Viruses with lipid envelopes must fuse their membranes with those of host cells to transfer their genomes and initiate infection. Depending on the virus, the membrane fusion process can occur at the plasma membrane, or at intracellular membranes following the internalization of virus particles. Not surprisingly, the viral proteins responsible for membrane fusion are highly diverse, as are the mechanisms by which the fusion processes can be triggered; however, common themes have emerged as our understanding of membrane fusion has developed. In particular, an expanding repertoire of high-resolution structures of viral fusion proteins (F proteins; VFPs) in pre- and postfusion conformations has driven the field forward. From these, we know that many VFPs undergo substantial conformational changes during fusion, forming highly stable rod-like structures to draw the membranes together (1–3). Despite this, for VFPs other than influenza HA (4–7), high-resolution structures for all three major static conformations adopted during the virus life cycle, uncleaved prefusion, primed prefusion and postfusion, have remained incomplete. These limitations have hindered our development of insights into the fusion mechanism of viruses that, unlike influenza virus, enter cells at the plasma membrane at neutral pH. In a groundbreaking report in PNAS (8), Welch et al. solve the high-resolution X-ray crystal structure of the cleaved, prefusion form of the F protein of parainfluenza virus 5 (PIV5). This alters the state of affairs by providing an essential missing link in the understanding of paramyxovirus entry. In conjunction with previous work by these laboratories (9, 10), the new structure now affords us with a complete set of all three major static conformations of paramyxovirus F proteins (Fig. 1), a tour de force representing many years of effort (9, 10). This constitutes another fundamental building block on which we can establish a broader appreciation of how different viruses prime their surface glycoproteins to mediate cell entry and initiate infection. In addition to advancing our molecular knowledge of virus biology, the present achievement will further the educated development of antiviral drugs designed to inhibit membrane fusion.
Fig. 1.
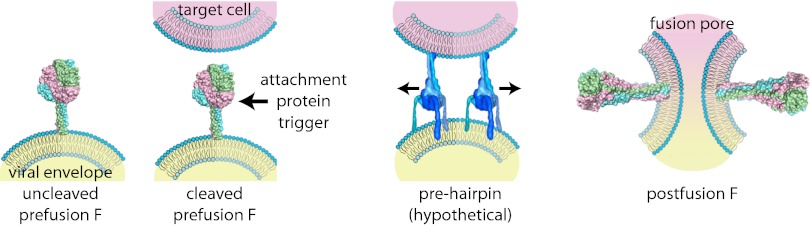
Cartoon of type I VFP-mediated viral entry, represented by example of the paramyxovirus F protein. Upon synthesis, uncleaved F assumes a metastable prefusion conformation (Left). Proteolytic maturation generates the fusion-ready prefusion form of the trimer (center, Left). Receptor binding by the associated attachment glycoprotein triggers major conformational changes of the primed F protein trimer. Refolding is considered to occur through a series of temporary conformations, including a hypothetical prehairpin intermediate (center, Right). Ultimately, the F protein assumes a thermodynamically highly stable postfusion conformation in which transmembrane domains and fusion peptides, and thus viral envelope and target membrane, are posited in close proximity (Right). Opening of a fusion pore enabling viral entry most likely requires concerted refolding of multiple F protein complexes. Structural renderings are based on original crystal structures [uncleaved prefusion F protein, Protein Data Bank (PDB) accession no. 2B9B; cleaved prefusion F protein from Welsh et al. (8); postfusion F protein, PDB accession no. 1ZTM) or hypothetical structural models (F protein prehairpin intermediate).
During the past decade, the expansion of our structural and mechanistic understanding of VFPs has allowed for their grouping into type I, II, and III classes (11, 12). We focus here on the type I VFPs that include influenza HA and paramyxovirus F proteins, as well as VFPs of other major human pathogens such as HIV Env and Ebola virus GP. Hallmarks of type I VFPs include initial synthesis and folding into an oligomeric precursor structure, which requires proteolytic processing into a mature form to prime the membrane fusion potential and virus infectivity (13–16). Each monomer is cleaved directly adjacent to an internal hydrophobic domain, the “fusion peptide,” which then forms the newly liberated N-terminal section of the membrane-anchored subunit. When it has been activated by proteolysis, the prefusion conformations must be triggered by external stimuli such as engagement of a cellular receptor in the case of paramyxoviruses, or the acidic environment of an endosome as found with influenza, to undergo the extensive structural rearrangements that drive the membrane fusion process (Fig. 1). Therefore, over the course of the virus replication cycle, the F proteins adopt at least three distinct static conformations, which are, along with the transitions from one structure to the next, critical for virus entry.
