Abstract
Recent studies have demonstrated dramatic shifts in metabolic supply-and-demand ratios during inflammation, a process resulting in localized tissue hypoxia within inflammatory lesions (“inflammatory hypoxia”). As part of the adaptive immune response, T cells are recruited to sites of inflammatory hypoxia. Given the profound effects of hypoxia on gene regulation, we hypothesized that T-cell differentiation is controlled by hypoxia. To pursue this hypothesis, we analyzed the transcriptional consequences of ambient hypoxia (1% oxygen) on a broad panel of T-cell differentiation factors. Surprisingly, these studies revealed selective, robust induction of FoxP3, a key transcriptional regulator for regulatory T cells (Tregs). Studies of promoter binding or loss- and gain-of-function implicated hypoxia-inducible factor (HIF)-1α in inducing FoxP3. Similarly, hypoxia enhanced Treg abundance in vitro and in vivo. Finally, Treg-intrinsic HIF-1α was required for optimal Treg function and Hif1a–deficient Tregs failed to control T-cell–mediated colitis. These studies demonstrate that hypoxia is an intrinsic molecular cue that promotes FoxP3 expression, in turn eliciting potent anti-inflammatory mechanisms to limit tissue damage in conditions of reduced oxygen availability.
Keywords: lymphocyte, metabolism, TGF-beta
During inflammation, affected tissues undergo profound changes in their metabolic supply-and-demand ratios (1). This change results from an increased metabolic demand of resident and infiltrating inflammatory cells, coupled with decreased oxygen supply (2). A major consequence of this is a sharp decline in oxygen availability, resulting in inflammation-associated hypoxia (i.e., inflammatory hypoxia). Inflammatory hypoxia is found in multiple pathologies such as inflammatory bowel disease (IBD), rheumatoid arthritis, or cancer (1, 3, 4).
Hypoxia elicits an orchestrated response in cells, tissues, and the entire organism to survive a hypoxic challenge (5). On a molecular level, this response is regulated by oxygen-dependent stabilization of hypoxia-inducible factor (HIF) transcription factors HIF-1α and HIF-2α (5). In the context of inflammatory disease, hypoxia has been shown to activate multiple anti-inflammatory mechanisms (e.g., the generation of extracellular adenosine) that dampen hypoxia-associated inflammation (1, 6–12). Moreover, hypoxia-elicited tissue protective mechanisms can be targeted pharmacologically by using prolyl-hydroxylase (PHD) inhibitors, which stabilize HIFs and protect against multiple pathologies including IBD (13, 14). Indeed, a recent study found that PHD inhibitors can be used safely and efficiently in the treatment of patients with renal anemia (15).
T cells are frequently recruited to sites of inflammation, where the resulting response is regulated by multiple cues, including the local cytokine milieu, interaction with epithelial cells, endothelial cells, and dendritic cells (16–18). The resulting contribution of T cells to the propagation or constraint of the inflammatory response is tightly regulated by the relative abundance of cytokine-producing effector T cells and regulatory T cells (Tregs). Significantly, defects in recruitment, function, or survival of Tregs at sites of inflammation result in overexuberant inflammatory responses (19–21).
Given the metabolic changes that occur at sites of inflammation, T cells frequently encounter hypoxia (22, 23). Previous studies have shown that hypoxia and HIF-1α can regulate multiple facets of T-cell biology, including development, proliferation, survival, and cytokine production (e.g., IFN-γ), with Hif1a deficiency associated with overproduction of proinflammatory cytokines (24–28). Despite these seminal studies, the full consequence of hypoxic regulation and HIFs on T-cell differentiation remains an area of intense investigation (22), with recent studies identifying a role for HIF-1α in promoting both Tregs and Th17 differentiation (29–31).
To gain insights into how T cells are regulated by hypoxia on a molecular level, we performed a screen to identify which, if any, transcription factors that direct T-cell differentiation were hypoxia responsive. This analysis revealed that FoxP3—the key transcription factor for the differentiation of regulatory T cells—was robustly induced by hypoxia. Further studies showed that oxygen availability promotes Tregs through a T-cell intrinsic HIF-1α pathway, to trigger a Treg-dependent negative feedback loop to limit the deleterious effects of inflammatory hypoxia.
Results
Hypoxia Selectively Induces FoxP3 Transcription.
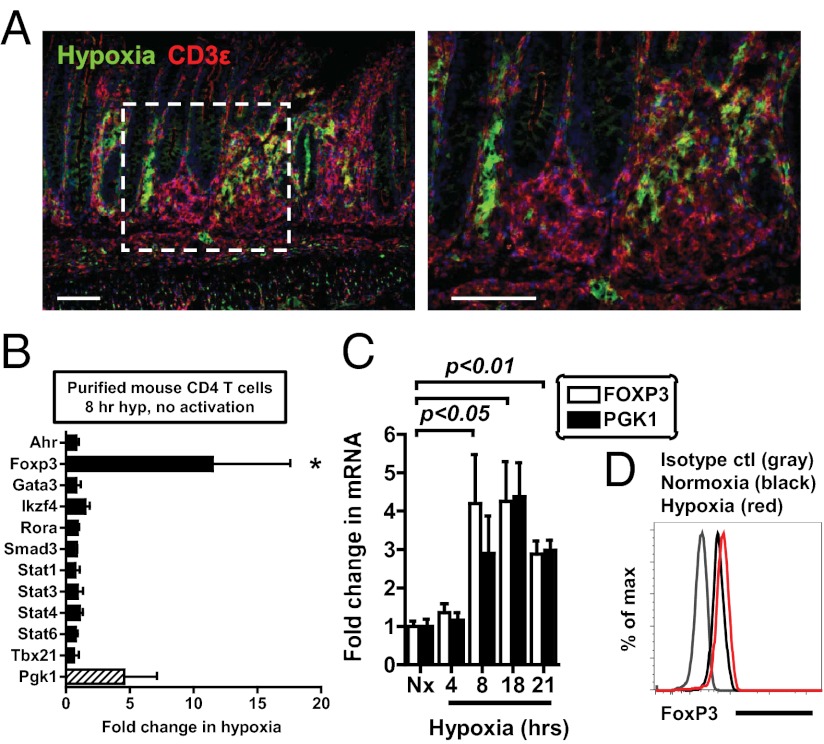
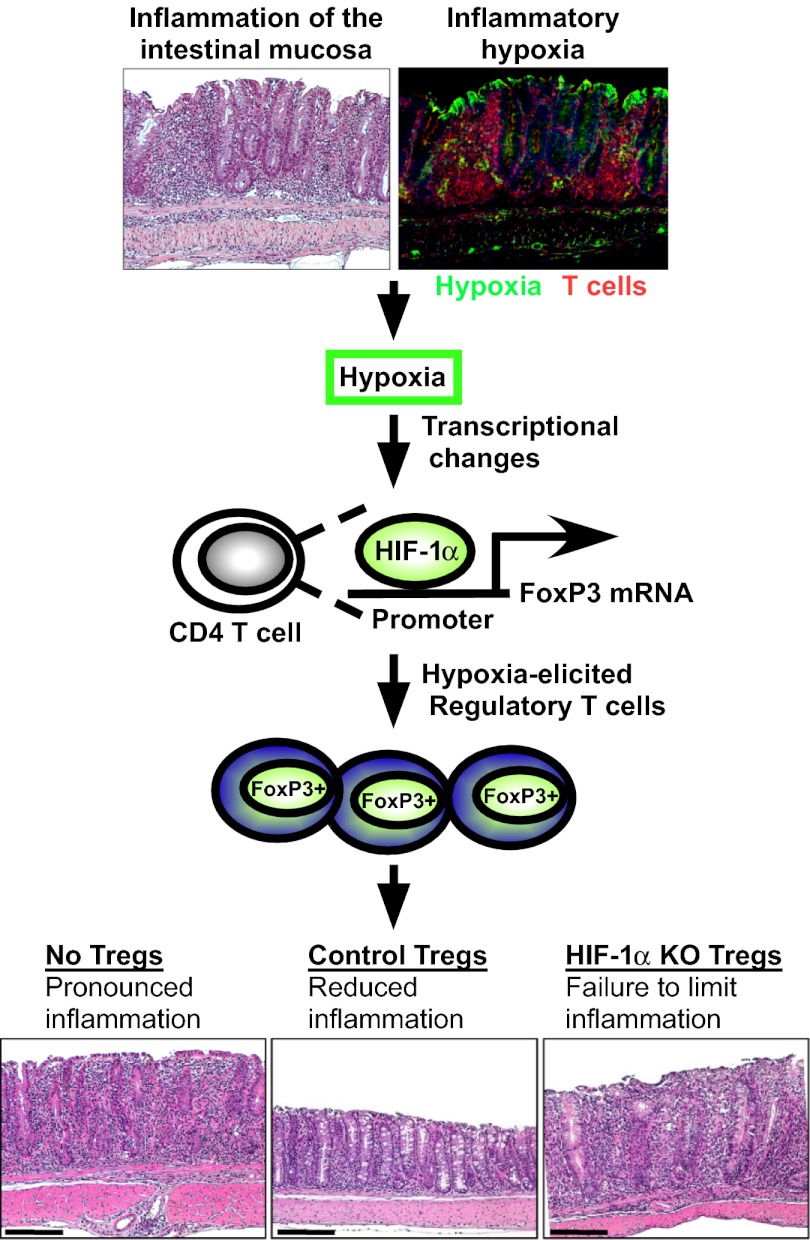
To study hypoxia as an environmental cue of the inflamed microenvironment, we used a T-cell–mediated model of colitis, in which CD45RBhigh CD4 T cells are adoptively transferred into lymphopenic Rag-deficient mice (32). As shown in Fig. 1A, mice with active colitis and robust T-cell infiltration revealed profound hypoxia-probe staining (green) in the colon, with hypoxia extending into the submucosal regions. Importantly, we observed the occurrence of CD3+ T cells (red) within hypoxic lesions, implicating low oxygen levels as an environmental stimulus for infiltrating T cells. Consistent with previous studies (1, 3), animals exposed to the colitis-inducing hapten 2,4,6-trinitrobenzenesulfonic acid (TNBS) also demonstrated profound extension of hypoxia staining from the superficial into the submucosal regions, with significant infiltration of T cells into these hypoxic microenvironments (Fig. S1). On the basis of these findings, we next tested the functional role of ambient hypoxia (1% oxygen for 8 h) on expression of a panel of transcription factors known to regulate T-cell differentiation. Strikingly, the Foxp3 gene was selectively induced 10-fold during culture of resting, primary mouse CD4 T cells under hypoxic conditions (Fig. 1B and Table S1). FOXP3 mRNA was also transcriptionally induced by hypoxia in human Jurkat cells, in parallel with a known hypoxia-inducible gene, PGK1 (Fig. 1C), and corresponding, although modest, changes in FoxP3 protein (Fig. 1D). Together, these studies indicate selective induction of FoxP3 during ambient hypoxia.
