Abstract
MUC5AC, a major gel-forming mucin expressed in the lungs, is secreted at increased rates in response to infectious agents, implying that mucins exert a protective role against inhaled pathogens. However, epidemiological and pathological studies suggest that excessive mucin secretion causes airways obstruction and inflammation. To determine whether increased MUC5AC secretion alone produces airway obstruction and/or inflammation, we generated a mouse model overexpressing Muc5ac mRNA ∼20-fold in the lungs, using the rCCSP promoter. The Muc5ac cDNA was cloned from mouse lungs and tagged internally with GFP. Bronchoalveolar lavage fluid (BALF) analysis demonstrated an approximate 18-fold increase in Muc5ac protein, which formed high-molecular-weight polymers. Histopathological studies and cell counts revealed no airway mucus obstruction or inflammation in the lungs of Muc5ac-transgenic (Muc5ac-Tg) mice. Mucus clearance was preserved, implying that the excess Muc5ac secretion produced an “expanded” rather than more concentrated mucus layer, a prediction confirmed by electron microscopy. To test whether the larger mucus barrier conferred increased protection against pathogens, Muc5ac-Tg animals were challenged with PR8/H1N1 influenza viruses and showed significant decreases in infection and neutrophilic responses. Plaque assay experiments demonstrated that Muc5ac-Tg BALF and purified Muc5ac reduced infection, likely via binding to α2,3-linked sialic acids, consistent with influenza protection in vivo. In conclusion, the normal mucus transport and absence of a pulmonary phenotype in Muc5ac-Tg mice suggests that mucin hypersecretion alone is not sufficient to trigger luminal mucus plugging or airways inflammation/goblet cell hyperplasia. In contrast, increased Muc5ac secretion appears to exhibit a protective role against influenza infection.
Keywords: Glycoproteins, mucociliary clearance, COPD
Airway mucus is a complex hydrophilic gel composed of gel-forming mucins, globular proteins, salt, and water. The gel-like properties of interpenetrating mucins serve to trap and clear inhaled pathogens, protecting the lungs against infection. Of the gel-forming mucins, only two, MUC5AC and MUC5B, are expressed in the lungs (1). Mucin protein cores of MUC5AC and MUC5B are decorated by a diverse repertoire of carbohydrate moieties, including sialic acid and sulfate residues, which interact with inhaled particulates, including pathogens. MUC5B is primarily secreted by submucosal glands, but also by airway goblet cells (2, 3), and is thought to play mostly a protective role. In contrast, MUC5AC, which is exclusively produced by goblet cells (4), has been thought to play more of a pathologic role because of greatly increased MUC5AC expression with disease states (5–8).
Clearance of mucus is an essential component of the innate defense required for respiratory health. Normally, a thin mucus layer on airway surfaces is efficiently transported by coordinated beating of cilia. Mucin hypersecretion has been speculated to compromise mucociliary clearance (MCC) and, therefore, a role for increased mucin secretion has been proposed in the pathogenesis of airway diseases (9, 10). Indeed, postmortem and airway biopsy studies support a significant pathophysiologic role for mucus hypersecretion in airways obstruction and inflammation observed in severe forms of asthma and chronic obstructive pulmonary disease (COPD) (11, 12).
Although mucin hypersecretion is associated with chronic lung diseases, it is unclear whether mucin hypersecretion alone can produce mucus obstruction and/or inflammation or whether mucin hypersecretion requires an inflammatory component or insufficient hydration to produce muco-obstructive disease. Indeed, it is possible that mucin hypersecretion per se is beneficial to the host, increasing the capacity to trap and clear inhaled foreign materials. Animal models of mucin hypersecretion, e.g., ovalbumin-sensitized mice (13) and the βENaC transgenic (14) mouse models, exhibit muco-obstructive airway disease associated with inflammatory cell infiltrates but these models have not permitted dissection of the relative roles for mucin hypersecretion vs. inflammation in disease pathogenesis. The increasing interest in mucin hypersecretion as a therapeutic target argues for studies designed to understand the role of mucin hypersecretion in disease pathogenesis.
