Abstract
Nerve myelination facilitates saltatory action potential conduction and exhibits spatiotemporal variation during development associated with the acquisition of behavioral and cognitive maturity. Although human cognitive development is unique, it is not known whether the ontogenetic progression of myelination in the human neocortex is evolutionarily exceptional. In this study, we quantified myelinated axon fiber length density and the expression of myelin-related proteins throughout postnatal life in the somatosensory (areas 3b/3a/1/2), motor (area 4), frontopolar (prefrontal area 10), and visual (areas 17/18) neocortex of chimpanzees (N = 20) and humans (N = 33). Our examination revealed that neocortical myelination is developmentally protracted in humans compared with chimpanzees. In chimpanzees, the density of myelinated axons increased steadily until adult-like levels were achieved at approximately the time of sexual maturity. In contrast, humans displayed slower myelination during childhood, characterized by a delayed period of maturation that extended beyond late adolescence. This comparative research contributes evidence crucial to understanding the evolution of human cognition and behavior, which arises from the unfolding of nervous system development within the context of an enriched cultural environment. Perturbations of normal developmental processes and the decreased expression of myelin-related molecules have been related to psychiatric disorders such as schizophrenia. Thus, these species differences suggest that the human-specific shift in the timing of cortical maturation during adolescence may have implications for vulnerability to certain psychiatric disorders.
Keywords: primate, hominids, neuropsychiatric illness, cortical development, behavioral maturation
Comparative studies suggest that human neurobiological development is unique. For example, humans differ from other primates in extending a rapid, fetal-like brain mass growth rate into the first postnatal year, thereby achieving relatively large adult brain size (1). Gene expression patterns related to postnatal development of the prefrontal cortex are delayed in humans compared with chimpanzees and macaque monkeys (2). In addition, synapse maturation (3, 4) and axon myelination (5, 6) occur during later life history stages in humans compared with macaques. Furthermore, recent volumetric data obtained by using in vivo MRI demonstrates that human neural development and aging differ from those of our close nonhuman primate relatives (7–9). Together, these observations indicate that a marked delay in the developmental schedule of human neocortex may play an important role in the growth of connections that contribute to our species-specific cognitive abilities by providing greater opportunities for social learning to influence the establishment of circuits.
The myelin lipid bilayer surrounding neuronal axons is crucial to normal brain function in vertebrates. Myelination results from the dynamic integration of neuron–oligodendrocyte signaling to promote saltatory action potential conduction (10). Specifically, myelination increases in response to electrical excitation and activity-dependent molecular cascades, protecting the axon from damage and significantly increasing nerve impulse transmission speed (6, 11). Myelination is important in establishing connectivity in the growing brain by facilitating rapid and synchronized information transfer across neural systems, which is essential to higher-order cognitive functions (6). Despite this crucial role, only limited data exist comparing the development of myelination in humans with our closest living relatives, chimpanzees (8).
Neocortical myelin development is disrupted in certain neuropsychiatric disorders that profoundly affect cognition in humans. Studies of patients with schizophrenia consistently indicate abnormalities in cortical white matter tracts (12–14) and decreased myelin-related mRNA and protein expression (15–18), particularly during adolescence (19). For example, expression of 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP) and myelin-associated glycoprotein (MAG) is dysregulated in patients with schizophrenia (20–22). CNP is crucial during the initial stage of myelin growth as oligodendrocytes extend processes toward axons (23). MAG regulates later stages of neuron–oligodendrocyte interactions, providing trophic support to established axons while inhibiting neurite outgrowth (24).
A comparison of the timing of the maturation of myelin in the cerebral cortex between humans and nonhuman primates may provide insight into the evolution of human cognitive development and our vulnerability to psychiatric disorders. To clarify whether myelin growth differs in the neocortex of humans and chimpanzees, we investigated the development of myelinated fiber length density (MFLD) in the primary somatosensory area (area 3b), primary motor area (area 4), most rostral part of the prefrontal cortex (the frontopolar region, area 10), and prestriate visual cortex (area 18/V2) from histological preparations. We also analyzed developmental changes in myelin-related protein expression (i.e., CNP and MAG) in somatosensory areas (areas 3b/3a/1/2), motor area (area 4), frontopolar area (area 10), and visual cortex (areas 17/V1 and 18/V2). Aside from studies of brain size growth (1), to our knowledge, this analysis represents the largest sample of chimpanzee neural development investigated to date.
Results
All cortical regions in human and chimpanzee samples (Table S1) showed increasing myelination with age (Figs. 1 and 2). In adulthood, somatosensory, motor, frontopolar, and visual areas displayed regional differences in myelination that were qualitatively similar in both species (Fig. S1).
