Abstract
Bacterial plasmid partitioning systems segregate plasmids into each daughter cell. In the well-understood ParMRC plasmid partitioning system, adapter protein ParR binds to centromere parC, forming a helix around which the DNA is externally wrapped. This complex stabilizes the growth of a filament of actin-like ParM protein, which pushes the plasmids to the poles. The TubZRC plasmid partitioning system consists of two proteins, tubulin-like TubZ and TubR, and a DNA centromere, tubC, which perform analogous roles to those in ParMRC, despite being unrelated in sequence and structure. We have dissected in detail the binding sites that comprise Bacillus thuringiensis tubC, visualized the TubRC complex by electron microscopy, and determined a crystal structure of TubR bound to the tubC repeat. We show that the TubRC complex takes the form of a flexible DNA–protein filament, formed by lateral coating along the plasmid from tubC, the full length of which is required for the successful in vitro stabilization of TubZ filaments. We also show that TubR from Bacillus megaterium forms a helical superstructure resembling that of ParR. We suggest that the TubRC DNA–protein filament may bind to, and stabilize, the TubZ filament by forming such a ring-like structure around it. The helical superstructure of this TubRC may indicate convergent evolution between the actin-containing ParMRC and tubulin-containing TubZRC systems.
Keywords: FtsZ, X-ray crystallography, DNA segregation
Low copy number plasmids often encode their own segregation machinery, ensuring that copies are partitioned into each daughter cell. These plasmid-partitioning systems organize replicated plasmids and actively separate them. The known plasmid-partitioning systems are minimalist and elegant. They consist of just three components: a DNA centromere, an adapter protein that binds the centromere, forming the centromeric complex, and a nucleotide triphosphate-dependent filament-forming protein, which produces the force to move the plasmids (1, 2).
Plasmid partitioning systems have been divided into types I–III, based upon the homology of their filament-forming proteins to known protein families (2, 3). Type I plasmid partitioning systems (4, 5) are based on deviant Walker A ATPases (6, 7), type II (8) on actin-like proteins (9), and type III (10–12) on tubulin/FtsZ-like proteins (13, 14). Although the structure of the filament implicated in segregation has been determined for all three systems (9, 14–16), only examples of type I and II centromeric complexes have so far been resolved (17–19).
The centromeric complex of the type II (actin-like) ParMRC plasmid partitioning system is formed by the binding of adapter protein ParR to parC, a series of parallel (direct), 11-base pair (bp) repeats (20). The superstructure this complex forms is helical, right-handed, and places the parC DNA on the exterior of the helix and ParR on the interior (18, 19). This arrangement clusters the interaction surfaces between ParM and ParR, which are located at the tip of the ParM filament and within the last few residues of ParR (18, 19). This clustering probably enables processive filament tip tracking by promoting binding between the filament end and the RC helix, ensuring that only parC-bound ParR is able to seed and grow, or at least stabilize filaments.
The centromeric complex of the type III (tubulin-like) TubZRC plasmid partitioning system is also composed of an adapter, TubR, and a DNA centromere, tubC. The rough disposition of four of the repeats making up Bacillus thuringiensis serovar israelensis pBtoxis (Bt) tubC, but not the TubR binding site or register, has been assigned by Tang and colleagues who demonstrated binding by Bt TubR to this region of pBtoxis (10, 21). Furthermore, the structure of the TubR adapter protein has been determined in the absence of DNA (13), revealing that it forms a recognition-helix dimer with a potential DNA binding surface and demonstrating that it is unrelated to type II ParR (18, 19). Finally, it has been shown that the TubRC complex is capable of recruiting TubZ (13, 21). A summary of previous work on the TubZRC system is provided in Table S1.
In this study we examine in detail the superstructures formed by TubRC complexes. We show that Bt tubC in fact contains seven high-affinity binding sites, the full region being required for proper in vitro activity. Furthermore we reveal that TubR coats tubC: the centromeric complex of TubZRC plasmid partitioning systems therefore takes the form of a DNA–protein filament. We present a 7.0-Å crystal structure revealing the superstructure of the flexible Bt TubRC filament and provide electron microscopy and a 3.5-Å crystal structure that imply a preferred conformation for the TubRC filament of Bacillus megaterium (Bm), allowing us to compare the superstructures of type III (tubulin-like) centromeric complexes to those of type II (actin-like) plasmid partitioning systems.
Results
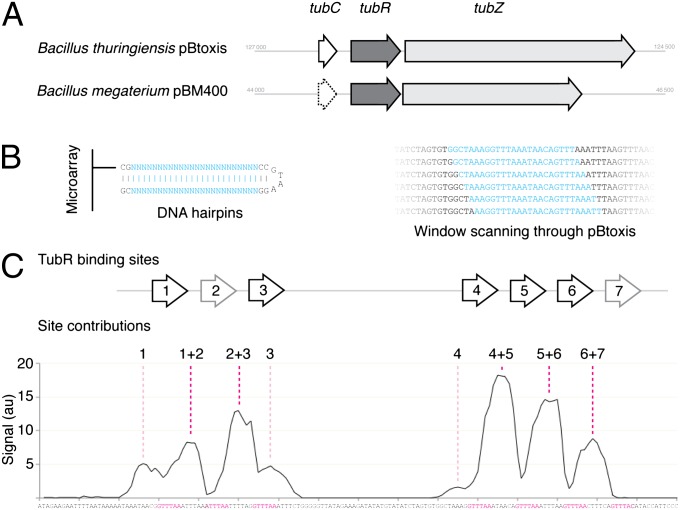
Bt tubC Dissected by Microarray.
