: 15–40%, P < 0.001) (Fig. 1B).
Fig. 1.
IL-2 and IL-17A mRNA expression and promoter methylation in CD4+ T cells. (A) IL-2 mRNA expression of naive, central memory (CM) and effector memory (EM) CD4+ T cells in response to stimulation with anti-CD3 and anti-CD28 antibodies (mean ± SD). (B) CpG-DNA methylation of the IL2 promoter in CD4+ T cells. Methylation index (MI) as assessed relative to methylated (100%) and unmethylated (0%) control DNA are shown (mean ± SD). (C) IL-17A mRNA expression of naive, CM and EM CD4+ T cells in response to stimulation with anti-CD3 and anti-CD28. (D) CpG-DNA methylation of the IL17A promoter in CD4+ T cells (mean ± SD).
IL-17A Expressing CD4+ T Cells Exhibit Reduced CpG-DNA Methylation of the IL17A Promoter.
We previously demonstrated that IL-17A expression in T cells from SLE patients is supported by epigenetic remodeling of the IL17A gene (15). To better understand the regulation of IL17A during T-cell lineage determination, we investigated CpG-DNA methylation in human naive, central memory, and effector memory CD4+ T cells. Naive and central memory CD4+ T cells express low levels of IL-17A mRNA compared with effector memory CD4+ T cells (Fig. 1C). IL-17A expression is reflected by IL17A promoter methylation. The IL17A promoter in naive and central memory CD4+ T cells is highly methylated (MI: 60–80%); effector memory CD4+ T cells are methylated to a significantly lower degree (MI: 15%, P < 0.001) (Fig. 1D).
CREMα Interacts with DNMT3a.
T cells from SLE patients express increased levels of CREMα, resulting in epigenetic remodeling of cytokine genes, including IL2 (8, 10, 13, 16, 17). We hypothesized that CREMα recruits de novo DNA methyltransferases to the IL2 promoter mediating epigenetic remodeling of IL2 in T cells from SLE patients (8). To confirm our previous findings (8) and to investigate the involvement of CREMα-mediated epigenetic remodeling during T-cell lineage commitment, we overexpressed CREMα and DNMT3a in the same cells, followed by coimmunoprecipitation of proteins with anti-CREMα antibodies. Jurkat T cells spontaneously express CREMα, which was increased by CREMα expression plasmids (Fig. S2A). We coimmunoprecipitated DNMT3a with CREMα in untreated Jurkat T cells and after forced expression of CREMα and/or DNMT3a (Fig. S2B). Thus, we repeated our experiments in HEK293T cells. Secondary to weak background expression of CREMα in HEK293T cells, we failed to coimmunoprecipitate DNMT3a with CREMα in untransfected cells (Fig. S2 B and C). Overexpression of DNMT3a or DNMT3a with CREMα allowed us to coimmunoprecipitate DNMT3a with CREMα (Fig. S2 B and C). This supports a direct physical interaction between CREMα and DNMT3a.
CREMα Mediates Increased CpG-DNA Methylation of the IL2 Gene Through DNMT3a.
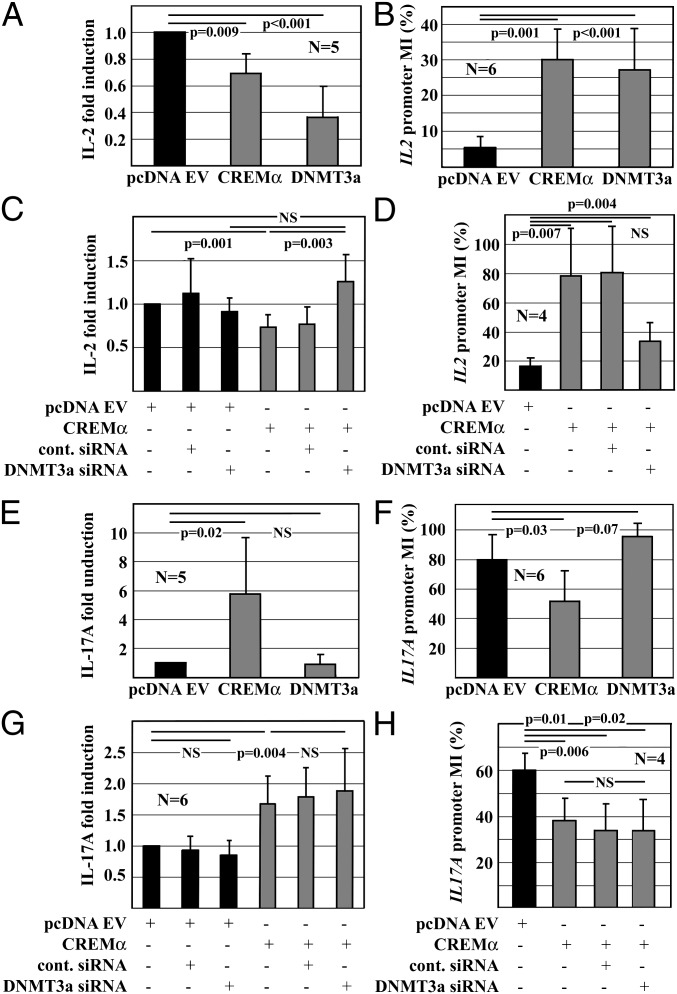