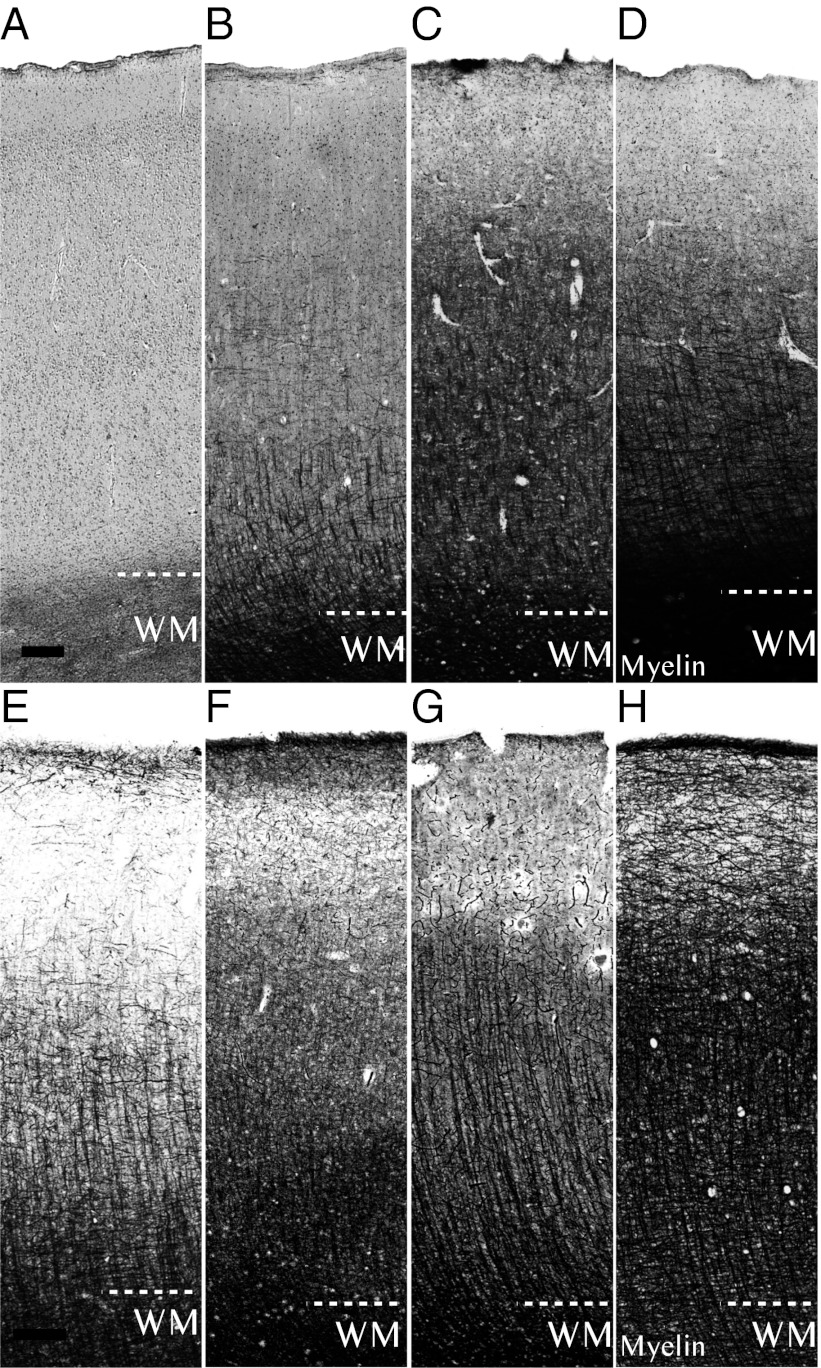
Fig. 1.
Developmental series of low-magnification photos of human and chimpanzee primary motor cortex. Sections from motor cortex (area 4) stained for myelinated axons (myelin) arranged by life-history stage. (A–D) Representative sections of human neocortical myelin as an A: infant (0–1 y), (B) child/juvenile (3–9 y), (C) adolescent/young adult (13–23 y), and (D) adult (≥28 y). (E–H) Sections of chimpanzee neocortical myelin as an E: infant (0–2 y), (F) juvenile (5–6 y), (G) adolescent (9–11 y), and (H) adult (≥17 y). White matter (WM) is demarcated at the bottom of the cortex. (Scale bar: 200 μm.)
Fig. 2.
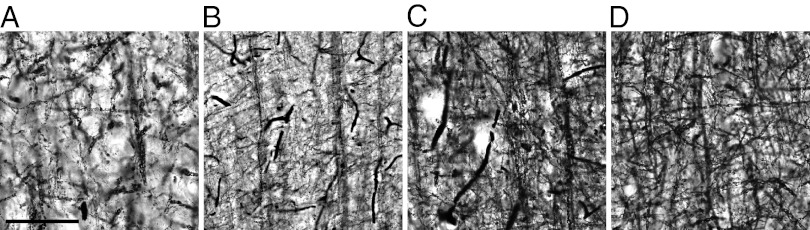
Developmental series of high-magnification photomicrographs of layer III in chimpanzee primary motor cortex. Representative photomicrographs of layer III of primary motor cortex (area 4) in chimpanzee tissue sections stained for myelin using the Gallyas preparation. Images are arranged by life-history stage: (A) infant (0–2 y), (B) juvenile (5–6 y), (C) adolescent (9–11 y), and (D) adult (≥17 y). (Scale bar: 50 μm.)