We initially set out to precisely define B. thuringiensis (Bt) tubC. The structure of the centromere is defined by the binding of its protein partner; therefore, we dissected the binding of Bt TubR to each independent site within this part of pBtoxis. We designed a microarray in which each experimental measurement consisted of a 24-bp double-stranded DNA sequence (Fig. 1B), formed by a hairpin stabilized through a tetraloop (22). This 24-base “window” was scanned through the plasmid in successive measurements; a 4-bp shift per measurement was used to conduct a coarse scan of a large area of pBtoxis, and single base pair shifts were then used for a high-resolution scan of the Bt tubC region (Fig. 1 B and C). Bt TubR bound the array in only a few places, and binding was sustained reproducibly with the movement of the experimental window. The Bt tubC region was readily identified as the sole site of strong, sustained binding.
Fig. 1.
Bt tubC is composed of seven repeats, which bind TubR in a cooperative fashion. (A) Schematic comparing the tubZRC loci of Bt pBtoxis and Bm pBM400. (B) Illustration of the DNA hairpins produced on a microarray to sample the sequence of pBtoxis, and a schematic indicating how this window was scanned through a region of the plasmid sequence by successive single base pair movements. The variable window is shown in cyan, and all other base pairs in black. (C) Plot of the recorded signal for each microarray spot in a 1-bp scan over the region of Bt tubC (bp 126688 to 126496). Each point is plotted over the 12th bp of the 24-bp hairpin, with the sequence shown below. The assigned binding sites for Bt TubR are shown above, and the corresponding sequences are colored magenta below. The site(s) resulting in each peak have been annotated above the graph.
Bt TubR Binds to tubC Cooperatively.
Peaks of binding by Bt TubR were clearly identifiable (Fig. 1C). Fourier analysis of this signal with respect to sequence confirmed the repeat length to be between 12 and 13 bp (Fig. S1) (10). We aligned the sequences producing peak signal allowing for a 12-bp repeat and produced a weighted consensus sequence of the repeats found in Bt tubC (Figs. S1 and S2). Two directly repeated sequences were required to produce each peak, troughs in binding occurring on centering of a single binding site (Fig. 1C and Fig. S2), implying that Bt TubR binding is highly cooperative, dependent on lateral stabilization. This mechanism is consistent with the known activity of TubR as a repressor (12), providing a mechanism by which affinity may be tuned. Stronger sites (such as sites 1, 3, and 4) were capable of supporting binding on adjacent DNA, indicated by low signal peaks in the absence of adjacent cognate sites, implying that spreading of Bt TubR along the plasmid is possible (Fig. 1C). We confirmed that such lateral spreading occurs in vitro by electrophoretic mobility shift assay (Fig. S3).
Bt tubC Is Seven TubR Binding Sites in Two Clusters.
Bt TubR binding was limited almost exclusively to the region of DNA 3′ from tubR. Whereas four direct repeats have previously been identified (21) we found that there are in fact two clusters of Bt TubR binding sites, the first comprising three adjacent sites (sites 1–3) and the second four (sites 4–7), which together we identify as the native Bt tubC (Fig. 1C). Each block binds Bt TubR independently in vitro (Fig. S3). Because the two blocks are separated by 54 bp, spreading of Bt TubR cannot coat all intervening DNA; however, it is well established in the case of ParR from Escherichia coli plasmid R1 that separate blocks of sites are linked by looping of the intervening DNA, so a single complex may be formed (18, 23). Evolution has selected for the production of an array of Bt tubC-bound TubR dimers; these must presumably therefore be required to perform the function of the TubRC centromeric complex.
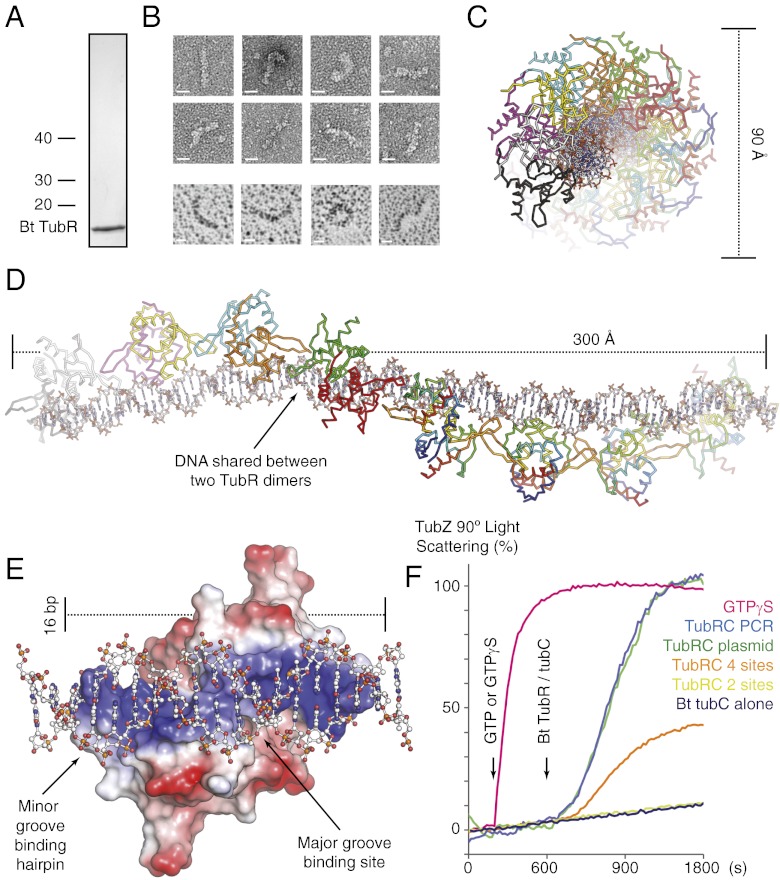
Bt TubRC Forms a DNA–Protein Filament in Vitro.