Next, we investigated whether CREMα recruits DNMT3a to the IL2 promoter during T-cell differentiation and whether this interaction plays a role during lineage commitment of CD4+ T cells. We overexpressed either CREMα or DNMT3a in primary human T cells from controls and determined IL-2 mRNA expression and IL2 promoter methylation. Because all investigated IL2 promoter regions showed similar methylation patterns (Fig. 1C), we concentrated on CNS3, covering the 300-bp core IL2 proximal promoter, including the –180 CRE site. Forced expression of either CREMα or DNMT3a resulted in a significant reduction of IL-2 mRNA expression (CREMα: P = 0.009; DNMT3a: P < 0.001) (Fig. 2A) and increased IL2 promoter CpG-DNA methylation (CREMα: P = 0.001; DNMT3a: P < 0.001) (Fig. 2B). To provide further evidence for an interaction between CREMα and DNMT3a, we cotransfected Jurkat T cells with a CREMα expression plasmid and DNMT3a siRNA knocking down DNMT3a (Fig. S3). CREMα overexpression resulted in reduced IL-2 mRNA expression (Fig. 2C; P = 0.001) and increased CpG-DNA methylation of the IL2 promoter (Fig. 2D; P = 0.007). These changes were reversed by siRNA mediated knock-down of DNMT3a (Fig. 2 C and D).
Fig. 2.
CREMα controls IL-2 and IL-17A expression on the epigenetic level. (A) IL-2 mRNA expression in primary human T cells 24 h after transfection with empty pcDNA3.1, CREMα, or DNMT3a expression plasmids. CREMα or DNMT3a mediate reduced IL-2 expression (mean ± SD). (B) CpG-DNA methylation (MI) of the IL2 promoter of primary human T cells 24 h after transfection. T cells that were transfected with pcDNA3.1 empty vector exhibited lower IL2 promoter methylation (MI: 5.8) compared with CREMα (MI: 30.1; P = 0.001) or DNMT3a (MI: 27.1; P < 0.001). (C) Jurkat T cells were cotransfected with pcDNA3.1 or CREMα and control siRNA or DNMT3a siRNA. Twenty-four hours after transfection, CREMα overexpression resulted in a significant reduction of IL-2 mRNA expression (lane 4; P = 0.001). Knock-down of DNMT3a reversed these effects (lane 6; P = 0.003). (D) Jurkat T cells were cotransfected with pcDNA3.1 or CREMα and control siRNA or DNMT3a siRNA and harvested after 24 h. CREMα resulted in increased IL2 promoter methylation (MI: 16.4 vs. 78.3; P = 0.007). DNMT3a knock-down reversed these effects (MI: 33.8%). (E) IL-17A expression in primary human T cells 24 h after transfection with pcDNA3.1, CREMα, or DNMT3a. CREMα resulted in increased IL-17A mRNA expression (P = 0.002), whereas DNMT3a did not show an effect. (F) CpG-DNA MI of the IL17A promoter was assessed 24 h after transfection with pcDNA3.1, CREMα, or DNMT3a. T cells that were transfected with pcDNA3.1 exhibited higher MIs (MI: 79.4) compared with cells transfected with CREMα (MI: 51.7; P = 0.03). DNMT3a resulted in complete methylation of the IL17A promoter (MI: 95.4; P = 0.07). (G) Jurkat T cells were cotransfected with pcDNA3.1 or CREMα and control siRNA or DNMT3a siRNA and harvested after 24 h. CREMα resulted in an increase of IL-17A mRNA expression (lane 4; P = 0.004). Knock-down of DNMT3a in combination with CREMα overexpression did not bring on additional effects (lane 6). (H) Jurkat T cells were cotransfected with pcDNA3.1 or CREMα and control siRNA or DNMT3a siRNA and harvested after 24 h. CREMα resulted in reduced IL17A promoter methylation (MI: 59.8 vs. 38.2; P = 0.006). DNMT3a knock-down did not have additional effects.
CREMα Mediates Reduced CpG-DNA Methylation of the IL17A Gene.