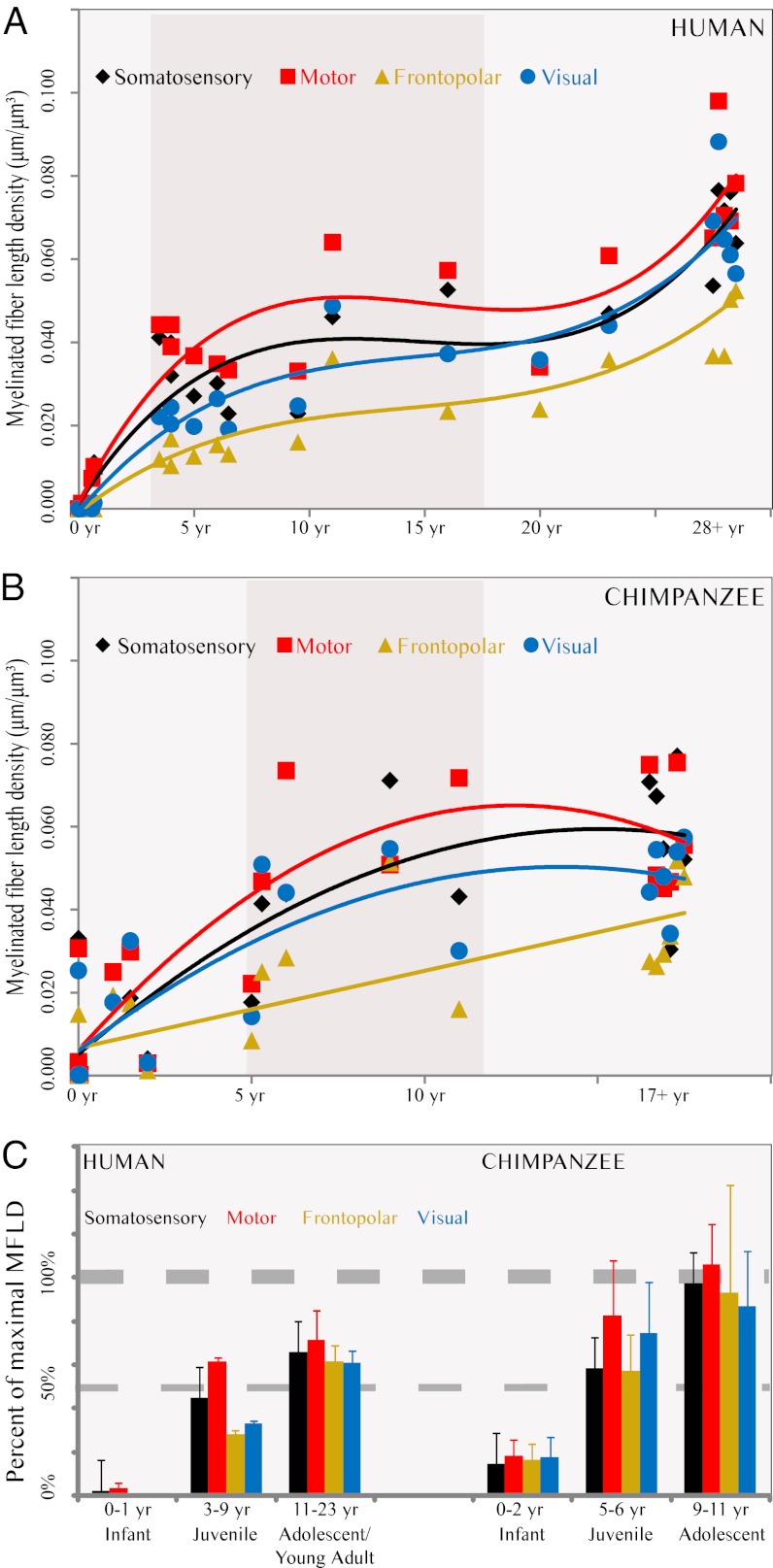
We used stereology to quantify MFLD within the gray matter of several cortical regions. MFLD in the cerebral cortex at birth in humans was extremely low (∼0.008 μm/μm3), but increased rapidly during infancy. After infancy, the rate of myelination slowed during childhood and adolescence, but exhibited continued growth until the end of the third decade. Specifically, mean MFLD during later adulthood (defined as ≥28 y) was significantly greater than in adolescence and early adulthood (11–23 y; Welch t, P = 0.000059; Mann–Whitney U, P = 0.00016). Accordingly, the developmental trajectory of cortical MFLD in the human sample was best fit by a cubic regression function for all regions (Fig. 3A and Table 1). Throughout life, human frontopolar cortex exhibited the lowest MFLD and the motor cortex had the highest average MFLD values.
Fig. 3.
Developmental trajectory of MFLD. Graphs show best-fit curves for MFLD data in humans (A; n = 24) and chimpanzees (B; n = 20) arranged by age in years. The shaded vertical area represents time between weaning and full sexual maturation. Diamonds represent somatosensory area (area 3b), squares represent motor area (area 4), triangles represent frontopolar area (area 10), and circles represent visual area (area 18). (C) Bar graph depicts mean percent of maximum mature adult MFLD across development in humans (Left) and chimpanzees (Right). Error bars represent SEM. The thin and thick horizontal dashed lines represent 50% and 100%, respectively, of maximum MFLD. Black represents somatosensory area (area 3b), red represents motor area (area 4), gold represents frontopolar area (area 10), and blue represents visual area (area 18).
Table 1.