To understand why Bt tubC had evolved an array of parallel repeats, we examined the complex formed by Bt TubR on its centromere using electron microscopy. We produced full-length, untagged Bt TubR and purified it to homogeneity (Fig. 2A); it was then bound to a PCR product encompassing the full Bt tubC region. DNA–protein filaments composed of blocky subunits were clearly visible in samples of the Bt TubRC complex (Fig. 2B). Bt TubRC filaments had an average width measuring 5–6 nm, consistent with the greatest width of a single TubR dimer, whereas the separation of the subunits (from Fourier transform) was 4–5 nm, suggesting that a single fiber of Bt tubC DNA was bound by a single row of TubR dimers. The filaments were typically extended, although some exhibited a higher degree of curvature, indicating that these complexes are flexible.
Fig. 2.
Bt TubR bound to tubC forms a DNA–protein filament that effects TubZ polymerization. (A) Coomassie-stained SDS/PAGE of purified Bt TubR. (B) Electron micrographs of negatively stained (Left) and rotary shadowed (Right) Bt TubR bound to full-length tubC showing the morphology of the DNA–protein filaments formed. (Scale bars, 10 nm.) (C and D) Crystal structure of Bt TubR (Cα ribbon representation, the first four dimers are colored by chain, the second four a continuum between blue at the N terminus and red at the C terminus) bound to two repeats of tubC (stick representation, C in white/CPK colors). Dimensions in angstroms are shown alongside the structure, whereas the filament has been rotated by 90° between the two plates. (E) Single Bt TubR dimer (surface charge representation, red negative, blue positive) from the structure, with B-DNA [ball and stick representation, C in white/Corey, Pauling, Koltun (CPK) coloring] extended from the 12-bp section used in refinement in order to show the interaction with the full 16-bp region covered by the dimer. (F) Effect of Bt TubRC on the polymerization of TubZ measured using 90° light scattering. GTP or GTPγS was added to all reactions at 200 s, and the complexes indicated to the Right of the graph were added at 600 s (color coded). The four- and two-site versions of Bt tubC encompassed binding sites 4–7 and 6–7.
Structure of Bt TubRC at 7 Å.
Unfortunately the Bt TubRC DNA–protein filaments were not sufficiently ordered for either helical or single-particle reconstruction; therefore, we obtained a higher resolution model by cocrystallizing Bt TubR with oligonucleotides from tubC. Cocrystals were obtained with Bt TubR and a 24-bp double-stranded oligonucleotide (tubC24). Diffraction was recorded to 7 Å. The structure shown in Fig. 2 C–E was solved by molecular replacement using PDB ID code 3M9A and B-form DNA (13) and verified by phasing selenium anomalous differences to confirm that the difference density obtained identified the selenomethionine sites (Fig. S4 and Table S2). Bt TubRC24 crystals were of space group C2, each asymmetric unit containing eight Bt TubR dimers and four tubC24 oligonucleotides. Electron density was surprisingly good (Fig. S4) and the positions after rigid body refinement resulted in close contacts but few clashes, implying a reasonable model; however, determination of the register of the DNA sequence lay beyond the resolution of our data.
Bt TubRC Crystals Contain a DNA–Protein Filament.
Bt TubRC24 forms an extended DNA–protein filament in our crystals. The DNA component, tubC24, describes a continuous linear fiber, curving in a slight right-handed superhelix, which forms the core of the complex. The outside of the filament is decorated with TubR molecules, forming an 8/1 helix with a rise of 38.4 Å, corresponding exactly to the 12-bp cognate repeat found in Bt tubC (Fig. 2 C and D). The coating of the outside of the DNA helix by Bt TubR enables the majority of crystal contacts to be formed between protein side chains rather than by DNA, as is expected because of the highly charged nature of the phosphate backbone. The Bt TubRC24 filament has very similar dimensions to the extended filaments observed by electron microscopy, and we conclude that this structure is representative of the predominant state of Bt TubRC in solution.
DNA Binding by Bt TubR.
Bt TubR bound predominantly within the major groove of tubC. The N termini of the paired “recognition” helices together protrude deep into the major groove where they are able to make contact with 5 bp. This is the only major groove contact made, and it is therefore likely to correspond to the sequence-specific component of Bt tubC. The consensus binding sequence GTTTAA is not a simple twofold however, suggesting that DNA conformation must also play a role. On either side of the major groove binding site, the phosphate backbone descends into a cleft between the short helix and loop N-terminal to the recognition helix and the β-hairpin of Bt TubR. The negative charge of the phosphates will be complemented by lysines 43, 54, and 79, and arginine 74, which form the sides and base of this cleft. Finally, the tip of the β-hairpin protrudes toward the minor groove where arginine 77, which clashes with the phosphate backbone after rigid body refinement, must intrude into the groove itself (Fig. 2E).
Currently, only one other structure of a recognition helix dimerized form of the winged helix-turn-helix fold bound to DNA is available, that of FadR from E. coli (24). The DNA binding mode is conserved between the two structures, although the minor groove binding β-hairpin is positioned slightly differently in FadR. The conformation of the DNA in the two structures deviates substantially, however. Both proteins extend the DNA helix relative to its conformation in standard B-DNA; however in the case of FadR, the DNA is bound closely within the minor grooves, bending the course of the fiber toward the surface of the protein, whereas Bt TubR binds less closely in the minor groove, the DNA bending slightly away from the protein. The minor groove is also elongated, possibly due to the interaction with the β-hairpin. The full length of DNA contacted by a single Bt TubR dimer is 16 bp, although the tubC repeat length is 12 bp (13). This requires 4 bp to be shared between adjacent dimers, which occurs at the site of protein–protein contact between the minor groove binding hairpins (Fig. 2 C–E). These β-hairpins from adjacent Bt TubR dimers are tightly paired in the minor groove, their opposition favoring the DNA curvature in the Bt TubRC24 structure.
