Abstract
Nitric oxide (NO) generated by neuronal NO synthase (nNOS) initiates penile erection, but has not been thought to participate in the sustained erection required for normal sexual performance. We now show that cAMP-dependent phosphorylation of nNOS mediates erectile physiology, including sustained erection. nNOS is phosphorylated by cAMP-dependent protein kinase (PKA) at serine(S)1412. Electrical stimulation of the penile innervation increases S1412 phosphorylation that is blocked by PKA inhibitors but not by PI3-kinase/Akt inhibitors. Stimulation of cAMP formation by forskolin also activates nNOS phosphorylation. Sustained penile erection elicited by either intracavernous forskolin injection, or augmented by forskolin during cavernous nerve electrical stimulation, is prevented by the NOS inhibitor l-NAME or in nNOS-deleted mice. Thus, nNOS mediates both initiation and maintenance of penile erection, implying unique approaches for treating erectile dysfunction.
Keywords: gasotransmitter, smooth muscle relaxation, endothelial NOS, cyclic GMP, phosphoantibody
Nitric oxide (NO) is well established as a mediator of penile erection (1, 2). Neuronal NO synthase (nNOS) is highly localized to the penile innervation (3, 4). Electrical stimulation of the cavernous nerve (CN) to the penis elicits penile erection, which is abolished with NOS inhibitors and markedly reduced in nNOS-α–deleted mice (nNOSα−/−) (5–7). Neuronal depolarization-induced production of NO reflects calcium entry activating calmodulin associated with nNOS to stimulate NO formation (8, 9). This activation is short-lived and thus is believed to account only for the initiation of penile erection (10, 11). Sessa and colleagues (12) established that increased blood flow and associated shear stress, acting via PI3-kinase, augments the activity of the serine protein kinase Akt, which phosphorylates vascular endothelial NOS (eNOS) at serine(S)1179, causing prolonged NO formation at resting intracellular calcium levels (13). The increased penile blood flow through cavernosal vessels initiated by nNOS activation similarly stimulates penile Akt to phosphorylate eNOS, thereby promoting sustained maximal penile erection (14).
nNOS possesses a phosphorylation consensus sequence at S1412 that closely resembles the sequence surrounding eNOS-S1179, and is thought to be a target for Akt in some systems (15–21). We wondered whether nNOS phosphorylation at S1412 might regulate penile erection. Using a highly selective antibody for phospho-S1412-nNOS (P-nNOS) we report electrically stimulated nNOS phosphorylation via cAMP-dependent protein kinase (PKA) and not through Akt. P-nNOS activity contributes to sustained erection in concert with phospho-eNOS stimulation, and the neuronal and endothelial NOS activities are independently regulated by separate signaling cascades. We propose a model integrating the posttranslational phospho-stimulation of normal erectile physiology.
Results
Electrical Stimulation of Penile Innervation Augments Phosphorylation of nNOS.
We developed a C-terminal antibody that selectively recognizes P-nNOS. Immunoreactivity is absent in cells overexpressing nNOS in which S1412 is mutated to alanine (Fig. S1). We also developed an antibody selective for unphosphorylated nNOS (unP-nNOS) that shows decreased affinity for P-nNOS but can be used at higher concentrations to detect total nNOS. A commercial N-terminus antibody that recognizes total nNOSα does not discriminate nNOS S1412 phosphorylation.
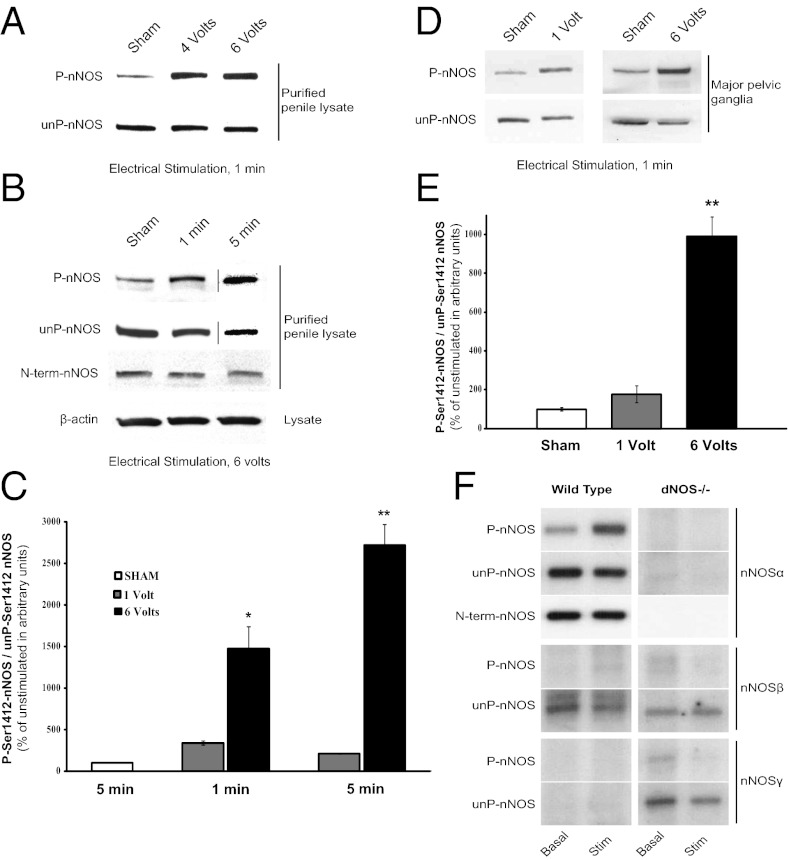
Using an established physiologic model of erectile function, we found electrical stimulation of the rat CN markedly increases P-nNOS immunoreactivity but not unP-nNOS in penile lysates (Fig. 1A). With 6-V stimulation, P-nNOS increases 15- or 27-fold following 1 or 5 min of stimulation, respectively (Fig. 1 B and C). Direct application of 1- or 6-V electrical stimulation to the rat major pelvic ganglion (MPG) increases P-nNOS in the neuronal tissue by two- or ninefold (Fig. 1 D and E).
Fig. 1.