Best-fit regression functions for myelinated fiber density data
| Function | Function | Adjusted R2 | P value |
| Human | |||
| Somatosensory | Cubic | 0.883 | 0.002 |
| Motor | Cubic | 0.884 | 0.001 |
| Frontopolar | Cubic | 0.878 | 0.034 |
| Visual | Cubic | 0.924 | 0.001 |
| Chimpanzee | |||
| Somatosensory | Quadratic | 0.733 | 0.061 |
| Motor | Quadratic | 0.722 | 0.006 |
| Frontopolar | Linear | 0.573 | 0.001 |
| Visual | Quadratic | 0.687 | 0.032 |
Values calculated by using hierarchical multiple regression analysis with adults as endpoint (Materials and Methods).
In contrast to humans, MFLD in chimpanzees displayed a consistent rate of increase until the time of puberty, at which time fibers became maximally dense. Thus, MFLD in adult chimpanzees (≥17 y) was not significantly different from juveniles (5–11 y; Welch t, P > 0.785; Mann–Whitney U, P > 0.848). Furthermore, the developmental trajectory of MFLD in the chimpanzee sample was best fit by a linear model for frontopolar cortex, and by quadratic regression functions for the other regions (Fig. 3B and Table 1). Overall, like humans, the frontopolar cortex exhibited the lowest MFLD and motor cortex had the highest average MFLD values throughout development.
To represent the relative degree of MFLD maturation at different developmental stages in each species, we calculated the percentage of values from the adult mean. Whereas human infants have less than 2% of maximal adult-like MFLD and achieve only ∼60% during adolescence, chimpanzee infants have ∼20% and attain nearly 96% maximal MFLD during adolescence (Fig. 3C and Table S2).
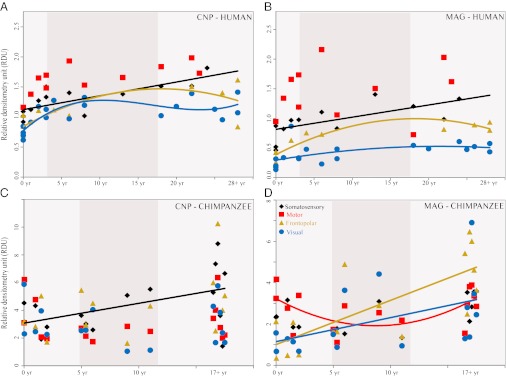
To examine whether the observed differences between humans and chimpanzees in the developmental progression of MFLD is related to species differences in the expression of proteins that are important in regulating myelination, we performed Western blot analyses of MAG and CNP. Overall, MAG and CNP protein expression tended to increase with age in both species, although some cortical regions did not exhibit a significant association with age in our samples (Fig. 4, Figs. S2 and S3, and Table S3). Notably, the age-related effects for MAG and CNP protein expression were gradual linear or quadratic increases, which differ from the distinctive pattern of extended childhood and continued postadolescent myelin growth observed in humans for MFLD.
Fig. 4.
Developmental trajectory of MAG and CNP protein. Graphs depict best-fit curves for densitometric analyses in relative densitometry units of CNP (A and C) and MAG (B and D) protein expression in humans (A and B; n = 23) and chimpanzees (C and D: n = 16) arranged by age in years. The shaded vertical area represents time between weaning and full sexual maturation. Trend lines represent a significant effect of age at the P ≤ 0.05 level. Diamonds represent somatosensory area (area 3b/3a/1/2), squares represent motor area (area 4), triangles represent frontopolar area (area 10), and circles represent visual area (areas 17 and 18). Note the different scales for relative densitometry units between species because Western blotting was performed on frozen human cortical samples, whereas, in chimpanzees, samples were formalin-fixed.
Discussion
Our data demonstrate that the developmental trajectory of neocortical myelination in humans is distinct compared with chimpanzees. Humans are born with fewer myelinated axons compared with chimpanzees, and prolong myelin growth well beyond adolescence. By using stereologic methods to analyze the length density of myelinated axon fibers within the gray matter of the cerebral cortex, we also corroborated previous observations based on histological and neuroimaging techniques, indicating that myelin develops in primary cortical areas before association cortical areas (5–7, 25–28). The infant, juvenile, and adolescent stages are periods of intense learning in primates. The convergence of neural growth with social and environmental factors during these periods mediates cognition in crucial ways, particularly given the extended lifespan and enriched cultural context of humans (29). Therefore, evolutionary modification of the developmental schedule of neural connectivity in humans might contribute to the formation of functional circuitry with greater plasticity, simultaneously contributing to unique species-specific pathological vulnerability to schizophrenia and other psychiatric disorders.
Myelination is ubiquitous throughout the nervous system of vertebrates and plays a dynamic role in regulating the functional activity of axons (10, 11). The myelin sheath is therefore crucial to normal neurological function, and the density of myelinated axons is commonly used to assess the relative maturity of brain regions. Traditionally, histological studies of myelin growth have been qualitative, making direct ontogenetic comparisons between species difficult (5, 6, 30–37). Nonetheless, previous studies (5, 6) described greater initial myelination of the cerebral cortex in neonatal macaque monkeys compared with humans. Our data provide further evidence that the lack of myelination in the newborn human neocortex is distinct from our closest living phylogenetic relatives, indicating that a greater fraction of mature adult-like neocortical myelination is achieved before birth in chimpanzees (∼20%) compared with humans (∼0%). The relative absence of myelin in the neonatal human cortex complements other data showing that, at the time of birth, human brains are a relatively small proportion of their adult size (27%) compared with chimpanzees (36%) (38), and that much of postnatal brain expansion results from the growth of white matter underlying the neocortex (8, 25). Together, these observations suggest that activity-mediated myelin growth early in human life has the capacity to be shaped by postnatal environmental and social interactions to a greater degree than in other primates, including chimpanzees.