Fig. 1.
Hypoxia specifically activates FoxP3 transcription. (A) To study hypoxia as an environmental cue of the inflamed microenvironment, CD45RBhigh T-cell–mediated colitis was used as model inflammatory disease. CD3+ T cells (red) infiltrate into areas of hypoxia (green) during colitis (Left, lower magnification; Right, higher magnification of white dashed box). Shown is immunofluorescence on colonic tissue from Rag1-deficient mice that received 5 × 105 CD45RBhigh CD4 T cells 10 wk prior, with tissue sections stained with hypoxyprobe (green) and nuclear counter staining with DAPI (blue). (Scale bar, 100 μm.) (B) qPCR analysis of mRNA expression in primary mouse CD4 T cells cultured in normoxia or hypoxia for 8 h. (C) Time course of FOXP3 (white bars) and PGK1 (black bars) mRNA in Jurkat T cells, measured by qPCR. (D) Flow cytometric analysis of FoxP3 protein in Jurkats exposed to hypoxia for 27 h (red) relative to normoxia (black) and an isotype control (gray). All plots show mean ± SEM, are representative of two to four independent experiments, and include statistical significance calculated by unpaired t test or ANOVA.
Hypoxia Stimulates FoxP3 Transcription Through HIF-1α.
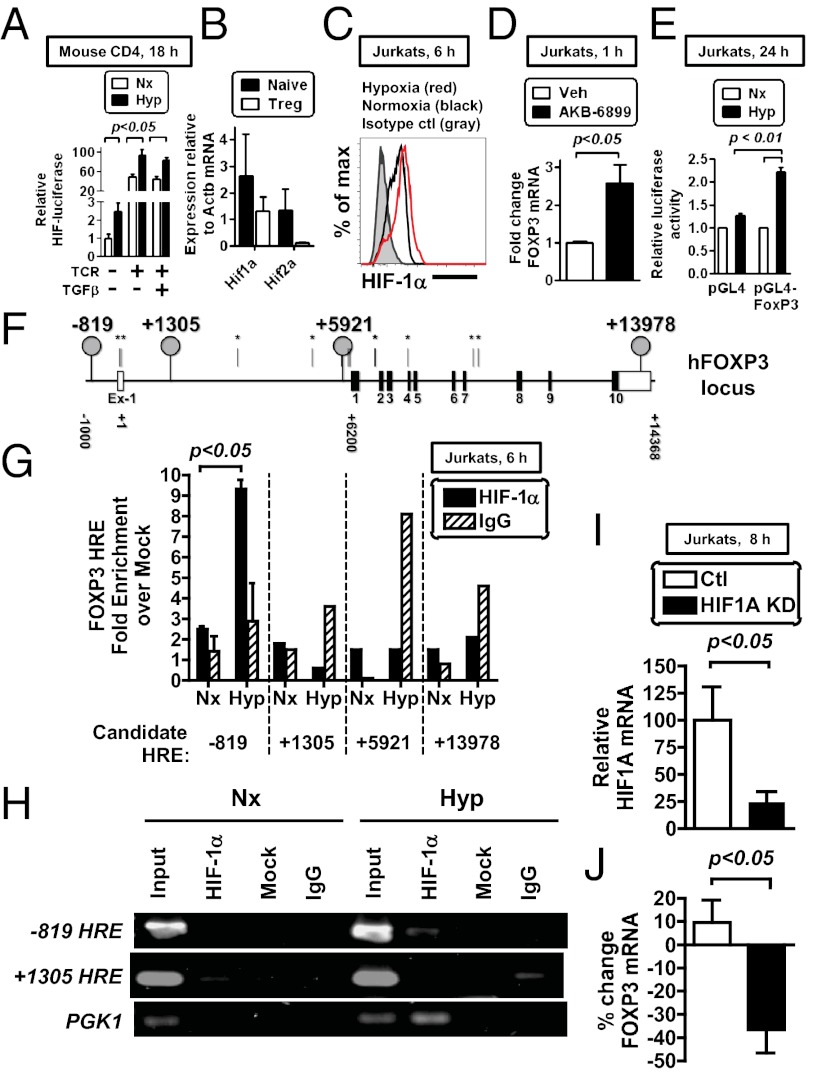
Early studies on hypoxia-inducible factor (HIF-1α) in T cells showed that HIF-1α protein was rapidly stabilized after T-cell receptor stimulation (33). To assess the relative impact of hypoxia and T-cell receptor on HIF-1α induction, we used transgenic mice that ubiquitously express a HIF-luciferase fusion protein (34). In these mice, the oxygen-dependent degradation domain of HIF-1α destabilizes luciferase, such that luciferase activity is increased only in conditions of HIF protein stabilization (such as in hypoxia) (34). When we cultured CD4 T cells from these mice in hypoxia in the absence of T-cell receptor (TCR) stimulation, hypoxia induced HIF-luciferase activity relative to normoxia (Fig. 2A). Whereas TCR stimulation increased HIF-luciferase activity relative to unstimulated cells in normoxia, hypoxia further enhanced the TCR-dependent HIF-luciferase induced in normoxia (Fig. 2A). Hypoxia also induced HIF-luciferase when cells were activated through their TCR and given exogenous TGF-β, a potent cue for Treg differentiation. These data reveal that hypoxia and TCR activation can function as independent variables influencing the magnitude of HIF induction and are consistent with previous studies (33, 35).
Fig. 2.
Hypoxia stimulates FoxP3 transcription through HIF-1α. (A) Magnetically enriched CD4 T cells from ODD-luciferase mice were cultured in either normoxia or hypoxia for 18 h, with some samples cultured with TCR stimulation (anti-CD3/CD28 beads), IL-2 ± TGF-β (0.75 ng/mL). Cells were then harvested and assessed for relative luciferase activity, with data presented as values standardized to total protein from four independent cultures. (B) mRNA expression in primary mouse naive CD4 T cells and Tregs relative to b-actin. (C) Flow cytometric analysis of HIF-1α protein in Jurkat cells in hypoxia for 6 h (red), in normoxia (black), or stained with an isotype control (gray). (D) Treatment of Jurkat cells with AKB-6899 (100 μM) for 1 h in normoxia induces FoxP3, measured by qPCR. (E) The human FOXP3 promoter is activated during hypoxia. Data show relative luciferase activity in Jurkat cells transfected either with empty pGL4.17 plasmid (left) or with a plasmid containing 900 bp of the proximal FOXP3 promoter (right), with cells cultured in normoxia (Nx) or hypoxia (Hyp) for 24 h posttransfection. Data show mean ± SEM from three independent experiments. (F) The human FOXP3 gene has many predicted HREs, shown as vertical lines (asterisks indicate sites without obvious conservation in the mouse). (G and H) HIF-1α protein binds directly to the FOXP3 promoter in Jurkat T cells cultured in hypoxia for 6 h, measured by ChIP followed by qPCR (G) or by gel electrophoresis (H), with primers designed to amplify the indicated HRE (location relative to TSS, see schematic). HIF-1α protein binding to the PGK1 promoter is a positive control. (I) Lentiviral knockdown of HIF-1α in Jurkat cells, showing HIF1A mRNA levels in control (white) or HIF-1α knockdown (black) Jurkats, measured by qPCR. (J) Percentage of change in FOXP3 mRNA in control (white) or HIF-1α knockdown (black) Jurkats relative to normoxia, measured by qPCR. Data in A depict mean ± SEM for four replicate cultures, from two independent experiments with statistical significance calculated by unpaired t test (comparing normoxia vs. hypoxia). All plots show mean ± SEM, are representative of two to three independent experiments, and include statistical significance calculated by unpaired t test.
Next, we analyzed the transcriptional pathway of FoxP3 induction. Hypoxia elicits a variety of transcriptional changes in cells, with HIF-1α and HIF-2α as two primary transcriptional mediators that regulate transcription through heterodimerization with HIF-1β and binding to hypoxia response elements (HREs) in a target gene (5). Within the FoxP3 gene, we identified multiple candidate HREs (defined as RCGTG), raising the possibility that FoxP3 is directly regulated by HIF. Given that our studies with the HIF-luciferase mice do not discriminate between oxygen-dependent regulation of HIF-1α and that of HIF-2α, we next measured the relative abundance of HIF-1α and HIF-2α in naive CD4 T cells and in Tregs. These studies revealed higher mRNA levels of HIF-1α than of HIF-2α in naive CD4 T cells (Fig. 2B) and almost complete repression of HIF-2α upon differentiation of a naive CD4 T-cell to a Treg. We therefore focused on HIF-1α, which was stabilized during hypoxic culture of Jurkat cells, a human T-cell line, before FoxP3 transcriptional induction (Fig. 2C).