TubRC Stabilizes TubZ Polymerization in Vitro.
To confirm that the TubRC complex we had discovered was relevant to the activity of the TubZRC plasmid partitioning system, we assayed its effect on TubZ polymerization by 90° light scattering. A recent study by Oliva and colleagues (25) has shown that TubRC increases polymerization of TubZ. We therefore mixed Bt TubZ with GTP below the critical point of filament formation measurable by light scattering. Bt TubRC complex was then added, which stabilized filament growth. A similar effect, with the same maximum polymerization, can be produced by replacing GTP with GTPγS, implying that the bar to growth is due to hydrolysis of the nucleotide and disassembly of the filament at a higher rate than growth (Fig. 2F). Given that the Bt TubRC complex was present at a substoichiometric level, this suggests that it might prevent depolymerization of TubZ filaments, possibly switching from treadmilling to elongation.
To determine whether or not the whole Bt TubRC complex is required for the action of the system, we performed the assay with Bt tubC containing plasmid and with linear DNA representing full-length tubC, four repeats (sites 4–7) and two repeats (sites 6 and 7). These Bt TubRC complexes were compared at a constant concentration of TubR binding sites. Bt TubRC complexes bearing only two repeats did not have any measurable effect on TubZ polymerization below stoichiometric levels, whereas both linear DNA and plasmid containing Bt tubC showed a similar substoichiometric effect. The four-repeat long Bt TubRC complex affected polymerization; however, this effect was substantially reduced relative to full-length tubC, requiring four times as much complex to match polymerization (Fig. 2F).
TubR from a tubZR Operon in pBM400.
Having defined tubC in the Bt TubZRC plasmid partitioning system and shown that Bt TubRC forms a flexible DNA–protein filament, the full length of which is required to match native in vitro efficacy, we moved on to consider whether or not there might be a preferred structural mode that the TubRC complex might take up, given that the flexible TubRC complex must presumably become ordered upon binding to the TubZ filament, which might explain the requirement for the length of tubC.
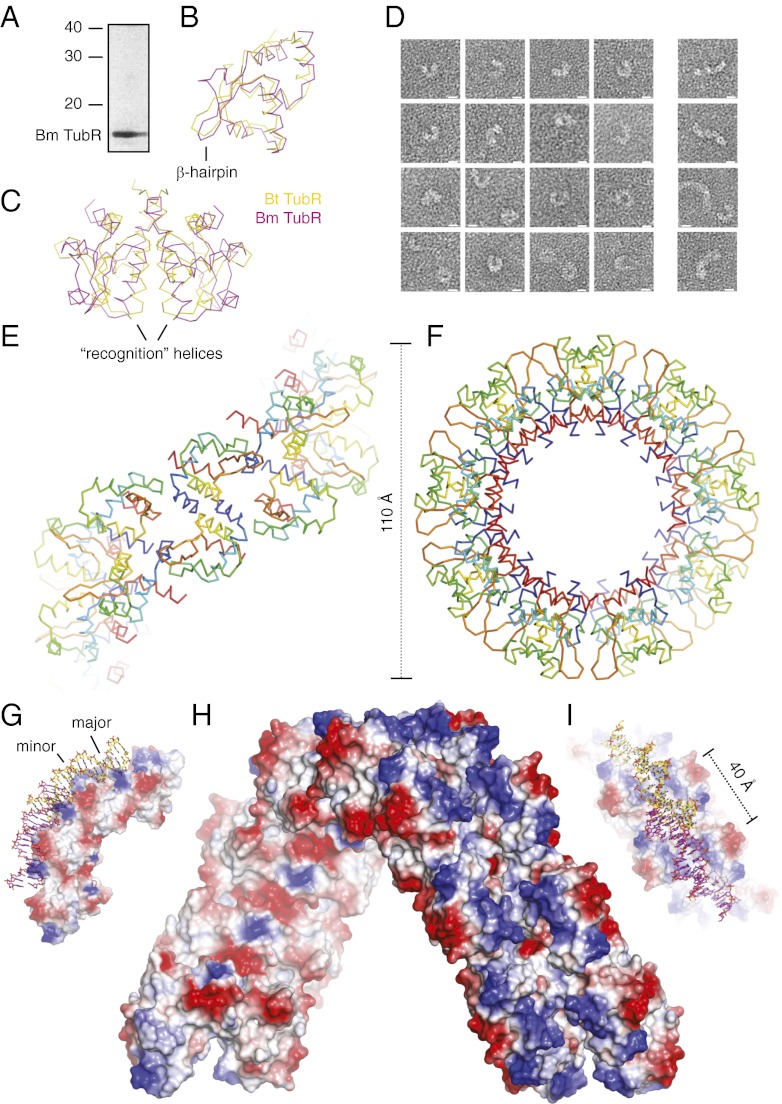
Fortuitously, in the course of our investigation of TubZRC plasmid partitioning systems we discovered an additional TubR homolog that adopts a more consistent preferred conformation as a DNA–protein complex, shedding light on this question. Bm TubR (UniProt Q848W2_BACMQ) lies within an operon containing a TubZ homolog in B. megaterium QM B1551 (Bm) plasmid pBM400 (Fig. S5). The gene encoding this TubR was synthesized and we overexpressed and purified the full-length protein without any tags or additions (Fig. 3A).