Later myelin growth also differentiates humans and chimpanzees. Our data demonstrate that, in chimpanzees, the density of myelinated axons reaches its maximum level by adolescence for most cortical regions. Notably, we found a gradual linear trend of increasing MFLD beyond adolescence in frontopolar cortex. This is similar to results from histological and neuroimaging studies of macaque monkeys indicating that the growth of cortical myelination and the volume of the underlying white matter are largely complete by puberty (5, 7, 8). In contrast, we found that pronounced increases in the density of myelinated axons in the human neocortex continue after adolescence and into the third decade, providing further evidence of extraordinary prolonged neocortical maturation. MRI studies also indicate that growth in the volume of the cortical white matter persists well beyond puberty (25–28, 39). Additionally, in humans, neocortical dendritic development and synaptogenesis exhibit heterogeneity across the processing hierarchy, with the greatest delay in maturation characterizing the prefrontal cortex (40–44). Synaptic pruning in human prefrontal cortex has been shown to continue until age 30 y (4). These neuroanatomical findings are congruent with recent evidence indicating widespread delay in the gene expression profile of the human prefrontal cortex compared with chimpanzees and macaque monkeys (2). In sum, these species differences suggest that neural development in humans is characterized by a number of features that prolong the process of neocortical maturation.
Given that humans and chimpanzees displayed divergent growth trends for myelinated axon density in the neocortex, we sought to explore the potential molecular mechanisms regulating these species differences by analyzing the expression of CNP and MAG, two proteins that contribute to distinct aspects of myelin development. CNP protein facilitates oligodendrocyte differentiation and process outgrowth early in the myelination process (45, 46), whereas MAG is important in oligodendrocyte–neuron signaling, such that it regulates axon caliber and contributes to axon growth cone collapse, maintaining established axons at the expense of novel growth (24, 47, 48). The results from Western blotting for CNP and MAG, however, did not reveal developmental changes in myelin-associated protein expression in humans that mirrored the cubic function of the MFLD curve. Indeed, the postnatal developmental regulation of CNP and MAG was modest compared with the more dramatic anatomical changes in MFLD in both species. The molecular mechanisms responsible for the evolution of prolonged myelination of the cerebral cortex in humans remain incompletely understood, and further research with other myelin-related genes, more sensitive techniques, and larger samples is needed.
The evolutionary significance of prolonged myelination in humans is uncertain. One possibility is that additional postpubertal growth of myelination is a byproduct of alterations to earlier life history stages in human evolution, such as an extended period of slow childhood growth (49). An alternative hypothesis is that there has been selection on changes to the neural circuitry mediating learning and memory following the time of sexual maturity. There are well-documented changes to executive and social cognitive functions that characterize adolescence in humans, presumably related to the growth of myelination and white matter tracts, including improvements in selective attention, decision-making, inhibitory control, working memory, and perspective-taking (50). Unfortunately, comparable data are not available to determine whether chimpanzees or other primates undergo such dramatic transformations of cognitive abilities after puberty.
Sustained postpubertal myelin growth, which is unique to humans, might be associated with our species-specific vulnerability to certain psychiatric diseases that display onset during adolescence and early adulthood. Previous research has identified abnormal myelination in a number of psychiatric diseases that may derive from the disruption of normal postnatal development (51). For example, schizophrenia is associated with abnormal myelination of the cortical white matter, particularly in the prefrontal cortex (52). Molecular studies also implicate disrupted myelin-related processes throughout the forebrain, such as CNP and MAG gene or protein expression, in the pathogenesis of schizophrenia (14–22).
Accumulating evidence suggests that adolescence and young adulthood constitute a novel period in the evolution of human neurobiological development (1–4, 7, 28, 49, 53). Our findings extend this observation to the ontogeny of myelination, which follows a distinctively delayed and prolonged pattern of development in humans. These results invite further exploration of the molecular and systems-level mechanisms that mediate plasticity and learning that are unique to the human species, as these are not only important for normal cognition and adaptive behavior, but may also be crucial in the emergence of certain psychiatric disorders.
Materials and Methods
Sample and Preparation.