Electrical stimulation increases P-nNOS in the penis and MPG. (A) Electrical stimulation of the rat CN causes voltage-dependent increase in penile P-nNOS. (B) The electrically stimulated increase in P-nNOS is also time-dependent, but the total nNOSα detected with an N-terminal antibody is unchanged, showing that nNOS protein is stable during stimulation. (C) Quantification of P-nNOS in arbitrary units is performed by densitometry. Each bar represents mean ± SE of P-nNOS/unP-nNOS expressed relative to unstimulated sham control. (D) Direct electrical stimulation of the MPG also increases P-nNOS in isolated nerve lysates, quantified in E. n = 4–7 animals per condition. *P < 0.05; **P < 0.001 compared with sham. (F) Representative blots show differential phosphorylation of nNOS isoforms in wild-type and double nNOS/eNOS- (dNOS−/−) deleted mice after direct MPG stimulation.
nNOS is alternatively spliced (22–24). The alternatively spliced forms are designated nNOS-β and nNOS-γ, whereas the predominant wild-type nNOS is designated nNOSα (25–27). Alternatively spliced nNOS isoforms retain some catalytic activity but lack the N-terminal PDZ domain that links nNOS to PSD95 and NMDA-glutamate receptors (25, 28, 29). We wondered whether nerve stimulation would influence phosphorylation of S1412 in the alternatively spliced isoforms (Fig. 1F). We detect very low levels of phosphorylated nNOSβ and nNOSγ in the mouse MPG, with no effect of electrical stimulation. Interestingly, unphosphorylated nNOSγ is increased in nNOSα−/− mice, perhaps reflecting some compensatory response (30).
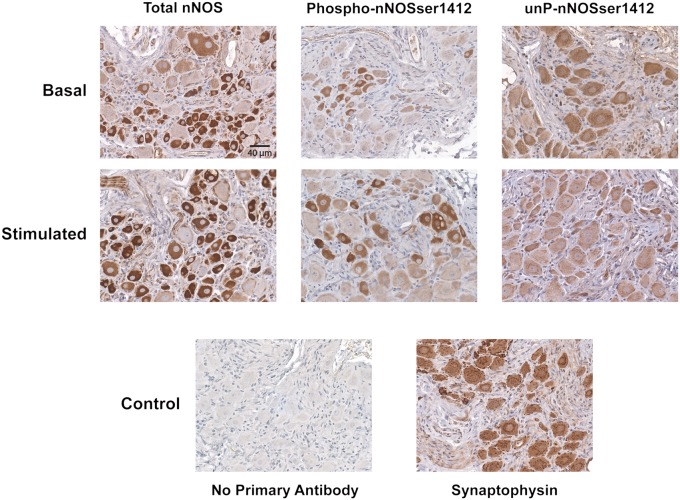
The antibodies to P-nNOS and unP-nNOS are suitable for immunohistochemistry. Both antibodies and the N-terminal nNOS antibody selectively stain MPG neuronal cell bodies and their processes in a pattern similar to the neuronal marker synaptophysin (Fig. 2). All three antibodies similarly stain cytosolic nNOS, with staining also evident at the plasma membrane.
Fig. 2.
Immunohistochemical localization of P-nNOS in MPG. The rat MPG was exposed and either sham or electrically stimulated (16 Hz, 4 V, 1 min), immediately dissected, and fixed. Paraffin-embedded serial sections were prepared for staining of total nNOS (N-terminal antibody), P-nNOS, and unP-nNOS (Magnification: 100×). Basal is sham-treated. Synaptophysin staining clearly identifies neuronal cell bodies in an untreated ganglion. No staining is seen when primary antibody is omitted. The differential phospho-staining was consistently apparent in multiple preparations from separate animals.
Electrical Stimulation Provides Prolonged Activation of nNOS Phosphorylation Mediated by PKA.
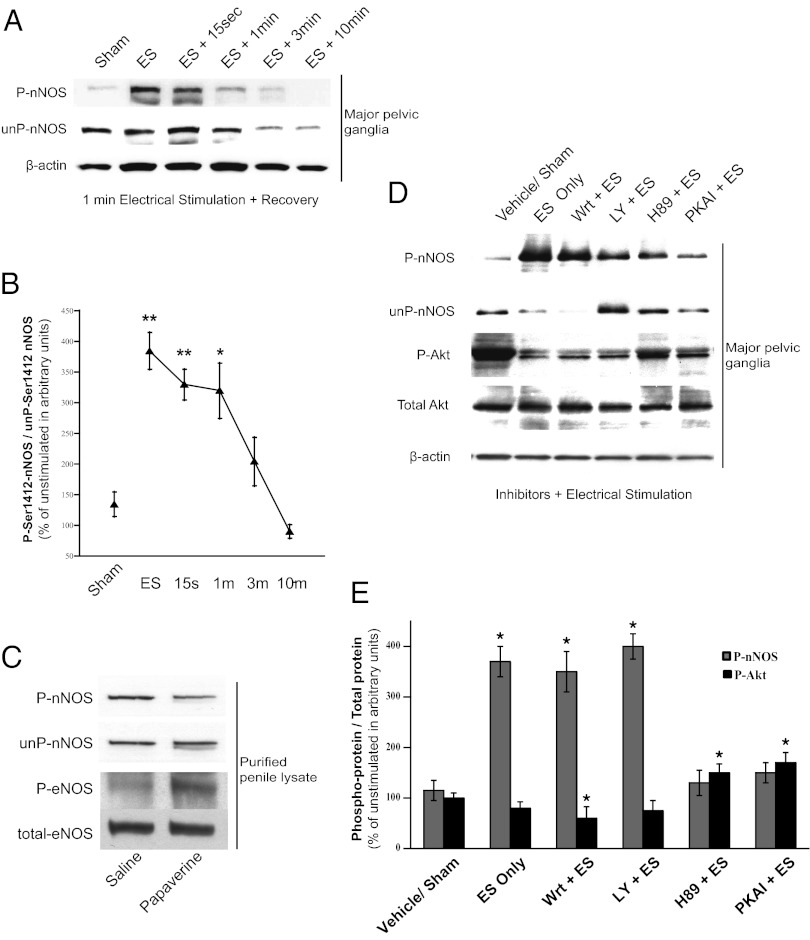
Physiologic depolarization-dependent increases in intracellular calcium that maximally activate nNOS are short lived, usually returning to baseline in seconds (31, 32). The increase of P-nNOS in rat MPG elicited by electrical stimulation is maintained for over a minute and then declines at 3 and 10 min (Fig. 3 A and B), similar to other persistent calcium-dependent signaling processes (33, 34). Although the proportion of P-nNOS increases with electrical stimulation, total nNOS normalized to β-actin is not significantly altered. Our prior work showed distinct eNOS-dependent stimulation of erection by papaverine, with remarkably decreased intracavernosal pressure (ICP) responses in eNOS−/− versus wild-type or nNOSα−/− mice. Papaverine dilates blood vessels, increasing flow and shear stress, which augments phospho-eNOS (12, 13) to produce penile erection. We investigated whether intracavernosal papaverine in the rat would also augment P-nNOS. Although papaverine increases phospho-eNOS (Fig. 3C), there is no change in nNOS phosphorylation (14). Although papaverine is a nonselective phosphodiesterase inhibitor, it does not increase cAMP production in penile tissue (35, 36). This finding suggests separate regulation of phosphorylation of eNOS and nNOS by distinct signaling pathways in normal erectile physiology.