Fig. 3.
Bm TubR forms helices of similar appearance in crystals and bound to DNA. (A) Coomassie-stained SDS/PAGE of purified Bm TubR. (B) Structural superimposition of monomers of Bt and Bm TubR (Cα ribbon representation, colored as indicated). (C) Structural superimposition of dimers of Bt and Bm TubR (Cα ribbon representation, colored as indicated). (D) Electron micrographs of negatively stained Bm TubR bound to Bt tubC DNA. (Scale bars, 10 nm.) (E and F) Crystal structure of Bm TubR helix (Cα ribbon representation, continuum between blue at the N terminus and red at the C terminus). Dimensions in angstroms are shown alongside the structure, whereas the helix has been rotated by 90° between the two plates. (G–I) Four Bm TubR dimers (surface charge representation, red negative, blue positive) with the DNA from Protein Data Bank (PDB) ID code 1HW2 (stick representation, C in yellow or magenta/CPK colors) shown after the two structures have been superimposed for the two central dimers. The two plates are rotated by 90° relative to one another. (H) Surface charge representation of the Bm TubR helix (red negative, blue positive).
Bm TubR Forms Ring-Like DNA–Protein Filaments.
To determine whether or not the Bm and Bt TubR–DNA complexes were comparable, we imaged the Bm TubR–DNA complex by electron microscopy. Whereas Bm TubR binds its intergenic DNA 5′ to tubZR with a slight preference over Bt tubC (Fig. S3), we were unable to identify the exact Bm TubR binding sites by microarray (its structure suggests an explanation for this). Electron microscopy of Bm TubR bound to DNA revealed DNA–protein filaments (Fig. 3D), the dimensions of which were similar to those recorded for Bt TubRC (Bm TubR formed identical DNA–protein filaments on intergenic, Bt tubC, and bulk DNA; tubC DNA was used here to allow direct comparison). The Bm TubR–DNA filament width averaged between 5 and 6 nm, and in general the filaments seemed similar in structure. In a notable contrast to the lack of a consistent preferred curvature by the Bt TubRC complex, however, although Bm TubR–DNA still produced relatively flexible filaments, they more often favored highly curved ring-like structures, with a typical diameter of 15–20 nm, mean 17.5, n = 43 (Fig. 3D). Bm TubR–DNA filaments adopted ring-like structures (defined as less than 15 nm separation between the tips of each end of the filament) and poorly ordered filaments in a ratio of 73.6:26.4 (n = 253), in contrast to Bt TubRC, for which the ratio was 10.7:89.3 (n = 242).
Bm TubR Is Structurally Homologous to Bt TubR.
To aid our understanding of Bm TubR, we crystallized the protein and recorded diffraction to 3.5 Å. The crystal structure was then solved by selenomethionine single anomalous dispersion (SeMet SAD). Bm TubR crystallized in space group H32, the asymmetric unit containing three protein molecules (Table S2). Bm TubR shares the same fold as Bt TubR, with a single chain backbone rmsd of 2.0 Å (Fig. 3B). Furthermore the quaternary structure of Bt TubR (13), in which the dimer is formed by twofold rotation about the recognition helix, is also recapitulated (Fig. 3C). In Bm TubR, the two monomers making up the dimer are splayed slightly, relative to those in Bt TubR, leaving a cleft between the recognition helices. Although it appears likely that this fissure may close slightly during DNA binding, the cleft may explain the observed lack of a clearly defined binding sequence. Superimposition of the surface of Bm TubR onto our structure of Bt TubRC shows that this cleft opens directly beneath the major groove: contacts with the DNA bases can therefore be expected to be substantially reduced. This implies that less base specific, more conformation-dependent minor groove binding may be expected to dominate, and that the overall protein–DNA contact area would also be considerably smaller, suggesting that larger numbers of dimers would be required to associate cooperatively for stable binding, whereas our microarrays cannot accommodate more than two dimers (Fig. S6). The putative DNA binding surface identified for Bt TubR is also conserved between the two proteins, a cluster of lysine and arginine residues characterizing the same face of the dimer (Fig. 3H) (13). The minor groove binding β-hairpin in Bm TubR is in a different conformation from that in Bt TubR; however, this region has higher B factors in both cases, and given that it is also slightly separated from the body of the protein, it may be flexible in vivo.
Bm TubR Helices Externalize the DNA Binding Site.
Importantly, the dimers within the crystal structure of Bm TubR form helical filaments (Fig. 3 E and F). The dimensions of these helices are similar to the Bm TubR–DNA rings visualized by electron microscopy, with a filament width of 48 Å and a maximum helical width of 155 Å. The helices place Bm TubR dimers back to back, allowing the wedge-shaped proteins to make significant contacts with one another. The mean buried interface between pairs of dimers is 399.5 Å2 in our structure of Bt TubRC, but this increases to 723.8 Å2 in the case of our structure of Bm TubR. The extended β-hairpins of adjacent dimers are paired with a small gap between each dimer, extending the groove between recognition helices around the entire helix. Significantly this superstructure displays the positively charged DNA binding surface on the exterior of these helices (Fig. 3H).
Discussion
Bm TubR Helices Are in Principle Compatible with DNA Wrapping.