Hypoxia stabilizes HIF-1α protein by inhibiting PHDs, enzymes that promote HIF-1α degradation in normoxia. HIF-1α can also be stabilized by pharmacological PHD inhibitors (8, 36, 37). To test whether HIF-1α stabilization by PHD inhibition was sufficient to transcriptionally induce FoxP3, we treated cells with the PHD inhibitor AKB-6899 and observed induction of FoxP3 mRNA. These data are consistent with direct HIF-1α activation of FoxP3 transcription independent of other hypoxia-elicited changes (Fig. 2D).
FoxP3 is subject to multiple levels of regulation. To test whether the FoxP3 promoter is itself responsive to hypoxia, we tested the ability of the FoxP3 promoter to be activated during hypoxia. Following transfection of a luciferase construct containing the human FOXP3 promoter into Jurkats, we observed that hypoxia increased luciferase activity, indicating that the FOXP3 promoter itself is hypoxia responsive (Fig. 2E). To determine whether FoxP3 was a direct transcriptional target of HIF-1α, we performed a chromatin immunoprecipitation (ChIP) analysis. Given the complex transcriptional regulation of FoxP3 (38), we performed ChIP for HIF-1α binding at 14 of the 19 putative HREs in the human FOXP3 locus. Among the 4 HREs in the human FOXP3 gene with positional orthologs in the mouse (Fig. 2F), we identified specific HIF-1α protein binding to a single consensus HRE located 819 bp 5′ of the transcriptional start site (TSS) (39) (Fig. 2 F and G). Specific HIF-1α binding to the FOXP3 HRE at −819 was further confirmed by agarose gel electrophoresis (Fig. 2H). Finally we tested the requirement for HIF-1α on FoxP3 induction by RNA interference. Stable knockdown of HIF-1α significantly impaired HIF-1α levels and the hypoxic induction of FoxP3 in Jurkats (Fig. 2 I and J). We noted a modest FoxP3 induction in this experiment, relative to that in Fig. 1C, that may be a result of culture selection posttransduction. In total, these data identify FoxP3 as a direct HIF-1α target gene.
Hypoxia Enhances the Relative Abundance of Tregs After in Vitro Activation.
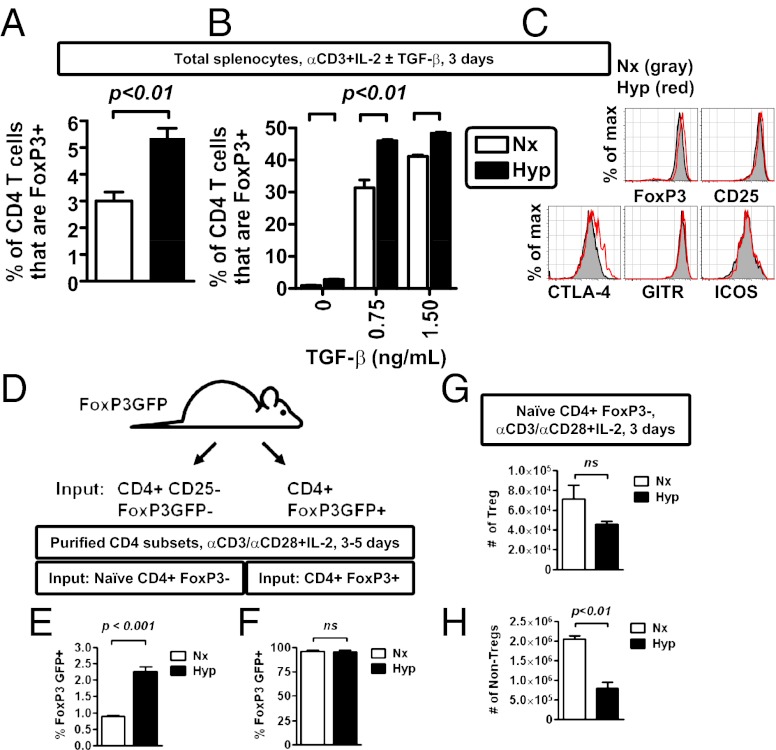
Hypoxic stimulation of FoxP3 mRNA suggested that hypoxia may enhance Treg abundance. To test this, we first analyzed Treg abundance by activating primary, murine T cells in bulk, unpurified splenocyte cultures under either normoxic or hypoxic conditions (1% ambient hypoxia). As anticipated, hypoxia increased the percentage of FoxP3+ cells after in vitro stimulation; this hypoxic enhancing effect occurred in cultures activated using either neutral (soluble anti-CD3 + IL-2) (Fig. 3A) or Treg-inducing (soluble anti-CD3 + IL-2 + TGF-β) conditions (Fig. 3B). Tregs elicited by hypoxic culture expressed multiple proteins characteristic of Tregs (Fig. 3C).
Fig. 3.
Hypoxia enhances the relative abundance of Tregs after in vitro activation. (A) Hypoxia enhances the frequency of FoxP3+ CD4 T cells after in vitro stimulation of primary mouse CD4 T cells with soluble anti-CD3 antibody (1 μg/mL) in bulk splenocyte culture, comparing cells cultured in normoxia (Nx, white bar) or hypoxia (Hyp, black bar). (B) Hypoxia enhances the frequency of FoxP3+ CD4 T cells after in vitro stimulation of primary mouse CD4 T cells with soluble anti-CD3 antibody (1 μg/mL), IL-2, and TGF-β1 in bulk splenocyte culture, comparing cells cultured in normoxia (Nx, white bar) or hypoxia (Hyp, black bar). (C) Flow cytometric analysis of protein expression within FoxP3+ CD4 T cells after 3 d of stimulation in hypoxia (red line) or normoxia (gray shading). (D) Experimental scheme to test the effect of hypoxia on the induction of FoxP3 expression in different T-cell subsets (Tregs vs. non-Tregs). (E and F) Hypoxia enhances de novo FoxP3 expression in non-Tregs (E), while having no effect on the relative abundance of existing Tregs (F). CD4 T cells fom FoxP3-GFP mice were sorted either as CD25-negative Foxp3GFP-negative (non-Tregs) or Foxp3GFP-positive cells (Tregs) and then stimulated with anti-CD3/CD28 microbeads and IL-2 for 3–5 d in either normoxia or hypoxia. Viable CD4 T cells were then analyzed for expression FoxP3 GFP by flow cytometry, with data depicting mean ± SEM for three to six replicate cultures, from two independent experiments. (G and H) Absolute cell counts for either Tregs (G) or non-Tregs (H) following stimulation of naive CD4, FoxP3− cells isolated from FoxP3GFP mice with anti-CD3/CD28 microbeads and IL-2 for 3 d in either normoxia or hypoxia. CD4 T cells were defined as viable, MHC class II negative, CD4+ events by flow cytometry, which were either FoxP3+ (Tregs, in G) or FoxP3− (non-Tregs, in H), with data depicting mean ± SEM for nine replicate cultures, from two independent experiments. All plots show mean ± SEM representative of two to four independent experiments, with flow cytometric results from a minimum of triplicate cultures (A and B), and include statistical significance calculated by unpaired t test.
One caveat to studies using bulk, unpurified splenocytes is that hypoxia may have both direct and indirect effects on CD4 T cells. To directly investigate the effects of hypoxia on the de novo generation of FoxP3-expressing cells, we sorted naive CD4+FoxP3− cells (defined as CD4+CD62LhiCD44lowCD25−FoxP3GFP−), using FoxP3-GFP reporter mice (40). In parallel, we also isolated existing Tregs (defined as CD4+ FoxP3GFP+ cells) (Fig. 3D). Isolated T cells were then subjected to in vitro stimulation in either normoxia or hypoxia. This analysis revealed that hypoxia enhanced the relative proportion of de novo-generated FoxP3+ T cells in vitro, while having little effect on the relative proportion of preexisting Tregs (Fig. 3 E and F). These data indicate that hypoxia increases the relative abundance of inducible Tregs during in vitro activation and differentiation of primary, naive CD4 T cells. In total, the above studies demonstrate that hypoxic culture is uniformly associated with a relative increase of FoxP3+ events among CD4+ T cells and that hypoxia can mediate this effect by directly regulating CD4 T cells.
Hypoxia Influences Proliferation and the Relative Abundance of Tregs After in Vitro Culture.
Hypoxia is known to influence rates of both proliferation and survival (22, 26), and hypoxic cultures routinely had reduced cellularity relative to normoxic cultures. Despite the increased frequency of de novo Tregs following hypoxic culture (Fig. 3E), when we calculated total cellularity for both Tregs and non-Tregs, we found that hypoxic cultures had Treg numbers that were relatively comparable to levels observed in normoxia (Fig. 3G). In contrast, hypoxic cultures had a reduced number of non-Tregs (defined as CD4+ FoxP3− cells) (Fig. 3H). Similar deficits in non-Treg abundance following hypoxic culture were also observed, using total splenocytes stimulated with soluble anti-CD3, IL-2, and TGF-β (Fig. S2). The reduction in cellularity and non-Tregs under hypoxic conditions may result from reduced proliferation in hypoxia, especially among non-Tregs (Fig. S2). On the basis of these data, in vitro cultures of activated T cells under hypoxic conditions are associated with a relative sparing of Tregs compared with non-Tregs, in turn resulting in an increased percentage of FoxP3+ cells among CD4 T cells. These results indicate that in vitro culture of activated T cells under hypoxic conditions enhances the proportion of Tregs relative to non-Tregs.
Hypoxia Enhances the Relative Treg Abundance in Vitro by a TGF-β–Dependent Mechanism.