Fig. 3.
Phosphorylation of nNOS-S1412 is distinct from phospho-eNOS regulation in penile erection. (A) Representative immunoblot shows sustained P-nNOS after electrical stimulation of rat MPG, quantified in B with mean ± SE. (C) P-nNOS is unchanged in rat penile lysates after intracavernosal injection of papaverine, but P-ser1179-eNOS is increased as previously reported. (D) Perigangliar injection of PKA inhibitors (H89, PKAI) decreases electrically stimulated P-nNOS in the MPG despite increasing Akt-S473 phosphorylation. PI3-kinase inhibitors (Wrt, LY) do not affect P-nNOS levels. (E) Mean ± SE for inhibitor experiments. ES, electrical stimulation. *P < 0.05; **P < 0.01 for treatment groups compared with control.
We used a pharmacologic approach to identify the kinase that phosphorylates nNOS at S1412. Ten minutes after periganglionic injection of inhibitors, the rat MPG and CN were electrically stimulated for 1 min, whereupon the ganglion-nerve preparation was snap-frozen for subsequent analysis (Fig. 3 D and E). Electrical stimulation elicits three- to fourfold augmentation of P-nNOS, which is prevented by treatment with PKA inhibitors H89 and PKA inhibitor peptide (PKAI). In contrast, wortmannin (Wrt) and LY294002 (LY), well-established inhibitors of PI3-kinase, fail to alter P-nNOS levels. The response of phospho-Akt to electrical stimulation is markedly different. Electrical stimulation fails to alter phospho-Akt levels, which are reduced by Wrt although not significantly decreased by LY. Surprisingly, H89 and PKAI significantly increase phospho-Akt.
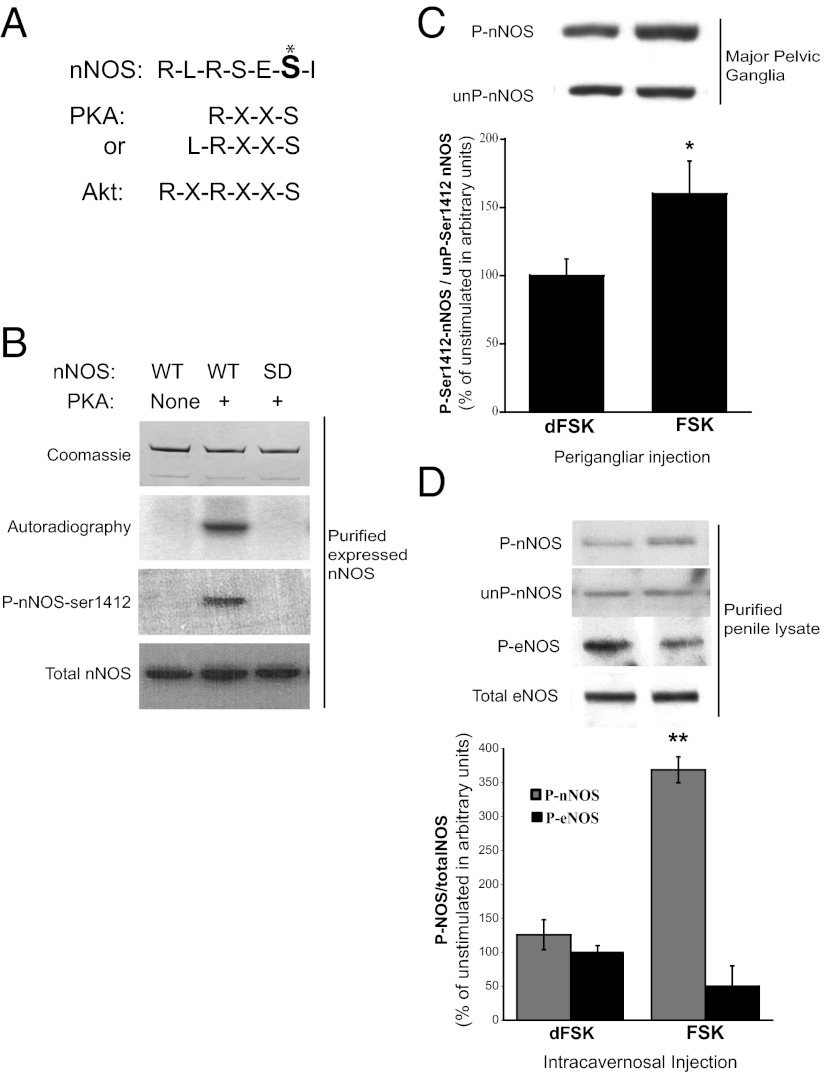
The ability of PKA inhibitors to abolish the increase in P-nNOS with electrical stimulation strongly implies that physiologic phosphorylation of S1412 is uniquely mediated by PKA. The sequence surrounding nNOS S1412 corresponds to a PKA consensus site (Fig. 4A), and although another PKA consensus sequence is present at S370, direct phosphorylation of that site has not been reported. Full-length nNOS purified from transfected HEK293 cells can be selectively phosphorylated with [32P]ATP and PKA. The phosphorylation is abolished in nNOS-S1412D mutants (Fig. 4B and Fig. S1). Kemp and colleagues (37, 38) reported that both Akt and PKA phosphorylate eNOS at S1179, which is comparable to S1412 of nNOS and supports our findings.