The length of DNA used to produce the ring-shaped Bm TubR–DNA filaments resolved by electron microscopy is consistent with DNA wrapping on the external surface. Ring diameter averaged 17.5 nm, implying a circumference of 55 nm, whereas the expected length of the PCR product would be 50 nm. The internal circumference would therefore be too short, whereas the external diameter would roughly match the expected length. Given the similarity in the dimensions of the Bm TubR–DNA rings, and the crystallographic helix, the most parsimonious explanation is that these structures are similar. If we assume that the DNA binding mode of Bm TubR is identical to that of Bt TubR, given their structural similarity, then superimposing the available structures of this class of DNA binding proteins (Bt TubRC/FadR–DNA) onto the structure of the Bm TubR helix will describe the path of the DNA. When this is carried out, it is clear that, although the surface is not immediately compatible with a continuous strand of DNA, only a relatively small distance (6.1 Å, Bt TubRC/3.4 Å, FadR–DNA) would separate the DNA backbones from adjacent dimers. FadR–DNA better matches the curve of the Bm TubR helix as the DNA from this structure curves in the same direction. Closer pairing of the minor groove binding β-hairpins would be required to bind DNA, which might be achieved through closure of the notch in the surface of Bm TubR or a slight rotation of the helix (Fig. 3 G–I and Fig. S7).
TubRC Is a DNA–Protein Filament.
Our results conclusively show that the centromeric complex of TubZRC plasmid partitioning systems forms a DNA–protein filament. The disposition of tubC has evolved to facilitate its formation, ensuring coverage of a minimum length of plasmid DNA; furthermore, the binding mechanism of TubR is highly cooperative, allowing coating, further favoring its formation. We have shown that such a filament is required for the activity of TubR upon TubZ polymerization in vitro, activity falling to nil as the length of the centromeric complex decreases, even though the total concentration of TubR–DNA binding pairs was held constant. It is clear, therefore, that a TubRC filament is essential for the activity of the centromeric complex. Why must this be the case?
Interaction with the centromeric complex must stabilize the assembly of its cognate filament, whereas the free adapter protein must be incapable of this activity. The formation of the complex must therefore either produce a novel binding site between its components with substantially higher affinity or cluster the adapter protein in space to achieve binding to the filament through higher avidity. In both the ParMRC and TubZRC plasmid partitioning systems, it would seem that the second mechanism appears to have won out over the first. This may well occur because this mechanism can take advantage of both the clustering of the adapter protein on an array of DNA binding sites and clustering of the subunits within the cognate protein filament. Because the superstructure of a linear filament of identical subunits consists of a repeated vector, it must form a helix of tighter or looser curvature. Close clustering in space by a linear filament will therefore, by definition, be achieved through the formation of tight helical structures.
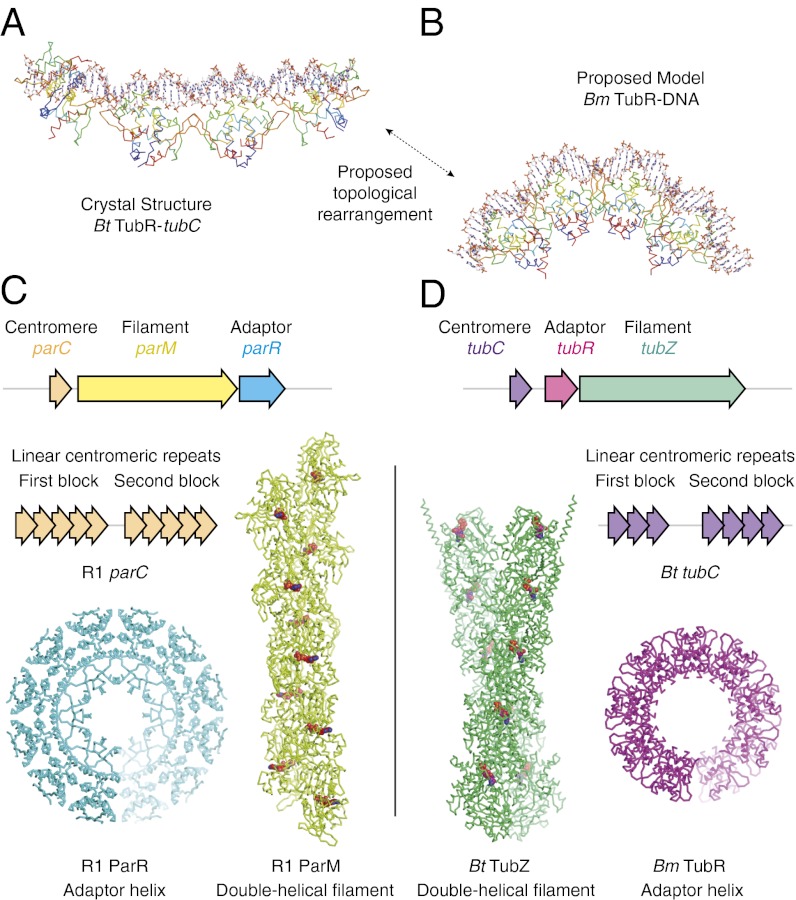
Although different TubZRC systems have so far been observed to have considerable similarity (25, 26), our Bt and Bm TubR complexes exhibit different curvatures. Bt TubRC crystallized as an extended DNA–protein filament with protein wrapping helically around the outside of the DNA, whereas the Bm TubR–DNA complex appears to form a ring or short helix with external DNA. Whereas it is possible that the two TubRC structures represent entirely diverged forms of the adapter complex, we think this unlikely; the Bt TubRC filament was clearly quite flexible in vitro, and it is in fact possible to reconcile the two structures as “flexible” and “curled” modes of a similar complex. The 12-bp repeat between major groove binding sites in the Bt TubRC filament is approximately conserved when DNA is superimposed upon the Bm TubR helix, so the binding periodicity would be similar (11–13 bp). The main structural change in DNA binding between the two would be rearrangement of the minor groove binding β-hairpins. These are roughly paired in both structures; however, the difference being that their orientation is reversed, providing a convenient switch between the two states. This proposed closure would neatly wrap the DNA and close up the space between adjacent TubR dimers (Fig. 4 A and B and Fig. S7).