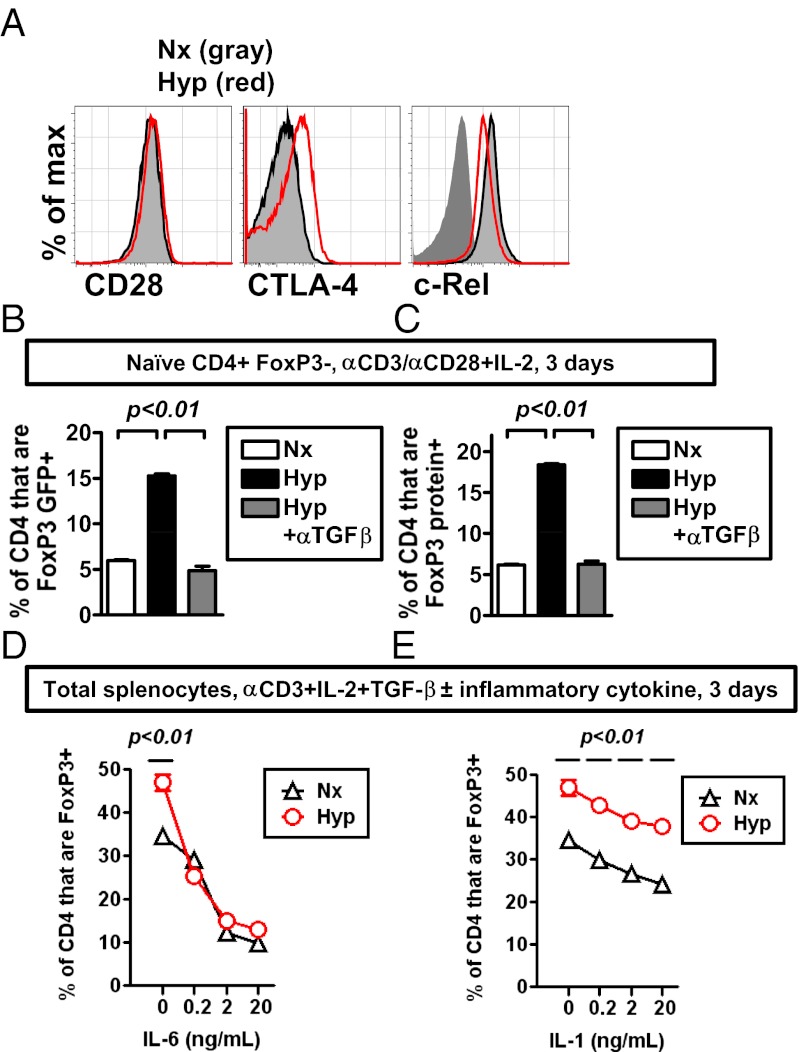
FoxP3 expression is known to be regulated by multiple factors, including TCR signaling, costimulation, NF-κB, and TGF-β (41). When we examined expression of costimulatory proteins (CD28 and its negatively regulating counterpart CTLA-4) and the NF-κB family member c-Rel, we found that hypoxic cultures had comparable expression levels relative to normoxic cultures (Fig. 4A). On the basis of these expression levels, hypoxic induction of FoxP3 does not appear to be due to alterations in costimulatory molecules or through enhanced expression of c-Rel.
Fig. 4.
Hypoxia enhances the relative abundance of Tregs in vitro by a TGF-β–dependent mechanism. (A) Flow cytometric analysis of CD28, CTLA-4 (cell surface) within viable CD4 FoxP3+ T cells, or c-Rel within viable CD4 T cells following culture for 3–5 d in either normoxia (black line with gray shading) or hypoxia (red line). Analysis of c-Rel includes an isotype control stain (dark gray). Results are representative of three independent experiments. (B and C) Hypoxic induction of FoxP3 mRNA and protein in vitro is TGF-β dependent. Sorted naive, FoxP3− CD4 T cells from FoxP3-GFP mice were stimulated in normoxia (white bars) or hypoxia (black or gray bars) for 3 d, with or without a blocking TGF-β antibody (αTGF-β, 10 μg/mL). Relative abundance of FoxP3 expression was measured by GFP fluorescence (B, where GFP expression is under control of an internal ribosome entry site linked to the FoxP3 gene) or by antibody detection of FoxP3 protein by flow cytometry (C). Data depict the percentage of CD4+ events that are either GFP+ (B) or FoxP3+ (C), where all CD4 T cells were pregated on viable, MHC class II negative events. (D and E) Hypoxic enhancement of Tregs by TGF-β and the effect of inflammatory cytokines on this induction. Bulk splenocytes were cultured with soluble anti-CD3 antibody (1 μg/mL), IL-2 (10 ng/mL), and TGF-β1 (0.75 ng/mL), with or without a series of dilutions of either IL-6 (G) or IL-1 (H). The frequency of FoxP3+ events among CD4+ T cells was measured by flow cytometry after 3 d of stimulation in hypoxia (Hyp) or normoxia (Nx). All plots show mean ± SEM representative of two to three independent experiments, with flow cytometric results from triplicate cultures (B–E), and include statistical significance calculated by unpaired t test or ANOVA.
Previous studies have shown a central role for TGF-β as a regulator for FoxP3 (42). The fact that TGF-β is a known HIF target gene introduces the possibility that hypoxia induction of Tregs involves a coordinated response involving HIF and TGF-β (43, 44). To test the role of TGF-β in the hypoxic induction of FoxP3-expressing cells, we used FoxP3 GFP reporter mice, in which GFP expression is directed by an internal ribosome entry site inserted within the endogenous Foxp3 locus, such that GFP expression corresponds directly to FoxP3 mRNA abundance (40). Following culture, we measured both GFP expression (as a measure of FoxP3 mRNA) and endogenous FoxP3 protein. Notably, TGF-β neutralization significantly attenuated hypoxia-dependent enhancement of both FoxP3 mRNA-expressing cells (measured by GFP expression) and FoxP3 protein-expressing cells following in vitro T-cell activation (Fig. 4 B and C). Together, these studies indicate that the in vitro hypoxic enhancement of Tregs requires TGF-β signaling for full hypoxic induction of FoxP3 mRNA and protein following T-cell activation and differentiation.
Hypoxia Enhances the Relative Abundance of Tregs in the Presence of IL-1 but Not IL-6.
Given the frequent overlap of inflammation and hypoxia, we tested whether the hypoxic enhancement of Tregs also occurred in the context of the inflammatory cytokines IL-6 and IL-1. When anti-CD3–stimulated bulk splenocytes were cultured with TGF-β, hypoxia enhanced the proportion of Tregs in culture (Fig. 4 D and E). Addition of increasing amounts of IL-6, a known antagonist of FoxP3 expression (45, 46), precipitously decreased the relative abundance of Tregs in both normoxia and hypoxia, such that the hypoxic enhancement of Treg abundance was lost with addition of IL-6 (Fig. 4D). In contrast, increasing amounts of IL-1 resulted in a modest reduction in the overall percentage of Tregs present in normoxia (Fig. 4E). Notably, hypoxic cultures retained a relative increase in the percentage of FoxP3+ T cells in the presence of IL-1 (Fig. 4E). In total, these data indicate that T-cell activation under hypoxic conditions can enhance the relative abundance of Tregs not only under neutral and Treg-promoting conditions, but also under select inflammatory conditions, dependent on the specific inflammatory milieu.
Hypoxia Is Not Sufficient to Induce de Novo Th17 Differentiation in the Absence of Th17 Differentiation Cues.
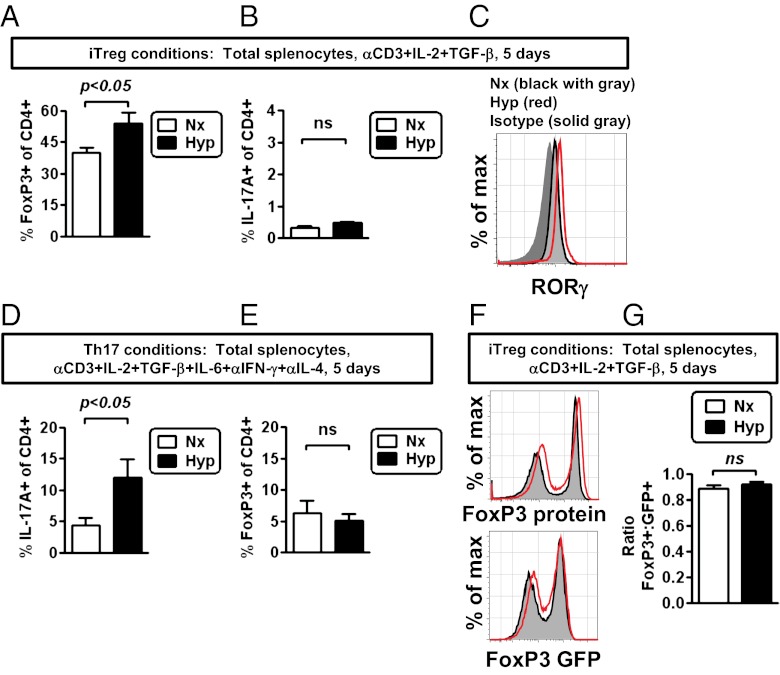
Recent studies have shown that HIF-1α is critical for Th17 differentiation in vitro and in vivo (30, 31). To test the impact of hypoxia on Th17 differentiation, relative to its effect on enhancing Treg abundance, we analyzed in vitro Treg and Th17 differentiation under normoxic and hypoxic conditions. When T cells were cultured under Treg differentiation conditions (IL-2, TGF-β), we found that hypoxia enhanced the differentiation of Tregs (Fig. 5A). In contrast, hypoxia had neglible effects on the induction of Th17 cells under Treg differentiation conditions (Fig. 5B). Consistent with a failure of hypoxia to induce Th17 differentiation using Treg differentiation conditions, hypoxia did not influence the expression of RORγ, a critical Th17 transcription factor, following hypoxic culture (Fig. 5C).
Fig. 5.