We previously reported that CREMα trans-activates the IL17A proximal promoter in T cells from SLE patients (15). In the present study, we investigated whether CREMα mediates epigenetic modifications to the IL17A promoter during T-cell differentiation. We overexpressed either CREMα or DNMT3a in primary human T cells from controls and determined IL-17A mRNA expression and IL17A promoter methylation. Because IL-17A is expressed at relatively low levels in T cells, forced expression of DNMT3a did not result in a significant reduction of IL-17A mRNA expression (Fig. 2E). However, the degree of CpG-DNA methylation from an MI of 79.45 was increased to almost 100% (Fig. 2F). Forced expression of CREMα resulted in a significant increase of IL-17A mRNA expression (P = 0.002; Fig. 2E). It is noteworthy that CREMα mediates a reduction of IL17A promoter CpG-DNA methylation (MI: 79.4 vs. 51.7%; P = 0.03; Fig. 2F). Cotransfection of Jurkat T cells with CREMα and DNMT3a siRNA did not alter the effects of CREMα on IL17A (Fig. 2 G and H).
CREM Promoter P1 Activity Is Controlled by CpG-DNA Methylation That Allows CREMα Expression in Effector Memory CD4+ Cells.
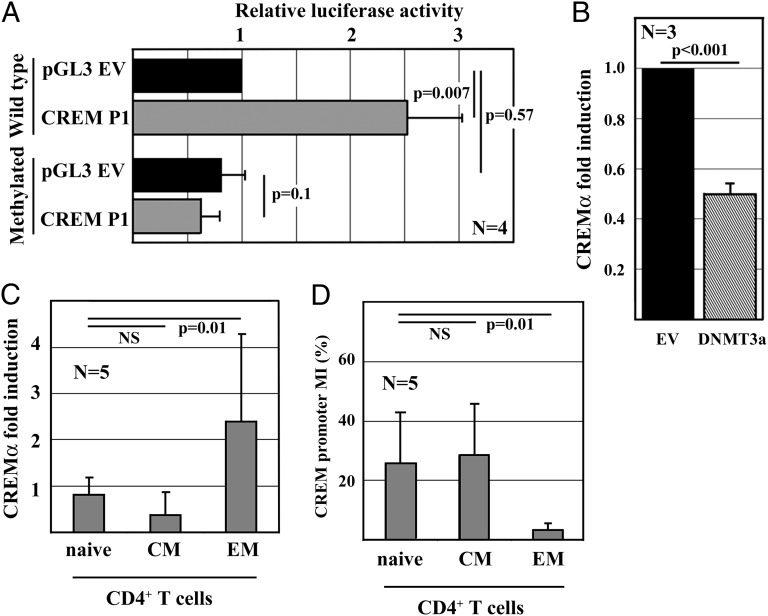
To investigate CpG-DNA methylation of the human CREM promoter P1, we defined one region of interest based on bioinformatic approaches. We determined 32 CpG sequences within the proximal 500 bp of the human CREM promoter P1 and asked whether CpG-DNA methylation of this region affects promoter activity (Fig. S1C). Enzymatic methylation of CREM P1 reporter constructs resulted in distinctly down-regulated promoter activity, whereas activity of the empty control reporter was not affected (Fig. 3A). Forced expression of DNMT3a in primary human T cells resulted in a reduction of CREMα mRNA expression (Fig. 3B). Thus our data suggest a role for DNA methylation in the regulation of CREMα expression.
Fig. 3.
CREMα mRNA expression and promoter methylation in CD4+ T cells. (A) pGL3-basic and CREM P1 reporter plasmids were methylated. Promoter activities of the unmethylated and the methylated reporters were assessed in primary human T cells (mean ± SD). (B) T cells were transfected with pcDNA3.1 or DNMT3a. DNMT3a resulted in reduced CREMα mRNA expression (P < 0.001). (C) CREMα mRNA expression in naive, central memory (CM) and effector memory (EM) CD4+ T cells in response to stimulation with anti-CD3 and anti-CD28 antibodies (mean ± SD). (D) CpG-DNA methylation (MI) of the CREM promoter P1 in CD4+ T cells (mean ± SD).
Naive, central memory, and effector memory CD4+ T cells exhibit distinct cytokine expression patterns. Because the IL-2 and IL-17A expression of effector memory CD4+ T cells share similarities with T cells from SLE patients, we asked whether CREMα also plays a role in lineage determination of CD4+ T cells under physiologic conditions. Thus, we determined CREMα mRNA expression and CREM promoter methylation in naive, central memory, and effector memory CD4+ T cells (Fig. 3 C and D). CREMα expression correlates with both IL-2 and IL-17A expression in a contrary fashion. Low CREMα expression levels in naive and central memory CD4+ T cells are associated with high IL-2 but low IL-17A mRNA expression. Conversely, CREMα is expressed by effector memory CD4+ T cells that express IL-17A but fail to produce IL-2. Together with the data presented here documenting functional interactions between CREMα with DNMT3a, this failure suggests an involvement of CREMα in the differentiation and lineage commitment of effector memory CD4+ T cells.