Fig. 4.
Bm TubR helix suggests further convergent evolution of type II and III partitioning systems. (A) Structure of Bt TubR (Cα ribbon representation, blue at N terminus, red at C terminus) bound to tubC (stick representation, C in white/CPK colors). (B) Structure of Bm TubR (Cα ribbon representation, blue at N terminus, red at C terminus) shown with the DNA (stick representation, C in white/CPK colors) from PDB ID code 1HW2 after superimposition of the protein chains. (C and D) Comparison of operon structure (3, 10, 12), centromeric structure (20, 21), filament superstructure (9, 14, 16), and adapter complex superstructure (18, 19, this study) for the (actin-like) ParMRC and (tubulin-like) TubZRC plasmid partitioning systems.
Why then would the two DNA–protein filaments have different curvatures in vitro? One possible cause could be that binding to the TubZ filament is required to stabilize the TubRC complex in one state or another. It seems quite plausible that interactions with the linear filament stabilize the relatively flexible structure of the TubRC centromeric complex. The internally clustered TubR dimers in a ring-like structure would seem more capable of making a protein–protein contact than the sparsely distributed dimers in the flexible, extended form, as this form does not present TubR on a consistent side of the filament. The internal diameter of the Bm TubR crystal structure, at ∼6.5 nm, is smaller than the diameter of the TubZ filament (14), at ∼10 nm; however, the rings observed by electron microscopy exhibit internal diameters between 5 and 10 nm, allowing sufficient flexibility to encompass TubZ filaments in theory. A switch between a flexible and a ring-like form might allow the TubRC complex to clamp onto a growing TubZ filament during partitioning. Further structural studies of the TubZRC system will be required to understand the mechanism of binding and it is worth mentioning that the interaction sites between TubZ and TubR currently remain unknown.
Convergent Evolution of Type II and III Centromeric Complexes?
It has not escaped our notice that an intriguing parallel emerges from this work. The structure of Bt tubC is strongly reminiscent of that of R1 parC, both consisting of two series of linear repeats separated by a linker, whereas the ring-like structure of Bm TubR is similar in superstructure to the known ParRC complex (Fig. 4C). This similarity adds to our earlier finding that TubZ, being tubulin related, forms actin- (and ParM)-like double helical filaments that are the smallest closed-symmetrical polymers possible and seem exquisitely tailored to the plasmid segregation task at hand (14). The observation of such similar superstructures, produced to perform similar functions by entirely unrelated proteins in different systems, suggests that convergent evolution may have taken place, driving both systems toward the same solution to the problem of binding a cytomotive filament, with entirely different protein and DNA building blocks.
Materials and Methods
Detailed information is provided in SI Materials and Methods. Briefly, all proteins were produced in E. coli and purified by chromatography; DNA was either synthesized (Bt tubC24), produced by PCR (all other linear DNA), or purified as a plasmid (ptubC); the Bt tubC aptamer microarray experiment was performed in conjunction with LC Sciences; electron microscopy was carried out using a 2% (wt/vol) uranyl acetate negative stain in an FEI T12 electron microscope; the sequence of Bt tubC24 was TTTAAGTTTAACTTTCAGTTTACA; Bt TubRC24 crystals were solved at 7 Å by molecular replacement and confirmed by selenomethionine SAD, whereas Bm TubR crystals were solved at 3.5 Å by selenomethionine SAD; 90° light scattering was carried out at 400 nm using 1.25 μM Bt TubZ, 500 nM TubR, and 125 nM of TubR binding sites in each tubC DNA, except for tubC repeats 4–7, where the concentration was doubled to make the difference from tubC24 clearly visible.
Supplementary Material
Acknowledgments
We thank Rachel Larsen, Joseph Pogliano (both University of California at San Diego), and Katherine Michie [Medical Research Council Laboratory of Molecular Biology (MRC-LMB)] for providing materials; Sebastian Eustermann (MRC-LMB) for his tetraloop sequence; Fabrice Gorrec and Sonja Kuhlman for their help at MRC-LMB crystallization facility; Chen Shaoxia, Qing Wang, and Colin Palmer for their aid with electron microscopy at MRC-LMB; Sonja Dunbar for summer work and Ramona Duman (both MRC-LMB) for help at the European Synchrotron Radiation Facility (ESRF); and LC Sciences, the ESRF, and Diamond Light Source for their excellent service and support. This work was supported by Medical Research Council Grant U105184326.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 4ASN, 4ASO, and 4ASS).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1210899109/-/DCSupplemental.