The study sample consisted of postmortem brains from a total of 33 humans (Homo sapiens, 16 male and 17 female) and 20 common chimpanzees (Pan troglodytes, 13 male and 7 female), ranging in age from birth to adulthood (Table S1). The human histological sample was drawn from the Yakovlev–Haleem slide collection (n = 24) at the National Museum of Health and Medicine. These sections were cut at 35 μm and stained for myelin with the Loyez method (6). Additional human samples were used for Western blotting and for examination of the effect of different myelin staining techniques on the results. These additional frozen and fixed human cortical tissue samples were obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders at the University of Maryland (Baltimore, MD; n = 9). Other frozen human samples that were used for Western blotting were obtained from the laboratory of Nenad Šestan (Yale University School of Medicine, New Haven, CT; full details provided in ref. 54). All human brain specimens originated from individuals free of neurological or psychiatric disorders. The same chimpanzee sample was used for histology and Western blotting, and it included formalin-fixed brains or dissected blocks collected from various research institutions (SI Materials and Methods and Table S1). The chimpanzee cortical samples were sectioned at 40 μm, every 10th section was stained for Nissl substance to reveal cytoarchitecture, and an adjacent 1-in-10 series was stained to visualize myelin using the Gallyas method (55). No neurological deficits were detected in any of the chimpanzees included in this study, and all brains appeared normal on routine inspection at necropsy. All histological sections from humans and chimpanzees appeared free of neuropathologic processes. Tissue samples were randomly coded to prevent observer bias in stereologic quantification and Western blotting; however, this was not feasible for the Yakovlev–Haleem slide collection. We did not examine sex differences because of limited tissue availability.
Stereology.
All quantification of MFLD was performed by the same observer (D.J.M.) by using Stereo Investigator software (MBF Bioscience). MFLD was calculated from sections stained for myelin at high magnification (≥60×; SI Materials and Methods) using a 6-μm SpaceBalls probe under Koehler illumination (56) in ∼90 sampling locations per region in each individual (17,550 total sampling locations; 9,450 in humans and 8,100 in chimpanzees). Curved portions of the cortical mantle were avoided when defining regions of interest for length density measurement.
Reliability of Results Between Different Myelin Staining Methods.
Because the stereologic quantification of MFLD in humans used sections stained for myelin with the Loyez method and the chimpanzee sections were stained with the Gallyas method, we sought to determine the reliability of results across these techniques. We compared MFLD between the different myelin stains in chimpanzee cortical sections from two regions (motor and frontopolar cortex) and in individuals of five different ages and found a high degree of correlation (Pearson adjusted R2 = 0.576, P = 0.007; Spearman adjusted R2 = 0.521, P = 0.011; n = 10; Fig. S4). Additionally, sections from human frontopolar cortex stained by using the Gallyas technique showed a significant cubic trajectory for MFLD, similar to that observed with the Loyez stain (adjusted R2 = 0.006, n = 9; Fig. S5). Chimpanzee sections stained using the Loyez technique show similar results for age-related changes in MFLD to those obtained with the Gallyas stain. Motor cortex was best fit by a quadratic model (adjusted R2 = 0.921, P = 0.006; n = 5; Fig. S6) and frontopolar cortex by a linear regression function (adjusted R2 = 0.921, P = 0.006; n = 5; Fig. S6).
Western Blot Analyses.
Western blotting was performed as described previously (57) with minor modifications. Fig. S3 shows results for Western blot validation using polyclonal anti-MAG (1:300; LifeSpan BioSciences) and monoclonal anti-CNP antibodies (1:300; Abcam). GAPDH (Imgenex) and β-actin (1:1,000; Santa Cruz Biotechnology) were used as loading controls to normalize expression levels. Densitometry analyses of Western blots were performed using Scion Image software (Fig. S3C). Full details of the Western blot assay are provided in SI Materials and Methods.
Statistical Methods.
The SPSS package was used for statistical analyses. For the calculation of best-fit regression curves depicting growth effects in MFLD and protein expression during development, we grouped older adult values together to summarize variability in each species because our aim was to focus on the trajectory of earlier developmental changes. Regression curves were calculated by using forward-selection multiple regression for linear, quadratic, and cubic functions (58). Age groups were determined based on life history stages (38, 49, 53), punctuated by the species-typical age at weaning (human, ∼3 y; chimpanzee, ∼5 y) and puberty (human, ∼11–14 y; chimpanzee, ∼8–10 y) (59, 60). Adult age was assigned as the age of the youngest postpubertal individual in the sample, except in humans in whom MFLD showed a significant difference between older (≥28 y) and younger (<28 y) adults (Results); mature adult age was thus assigned at 28 y. P values lower than 0.05 were considered significant.
Supplementary Material
Acknowledgments
The authors thank Drs. William Hopkins and Joseph Erwin for facilitating access to brain specimens; and Drs. Robin Bernstein, Shannon McFarlin, and James Steiger for helpful discussion related to this research. This work was supported by National Science Foundation Grants BCS-0515484, BCS-0549117, BCS-0824531, and DGE-0801634; National Institutes of Health Grants NS-42867, RR-00165, and U01 MH081896; and James S. McDonnell Foundation Grants 22002078, 220020165, and 220020293. A.M.M.S. is supported by a fellowship from the Portuguese Foundation for Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1117943109/-/DCSupplemental.