CpG-DNA Methylation and mRNA Expression of CREM, IL2, and IL17A of SLE T Cells Reflect the Effector Memory CD4+ T-Cell Phenotype.
Although CREMα is overexpressed in T cells from SLE patients compared with healthy controls (10, 13, 18), it remains unknown whether CREMα mRNA expression reflects SLE disease activity as measured by the systemic lupus erythematosus disease activity index (SLEDAI). Thus, we examined the mRNA expression of CREMα, IL-2 and IL-17A, and CpG-DNA methylation of the promoters of CREM, IL2, and IL17A in T cells from SLE patients with active (SLEDAI: 8–14) and inactive (SLEDAI: 0–4) disease vs. age-, sex-, and ethnicity-matched healthy controls (Table S1). Because patients with RA produce increased levels of proinflammatory cytokines, including IL-2 (5, 19, 20), we included T cells from RA patients with active disease in our study (Table S2, Fig. S4).
CREMα.
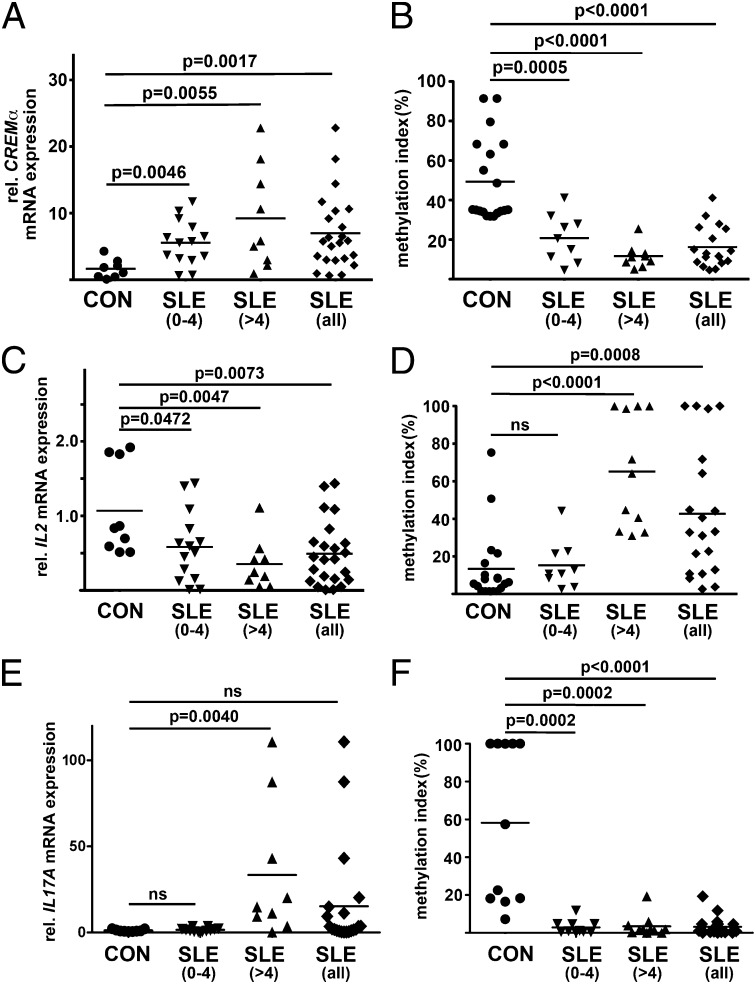
mRNA is expressed by T cells from SLE patients at higher levels compared with healthy controls (Fig. 4A) and patients with RA (Fig. S4) following a disease activity–dependent trend (Fig. 5A; SLEDAI 0–4: P = 0.0046; SLEDAI > 4: P = 0.0055). CREMα mRNA expression levels are associated with reciprocal CpG-DNA methylation patterns of the CREM promoter P1. T cells from healthy controls or from patients with RA exhibit higher CpG-DNA methylation levels compared with SLE patients in remission and with active disease following a trend that reflects disease activity (Fig. 4B; SLEDAI 0–4: P = 0.0005; Fig. S4B; SLEDAI > 4: P < 0.0001).
Fig. 4.