Fig. 4.
cAMP/PKA directly phosphorylates nNOS-S1412. (A) Comparison of nNOS C-terminus amino acid sequence (S1412, marked *) with PKA and Akt kinase target-consensus sequences. (B) Direct in vitro phosphorylation of purified nNOS by PKA is abolished in S1412D mutant nNOS. (C) Perigangliar or (D) intracavernosal injection of FSK increases P-nNOS but not P-ser1179-eNOS, but inactive dFSK has no effect. *P < 0.05; **P < 0.01 for FSK treatment compared with dFSK.
To investigate the role of PKA in S1412-nNOS phosphorylation in the intact penis, we performed injections of forskolin (FSK), a potent and selective activator of adenylyl cyclase, beneath the rat MPG, and monitored P-nNOS in ganglion/CN preparations (Fig. 4C). Perigangliar FSK elicits a substantial increase in neuronal P-nNOS, but the inactive derivative deoxy-forskolin (dFSK) does not. No change in P-nNOS is detected with dFSK treatment. We also examined penile P-nNOS levels following intracavernosal injection of FSK or dFSK (Fig. 4D). FSK triples penile P-nNOS, but dFSK has no effect. In contrast, FSK does not influence penile phospho-eNOS levels. The unexpected absence of increased P-eNOS in this preparation may reflect the shorter time course before tissue collection (30–90 s for FSK, and 5–7 min for papaverine). Thus, in the MPG and penile tissue P-nNOS immunoreactivity reflects PKA phosphorylation of nNOS at S1412.
Neurally Evoked Penile Erection Is Mediated by PKA Phosphorylation of nNOS.
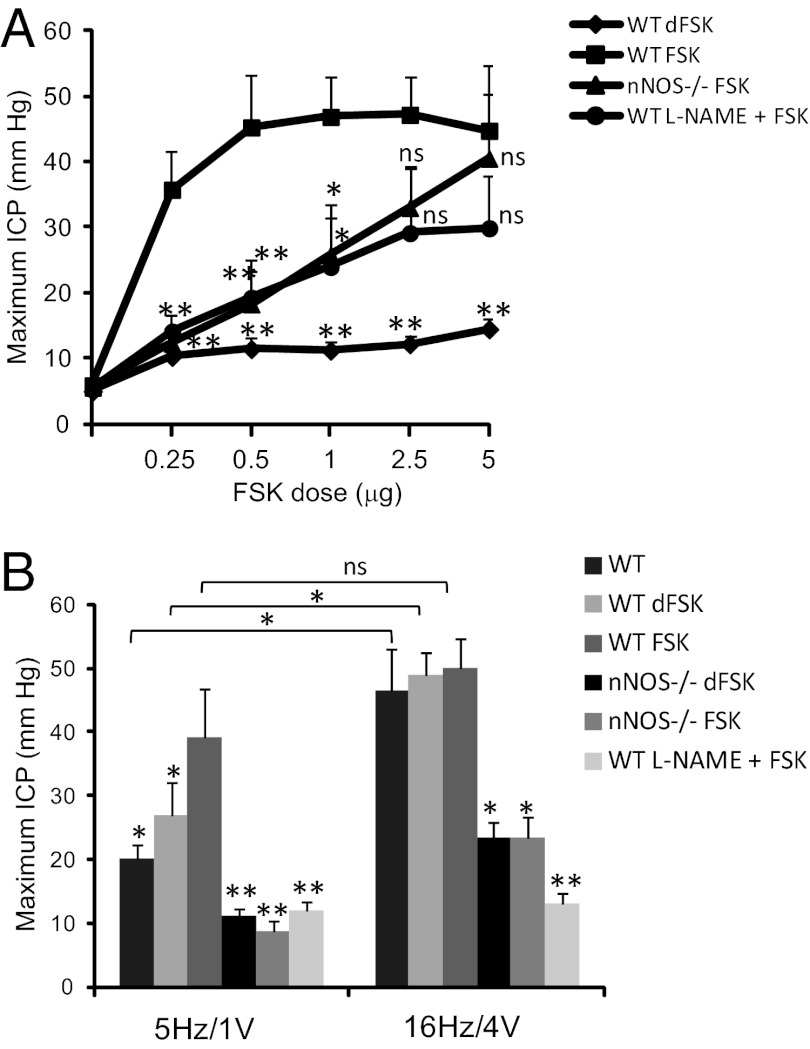
We explored whether PKA phosphorylation of nNOS is required for penile erection evoked by nerve stimulation. First we examined the influence of FSK. As little as 0.25 μg FSK injected intracavernosally in the mouse elicits a four- to fivefold increase in ICP, with only modest increase at higher doses (Fig. 5A and Fig. S2), but the inactive dFSK has no effect. The effect of low doses of FSK (0.25–1 μg) is markedly reduced in nNOSα−/− mice or following treatment with the NOS inhibitor l-nitro-arginine-methylester (l-NAME). The increased ICP at high doses of FSK (2.5–5 μg) is not influenced by nNOS deletion or inhibition, consistent with known nNOS-independent actions of FSK mediating smooth muscle relaxation. The similar changes in ICP at higher doses of FSK suggest that different signaling pathways mediate low- and high-dose FSK effects and that the smooth muscle contractile apparatus is not modified in the nNOSα−/− mice. The FSK effect on penile erection is evident in measurements of both maximal ICP (Fig. 5A) and for the integrated total pressure over time (area under the curve) (Fig. S3A). Previously, Andersson and colleagues (39) reported increased ICP elicited by FSK with no alteration following treatment with l-NAME; however, their studies used a lower dose of l-NAME (50 mg/kg) than was used in our experiments (100 mg/kg).
Fig. 5.
nNOS mediates the FSK-induced increase in ICP and enhances the neurogenic erectile response. (A) ICP increases with intrapenile injection of FSK in WT mice. FSK-stimulated ICP is significantly reduced by pretreatment with l-NAME and in nNOSα−/− mice. The inactive compound dFSK does not affect ICP. (B) Immediately following FSK or dFSK treatment, CN electrical stimulation was performed using low (5 Hz/1 V) or maximal (16 Hz/4 V) parameters. Electrically stimulated maximum ICP is similar in untreated wild-type mice and in mice treated with dFSK. FSK injection significantly increases the submaximal electrical stimulation response, but there is minimal response in animals treated with l-NAME or in nNOSα−/− animals. Data are mean ± SE for n = 6–9 animals. *P < 0.05 vs. wild-type FSK; **P < 0.001 vs. wild-type FSK. For 5 Hz vs. 16 Hz comparisons, *P < 0.05 by Student’s t test. ns, not significant.