Hypoxia induces Tregs, not Th17 cells, under Treg differentiation conditions. (A and B) Hypoxia enhances the frequency of FoxP3+ CD4 T cells after in vitro stimulation of CD4 T cells in bulk splenocyte culture, with soluble anti-CD3 antibody (1 μg/mL), IL-2, and TGF-β1, comparing cells cultured in normoxia (Nx, white bar) or hypoxia (Hyp, black bar). Total splenocytes were cultured in normoxia (white bar) or hypoxia (black bar), using Treg differentiation conditions for 5 d, at which time cells were restimulated with PMA, ionomycin, and monensin for 5 h. Cells were then stained for FoxP3 and IL-17A expression, with data depicting the percentage of viable, activated CD4 T cells that express either FoxP3 (A) or IL-17A (B). (C) Flow cytometric analysis of RORγ expression within viable CD4 T cells, comparing relative expression in normoxic (black line) or hypoxic (red line) cultures relative to an isotype control stain (gray area). Results are representative of two independent experiments. (D and E) Hypoxia enhances differentiation of IL-17A+ cells after in vitro stimulation of CD4 T cells in bulk splenocyte culture, containing Th17-inducing conditions. Total splenocytes were cultured in normoxia (white bar) or hypoxia (black bar) under Th17 differentiation conditions for 5 d, at which time cells were restimulated with PMA, ionomycin, and monensin for 5 h. Cells were then stained for FoxP3 and IL-17A expression, with data depicting the percentage of viable, activated CD4 T cells that express either IL-17A (D) or FoxP3 (E). (F) Flow cytometric analysis of FoxP3 protein expression (Upper) or GFP expression (Lower) (a transcriptional reporter for FoxP3 expression) from FoxP3-GFP cells, following 5 d of stimulation in hypoxia (red line) or normoxia (gray area). (G) Ratio comparing the frequency of FoxP3 protein-expressing cells relative to the frequency of GFP-positive cells, using FoxP3-GFP transcriptional reporter mice, based on flow cytometric data as shown in F. Results are representative of two independent experiments. In A, B, and D, data depict mean ± SEM for four to six replicate cultures from two independent experiments and include statistical significance calculated by unpaired t test (comparing normoxia vs. hypoxia).
Hypoxia Enhances Th17 Differentiation in the Presence of Th17 Differentiation Cues.
Whereas Th17 differentiation was not enhanced under Treg differentiation conditions, we next tested what consequence hypoxia had on Th17 differentiation in the presence of Th17 differentiating cytokines (TGF-β and IL-6, plus antibodies against IFN-γ and IL-4). When T cells were cultured in hypoxia under Th17 differentiation conditions, we found that hypoxia enhanced the differentiation of Th17 cells (Fig. 5D). In these same conditions, hypoxia neither significantly enhanced nor repressed the relative abundance of Tregs (Fig. 5E). These data indicate that hypoxia can enhance Th17 differentiation, given the presence of optimal Th17 differentiation cytokines.
Hypoxia Does Not Result in FoxP3 Protein Degradation Under Treg Differentiation Conditions.
In addition to showing data that HIF-1α is important for Th17 differentiation, Dang et al. showed that HIF-1α may actually reduce FoxP3 expression through proteasomal degradation (31). Although we had not routinely seen an overall reduction in FoxP3 protein expression on an individual cell basis (Fig. 3C), we tested whether hypoxia might result in a significant discordance in the frequency of FoxP3 mRNA-expressing cells relative to FoxP3 protein-expressing cells. To do this, we again made use of FoxP3 GFP reporter mice, in which GFP serves as a transcriptional reporter for FoxP3 mRNA (40). In these mice, GFP expression serves as a transcriptional reporter of FoxP3 mRNA, given the placement of the GFP gene downstream of an internal ribosomal entry site within the endogenous Foxp3 locus. Next, we measured the frequency of GFP-expressing cells (i.e., FoxP3 mRNA-expressing cells from the FoxP3-GFP reporter mice) relative to the frequency of FoxP3 protein-expressing cells, by direct detection of FoxP3 protein (Fig. 5F). There was a tight correlation between the frequency of GFP+ and FoxP3+ cells in both normoxia and hypoxia (Fig. 5G), indicating that almost all cells that expressed FoxP3 mRNA (detected here by GFP) also expressed FoxP3 protein. In addition, the relative expression levels of GFP and FoxP3 protein on a per cell basis were similar between normoxia and hypoxia. The strong correlation between a transcriptional reporter and the endogenous protein, in combination with the normal FoxP3 expression in hypoxia (Fig. 3F), argues that hypoxia per se is not sufficient to induce proteasomal degradation of FoxP3 protein (31).
Hypoxia and HIF Signaling Enhance Treg Abundance in Vivo.
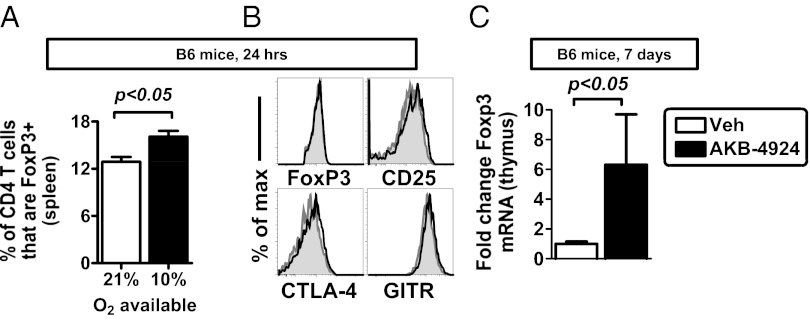
To examine the role of tissue hypoxia on FoxP3 induction and Treg abundance in vivo, we first used murine whole-body hypoxia. As predicted from our in vitro studies, we observed an increased percentage of Tregs in hypoxia-treated animals (Fig. 6A) (10% oxygen for 24 h). These Tregs had comparable protein expression profiles to those of Tregs in control mice (Fig. 6B). Next, we examined the consequence of pharmacologic HIF-1α stabilization on Treg abundance in vivo. Mice treated with a PHD inhibitor had increased FoxP3 mRNA in the thymus, although FoxP3 mRNA was not increased in the spleen (Fig. 6C and Fig. S3). These data indicate that whole-body hypoxia or pharmacologic HIF-1α stabilization can enhance FoxP3 expression and Treg abundance in vivo.
Fig. 6.
Hypoxia and HIF signaling enhance Treg abundance in vivo. (A) Whole-body hypoxia (10% O2) increases Treg abundance in the spleen after 24 h. Data show mean ± SEM, indicating percentage of live CD4 T cells that are FoxP3+, n = 4–5 mice per group, with data representative of two independent experiments and analyzed by unpaired t test. (B) Phenotype of Tregs after whole-body hypoxia, with histogram overlays comparing protein expression in Tregs in 21% (gray) or 10% O2 availability (black). (C) Treatment of mice with a PHD inhibitor increases FoxP3 mRNA expression in the thymus, measured by qPCR in thymus, in B6 mice treated with vehicle (control) or with AKB-4924. Data show mean ± SEM, n = 5 mice per group, representative of two independent experiments. Statistically significant differences are indicated, calculated by unpaired t test.
T-Cell Intrinsic HIF-1α Is Required for Optimal Treg Suppressive Function.
Whereas our studies to this point focused on the contribution of hypoxia on Tregs, we next sought to test the genetic contribution of T-cell–intrinsic HIF-1α within Tregs. To do this, we isolated Tregs from mice containing a specific deletion of Hif1a (Hif1a flox/flox LckCre mice) and compared them to control Tregs. Hif1a–deficient animals had comparable numbers of Tregs to those of their littermate controls and Tregs isolated from these animals were of equivalent purity, with comparable protein expression among common Treg markers (Fig. S4).
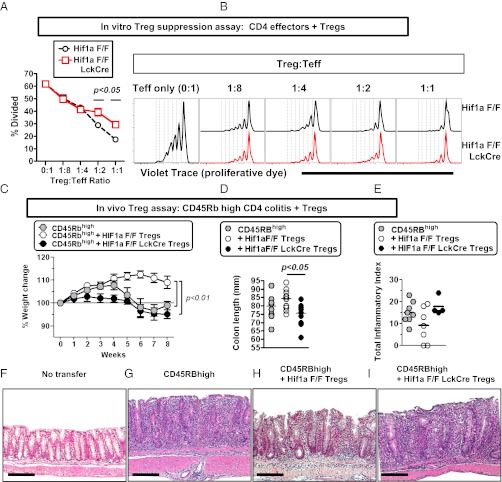
First, we tested the suppressive capacity of Hif1a-deficient Tregs under conditions of normoxia and hypoxia, by using a well-established in vitro suppression assay for Treg function. When we tested the relative ability of Hif1a-deficient Tregs to limit proliferation of violet trace-labeled CD4 T cells, we found that Hif1a-deficient Tregs had a significant defect in their suppressive function (Fig. 7 A and B). This deficit in suppressive activity of Hif1a–deficient Tregs was observed in conditions of robust T-cell proliferation (normoxia); in contrast, Hif1a–deficient Tregs were able to mediate more modest suppressive effects required to limit reduced effector T-cell proliferation in conditions of hypoxia (Fig. S4). These data indicate that HIF-1α plays a critical role in promoting optimal suppressive function of Tregs in vitro.
Fig. 7.
Treg-intrinsic HIF-1α is required for optimal Treg suppressive function in vitro and in vivo. (A and B) HIF-1α is required for optimal suppressive function of regulatory T cells in normoxia. Shown is in vitro Treg suppression assay using different ratios of CD4+ CD25+ T cells from Hif1a flox/flox (F/F) or Hif1a flox/flox LckCre+ mice cocultured with Violet Trace-labeled CD4+ CD25− T cells from Hif1a flox/flox mice, irradiated APCs, and soluble anti-CD3 (1 μg/mL). T-cell proliferation was assessed 4 d poststimulation, with data obtained from four independent cultures, with cells isolated from two independent mice. Data depict (A) the percentage of CD4+ CD25− T cells that have undergone at least one round of division, showing mean ± SEM for four independent cultures, and (B) proliferation of CD4+ CD25− T cells as measured by Violet Trace dilution at multiple different ratios of effector T cells to regulatory T cells, with control Treg cultures plotted in black (upper) and Hif1a–deficient Treg cultures plotted in red (lower). Statistically significant differences are indicated, calculated by unpaired t test. (C–I) Rag1−/− mice were adoptively transferred with CD4+ CD45RBhigh cells with or without CD4+ CD25+ Tregs from control mice or mice deficient in Hif1a in T cells (Hif1a F/F LckCre). Progression to colitis was monitored weekly by weight loss (C) or at the time of harvest by measuring colon length (D) or by histological scoring (E). Examples of histology in each of the groups are provided in F–I, corresponding to mice without T-cell transfer (F) or receiving CD45RBhigh cells without Tregs (G), CD45RBhigh cells + control Tregs (H), or CD45RBhigh cells + Hif1a–deficient Tregs (I). Data indicate mean ± SEM (n = 9–13 mice per group), from three independent experiments except for E, which shows data from n = 4–8 mice per group from two independent experiments. For Hif1a genotype, F refers to floxed. (Scale bars in F–I, 200 μm.) Statistically significant differences are indicated, calculated by unpaired t test or by ANOVA with Tukey’s posttest correction.