CREM, IL2, and IL17A mRNA expression and promoter methylation in T cells from SLE patients. T cells from SLE patients and healthy controls were assessed for the expression of (A) CREMα, (C) IL-2, and (E) IL-17A mRNA. Controls were compared with SLE patients with low disease activity (SLEDAI 0–4) and active disease (SLEDAI > 4). T cells from SLE patients and controls were screened for CpG-DNA methylation of the proximal promoter of (B) CREM, (D) IL2, and (F) IL17A.
Fig. 5.
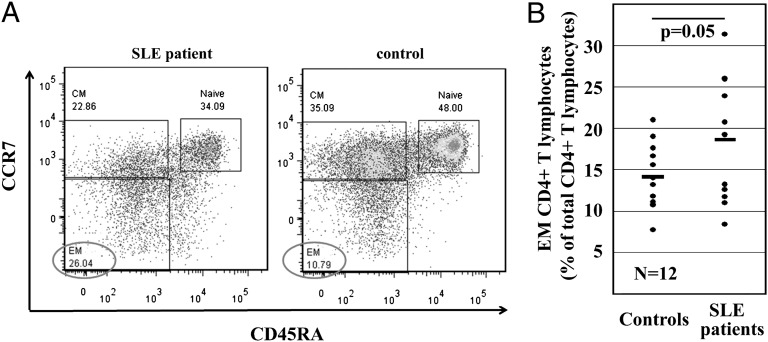
SLE patients exhibit a higher percentage of effector CD4+ T cells. (A) A representative pair of age, sex, and ethnicity matched SLE patient and the corresponding healthy control. T cells were stained for CD3, CD4, CD45RA, and CCR7 to define naive, central memory (CM), and effector memory (EM) CD4+ T cells. (B) Frequency of EM CD4+ T cells in total CD4+ T cells from SLE patients (SLEDAI: 4–8) and controls.
IL-2.
mRNA is expressed by T cells from SLE patients at lower levels compared with healthy controls and patients with RA. IL-2 mRNA expression follows a trend that reflects disease activity with lower IL-2 expression in patients with higher SLEDAI scores (Fig. 4C; SLEDAI 0–4: P = 0.0472; Fig. S4C; SLEDAI > 4: P = 0.0047). IL-2 mRNA expression levels are associated with reciprocal CpG-DNA methylation patterns of the IL2 proximal promoter. T cells from healthy controls, patients with RA, and SLE patients in remission exhibit comparable CpG-DNA methylation levels, whereas patients with active disease exhibit significantly more CpG-DNA methylation (Fig. 4D; SLEDAI 0–4: Fig. S4D; NS; SLEDAI > 4: P < 0.0001).
T cells from SLE patients with active disease express significantly more IL-17A mRNA compared with SLE patients in remission, RA patients, and healthy controls (Fig. S4E; SLEDAI 0–4: NS; SLEDAI > 4: P = 0.004). The degree of CpG-DNA methylation of the IL17A proximal promoter is reduced in T cells from all SLE patients compared with healthy controls and RA patients (Fig. 4F; SLEDAI 0–4: P = 0.0002; Fig. S4F; SLEDAI > 4: P = 0.0002).
Effector Memory CD4+ T Cells Are Enriched in SLE Patients.
CREMα is involved in chromatin remodeling during lineage determination in CD4+ T cells from healthy controls, and T cells from SLE patients fail to express IL-2 while overexpressing IL-17A. Thus, we asked whether SLE patients present an enrichment of effector memory phenotypes in their CD4+ T-cell repertoire compared with healthy controls. Twelve SLE patients with varying disease activity and healthy controls were screened for the relative frequency of effector memory T cells within the CD4+ T-cell fraction. Naive CD4+ T cells were defined as CD3+CD45RA+CCR7−, central memory as CD3+CD45RA−CCR7+, and effector memory as CD3+CD45RA−CCR7−. Because effector memory CD4+ T cells were enriched in SLE patients (P = 0.05) (Fig. 5), we propose a potential role for CREMα in chromatin remodeling of IL2 and IL17A and an enrichment of effector memory CD4+ T cells in SLE patients.
Discussion
In this report, we provide evidence that CREMα mediates epigenetic remodeling of IL2 and IL17A during CD4+ T-cell differentiation toward central and effector memory CD4+ T phenotypes through interaction with DNMT3a. We demonstrate that the CREM proximal promoter undergoes epigenetic remodeling that regulates gene expression in health and disease.
IL-2 plays a key role during T-cell activation and proliferation in vivo and in vitro, but also exerts immune-regulatory functions. IL-2 or IL-2 receptor–deficient mice develop lymphoproliferative disorders, resulting in autoimmune disease caused by impaired differentiation and subsequently reduced numbers of FoxP3+ regulatory T cells (21–25). IL-2 signaling is central for the later stages of T-cell development, particularly during the expansion of effector populations in nonlymphoid tissues and for effector functions during secondary T-cell responses (25, 26).