To distinguish between the possible roles of mucin hypersecretion in augmenting host defense vs. disease promoting muco-obstructive lung disease, a transgenic mouse model overexpressing Muc5ac in murine airways was generated. This model was used to ask whether mucin hypersecretion alone was sufficient to cause mucus plugging, drive goblet cell metaplasia, and/or cause airway inflammation. In parallel, we asked whether increased Muc5ac secretion provided benefit against inhaled pathogens, using a well-characterized influenza virus challenge model.
Results
Muc5ac-GFP cDNA Cloning and Expression.
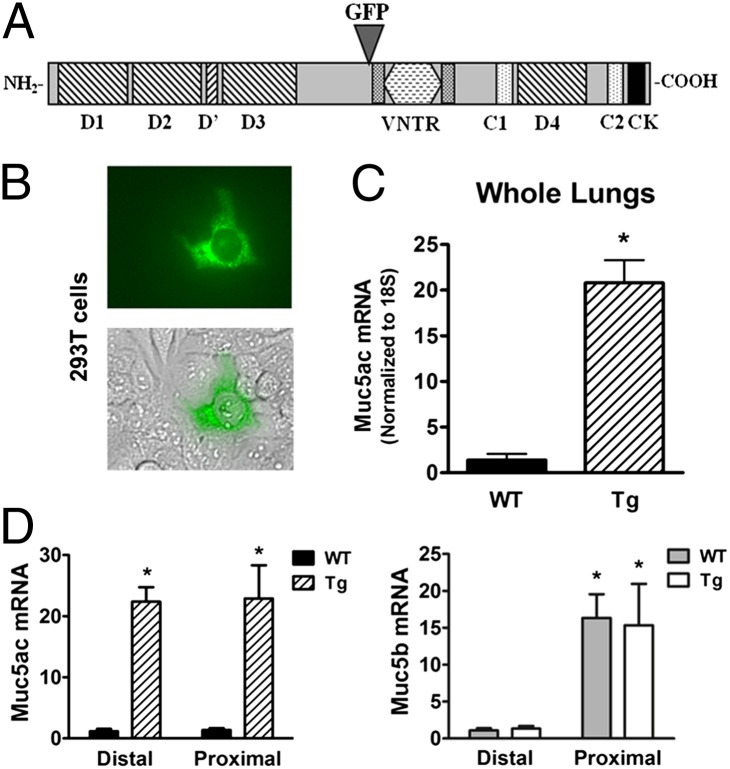
Muc5ac, like most secreted mucins, possesses a large central domain composed of variable numbers of tandem repeats (VNTRs), flanked by nonrepeat domains analogous to the von Willebrand factor domains that are essential for mucin function (Fig. 1A). Because secreted mucins use their N and C termini for multimerization and dimerization, respectively, GFP tagging required internal insertion. The entire Muc5ac cDNA was cloned, and the GFP tag was inserted immediately 5′ of the VNTR. The fusion product exhibited a final length of 9.1 kb (Fig. S1) and was placed under the control of the rat Clara cell secretory protein (rCCSP) promoter. Clara cells possess the entire secretory machinery to ensure posttranslational maturation of mucins and populate the large and small murine airways (15). Consequently, the rCCSP promoter selectively directs expression in the airways in a mucin-secretion–competent cell.
Fig. 1.
Muc5ac cloning and expression. Muc5ac cDNA was cloned from mRNA extracted from mouse lungs. (A) Diagram for Muc5ac peptide domains and localization of insertion of GFP tag. (B) Native GFP fluorescence from 293T cells transiently transfected with the CCSP-Muc5ac-GFP plasmid. (C) Fold increase mRNA expression in the whole lungs of transgenic (Tg) compared with wild-type (WT) mice. Values are means ± SE (n = 5); *P < 0.0005. (D) Muc5ac and Muc5b mRNA pattern of expression in WT and Muc5ac-Tg lungs, presented as a fold increase over WT expression in the distal lung. Proximal samples excluded trachea and submucosal glands. Values are means ± SE (n = 4); *P < 0.01.