References
- 1.Leigh SR. Brain growth, life history, and cognition in primate and human evolution. Am J Primatol. 2004;62:139–164. doi: 10.1002/ajp.20012. [DOI] [PubMed] [Google Scholar]
- 2.Somel M, et al. Transcriptional neoteny in the human brain. Proc Natl Acad Sci USA. 2009;106:5743–5748. doi: 10.1073/pnas.0900544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 4.Petanjek Z, et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. doi: 10.1073/pnas.1105108108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibson KR. Sequence of Myelinization in the Brain of Macaca mulatta. Berkeley: University of California; 1970. [Google Scholar]
- 6.Yakovlev PI, Lecours A. The myelogenetic cycles of regional maturation of the brain. In: Minkowski A, editor. Regional Development of the Brain in Early Life. Oxford: Blackwell Science; 1967. pp. 3–70. [Google Scholar]
- 7.Knickmeyer RC, et al. Maturational trajectories of cortical brain development through the pubertal transition: Unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakai T, et al. Differential prefrontal white matter development in chimpanzees and humans. Curr Biol. 2011;21:1397–1402. doi: 10.1016/j.cub.2011.07.019. [DOI] [PubMed] [Google Scholar]
- 9.Sherwood CC, et al. Aging of the cerebral cortex differs between humans and chimpanzees. Proc Natl Acad Sci USA. 2011;108:13029–13034. doi: 10.1073/pnas.1016709108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simons M, Trajkovic K. Neuron-glia communication in the control of oligodendrocyte function and myelin biogenesis. J Cell Sci. 2006;119:4381–4389. doi: 10.1242/jcs.03242. [DOI] [PubMed] [Google Scholar]
- 11.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis KL, et al. White matter changes in schizophrenia: Evidence for myelin-related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 13.Schlösser RG, et al. White matter abnormalities and brain activation in schizophrenia: A combined DTI and fMRI study. Schizophr Res. 2007;89:1–11. doi: 10.1016/j.schres.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Höistad M, et al. Linking white and grey matter in schizophrenia: Oligodendrocyte and neuron pathology in the prefrontal cortex. Front Neuroanat. 2009;3:9. doi: 10.3389/neuro.05.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dracheva S, et al. Myelin-associated mRNA and protein expression deficits in the anterior cingulate cortex and hippocampus in elderly schizophrenia patients. Neurobiol Dis. 2006;21:531–540. doi: 10.1016/j.nbd.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 16.Hakak Y, et al. Genome-wide expression analysis reveals dysregulation of myelination-related genes in chronic schizophrenia. Proc Natl Acad Sci USA. 2001;98:4746–4751. doi: 10.1073/pnas.081071198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullumsmith RE, et al. Expression of transcripts for myelination-related genes in the anterior cingulate cortex in schizophrenia. Schizophr Res. 2007;90:15–27. doi: 10.1016/j.schres.2006.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mitkus SN, et al. Expression of oligodendrocyte-associated genes in dorsolateral prefrontal cortex of patients with schizophrenia. Schizophr Res. 2008;98:129–138. doi: 10.1016/j.schres.2007.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris LW, et al. Gene expression in the prefrontal cortex during adolescence: Implications for the onset of schizophrenia. BMC Med Genomics. 2009;2:28. doi: 10.1186/1755-8794-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barley K, Dracheva S, Byne W. Subcortical oligodendrocyte- and astrocyte-associated gene expression in subjects with schizophrenia, major depression and bipolar disorder. Schizophr Res. 2009;112:54–64. doi: 10.1016/j.schres.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 21.Davis KL, Haroutunian V. Global expression-profiling studies and oligodendrocyte dysfunction in schizophrenia and bipolar disorder. Lancet. 2003;362:758. doi: 10.1016/S0140-6736(03)14297-3. [DOI] [PubMed] [Google Scholar]
- 22.Hof PR, et al. Loss and altered spatial distribution of oligodendrocytes in the superior frontal gyrus in schizophrenia. Biol Psychiatry. 2003;53:1075–1085. doi: 10.1016/s0006-3223(03)00237-3. [DOI] [PubMed] [Google Scholar]
- 23.Edgar JM, et al. Early ultrastructural defects of axons and axon-glia junctions in mice lacking expression of Cnp1. Glia. 2009;57:1815–1824. doi: 10.1002/glia.20893. [DOI] [PubMed] [Google Scholar]
- 24.Quarles RH. Myelin-associated glycoprotein (MAG): Past, present and beyond. J Neurochem. 2007;100:1431–1448. doi: 10.1111/j.1471-4159.2006.04319.x. [DOI] [PubMed] [Google Scholar]
- 25.Groeschel S, Vollmer B, King MD, Connelly A. Developmental changes in cerebral grey and white matter volume from infancy to adulthood. Int J Dev Neurosci. 2010;28:481–489. doi: 10.1016/j.ijdevneu.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw P, et al. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nat Neurosci. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- 29.Herrmann E, Call J, Hernàndez-Lloreda MV, Hare B, Tomasello M. Humans have evolved specialized skills of social cognition: The cultural intelligence hypothesis. Science. 2007;317:1360–1366. doi: 10.1126/science.1146282. [DOI] [PubMed] [Google Scholar]