We explored the interaction between electrical stimulation and FSK treatment (Fig. 5B and Fig. S3B). Modest electrical stimulation of the CN and MPG alone (5 Hz/1 V) elicits a nearly twofold increase in ICP. After injection of 5 μg intracavernosal FSK and return to ICP baseline, the response to low-voltage electrical stimulation is augmented fourfold. We cannot rule out the possibility that this synergy is the result of an effect on smooth muscle; however, the electrical stimulation is performed at a time point when ICP has returned to baseline but P-nNOS remains elevated. In nNOSα−/− mice, or after treating with l-NAME, no FSK-dependent increase in penile erection is evident, suggesting that P-nNOS may mediate the persistent effect of FSK. With maximal electrical stimulation (16 Hz/4 V), ICP is similar to that elicited by modest stimulation plus FSK, although no additive effect is observed. nNOS deletion and l-NAME treatment markedly reduce penile erection with maximal stimulation. As we previously reported, nNOSβ mediates the NO-dependent change in ICP with maximal electrical stimulation in nNOSα−/− mice. However, nNOSβ activity is not sufficient to produce erectile function with submaxmial electrical stimulation and nNOSβ is not phosphorylated by PKA, so it cannot mediate the increased response after FSK treatment seen in wild-type animals expressing nNOSα.
Discussion
In the present study we demonstrate a major role for PKA phosphorylation of nNOS at S1412 in mediating penile erection. Using a highly specific antibody to phospho-nNOS-S1412, we showed that electrical stimulation of the CN markedly augments phosphorylation of nNOS in both MPG and penile tissue preparations. Moreover, cAMP activation of PKA is responsible for that phosphorylation, because FSK—which leads to generation of cAMP and PKA activation—markedly increases and PKA inhibitors decrease P-nNOS levels. FSK-mediated penile erection includes a component mediated specifically by nNOS, as the effect is similarly abolished by treatment with NOS inhibitors and in nNOSα−/− animals. In vitro studies have shown increased NO production at resting calcium levels with nNOS serine-1412 phosphorylation (40).
Previously we established two phases for penile erection. Psychogenic- and reflex-mediated stimuli lead to depolarization of the CNs, causing increased intracellular calcium that binds calmodulin to activate nNOS (41). Because calcium entry is a brief and tightly regulated event, this stimulation can only cause a brief increase in neuronal NO-dependent blood flow. This increased blood flow, however, activates endothelial PI3-kinase to stimulate Akt, phosphorylate and activate eNOS, and provide persistent NO production and sustained penile erection (14, 42, 43). Our findings here indicate that neuronal stimulation increases cAMP to activate PKA, which phosphorylates nNOS at S1412, stimulating nNOS catalytic activity (Fig. 6). This covalent phospho-modification can last substantially longer than the neuronal calcium transient and so, in coordination with phospho-eNOS, phospho-nNOS may contribute to sustained erection. We conjecture that activity dependent calcium-stimulated adenylyl cyclase could mediate cAMP/PKA activation in the CN.
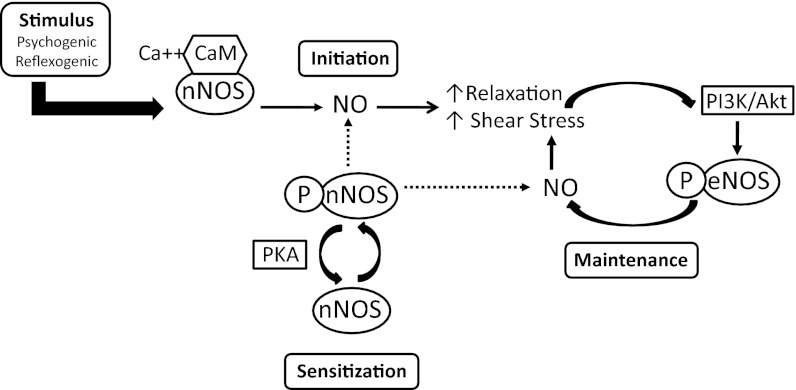
Fig. 6.
A model integrating nNOS and endothelial NOS (eNOS) regulation in initiation and maintenance of erectile function. Initiating neural stimuli promote NO production by nNOS and nNOS sensitization by PKA phosphorylation of S1412. Initial smooth muscle relaxation and increased penile blood flow stimulates PI3-kinase/Akt phosphorylation of eNOS-S1179. Phosphorylation and activation of nNOS and eNOS produces NO at resting intracellular calcium levels. Maintenance of maximum erectile response may be sustained by synergistic NO production from P-nNOS and P-eNOS. Dotted lines represent the proposed contribution of P-nNOS in this model.
Ways in which alterations of nNOS and eNOS interact in coordinated neurovascular erectile physiology are not clear. Moreover, the influence of nNOS phosphorylation on its usual regulation by calcium/calmodulin is uncertain, although there is evidence that eNOS phosphorylation by Akt sensitizes it to the effects of calcium (13, 44, 45) and eNOS also associates with calmodulin. eNOS phosphorylation renders it more sensitive to resting intracellular calcium concentrations, providing a feed-forward augmentation of enzymatic activity. It is possible that nNOS phosphorylation by PKA likewise increases its sensitivity to resting calcium/calmodulin, thereby prolonging nNOS activation (40). It is known that Akt can phosphorylate nNOS at S1412. Our findings establish that PKA also directly phosphorylates and activates nNOS-S1412 in a physiologically meaningful way similar to endothelial PKA phosphorylation of eNOS-S1177 (37). Rameau et al. (17, 21) used immunofluorescent staining techniques to show that NMDA stimulation of cortical neuronal cultures enhances phosphorylation of nNOS at S1412 in specific and localized synaptic signaling. Conceivably, Akt and PKA phosphorylation of nNOS interact in a regulated fashion. Further studies will determine the coordinated regulation of stimulatory and inhibitory phosphorylation sites for cAMP-dependent penile NO production.
FSK has heretofore been thought to stimulate penile erection by activating PKA in smooth muscle of the penis (46–48), and that is also the likely mechanism in our higher-dose FSK experiments. Our findings suggest that FSK also acts by stimulating nNOS, enhancing the neurovascular coordination of sustained NO release and suggesting novel therapeutic approaches to erectile dysfunction. Current therapeutic agents selectively inhibit forms of phosphodiesterase that act primarily upon cGMP in the erectile smooth muscle. Our findings imply that drugs inhibiting the metabolism of both cAMP and cGMP will act synergistically by respectively enhancing NO generation and preventing cGMP degradation.