CD4+ T-cell subsets are defined by specific cytokine expression patterns. Although naive CD4+ T cells express a number of cytokines, including low levels of the effector cytokines IFN-γ and IL-17, specialized T-cell subsets lose this ability and express defined cytokine patterns. During the differentiation toward highly specialized CD4+ T-helper populations, cytokine genes undergo epigenetic remodeling controlled by transcription factor cascades. However, the molecular mechanisms that orchestrate these events are poorly understood (4–6). We demonstrate that central memory CD4+ T cells are capable of IL-2 expression but fail to produce IL-17A, whereas effector memory phenotypes fail to express IL-2 but produce IL-17A.
We previously demonstrated that CREMα acts as an epigenetic modulator in T cells from SLE patients. In addition to its trans-activating or trans-repressing effects on promoters, CREMα also mediates epigenetic remodeling of IL2 through the recruitment of DNMT3a (8) and HDAC1 (8, 16, 17). These findings are in line with recent reports on the transcription factor B-cell maturation protein (Blimp-)1. Blimp-1 recruits histone deacetylases and methyltransferases to B-cell–specific genes (27). It also plays a central role in T-cell homeostasis and Blimp-1–deficient mice develop a significant disbalance toward effector memory T-cell phenotypes. Thus, we investigated whether CREMα exerts regulatory functions during (physiological) T-cell lineage programming (25, 28, 29). Indeed, the IL-2 expression capacity of naive and central memory CD4+ T cells is mirrored by lower CpG-DNA methylation levels of the IL2 promoter compared with effector memory CD4+ T cells. Conversely, the IL17A promoter of effector memory CD4+ T cells is methylated to a lower degree in IL-17A expressing effector memory CD4+ T cells compared with naive and central memory CD4+ T cells. Forced expression of CREMα in primary human T cells mediated cytokine expression patterns that are similar to those of effector memory phenotypes. This may, in part, be due to trans-activating effects of CREMα on IL17A promoter activity (8, 15, 15) and trans-repressing effects on the IL2 promoter through replacement of the activating transcription factor pCREB (12). However, here we demonstrate additional effects of CREMα on CpG-DNA methylation of the IL2 and IL17A promoter during T-cell priming. Forced expression of CREMα mediates an increase of DNA methylation within the IL2 promoter while reducing DNA methylation of the IL17A promoter, mirroring the situation in effector memory CD4+ T cells. This is of special interest, because effector memory CD4+ T cells express significantly more CREMα compared with naive and central memory CD4+ T cells. Silencing of DNMT3a, an epigenetic modulator that can physically interact with CREMα, with siRNAs reverses CREMα effects on the IL2 promoter methylation, documenting the possible physiological impact of this mechanism in vitro.
T cells from SLE patients exhibit cytokine expression profiles that are comparable to effector memory CD4+ T cells. CREMα expression is increased in T cells from SLE patients, reflecting disease activity and mirrored by demethylation of the CREM promoter P1. Furthermore, T cells from SLE patients in remission express reduced levels of IL-2 mRNA compared with controls, and T cells from active SLE patients fail to express IL-2. IL-17A mRNA is not expressed in T cells from controls and patients in remission, whereas T cells from patients with active SLE express IL-17A. DNA methylation does not completely mirror this trend, suggesting there may be additional mechanisms involved in the deregulation of IL2 and IL17A in SLE T cells. CpG-DNA methylation of the IL2 promoter is comparable in T cells from patients in remission or controls but significantly reduced in T cells from active patients. The IL17A promoter is demethylated in T cells from patients with active or inactive disease. These findings may reflect dose-dependent effects of CREMα on both the IL2 and IL17A promoters. Data from our group suggest that CREMα could primarily mediate trans-activating effects on the IL2 promoter at low levels and epigenetic remodeling in active disease (8, 12, 16, 17). In the case of IL17A, CREMα could mediate active demethylation or inhibit (re)methylation of the promoter, whereas higher doses may be necessary for transactivation (15).