- 30.Abrahám H, et al. Myelination in the human hippocampal formation from midgestation to adulthood. Int J Dev Neurosci. 2010;28:401–410. doi: 10.1016/j.ijdevneu.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 31.Benes FM. Myelination of cortical-hippocampal relays during late adolescence. Schizophr Bull. 1989;15:585–593. doi: 10.1093/schbul/15.4.585. [DOI] [PubMed] [Google Scholar]
- 32.Benes FM, Turtle M, Khan Y, Farol P. Myelination of a key relay zone in the hippocampal formation occurs in the human brain during childhood, adolescence, and adulthood. Arch Gen Psychiatry. 1994;51:477–484. doi: 10.1001/archpsyc.1994.03950060041004. [DOI] [PubMed] [Google Scholar]
- 33.Brody BA, Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. I. An autopsy study of myelination. J Neuropathol Exp Neurol. 1987;46:283–301. doi: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Gilles FH. Myelination in the neonatal brain. Hum Pathol. 1976;7:244–248. doi: 10.1016/s0046-8177(76)80035-4. [DOI] [PubMed] [Google Scholar]
- 35.Hildebrand C, Remahl S, Persson H, Bjartmar C. Myelinated nerve fibres in the CNS. Prog Neurobiol. 1993;40:319–384. doi: 10.1016/0301-0082(93)90015-k. [DOI] [PubMed] [Google Scholar]
- 36.Kinney HC, Brody BA, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. J Neuropathol Exp Neurol. 1988;47:217–234. doi: 10.1097/00005072-198805000-00003. [DOI] [PubMed] [Google Scholar]
- 37.Preuss TM, Goldman-Rakic PS. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991;310:429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- 38.Robson SL, Wood BA. Hominin life history: Reconstruction and evolution. J Anat. 2008;212:394–425. doi: 10.1111/j.1469-7580.2008.00867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartzokis G, et al. Lifespan trajectory of myelin integrity and maximum motor speed. Neurobiol Aging. 2010;31:1554–1562. doi: 10.1016/j.neurobiolaging.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Elston GN, Oga T, Fujita I. Spinogenesis and pruning scales across functional hierarchies. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 42.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- 43.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 44.Travis K, Ford K, Jacobs B. Regional dendritic variation in neonatal human cortex: A quantitative Golgi study. Dev Neurosci. 2005;27:277–287. doi: 10.1159/000086707. [DOI] [PubMed] [Google Scholar]
- 45.Gravel M, et al. 2′,3′-Cyclic nucleotide 3′-phosphodiesterase: A novel RNA-binding protein that inhibits protein synthesis. J Neurosci Res. 2009;87:1069–1079. doi: 10.1002/jnr.21939. [DOI] [PubMed] [Google Scholar]
- 46.Lee PR, Fields RD. Regulation of myelin genes implicated in psychiatric disorders by functional activity in axons. Front Neuroanat. 2009;3:4. doi: 10.3389/neuro.05.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li M, et al. Myelin-associated glycoprotein inhibits neurite/axon growth and causes growth cone collapse. J Neurosci Res. 1996;46:404–414. doi: 10.1002/(SICI)1097-4547(19961115)46:4<404::AID-JNR2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 48.Yin X, et al. Myelin-associated glycoprotein is a myelin signal that modulates the caliber of myelinated axons. J Neurosci. 1998;18:1953–1962. doi: 10.1523/JNEUROSCI.18-06-01953.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bogin B. Evolutionary perspective on human growth. Annu Rev Anthropol. 1999;28:109–153. doi: 10.1146/annurev.anthro.28.1.109. [DOI] [PubMed] [Google Scholar]
- 50.Blakemore SJ, Choudhury S. Development of the adolescent brain: implications for executive function and social cognition. J Child Psychol Psychiatry. 2006;47:296–312. doi: 10.1111/j.1469-7610.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 51.Bartzokis G. Schizophrenia: Breakdown in the well-regulated lifelong process of brain development and maturation. Neuropsychopharmacology. 2002;27:672–683. doi: 10.1016/S0893-133X(02)00364-0. [DOI] [PubMed] [Google Scholar]
- 52.Fields RD. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008;31:361–370. doi: 10.1016/j.tins.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leigh SR, Park PB. Evolution of human growth prolongation. Am J Phys Anthropol. 1998;107:331–350. doi: 10.1002/(SICI)1096-8644(199811)107:3<331::AID-AJPA9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 54.Kang HJ, et al. Spatio-temporal transcriptome of the human brain. Nature. 2011;478:483–489. doi: 10.1038/nature10523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pistorio AL, Hendry SH, Wang X. A modified technique for high-resolution staining of myelin. J Neurosci Methods. 2006;153:135–146. doi: 10.1016/j.jneumeth.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 56.Mouton PR, Gokhale AM, Ward NL, West MJ. Stereological length estimation using spherical probes. J Microsc. 2002;206:54–64. doi: 10.1046/j.1365-2818.2002.01006.x. [DOI] [PubMed] [Google Scholar]
- 57.Sherwood CC, et al. Neocortical synaptophysin asymmetry and behavioral lateralization in chimpanzees (Pan troglodytes) Eur J Neurosci. 2010;31:1456–1464. doi: 10.1111/j.1460-9568.2010.07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zar JH. Biostatistical Analysis. 5th Ed. Upper Saddle River, NJ: Prentice Hall; 2010. [Google Scholar]
- 59.Kennedy GE. From the ape’s dilemma to the weanling’s dilemma: Early weaning and its evolutionary context. J Hum Evol. 2005;48:123–145. doi: 10.1016/j.jhevol.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 60.Littleton J. Fifty years of chimpanzee demography at Taronga Park Zoo. Am J Primatol. 2005;67:281–298. doi: 10.1002/ajp.20185. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.