Materials and Methods
Reagents.
FSK, dFSK, papaverine, Wrt, LY, H89, PKAI, l-NAME were from Sigma-Aldrich; commercial anti–nNOS-S1412 antibody were from Abcam; nNOS N-terminus antibody, anti-total Akt, anti-phospho-Akt-S473 were from Cell Signaling Technology.
Animal Models.
Male Sprague–Dawley rats (300–325 g; Charles River Breeding Laboratories) or 8- to 10-wk-old C57BL6/J (wild-type; Jackson Laboratories), and nNOSα−/− mice (S.H.S. laboratory, Johns Hopkins University, Baltimore, MD) were anesthetized by intraperitoneal injection of ketamine (50 mg/kg)/xylazine (5 mg/kg). MPG and CN were identified/isolated via midline suprapubic incision for electrical stimulation of the CN. To monitor ICP in mice, the penis was denuded of skin and right corpus cavernosum was pierced with 30-gauge needle attached to PE-50 tubing connected to a pressure transducer (Harvard Apparatus) as previously described (49). Response parameters were recorded using a data acquisition (DI-190; Dataq Instruments) and calculated using MATLAB software (Mathworks). All experiments were approved by the Johns Hopkins University Institutional Animal Care and Use Committee (IACUC).
Electrically Induced Penile Erection Studies.
In anesthetized animals, electrical stimulation of penile erection was performed by placing a bipolar platinum electrode hook around the CN, as previously described (5). The electrode was attached to a Grass Instruments S48 stimulator. Stimulation parameters are indicated in results for various experiments. Typical maximum stimulation in rats was 16 Hz at 6 V with square-wave duration of 5 ms. Except for time-course experiments, duration of electro-stimulation was 1 min. In dephosphorylation experiments, rat CN was electrically stimulated at maximal parameters for 1 min, then both the MPG and penis were collected 15 s to 10 min after termination of stimulation.
Preparation of Protein Extracts, Western Immunoblot, and Phospho-Labeling.
Samples were prepared as previously described (14). Briefly, frozen tissue was minced then homogenized (penis) or sonicated (MPG) in 8 vol of ice-cold buffer containing 50 mM Tris, pH 7.5, 2 mM EDTA, 2 mM EGTA, 150 mM NaCl, 1% Triton X-100, and 10% (vol/vol) glycerol, with phosphatase and protease inhibitors [50 mM NaF /5 mM sodium pyrophosphate/30 mM β-glycerophosphate (BGP)/1 mM sodium orthovanadate /2× Sigma-Aldrich phosphatase inhibitor mixture/2 μg/mL aprotinin/10 μg/mL leupeptin/1 mM Pefabloc]. After centrifuging at 16,000 × g for 30 min, soluble protein was determined by BCA assay (ThermoScientific). Protein (1–3 mg) was added to 40 μL of packed 2′,5′-ADP-Sepharose for purification of penile NOS or 50–100 μg of total protein was used directly for MPG blots. For purification, after 3–4 h incubation, the beads were washed with PBS/400 mM NaCl/1% Triton X-100; PBS/2% Triton X-100; and finally PBS alone. Bound protein was directly eluted in 30 μL SDS loading buffer (62.5 mM Tris, pH 6.8/2% SDS/10% glycerol/2 mM EDTA) at 100 °C for 3 min. Samples were separated on 4–20% gradient gels (BioRad), then transferred to PVDF (Millipore), blocked with Superblok-PBS (ThermoScientific), and probed for phospho-proteins overnight at 4 °C with the indicated antibodies. Then, blots were stripped for 20 min at room temperature (Restore; ThermoScientific) and reprobed for total protein. Results were quantified by densitometry, and the ratio of phospho- to total or unphospho-protein was determined in arbitrary units expressed relative to the ratio for sham-treated animals prepared and blotted at the same time. In phospho-labeling experiments, wild-type or S1412D nNOS from transfected HEK293 cells was purified on 2′,5′-ADP-Sepharose, eluted with NADPH, and incubated with PKA catalytic subunit in reaction buffer containing [32P]ATP. The reaction was stopped with SDS loading buffer and run on a gradient gel, as above, for Coomassie blue staining and autoradiography. A fraction of the same reaction was prepared for Western blot with P-nNOS antibodies, as described above.
Pharmacologically Induced Penile Erection Studies.
In rats, papaverine hydrochloride, FSK, or dFSK at the indicated concentration, was injected intracavernosally, as described previously (14, 50). Penes or MPG were collected during maximal ICP, snap-frozen, and prepared for Western blotting. In mice, FSK or dFSK was administered via a second 30-gauge needle inserted in the left corpus cavernosum; ICP was monitored as described above. Some animals were pretreated with l-NAME (100 mg/kg, i.p.) 30 min before FSK treatment. After FSK dose–response injections, and at least 5 min after ICP returned to baseline, the CN was electrically stimulated for 1 min at minimal parameters of 5 Hz/1 V and then at 16 Hz/4 V with a 3-min rest between electro-stimulations. This process was done to confirm l-NAME effect and nNOS−/− phenotype, and to identify changes in electrically stimulated erection after FSK injection.
Pharmacologic Inhibition of nNOS Phosphorylation with Perigangliar Injection.
The CN and MPG were exposed and prepared as above. PI3-kinase inhibitors (1 μM Wrt or 50 μM LY), PKA inhibitors (30 μM H89 or 60 μM PKAI), or vehicle (0.1% dimethyl sulfoxide or ethanol) were injected directly beneath the MPG using a 30-gauge needle attached to a Hamilton syringe. After 10 min, the CN was electrically stimulated at 16 Hz/6 V for 1 min. The MPG was then immediately excised, rinsed in PBS, snap-frozen, and processed for phospho-protein analysis.
Immunohistochemistry.