Although we achieved further insight on the involvement of CREMα in some of the molecular mechanisms that contribute to the T-cell phenotype of SLE, a number of questions remain: (i) Is CREMα is responsible for the recruitment of mediators that actively demethylate CpG-DNA or does CREMα inhibit the remethylation of the IL17A promoter remains to be elucidated? Also, the question of how CREMα mediates antithetic effects on IL2 and IL17A has not been sufficiently answered. The recruitment or colocalization with transcription factors that allow epigenetic remodeling of one gene and the inhibition of transcription factor binding at the other gene may explain diametric effects on chromatin conformation. (ii) Although increased IL-17A expression is a hallmark of T cells from SLE patients, it is not unique to this autoimmune disease. Increased expression of IL-17A from T cells has been demonstrated in other autoimmune disorders, including RA. However, in RA, increased expression of IL-17A is accompanied by unaltered IL-2 mRNA expression (19). In agreement with this, we determined reduced CpG-DNA methylation of the IL17A promoter but unaltered IL2 promoter methylation in T cells from patients with RA. This could be a reflection of diverse pathomechanisms in SLE and RA. The up-regulation of IL-17A together with unaltered IL-2 expression in RA could be a result of increased Stat3 activation through IL-6 or attenuated IL-23 receptor signaling in the absence of increased CREMα expression (20). (iii) We document a mechanism that contributes to T-cell lineage determination and an enrichment of effector memory phenotypes in SLE. However, additional CD4+ cell subsets display impaired CpG-DNA methylation patterns in SLE patients. So-called “senescent” T cells are enriched in the elderly and in patients with autoimmune disorders and are characterized by CpG-DNA hypomethylation, reduced CD28 surface expression, and a significant shortening of telomere DNA (30). Recently, Chen et al. (2009) demonstrated that senescent CD4+CD28– T cells express genes that are usually suppressed by CpG-DNA methylation in “regular” CD4+ T cells, including killer cell Ig-like receptors (KIR), perforin, and the signaling molecule CD70 (31). The activation of these genes contributes to autoimmune phenomena in SLE and other disorders (5, 31). The question of whether premature immune-senescence in SLE and the enrichment of effector memory phenotypes are mechanistically linked remains speculative and warrants further studies.
Conclusions
We provide evidence for the involvement of CREMα in CD4+ cell homeostasis by its contribution to the priming of effector memory CD4+ T cells. CREMα expression is regulated on the epigenetic level by CpG-DNA methylation of the CREM promoter P1. Increased CREMα expression mediates (i) epigenetic remodeling of IL2 through DNMT3a recruitment, resulting in increased DNA methylation, and (ii) trans-activation and demethylation of the IL17A promoter. Altered IL-2/IL-17A expression reflecting effector memory T-cell phenotypes is a hallmark of autoimmune disorders, including SLE. This makes CREMα not only an interesting biomarker for disease activity in SLE, but also a potential candidate for future target-directed treatment.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AI42269, R01 AI49954, and R01 AI85567 (to G.C.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210129109/-/DCSupplemental.
References
- 1.Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11:620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Zielinski CE, et al. Pathogen-induced human TH17 cells produce IFN-γ or IL-10 and are regulated by IL-1β. Nature. 2012;484:514–518. doi: 10.1038/nature10957. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 4.Wilson CB, Rowell E, Sekimata M. Epigenetic control of T-helper-cell differentiation. Nat Rev Immunol. 2009;9:91–105. doi: 10.1038/nri2487. [DOI] [PubMed] [Google Scholar]
- 5.Hedrich CM, Tsokos GC. Epigenetic mechanisms in systemic lupus erythematosus and other autoimmune diseases. Trends Mol Med. 2011;17:714–724. doi: 10.1016/j.molmed.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brenner C, Fuks F. A methylation rendezvous: Reader meets writers. Dev Cell. 2007;12:843–844. doi: 10.1016/j.devcel.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 7.Smallwood A, Estève PO, Pradhan S, Carey M. Functional cooperation between HP1 and DNMT1 mediates gene silencing. Genes Dev. 2007;21:1169–1178. doi: 10.1101/gad.1536807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hedrich CM, Rauen T, Tsokos GC. cAMP-responsive element modulator (CREM)α protein signaling mediates epigenetic remodeling of the human interleukin-2 gene: Implications in systemic lupus erythematosus. J Biol Chem. 2011;286:43429–43436. doi: 10.1074/jbc.M111.299339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Don J, Stelzer G. The expanding family of CREB/CREM transcription factors that are involved with spermatogenesis. Mol Cell Endocrinol. 2002;187:115–124. doi: 10.1016/s0303-7207(01)00696-7. [DOI] [PubMed] [Google Scholar]