HIF-1α Is Required Within Tregs to Suppress T-Cell–Mediated Colitis.
Given the potential role of HIF-1α in regulating both effector T-cell and regulatory T-cell function, we next tested the Treg-intrinsic requirement for HIF-1α for limiting inflammation in vivo. To do this, we used the CD4 T-cell CD45RBhigh adoptive transfer model of colitis into Rag1-deficient mice, a model previously shown to be driven by pathogenic effector T cells (32), and a disease process that can be controlled by the adoptive transfer of regulatory T cells (47). To specifically isolate the genetic contribution of HIF-1α only to Tregs, control (Hif1a F/F) Tregs or Hif1a–deficient (Hif1a F/F LckCre) Tregs were cotransferred with wild-type CD45RBhigh CD4 T cells at a physiologically relevant 1:4 ratio of Treg:CD45RBhigh CD4 T cells. Whereas control Tregs limited onset of colitis, as revealed by protection against weight loss (Fig. 7C), protection against colon shortening (Fig. 7D), and protection against histological damage (Fig. 7 E–I), mice receiving Hif1a–deficient Tregs failed to limit colitis as evidenced by failure to protect against weight less, decreased colon lengths, and a trend toward worse histological damage. In total, these data indicate that Treg-intrinsic HIF-1α is required for optimal Treg suppressive function in vivo, particularly in the context of inflammatory bowel disease.
Discussion
Hypoxia is a common occurrence in a variety of inflammatory and pathological conditions and has long been appreciated to induce adaptive responses at the cellular, tissue, and organismal levels (1, 5). These hypoxia-elicited changes in gene expression elicit adaptive responses that promote adaptation to limited oxygen availability. Particularly during inflammatory hypoxia, HIF-dependent transcription can activate multiple anti-inflammatory mechanisms (13, 14, 22, 48). One notable example of this is the coordinated induction of the extracellular adenosine pathway (7), a pathway that in the context of T cells can both promote Treg differentiation and function as a Treg effector mechanism (9, 49). Here, we hypothesized that hypoxia-elicited transcription could directly influence T-cell differentiation and function. To test this, we screened key transcription factors for T-cell differentiation and observed a selective and robust induction of FoxP3 with hypoxia. We further present data that FoxP3 is a direct HIF-1α target gene, that hypoxia through HIF-1α promotes the abundance and function of Tregs in vitro and in vivo, and that Treg-intrinsic HIF-1α is critical in constraining inflammation. In total, our data indicate this pathway is important in limiting tissue inflammation during inflammatory hypoxia, particularly during intestinal inflammation.
The molecular regulation of FoxP3 is coordinated through multiple levels of positive and negative regulation. Although FoxP3 expression is enhanced by costimulatory molecules (e.g., CD28) and TGF-β (42, 50), expression can be restricted by methylation and by strong T-cell receptor signaling that activates the PI3 kinase pathway (41, 51, 52). Significantly, these distinct developmental and microenvironmental cues coordinate Foxp3 expression through multiple, conserved transcriptional elements in the Foxp3 locus, work elegantly revealed through targeted deletion of individual, conserved enhancer elements (38). Within this larger context, our data indicate that hypoxia promotes FoxP3 and regulatory T-cell function through at least three distinct mechanisms: (i) direct transcriptional activation of FoxP3 mRNA by HIF-1α early after hypoxia, (ii) a hypoxia-driven, TGF-β–dependent process required for full hypoxic induction of FoxP3 mRNA and protein following T-cell activation and differentiation, and (iii) a Treg-intrinsic role for HIF-1α that is required for optimal suppressive function of Tregs in vitro and in vivo. Given the inhibitory effects of hypoxia on proliferation and survival, hypoxia is also associated with an enhanced relative abundance of Tregs in comparison with non-Tregs; we have observed the enhanced relative abundance of FoxP3+ cells under hypoxic conditions both in vitro and in vivo, demonstrating that hypoxia generally enhances the relative abundance of Tregs. In this context, it is possible that hypoxic microenvironments arising during inflammation may function as foci that trigger a cascade of inhibitory mechanisms, characterized by an increased ratio of inhibitory to effector T cells and the activation of additional anti-inflammatory pathways, such as the generation of extracellular adenosine, an inhibitory mechanism used by Tregs (1, 7, 9). The multilayer impact of hypoxia and HIF-1α on Tregs, in both hypoxia and normoxia, is consistent with previous reports demonstrating the role of hypoxia on T-cell function and of HIF-1α–dependent regulation of T cells in both hypoxia and normoxia (22, 30, 31).
Early studies of HIF-1α in T cells led to the concept that a primary function of hypoxia and HIF-1α in T cells is to constrain T-cell–mediated inflammation, a concept pioneered by Sitkovsky (28, 53). Since then, the role of HIF-1α in T-cell differentiation has been the subject of increasing investigation, with different groups finding divergent roles for HIF-1α. Our data, demonstrating that hypoxia, via HIF-1α, promotes regulatory T cells is consistent with a previous report by Ben-Shoshan et al., in which they identified HIF-1α–dependent hypoxic induction of FoxP3 in Jurkat T cells (29). The authors showed that hypoxia increased FoxP3 expression in mouse splenocytes and human peripheral blood mononuclear cells and that transfection of HIF-1α in mouse cells resulted in increased FoxP3 expression in vitro and in vivo (29). Although this paper identified hypoxic induction of FoxP3-expressing cells, the in vivo significance of this pathway, in terms of both pharmacologic manipulation and loss-of-function, was unknown. Our study has further investigated the hypoxic regulation of FoxP3, to demonstrate that HIF-1α binds directly to the FOXP3 promoter, that hypoxic induction of FoxP3 requires both HIF-1α and TGF-β, and that loss of Hif1a within Tregs results in a Treg-intrinsic deficit in controlling intestinal inflammation. Whereas Hif1a–deficient Tregs are not completely devoid of suppressive function, our in vitro and in vivo studies demonstrate that HIF-1α within Tregs is required for optimal Treg function. These observations are important because they provide genetic evidence that HIF-1α has a Treg-intrinsic role in limiting inflammation. Beyond this study, it is intriguing to note that recent data describing a hypomorphic FoxP3 allele (encoded by a FoxP3-GFP fusion protein) demonstrated that this fusion protein had a reduced interaction with HIF-1α, an enhanced interaction with IRF4, and selective deficits in Treg function (54).
In contrast to data indicating that hypoxia and HIF-1α promote Tregs, two papers recently reported that HIF-1α is essential for the generation of proinflammatory Th17 cells in vitro and in vivo (30, 31). Further, the authors concluded that HIF-1α promotes Th17 differentiation and limits Treg differentiation, identifying HIF-1α as a central regulator of the Treg/Th17 balance. This conclusion was based on the observation that deletion of Hif1a impaired Th17 differentiation while enhancing generation of Tregs (30, 31). Although the precise molecular basis for this observation was different between the two papers, both groups showed that HIF-1α was required for induction of experimental autoimmune encephalitis, a Th17-driven model of autoimmunity in vivo.
In the study by Shi et al., the authors focused on how T-cell differentiation and glycolysis were regulated under normoxic conditions (30). First, they demonstrated that in vitro Th17 differentiation was associated with HIF-1α–dependent induction of glycolytic activity. The authors then showed that Hif1a–deficient T cells were impaired in becoming Th17 cells in vitro and in vivo and instead were more efficient at becoming Tregs in Th17-inducing conditions. This altered differentiation appeared to result from increased FoxP3 expression coupled with impaired expression of the IL-23 receptor, a cytokine receptor important for expansion and survival of Th17 cells (30, 55). Importantly, these studies were uniformly done in the context of normoxia. In contrast to their findings, we did not observe an increase of normoxic FoxP3 levels in Hif1a–deficient Tregs. Consistent with previous studies, we found that HIF-1α increases FoxP3 mRNA and Treg abundance.
The role of HIF-1α in Th17 differentiation was also the focus of a study by Dang et al. In this paper, the authors show that HIF-1α influences the Th17/Treg balance (i) by the induction of RORγt and subsequent transactivation of Th17-associated genes, (ii) while limiting FoxP3 protein through HIF-1α–mediated protein degradation (31). Beyond showing that HIF-1α is necessary and sufficient for Th17 differentiation in vitro, the authors also showed that hypoxia, which will induce HIF-1α, enhanced Th17 differentiation, while decreasing FoxP3 protein through a proteasome-dependent mechanism of degradation.
To understand the apparent differences between our study and those above showing a critical role for HIF-1α in Th17 cells, we tested the role of hypoxia on the generation of Tregs and Th17 cells. Consistent with Dang et al., we found that culturing T cells in Th17-inducing conditions in hypoxia enhances Th17 cell generation. However, when we cultured T cells in Treg-inducing culture conditions, we found that hypoxia is completely unable to induce Th17 differentiation and instead enhances Treg differentiation. Also, in contrast to Dang et al., we found no evidence of reduced FoxP3 protein within Tregs cultured in hypoxia, using a state-of-the-art reporter mouse that allows us to investigate mRNA and protein expression analyses by flow cytometry. Previous studies had shown that hypoxia—as occurs during conditions of intestinal inflammation (1)—induces TGF-β (43), thereby setting up a microenviroment that strongly favors Treg differentiation. Indeed, although HIF-1α may play a prominent role in Th17-mediated autoimmune diseases (such as experimental autoimmune encephalitis) (30, 31), when we directly tested the role of Treg-intrinsic Hif1a in vivo, we found that Hif1a–deficient Tregs fail to limit T-cell–mediated colitis, demonstrating that HIF-1α plays a Treg-intrinsic role in limiting inflammation.