Whole MPGs with underlying prostate tissue were removed from adult rats immediately after electrical stimulation of CN (16 Hz, 6 V, 1 min), and immediately fixed in ice-cold formalin (containing 50 mM BGP, 5 mM sodium orthovanadate, 5 mM sodium pyrophosphate, and 1× Sigma-Aldrich phosphatase-inhibitor mixture) for 15 min, then 1 h at room temperature before storing in 70% ethanol. Tissue was paraffin-embedded, cut in 10-μm transverse sections, and mounted on glass slides. Sections were baked at 60 °C, deparaffinized, and boiled in citrate buffer for 20 min, then permeabilized in 0.4% Triton X-100 for 15 min, quenched in 2% H2O2 in PBS for 15 min, and blocked with PBS containing 5% goat serum/10 mM BGP/5% BSA for 2 h. Indicated antibodies were incubated overnight at 4 °C PBS containing 0.2% BSA/1% normal goat serum/5 mM BGP:total nNOS (1:300), affinity-purified phospho-nNOS-S1412 (1:150) or unphospho-nNOS-S1412 (1:5), or synaptophysin (1:5). Staining was visualized using the Elite Vectastain kit (Vector Laboratories) and counterstained with hematoxylin before dehydrating and cover-slipping. Controls without primary antibody were run for each set of slides. Stimulated and baseline sections were stained and developed simultaneously on the same slide.
Statistical Evaluation.
Analysis was performed with MATLAB or KaleidaGraph (Synergy Software) using one-way ANOVA followed by Holm–Sidak or Newman–Keuls multiple comparison or t test when appropriate. Data are expressed as mean ± SE. n are listed within figure legends. P values < 0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by US Public Health Service Grants R01DK067223 (to A.L.B.) and MH018501 (to S.H.S.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1213790109/-/DCSupplemental.
References
- 1.Burnett AL. Nitric oxide in the penis—Science and therapeutic implications from erectile dysfunction to priapism. J Sex Med. 2006;3:578–582. doi: 10.1111/j.1743-6109.2006.00270.x. [DOI] [PubMed] [Google Scholar]
- 2.Ignarro LJ. Nitric oxide as a unique signaling molecule in the vascular system: A historical overview. J Physiol Pharmacol. 2002;53:503–514. [PubMed] [Google Scholar]
- 3.Burnett AL, et al. Immunohistochemical localization of nitric oxide synthase in the autonomic innervation of the human penis. J Urol. 1993;150:73–76. doi: 10.1016/s0022-5347(17)35401-0. [DOI] [PubMed] [Google Scholar]
- 4.Stanarius A, et al. Immunocytochemical distribution of nitric oxide synthase in the human corpus cavernosum: An electron microscopical study using the tyramide signal amplification technique. Urol Res. 2001;29:168–172. doi: 10.1007/s002400100181. [DOI] [PubMed] [Google Scholar]
- 5.Burnett AL, Lowenstein CJ, Bredt DS, Chang TS, Snyder SH. Nitric oxide: A physiologic mediator of penile erection. Science. 1992;257:401–403. doi: 10.1126/science.1378650. [DOI] [PubMed] [Google Scholar]
- 6.Trigo-Rocha F, et al. Nitric oxide and cGMP: Mediators of pelvic nerve-stimulated erection in dogs. Am J Physiol. 1993;264:H419–H422. doi: 10.1152/ajpheart.1993.264.2.H419. [DOI] [PubMed] [Google Scholar]
- 7.Trigo-Rocha F, et al. Sodium nitroprusside: Physiologic effects as a nitric oxide donor in three species. Int J Impot Res. 1995;7:49–56. [PubMed] [Google Scholar]
- 8.Bredt DS. Endogenous nitric oxide synthesis: Biological functions and pathophysiology. Free Radic Res. 1999;31:577–596. doi: 10.1080/10715769900301161. [DOI] [PubMed] [Google Scholar]
- 9.Mungrue IN, Bredt DS. nNOS at a glance: Implications for brain and brawn. J Cell Sci. 2004;117:2627–2629. doi: 10.1242/jcs.01187. [DOI] [PubMed] [Google Scholar]
- 10.Toda N, Okamura T. The pharmacology of nitric oxide in the peripheral nervous system of blood vessels. Pharmacol Rev. 2003;55:271–324. doi: 10.1124/pr.55.2.3. [DOI] [PubMed] [Google Scholar]
- 11.Ayajiki K, Hayashida H, Tawa M, Okamura T, Toda N. Characterization of nitrergic function in monkey penile erection in vivo and in vitro. Hypertens Res. 2009;32:685–689. doi: 10.1038/hr.2009.84. [DOI] [PubMed] [Google Scholar]
- 12.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 14.Hurt KJ, et al. Akt-dependent phosphorylation of endothelial nitric-oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2002;99:4061–4066. doi: 10.1073/pnas.052712499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen ZP, et al. Effect of exercise intensity on skeletal muscle AMPK signaling in humans. Diabetes. 2003;52:2205–2212. doi: 10.2337/diabetes.52.9.2205. [DOI] [PubMed] [Google Scholar]
- 16.Mount PF, et al. Phosphorylation of neuronal and endothelial nitric oxide synthase in the kidney with high and low salt diets. Nephron, Physiol. 2006;102:36–50. doi: 10.1159/000089092. [DOI] [PubMed] [Google Scholar]
- 17.Rameau GA, et al. Biphasic coupling of neuronal nitric oxide synthase phosphorylation to the NMDA receptor regulates AMPA receptor trafficking and neuronal cell death. J Neurosci. 2007;27:3445–3455. doi: 10.1523/JNEUROSCI.4799-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carreras MC, et al. The biological significance of mtNOS modulation. Front Biosci. 2007;12:1041–1048. doi: 10.2741/2124. [DOI] [PubMed] [Google Scholar]
- 19.Gingerich S, Krukoff TL. Activation of ERbeta increases levels of phosphorylated nNOS and NO production through a Src/PI3K/Akt-dependent pathway in hypothalamic neurons. Neuropharmacology. 2008;55:878–885. doi: 10.1016/j.neuropharm.2008.06.058. [DOI] [PubMed] [Google Scholar]