- 10.Juang YT, et al. Transcriptional activation of the cAMP-responsive modulator promoter in human T cells is regulated by protein phosphatase 2A-mediated dephosphorylation of SP-1 and reflects disease activity in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:1795–1801. doi: 10.1074/jbc.M110.166785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juang YT, et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J Clin Invest. 2005;115:996–1005. doi: 10.1172/JCI200522854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katsiari CG, Tsokos GC. Transcriptional repression of interleukin-2 in human systemic lupus erythematosus. Autoimmun Rev. 2006;5:118–121. doi: 10.1016/j.autrev.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Solomou EE, Juang YT, Gourley MF, Kammer GM, Tsokos GC. Molecular basis of deficient IL-2 production in T cells from patients with systemic lupus erythematosus. J Immunol. 2001;166:4216–4222. doi: 10.4049/jimmunol.166.6.4216. [DOI] [PubMed] [Google Scholar]
- 14.Tsokos GC. Systemic lupus erythematosus. N Engl J Med. 2011;365:2110–2121. doi: 10.1056/NEJMra1100359. [DOI] [PubMed] [Google Scholar]
- 15.Rauen T, Hedrich CM, Juang YT, Tenbrock K, Tsokos GC. cAMP-responsive element modulator (CREM)α protein induces interleukin 17A expression and mediates epigenetic alterations at the interleukin-17A gene locus in patients with systemic lupus erythematosus. J Biol Chem. 2011;286:43437–43446. doi: 10.1074/jbc.M111.299313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tenbrock K, Juang YT, Tolnay M, Tsokos GC. The cyclic adenosine 5′-monophosphate response element modulator suppresses IL-2 production in stimulated T cells by a chromatin-dependent mechanism. J Immunol. 2003;170:2971–2976. doi: 10.4049/jimmunol.170.6.2971. [DOI] [PubMed] [Google Scholar]
- 17.Tenbrock K, Juang YT, Leukert N, Roth J, Tsokos GC. The transcriptional repressor cAMP response element modulator alpha interacts with histone deacetylase 1 to repress promoter activity. J Immunol. 2006;177:6159–6164. doi: 10.4049/jimmunol.177.9.6159. [DOI] [PubMed] [Google Scholar]
- 18.Kyttaris VC, Wang Y, Juang YT, Weinstein A, Tsokos GC. CAMP response element modulator a expression in patients with systemic lupus erythematosus. Lupus. 2006;15:840–844. doi: 10.1177/0961203306069985. [DOI] [PubMed] [Google Scholar]
- 19.Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–3545. doi: 10.1172/JCI36389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hazlett J, Stamp LK, Merriman T, Highton J, Hessian PA. IL-23R rs11209026 polymorphism modulates IL-17A expression in patients with rheumatoid arthritis. Genes Immun. 2012;13:282–287. doi: 10.1038/gene.2011.80. [DOI] [PubMed] [Google Scholar]
- 21.D’Cruz LM, Klein L. Development and function of agonist-induced CD25+Foxp3+ regulatory T cells in the absence of interleukin 2 signaling. Nat Immunol. 2005;6:1152–1159. doi: 10.1038/ni1264. [DOI] [PubMed] [Google Scholar]
- 22.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki H, et al. Deregulated T cell activation and autoimmunity in mice lacking interleukin-2 receptor beta. Science. 1995;268:1472–1476. doi: 10.1126/science.7770771. [DOI] [PubMed] [Google Scholar]
- 24.Willerford DM, et al. Interleukin-2 receptor alpha chain regulates the size and content of the peripheral lymphoid compartment. Immunity. 1995;3:521–530. doi: 10.1016/1074-7613(95)90180-9. [DOI] [PubMed] [Google Scholar]
- 25.Kallies A. Distinct regulation of effector and memory T-cell differentiation. Immunol Cell Biol. 2008;86:325–332. doi: 10.1038/icb.2008.16. [DOI] [PubMed] [Google Scholar]
- 26.Mescher MF, et al. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 27.Yu J, Angelin-Duclos C, Greenwood J, Liao J, Calame K. Transcriptional repression by blimp-1 (PRDI-BF1) involves recruitment of histone deacetylase. Mol Cell Biol. 2000;20:2592–2603. doi: 10.1128/mcb.20.7.2592-2603.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kallies A, et al. Transcriptional repressor Blimp-1 is essential for T cell homeostasis and self-tolerance. Nat Immunol. 2006;7:466–474. doi: 10.1038/ni1321. [DOI] [PubMed] [Google Scholar]
- 29.Martins GA, et al. Transcriptional repressor Blimp-1 regulates T cell homeostasis and function. Nat Immunol. 2006;7:457–465. doi: 10.1038/ni1320. [DOI] [PubMed] [Google Scholar]
- 30.Honda M, et al. Telomere shortening and decreased replicative potential, contrasted by continued proliferation of telomerase-positive CD8+CD28(lo) T cells in patients with systemic lupus erythematosus. Clin Immunol. 2001;99:211–221. doi: 10.1006/clim.2001.5023. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Gorelik GJ, Strickland FM, Richardson BC. Decreased ERK and JNK signaling contribute to gene overexpression in “senescent” CD4+CD28- T cells through epigenetic mechanisms. J Leukoc Biol. 2010;87:137–145. doi: 10.1189/jlb.0809562. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.