Although Th17 cells may use HIF-1α to promote their metabolic and differentiation requirements, it is important to note that Th17 cells are not unique in their expression of HIF-1α. Early studies by Lukashev et al. clearly demonstrated that HIF-1α protein is stabilized following T-cell receptor engagement (33), results we have corroborated here. In addition, although Dang et al. found that Th17 cells had the highest levels of HIF-1α mRNA, inducible Tregs also had elevated levels of HIF-1α relative to naive CD4 T cells, levels much higher than in either Th1 cells or Th2 cells (31). Furthermore, TGF-β treatment of activated CD4 T cells stabilized HIF-1α protein, albeit to a lesser extent than that observed in Th17 culture conditions (31).
Whereas HIF-1α may facilitate divergent outcomes of differentiation, recent studies on HIF-1α in T cells have surprisingly overlooked hypoxia-induced TGF-β signaling as a common mechanism underlying the effects of hypoxia on CD4 T cells. TGF-β is known to be a downstream effector of hypoxia: TGF-β is induced at the mRNA level in hypoxia, and the mature TGF-β protein is induced through posttranslational mechanisms, including hypoxic induction of furin mRNA and furin protein relocalization to the cell membrane to cleave cell surface-bound TGF-β (43, 44, 56). Further, TGF-β itself can enhance HIF-1α expression through inhibition of PHD2, a critical negative regulator of HIF-1α protein stability (57).
Given that TGF-β promotes Tregs and Th17 cells, and the decision between a Treg and a Th17 cell is influenced both by the relative amount of TGF-β and by the presence of inflammatory cytokines (e.g., IL-6 and IL-1 promote Th17 differentiation, limiting Tregs) (58), we postulate that the overall inductive role of hypoxia on T-cell differentiation will be an integration of HIF-1α, TGF-β, and the surrounding cytokine environment. The use of hypoxia and HIF-1α to enhance both Tregs and Th17 cells is particularly fascinating given that both of these cell types play integral parts in maintaining tissue homeostasis within the gut (59, 60), a site that is characterized by hypoxia (both basally and during inflammation) (1, 3).
On the basis of these insights, we postulate that HIF-1α is involved in both Treg and Th17 differentiation. Indeed, the observation that Tregs may use a transcriptional regulator expressed in proinflammatory T cells is well established in the literature: Tregs use Stat3, a key transcription factor required for the generation of Th17 cells, to limit Th17-mediated inflammation in the gastrointestinal tract (61), an observation made possible only through genetic ablation of Stat3 specifically within Tregs. Beyond this seminal study, Treg-intrinsic roles for transcriptional regulators initially defined in effector CD4 T cells have now been observed for multiple transcription factors, including IRF4, T-bet, Blimp-1, GATA3, Bcl-6, and c-Rel (62–69). On the basis of this expanding literature, we hypothesize that HIF-1α regulates multiple T-cell fates; although some of these effects might be elicited by strong metabolic demands (e.g., induced by robust proliferation or inflammatory cytokines like IL-6 in the context of Th17 differentiation), other effects might be elicited by unique microenvironmental cues (e.g., hypoxia driving Treg generation).
Taken together, our data indicate that oxygen availability can function as a local cue that influences T-cell differentiation, with a prominent outcome being enhanced Treg abundance through HIF-1α–dependent regulation of FoxP3. Our data further indicate that manipulating HIF signaling has the potential to limit inflammation at both a local and a systemic level, offering unique opportunities to shape the outcome of diseases characterized by pathologic inflammation. Future challenges will include the translation of our findings from studies in mice or in culture systems of human immune cells into the treatment of patients. However, it is of significance to point out that pharmacologic strategies to induce HIF stabilization have recently been tested in patients (15), thereby setting the stage to use PHD inhibitors to treat patients suffering from inflammatory hypoxia, such as occurs in the setting of inflammatory bowel disease.
Materials and Methods
Mice.
C57BL/6J, B6.Rag1−/−, Hif1a-flox/flox, LckCre, ODD-luciferase, Foxp3-GFP, and Ubc-GFP mice were obtained from Jackson Laboratory. Hif1a-flox/flox × LckCre mice were F2 progeny of Hif1a-flox/flox and LckCre mice. All experiments were approved by the Institutional Animal Care and Use Committee of the University of Colorado Denver, in accordance with the National Institutes of Health guidelines for use of live animals. The University of Colorado Denver, Anschutz Medical Campus is accredited by the American Association for Accreditation of Laboratory Animal Care. Further details are in SI Materials and Methods.
T-Cell Purification and Culture.
T cells were purified by magnetic bead enrichment and FACS, with naive CD4 T cells defined as CD62Lhigh CD44low. Bulk splenocytes were stimulated with 1 μg/mL soluble anti-CD3ε (145-2C11; eBioscience) or naive CD4 T cells were stimulated with anti-CD3/anti-CD28 microbeads (Dynabeads Mouse T-Activator CD3/CD28; Invitrogen) with 10 ng/mL IL-2, with analysis at day 3 poststimulation. In vitro Treg suppression assays were done as described in ref. 70. Th17 differentiation used bulk splenocytes cultured with soluble anti-CD3, IL-2 (10 ng/mL), TGF-β (5 ng/mL), IL-6 (10 ng/mL), anti–IFN-γ (10 μg/mL; XMG1.2), and anti–IL-4 (10 μg/mL; 11B11). Further details are in SI Materials and Methods.
Luciferase Assays.
Luciferase assays were done using standard luciferase (Dual Luciferase Assay Kit; Promega) from plasmid-transfected Jurkat cells or magnetically enriched CD4 T cells from ODD-luciferase mice (34) as detailed in SI Materials and Methods.
Lentiviral HIF-1α Knockdown.
Jurkat cells were subjected to lentiviral transduction and selected for HIF-1α knockdown as detailed in SI Materials and Methods.
Hypoxia and HIF Stabilization.
Cells were subjected to hypoxia (1% O2) in a humidified hypoxia chamber (Coy Laboratory Products) or treated with PHD inhibitor AKB-6899 (100 μM). For in vivo hypoxia, mice were exposed to hypobaric pressure (mimicking ∼10% atmospheric O2 by volume) or injected with PHD inhibitor AKB-4924 (5 mg/kg, every day for 6 d) (kind gift of Robert Shalwitz, Akebia Therapeutics, Cincinnati, OH). Further details are in SI Materials and Methods.
RNA Isolation, Real-Time PCR, ChIP, and Primers.
Total RNA was extracted by TRIzol, followed by cDNA synthesis and quantitative real-time reverse-transcriptase PCR (qPCR). ChIP assay was done using standard protocols. Antibodies and primers are in SI Materials and Methods.
Antibodies and Flow Cytometric Analysis.
Antibodies (eBioscience) and flow cytometric analysis (LSR II with FACSDiva software; BD) were done using standard protocols for detection of intracellular antigens as detailed in SI Materials and Methods.
CD45RBhigh Colitis.
To induce colitis, Rag1-deficient mice were adoptively transferred with a 1:4 ratio of Tregs:CD45RBhigh CD4 T cells, using control or Hif1a-deficient CD4+ CD25+ Tregs. Mice were weighed weekly, with tissues analyzed for colitis at harvest. Further details are in SI Materials and Methods.
Data Analysis.
Analysis was done using Prism 4.0c (GraphPad Software), FlowJo (TreeStar), or MacVector. Statistical analyses were performed by unpaired t tests or one-way ANOVA and posttest Tukey’s correction as indicated. Further details are in SI Materials and Methods.
Supplementary Material
Acknowledgments
The authors thank Ian Connor, Elizabeth Kelly-McKnight, and Sonia Soto for technical assistance; Kristann Magee for expert technical assistance with mice; Dr. Lenny Dragone for Jurkats; Dr. Robert Shalwitz for the kind gift of PHD inhibitors; and Dr. Linda van Dyk for critical discussion. This research was funded by National Institutes of Health Grants R01-HL0921, R01-DK083385, and R01-HL098294 (to H.K.E.); R01-DK50189 and R01-HL60569 (to S.P.C.); and Crohn’s and Colitis Foundation grants (to E.N.M., L.E.G., E.L.C., and H.K.E.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
See Author Summary on page 16418 (volume 109, number 41).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1202366109/-/DCSupplemental.