- 20.El-Mas MM, Fan M, Abdel-Rahman AA. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira JM, Burnett AL, Rameau GA. Activity-dependent regulation of surface glucose transporter-3. J Neurosci. 2011;31:1991–1999. doi: 10.1523/JNEUROSCI.1850-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MA, Cai L, Hübner N, Lee YA, Lindpaintner K. Tissue- and development-specific expression of multiple alternatively spliced transcripts of rat neuronal nitric oxide synthase. J Clin Invest. 1997;100:1507–1512. doi: 10.1172/JCI119673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eliasson MJ, Blackshaw S, Schell MJ, Snyder SH. Neuronal nitric oxide synthase alternatively spliced forms: prominent functional localizations in the brain. Proc Natl Acad Sci USA. 1997;94:3396–3401. doi: 10.1073/pnas.94.7.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Putzke J, Seidel B, Huang PL, Wolf G. Differential expression of alternatively spliced isoforms of neuronal nitric oxide synthase (nNOS) and N-methyl-D-aspartate receptors (NMDAR) in knockout mice deficient in nNOS alpha (nNOS alpha(Delta/Delta) mice) Brain Res Mol Brain Res. 2000;85:13–23. doi: 10.1016/s0169-328x(00)00220-5. [DOI] [PubMed] [Google Scholar]
- 25.Brenman JE, et al. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- 26.Iwasaki T, et al. Characterization of mouse nNOS2, a natural variant of neuronal nitric-oxide synthase produced in the central nervous system by selective alternative splicing. J Biol Chem. 1999;274:17559–17566. doi: 10.1074/jbc.274.25.17559. [DOI] [PubMed] [Google Scholar]
- 27.Kolesnikov YA, et al. Functionally differentiating two neuronal nitric oxide synthase isoforms through antisense mapping: Evidence for opposing NO actions on morphine analgesia and tolerance. Proc Natl Acad Sci USA. 1997;94:8220–8225. doi: 10.1073/pnas.94.15.8220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Watanabe Y, et al. Post-synaptic density-95 promotes calcium/calmodulin-dependent protein kinase II-mediated Ser847 phosphorylation of neuronal nitric oxide synthase. Biochem J. 2003;372:465–471. doi: 10.1042/BJ20030380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- 30.Hurt KJ, et al. Alternatively spliced neuronal nitric oxide synthase mediates penile erection. Proc Natl Acad Sci USA. 2006;103:3440–3443. doi: 10.1073/pnas.0511326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaffe DB, et al. A model for dendritic Ca2+ accumulation in hippocampal pyramidal neurons based on fluorescence imaging measurements. J Neurophysiol. 1994;71:1065–1077. doi: 10.1152/jn.1994.71.3.1065. [DOI] [PubMed] [Google Scholar]
- 32.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: Temporal specificity in ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: A Ca(2+)- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 34.Wu J, Huang KP, Huang FL. Participation of NMDA-mediated phosphorylation and oxidation of neurogranin in the regulation of Ca2+- and Ca2+/calmodulin-dependent neuronal signaling in the hippocampus. J Neurochem. 2003;86:1524–1533. doi: 10.1046/j.1471-4159.2003.01963.x. [DOI] [PubMed] [Google Scholar]
- 35.Jeremy JY, Ballard SA, Naylor AM, Miller MA, Angelini GD. Effects of sildenafil, a type-5 cGMP phosphodiesterase inhibitor, and papaverine on cyclic GMP and cyclic AMP levels in the rabbit corpus cavernosum in vitro. Br J Urol. 1997;79:958–963. doi: 10.1046/j.1464-410x.1997.00206.x. [DOI] [PubMed] [Google Scholar]
- 36.Miller MA, Morgan RJ, Thompson CS, Mikhailidis DP, Jeremy JY. Effects of papaverine and vasointestinal polypeptide on penile and vascular cAMP and cGMP in control and diabetic animals: An in vitro study. Int J Impot Res. 1995;7:91–100. [PubMed] [Google Scholar]
- 37.Michell BJ, et al. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 38.Michell BJ, et al. Identification of regulatory sites of phosphorylation of the bovine endothelial nitric-oxide synthase at serine 617 and serine 635. J Biol Chem. 2002;277:42344–42351. doi: 10.1074/jbc.M205144200. [DOI] [PubMed] [Google Scholar]
- 39.Mizusawa H, Hedlund P, Håkansson A, Alm P, Andersson KE. Morphological and functional in vitro and in vivo characterization of the mouse corpus cavernosum. Br J Pharmacol. 2001;132:1333–1341. doi: 10.1038/sj.bjp.0703938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adak S, et al. Neuronal nitric-oxide synthase mutant (Ser-1412 —> Asp) demonstrates surprising connections between heme reduction, NO complex formation, and catalysis. J Biol Chem. 2001;276:1244–1252. doi: 10.1074/jbc.M006857200. [DOI] [PubMed] [Google Scholar]
- 41.Bredt DS, Snyder SH. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci USA. 1990;87:682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Musicki B, et al. Erection capability is potentiated by long-term sildenafil treatment: Role of blood flow-induced endothelial nitric-oxide synthase phosphorylation. Mol Pharmacol. 2005;68:226–232. doi: 10.1124/mol.104.010678. [DOI] [PubMed] [Google Scholar]
- 43.Wen J, et al. A2B adenosine receptor contributes to penile erection via PI3K/AKT signaling cascade-mediated eNOS activation. FASEB J. 2011;25:2823–2830. doi: 10.1096/fj.11-181057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–R12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 45.Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179) J Biol Chem. 2001;276:30392–30398. doi: 10.1074/jbc.M103702200. [DOI] [PubMed] [Google Scholar]
- 46.Mulhall JP, et al. Intracavernosal forskolin: Role in management of vasculogenic impotence resistant to standard 3-agent pharmacotherapy. J Urol. 1997;158:1752–1758; discussion 1758–1759. doi: 10.1016/s0022-5347(01)64118-1. [DOI] [PubMed] [Google Scholar]
- 47.Cahn D, Melman A, Valcic M, Christ GJ. Forskolin: A promising new adjunct to intracavernous pharmacotherapy. J Urol. 1996;155:1789–1794. doi: 10.1016/s0022-5347(01)66199-8. [DOI] [PubMed] [Google Scholar]
- 48.Becker AJ, et al. Possible role of bradykinin and angiotensin II in the regulation of penile erection and detumescence. Urology. 2001;57:193–198. doi: 10.1016/s0090-4295(00)00881-5. [DOI] [PubMed] [Google Scholar]
- 49.Sezen SF, Burnett AL. Intracavernosal pressure monitoring in mice: responses to electrical stimulation of the cavernous nerve and to intracavernosal drug administration. J Androl. 2000;21:311–315. [PubMed] [Google Scholar]
- 50.Burnett AL, et al. Nitric oxide-dependent penile erection in mice lacking neuronal nitric oxide synthase. Mol Med. 1996;2:288–296. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.