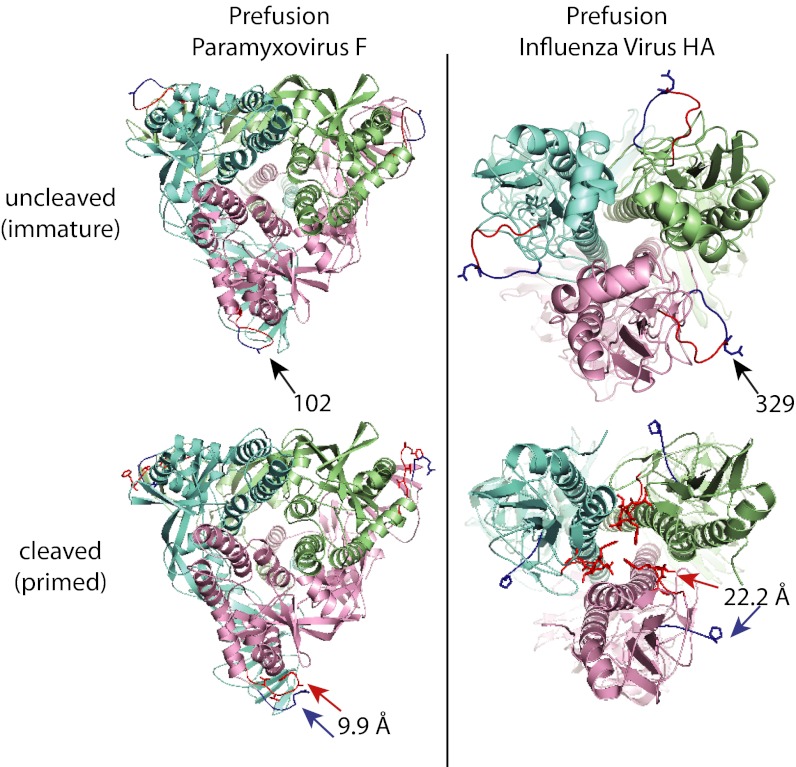
The structural rearrangements that accompany cleavage activation of influenza HA are revealing with regard to the subsequent triggering of fusion by acidification of endosomes. In particular, following cleavage of the external loop structure of uncleaved HA, part of the fusion peptide inserts into the trimer interior and forms contacts with a number of highly conserved ionizable residues (6). This requires considerable movement of the newly liberated N terminus (Fig. 2). Subsequent exposure to low pH conditions of the endosome is then thought to protonate these ionizable residues, driving the fusion peptide from its buried position and propelling it toward the target membrane (6, 17). Although this provides a compelling case for modeling the fusion strategy for viruses that use low pH as a trigger, it leaves unanswered the question of whether VFPs of viruses with pH-independent entry mechanisms, such as members of the Paramyxovirinae subfamily, use similar strategies to temporarily shield the fusion peptide domain after cleavage and, if so, how the buried fusion peptide becomes expelled.
Fig. 2.
Ribbon representations of immature and mature prefusion paramyxovirus F protein [Left, PDB accession no. 2B9B and Welsh et al. (8)] and influenza virus HA (Right, PDB accession nos. 1HA0 and 2IBX) protein trimers, colored by monomer. Residues immediately upstream to the cleavage sites are colored dark blue, whereas residues immediately downstream representing the N-terminal sections of the fusion peptides are shown in red. Numbers and arrows (Upper) specify residue upstream to the cleavage sites in one of the monomers. Upon proteolytic maturation, only minor relocation of the newly generated N- and C-termini are observed in paramyxovirus F protein, whereas the N-terminal region of the influenza virus HA fusion peptide inserts deeply into the trimer interior (arrows, Lower). Numbers represent distance between chain termini after cleavage.
Addressing this question, the structure of PIV5 fusion reported by Welch et al. (8) supports the concept that paramyxoviruses, and possibly all VFPs associated with viruses that fuse membranes at neutral pH, use a fundamentally different approach from influenza virus HA for cleavage activation of fusion potential. Unlike influenza HA, which mediates attachment and membrane fusion, PIV5 encodes separate attachment and membrane F proteins. Cell entry is achieved by a cooperative process involving attachment of PIV5 HN to a cellular receptor and subsequent interaction with primed F protein to promote the structural changes that drive the fusion process.
For PIV5 fusion, the bulk of the hydrophobic fusion peptide is buried in immature, uncleaved F (10). Upon proteolytic maturation, only minor rearrangements of the newly liberated N terminus were observed, whereas residues adjacent to the cleavage site show little movement and remain largely solvent-protected. The greatest increase in structural flexibility was noted for the arginine residue at the newly generated C terminus (Fig. 2). Conceivably, these comparably minor rearrangements could be sufficient to allow productive interaction of the mature PIV5 F protein with the paramyxovirus attachment protein, resulting in the formation of primed, functional fusion complex heterooligomers. For PIV5 and other members of the paramyxovirus family (18), receptor binding by the attachment protein can then functionally replace acidification of influenza virus HA, triggering the conformational rearrangements of the associated F proteins that propel the fusion peptide toward the target membrane. At present, it remains unclear whether expulsion of the paramyxovirus fusion peptide from its buried position is a consequence of attachment protein-induced fusion refolding, or whether, rather, the receptor-complexed attachment protein directly acts on the F protein fusion loop. In the latter scenario, relocating the fusion peptide domain from its protected position may, in fact, initiate the major conformational changes of the F protein trimer.