References
- 1.Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kominsky DJ, Campbell EL, Colgan SP. Metabolic shifts in immunity and inflammation. J Immunol. 2010;184:4062–4068. doi: 10.4049/jimmunol.0903002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karhausen J, et al. Epithelial hypoxia-inducible factor-1 is protective in murine experimental colitis. J Clin Invest. 2004;114:1098–1106. doi: 10.1172/JCI21086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng CT, et al. Synovial tissue hypoxia and inflammation in vivo. Ann Rheum Dis. 2010;69:1389–1395. doi: 10.1136/ard.2009.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semenza GL. Oxygen homeostasis. Wiley Interdiscip Rev Syst Biol Med. 2010;2:336–361. doi: 10.1002/wsbm.69. [DOI] [PubMed] [Google Scholar]
- 6.Cronstein BN, Daguma L, Nichols D, Hutchison AJ, Williams M. The adenosine/neutrophil paradox resolved: Human neutrophils possess both A1 and A2 receptors that promote chemotaxis and inhibit O2 generation, respectively. J Clin Invest. 1990;85:1150–1157. doi: 10.1172/JCI114547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eltzschig HK, et al. Coordinated adenine nucleotide phosphohydrolysis and nucleoside signaling in posthypoxic endothelium: Role of ectonucleotidases and adenosine A2B receptors. J Exp Med. 2003;198:783–796. doi: 10.1084/jem.20030891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rosenberger P, et al. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat Immunol. 2009;10:195–202. doi: 10.1038/ni.1683. [DOI] [PubMed] [Google Scholar]
- 9.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aherne CM, Kewley EM, Eltzschig HK. The resurgence of A2B adenosine receptor signaling. Biochim Biophys Acta. 2011;1808:1329–1339. doi: 10.1016/j.bbamem.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morote-Garcia JC, Rosenberger P, Kuhlicke J, Eltzschig HK. HIF-1-dependent repression of adenosine kinase attenuates hypoxia-induced vascular leak. Blood. 2008;111:5571–5580. doi: 10.1182/blood-2007-11-126763. [DOI] [PubMed] [Google Scholar]
- 12.Morote-Garcia JC, Rosenberger P, Nivillac NM, Coe IR, Eltzschig HK. Hypoxia-inducible factor-dependent repression of equilibrative nucleoside transporter 2 attenuates mucosal inflammation during intestinal hypoxia. Gastroenterology. 2009;136:607–618. doi: 10.1053/j.gastro.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Robinson A, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cummins EP, et al. The hydroxylase inhibitor dimethyloxalylglycine is protective in a murine model of colitis. Gastroenterology. 2008;134:156–165. doi: 10.1053/j.gastro.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Bernhardt WM, et al. Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol. 2010;21:2151–2156. doi: 10.1681/ASN.2010010116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Belkaid Y, Oldenhove G. Tuning microenvironments: Induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Laukoetter MG, et al. JAM-A regulates permeability and inflammation in the intestine in vivo. J Exp Med. 2007;204:3067–3076. doi: 10.1084/jem.20071416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stefanidakis M, Newton G, Lee WY, Parkos CA, Luscinskas FW. Endothelial CD47 interaction with SIRPgamma is required for human T-cell transendothelial migration under shear flow conditions in vitro. Blood. 2008;112:1280–1289. doi: 10.1182/blood-2008-01-134429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sather BD, et al. Altering the distribution of Foxp3(+) regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. 2007;204:1335–1347. doi: 10.1084/jem.20070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 21.Oldenhove G, et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity. 2009;31:772–786. doi: 10.1016/j.immuni.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sitkovsky M, Lukashev D. Regulation of immune cells by local-tissue oxygen tension: HIF1 alpha and adenosine receptors. Nat Rev Immunol. 2005;5:712–721. doi: 10.1038/nri1685. [DOI] [PubMed] [Google Scholar]
- 23.Eltzschig HK, Eckle T. Ischemia and reperfusion—from mechanism to translation. Nat Med. 2011;17:1391–1401. doi: 10.1038/nm.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Biju MP, et al. Vhlh gene deletion induces Hif-1-mediated cell death in thymocytes. Mol Cell Biol. 2004;24:9038–9047. doi: 10.1128/MCB.24.20.9038-9047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neumann AK, et al. Hypoxia inducible factor 1 alpha regulates T cell receptor signal transduction. Proc Natl Acad Sci USA. 2005;102:17071–17076. doi: 10.1073/pnas.0506070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Caldwell CC, et al. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol. 2001;167:6140–6149. doi: 10.4049/jimmunol.167.11.6140. [DOI] [PubMed] [Google Scholar]
- 27.Lukashev D, et al. Cutting edge: Hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol. 2006;177:4962–4965. doi: 10.4049/jimmunol.177.8.4962. [DOI] [PubMed] [Google Scholar]
- 28.Thiel M, et al. Targeted deletion of HIF-1alpha gene in T cells prevents their inhibition in hypoxic inflamed tissues and improves septic mice survival. PLoS ONE. 2007;2:e853. doi: 10.1371/journal.pone.0000853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ben-Shoshan J, Maysel-Auslender S, Mor A, Keren G, George J. Hypoxia controls CD4+CD25+ regulatory T-cell homeostasis via hypoxia-inducible factor-1alpha. Eur J Immunol. 2008;38:2412–2418. doi: 10.1002/eji.200838318. [DOI] [PubMed] [Google Scholar]
- 30.Shi LZ, et al. HIF1alpha-dependent glycolytic pathway orchestrates a metabolic checkpoint for the differentiation of TH17 and Treg cells. J Exp Med. 2011;208:1367–1376. doi: 10.1084/jem.20110278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dang EV, et al. Control of T(H)17/T(reg) balance by hypoxia-inducible factor 1. Cell. 2011;146:772–784. doi: 10.1016/j.cell.2011.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Powrie F, et al. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 33.Lukashev D, Caldwell C, Ohta A, Chen P, Sitkovsky M. Differential regulation of two alternatively spliced isoforms of hypoxia-inducible factor-1 alpha in activated T lymphocytes. J Biol Chem. 2001;276:48754–48763. doi: 10.1074/jbc.M104782200. [DOI] [PubMed] [Google Scholar]
- 34.Safran M, et al. Mouse model for noninvasive imaging of HIF prolyl hydroxylase activity: Assessment of an oral agent that stimulates erythropoietin production. Proc Natl Acad Sci USA. 2006;103:105–110. doi: 10.1073/pnas.0509459103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura H, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 36.Fraisl P, Aragonés J, Carmeliet P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat Rev Drug Discov. 2009;8:139–152. doi: 10.1038/nrd2761. [DOI] [PubMed] [Google Scholar]
- 37.Eckle T, Köhler D, Lehmann R, El Kasmi KC, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: A new paradigm for ischemic preconditioning. Circulation. 2008;118:166–175. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- 38.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mantel PY, et al. Molecular mechanisms underlying FOXP3 induction in human T cells. J Immunol. 2006;176:3593–3602. doi: 10.4049/jimmunol.176.6.3593. [DOI] [PubMed] [Google Scholar]
- 40.Haribhai D, et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J Immunol. 2007;178:2961–2972. doi: 10.4049/jimmunol.178.5.2961. [DOI] [PubMed] [Google Scholar]
- 41.Kim JM, Rudensky A. The role of the transcription factor Foxp3 in the development of regulatory T cells. Immunol Rev. 2006;212:86–98. doi: 10.1111/j.0105-2896.2006.00426.x. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Kitani A, Strober W. Molecular mechanisms regulating TGF-beta-induced Foxp3 expression. Mucosal Immunol. 2010;3:230–238. doi: 10.1038/mi.2010.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falanga V, et al. Hypoxia upregulates the synthesis of TGF-beta 1 by human dermal fibroblasts. J Invest Dermatol. 1991;97:634–637. doi: 10.1111/1523-1747.ep12483126. [DOI] [PubMed] [Google Scholar]
- 44.McMahon S, Grondin F, McDonald PP, Richard DE, Dubois CM. Hypoxia-enhanced expression of the proprotein convertase furin is mediated by hypoxia-inducible factor-1: Impact on the bioactivation of proproteins. J Biol Chem. 2005;280:6561–6569. doi: 10.1074/jbc.M413248200. [DOI] [PubMed] [Google Scholar]
- 45.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 46.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mottet C, Uhlig HH, Powrie F. Cutting edge: Cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 48.Grenz A, et al. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zarek PE, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bour-Jordan H, Bluestone JA. Regulating the regulators: Costimulatory signals control the homeostasis and function of regulatory T cells. Immunol Rev. 2009;229:41–66. doi: 10.1111/j.1600-065X.2009.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sauer S, et al. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proc Natl Acad Sci USA. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lal G, Bromberg JS. Epigenetic mechanisms of regulation of Foxp3 expression. Blood. 2009;114:3727–3735. doi: 10.1182/blood-2009-05-219584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sitkovsky MV. T regulatory cells: Hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol. 2009;30:102–108. doi: 10.1016/j.it.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 54.Darce J, et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity. 2012;36:731–741. doi: 10.1016/j.immuni.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGeachy MJ, et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat Immunol. 2009;10:314–324. doi: 10.1038/ni.1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arsenault D, Lucien F, Dubois CM. Hypoxia enhances cancer cell invasion through relocalization of the proprotein convertase furin from the trans-Golgi network to the cell surface. J Cell Physiol. 2012;227:789–800. doi: 10.1002/jcp.22792. [DOI] [PubMed] [Google Scholar]
- 57.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. J Biol Chem. 2006;281:24171–24181. doi: 10.1074/jbc.M604507200. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L, et al. TGF-beta-induced Foxp3 inhibits T(H)17 cell differentiation by antagonizing RORgammat function. Nature. 2008;453:236–240. doi: 10.1038/nature06878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.O’Connor W, Jr, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10:603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 2010;140:845–858. doi: 10.1016/j.cell.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 61.Chaudhry A, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009;326:986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cretney E, et al. The transcription factors Blimp-1 and IRF4 jointly control the differentiation and function of effector regulatory T cells. Nat Immunol. 2011;12:304–311. doi: 10.1038/ni.2006. [DOI] [PubMed] [Google Scholar]
- 63.Zheng Y, et al. Regulatory T-cell suppressor program co-opts transcription factor IRF4 to control T(H)2 responses. Nature. 2009;458:351–356. doi: 10.1038/nature07674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch MA, et al. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nat Immunol. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang Y, Su MA, Wan YY. An essential role of the transcription factor GATA-3 for the function of regulatory T cells. Immunity. 2011;35:337–348. doi: 10.1016/j.immuni.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wohlfert EA, et al. GATA3 controls Foxp3+ regulatory T cell fate during inflammation in mice. J Clin Invest. 2011;121:4503–4515. doi: 10.1172/JCI57456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chung Y, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Linterman MA, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ruan Q, et al. The Th17 immune response is controlled by the Rel-RORγ-RORγ T transcriptional axis. J Exp Med. 2011;208:2321–2333. doi: 10.1084/jem.20110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tao R, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13:1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]