References
- 1.Gerdes K, Howard M, Szardenings F. Pushing and pulling in prokaryotic DNA segregation. Cell. 2010;141:927–942. doi: 10.1016/j.cell.2010.05.033. [DOI] [PubMed] [Google Scholar]
- 2.Salje J. Plasmid segregation: How to survive as an extra piece of DNA. Crit Rev Biochem Mol Biol. 2010;45:296–317. doi: 10.3109/10409238.2010.494657. [DOI] [PubMed] [Google Scholar]
- 3.Gerdes K, Møller-Jensen J, Bugge Jensen R. Plasmid and chromosome partitioning: Surprises from phylogeny. Mol Microbiol. 2000;37:455–466. doi: 10.1046/j.1365-2958.2000.01975.x. [DOI] [PubMed] [Google Scholar]
- 4.Austin S, Abeles A. Partition of unit-copy miniplasmids to daughter cells. II. The partition region of miniplasmid P1 encodes an essential protein and a centromere-like site at which it acts. J Mol Biol. 1983;169:373–387. doi: 10.1016/s0022-2836(83)80056-4. [DOI] [PubMed] [Google Scholar]
- 5.Ogura T, Hiraga S. Partition mechanism of F plasmid: Two plasmid gene-encoded products and a cis-acting region are involved in partition. Cell. 1983;32:351–360. doi: 10.1016/0092-8674(83)90454-3. [DOI] [PubMed] [Google Scholar]
- 6.Leonard TA, Butler PJ, Löwe J. Bacterial chromosome segregation: Structure and DNA binding of the Soj dimer—a conserved biological switch. EMBO J. 2005;24:270–282. doi: 10.1038/sj.emboj.7600530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barillà D, Carmelo E, Hayes F. The tail of the ParG DNA segregation protein remodels ParF polymers and enhances ATP hydrolysis via an arginine finger-like motif. Proc Natl Acad Sci USA. 2007;104:1811–1816. doi: 10.1073/pnas.0607216104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerdes K, Larsen JE, Molin S. Stable inheritance of plasmid R1 requires two different loci. J Bacteriol. 1985;161:292–298. doi: 10.1128/jb.161.1.292-298.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van den Ent F, Møller-Jensen J, Amos LA, Gerdes K, Löwe J. F-actin-like filaments formed by plasmid segregation protein ParM. EMBO J. 2002;21:6935–6943. doi: 10.1093/emboj/cdf672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang M, Bideshi DK, Park HW, Federici BA. Minireplicon from pBtoxis of Bacillus thuringiensis subsp. israelensis. Appl Environ Microbiol. 2006;72:6948–6954. doi: 10.1128/AEM.00976-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tinsley E, Khan SA. A novel FtsZ-like protein is involved in replication of the anthrax toxin-encoding pXO1 plasmid in Bacillus anthracis. J Bacteriol. 2006;188:2829–2835. doi: 10.1128/JB.188.8.2829-2835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsen RA, et al. Treadmilling of a prokaryotic tubulin-like protein, TubZ, required for plasmid stability in Bacillus thuringiensis. Genes Dev. 2007;21:1340–1352. doi: 10.1101/gad.1546107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ni L, Xu W, Kumaraswami M, Schumacher MA. Plasmid protein TubR uses a distinct mode of HTH-DNA binding and recruits the prokaryotic tubulin homolog TubZ to effect DNA partition. Proc Natl Acad Sci USA. 2010;107:11763–11768. doi: 10.1073/pnas.1003817107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aylett CHS, Wang Q, Michie KA, Amos LA, Löwe J. Filament structure of bacterial tubulin homologue TubZ. Proc Natl Acad Sci USA. 2010;107:19766–19771. doi: 10.1073/pnas.1010176107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hui MP, et al. ParA2, a Vibrio cholerae chromosome partitioning protein, forms left-handed helical filaments on DNA. Proc Natl Acad Sci USA. 2010;107:4590–4595. doi: 10.1073/pnas.0913060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orlova A, et al. The structure of bacterial ParM filaments. Nat Struct Mol Biol. 2007;14:921–926. doi: 10.1038/nsmb1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schumacher MA, Funnell BE. Structures of ParB bound to DNA reveal mechanism of partition complex formation. Nature. 2005;438:516–519. doi: 10.1038/nature04149. [DOI] [PubMed] [Google Scholar]
- 18.Møller-Jensen J, Ringgaard S, Mercogliano CP, Gerdes K, Löwe J. Structural analysis of the ParR/parC plasmid partition complex. EMBO J. 2007;26:4413–4422. doi: 10.1038/sj.emboj.7601864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schumacher MA, et al. Segrosome structure revealed by a complex of ParR with centromere DNA. Nature. 2007;450:1268–1271. doi: 10.1038/nature06392. [DOI] [PubMed] [Google Scholar]
- 20.Dam M, Gerdes K. Partitioning of plasmid R1. Ten direct repeats flanking the parA promoter constitute a centromere-like partition site parC, that expresses incompatibility. J Mol Biol. 1994;236:1289–1298. doi: 10.1016/0022-2836(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 21.Tang M, Bideshi DK, Park HW, Federici BA. Iteron-binding ORF157 and FtsZ-like ORF156 proteins encoded by pBtoxis play a role in its replication in Bacillus thuringiensis subsp. israelensis. J Bacteriol. 2007;189:8053–8058. doi: 10.1128/JB.00908-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eustermann S, et al. The DNA-binding domain of human PARP-1 interacts with DNA single-strand breaks as a monomer through its second zinc finger. J Mol Biol. 2011;407:149–170. doi: 10.1016/j.jmb.2011.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salje J, Löwe J. Bacterial actin: Architecture of the ParMRC plasmid DNA partitioning complex. EMBO J. 2008;27:2230–2238. doi: 10.1038/emboj.2008.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Heath RJ, Li Z, Rock CO, White SW. The FadR.DNA complex. Transcriptional control of fatty acid metabolism in Escherichia coli. J Biol Chem. 2001;276:17373–17379. doi: 10.1074/jbc.M100195200. [DOI] [PubMed] [Google Scholar]
- 25.Oliva MA, Martin-Galiano AJ, Sakaguchi Y, Andreu JM. Tubulin homolog TubZ in a phage-encoded partition system. Proc Natl Acad Sci USA. 2012;109:7711–7716. doi: 10.1073/pnas.1121546109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen Y, Erickson HP. In vitro assembly studies of FtsZ/tubulin-like proteins (TubZ) from Bacillus plasmids: Evidence for a capping mechanism. J Biol Chem. 2008;283:8102–8109. doi: 10.1074/jbc.M709163200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.