The complete set of structural data available now for the major static conformations of the paramyxovirus F protein sets the stage to address these questions experimentally and ultimately develop a full mechanistic pathway of paramyxovirus entry. Without doubt, the continued accumulation of structural, biochemical, and functional data on a range of pH-dependent and pH-independent VFPs will allow for extension and modification of our views of cell entry by enveloped viruses; however, we now have a new key set of fundamental structures on which to build and develop this understanding.
Footnotes
The authors declare no conflict of interest.
See companion article on page 16672.
References
- 1.Harrison SC. Viral membrane fusion. Nat Struct Mol Biol. 2008;15:690–698. doi: 10.1038/nsmb.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skehel JJ, Wiley DC. Coiled coils in both intracellular vesicle and viral membrane fusion. Cell. 1998;95:871–874. doi: 10.1016/s0092-8674(00)81710-9. [DOI] [PubMed] [Google Scholar]
- 3.Plemper RK. Cell entry of enveloped viruses. Curr Opin Virol. 2011;1:92–100. doi: 10.1016/j.coviro.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bizebard T, et al. Structure of influenza virus haemagglutinin complexed with a neutralizing antibody. Nature. 1995;376:92–94. doi: 10.1038/376092a0. [DOI] [PubMed] [Google Scholar]
- 5.Bullough PA, Hughson FM, Skehel JJ, Wiley DC. Structure of influenza haemagglutinin at the pH of membrane fusion. Nature. 1994;371:37–43. doi: 10.1038/371037a0. [DOI] [PubMed] [Google Scholar]
- 6.Chen J, et al. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell. 1998;95:409–417. doi: 10.1016/s0092-8674(00)81771-7. [DOI] [PubMed] [Google Scholar]
- 7.Wilson IA, Skehel JJ, Wiley DC. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 A resolution. Nature. 1981;289:366–373. doi: 10.1038/289366a0. [DOI] [PubMed] [Google Scholar]
- 8.Welch BD, et al. Structure of the cleavage-activated prefusion form of the parainfluenza virus 5 fusion protein. Proc Natl Acad Sci USA. 2012;109:16672–16677. doi: 10.1073/pnas.1213802109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baker KA, Dutch RE, Lamb RA, Jardetzky TS. Structural basis for paramyxovirus-mediated membrane fusion. Mol Cell. 1999;3:309–319. doi: 10.1016/s1097-2765(00)80458-x. [DOI] [PubMed] [Google Scholar]
- 10.Yin HS, Wen X, Paterson RG, Lamb RA, Jardetzky TS. Structure of the parainfluenza virus 5 F protein in its metastable, prefusion conformation. Nature. 2006;439:38–44. doi: 10.1038/nature04322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kielian M, Rey FA. Virus membrane-fusion proteins: More than one way to make a hairpin. Nat Rev Microbiol. 2006;4:67–76. doi: 10.1038/nrmicro1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White JM, Delos SE, Brecher M, Schornberg K. Structures and mechanisms of viral membrane fusion proteins: Multiple variations on a common theme. Crit Rev Biochem Mol Biol. 2008;43:189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Homma M, Ouchi M. Trypsin action on the growth of Sendai virus in tissue culture cells. 3. Structural difference of Sendai viruses grown in eggs and tissue culture cells. J Virol. 1973;12:1457–1465. doi: 10.1128/jvi.12.6.1457-1465.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klenk HD, Rott R, Orlich M, Blödorn J. Activation of influenza A viruses by trypsin treatment. Virology. 1975;68:426–439. doi: 10.1016/0042-6822(75)90284-6. [DOI] [PubMed] [Google Scholar]
- 15.Lazarowitz SG, Choppin PW. Enhancement of the infectivity of influenza A and B viruses by proteolytic cleavage of the hemagglutinin polypeptide. Virology. 1975;68:440–454. doi: 10.1016/0042-6822(75)90285-8. [DOI] [PubMed] [Google Scholar]
- 16.Scheid A, Choppin PW. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974;57:475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- 17.Thoennes S, et al. Analysis of residues near the fusion peptide in the influenza hemagglutinin structure for roles in triggering membrane fusion. Virology. 2008;370:403–414. doi: 10.1016/j.virol.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Plemper RK, Brindley MA, Iorio RM. Structural and mechanistic studies of measles virus illuminate paramyxovirus entry. PLoS Pathog. 2011;7:e1002058. doi: 10.1371/journal.ppat.1002058. [DOI] [PMC free article] [PubMed] [Google Scholar]