Although 293T cells are not mucin-producing cells, this cell line was transiently transfected with Muc5ac-GFP cDNA to test the function of the GFP tag. Sporadic green fluorescent cells were detected after 24 h (Fig. 1B), which confirmed the proper insertion and function of GFP. Subsequently, blastocyst injections to generate transgenic mice overexpressing Muc5ac-GFP were performed in a C57BL/6 background. Founders were analyzed for mRNA and protein expression. A transgenic line that showed an approximate 20-fold increase in Muc5ac mRNA expression by quantitative PCR using whole lung extracts was selected for further study (P < 0.0005) (Fig. 1C).
The expression profile of endogenous Muc5ac and Muc5b was first analyzed in WT mice, using primers with comparable efficiency (each approaching 2) and normalizing to 18S. The levels of expression of endogenous Muc5ac were low and similar in proximal vs. distal airways (Fig. 1D). In contrast, Muc5b was the dominant secreted mucin in the WT strain but was expressed at much higher levels proximally than distally. Muc5ac-transgenic (Muc5ac-Tg) mice overexpressed Muc5ac mRNA by 21.3- and 22.1-fold for distal and proximal regions, respectively. Interestingly, mRNA measurements revealed that the levels of Muc5b transcripts were not affected by Muc5ac overexpression in either distal or proximal regions. Importantly, these data demonstrate that total secreted mucin mRNA levels in Muc5ac-Tg mice were greatly increased in distal airways (mainly due to expressed transgenic Muc5ac), whereas total secreted mucin mRNA levels approximately doubled in proximal airways (i.e., Muc5ac levels equalled Muc5b levels).
Muc5ac-GFP Secretion and Polymer Formation.
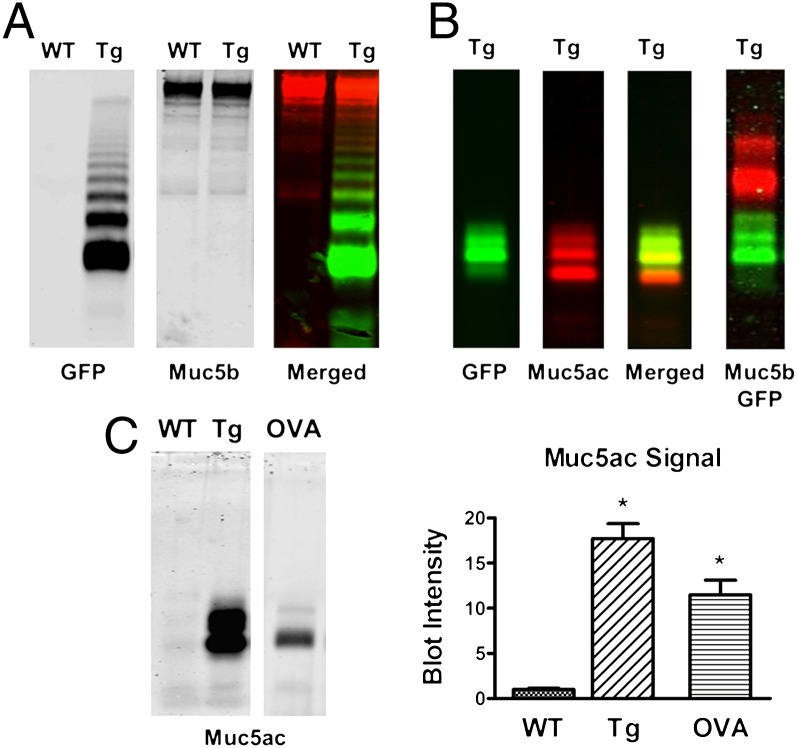
We next measured the levels of Muc5ac protein in transgenic vs. WT lungs and investigated whether the transgenic Muc5ac protein was secreted into the airway lumen. To test for proper maturation/assembly of secreted Muc5ac-GFP, we analyzed unreduced bronchoalveolar lavage fluid (BALF), using agarose Western blots, HPLC/light scattering, and CsCl density gradients. Lungs were lavaged and equal volumes of BALF were analyzed by Western blots, using GFP and/or specific mucin antibodies. Fig. 2A demonstrates a ladder-like migration pattern of unreduced Muc5ac-Tg BALF visualized with a GFP antibody, consistent with the secretion of high-molecular-weight multimers. Muc5b protein levels were unaffected by transgene expression (consistent with RNA data; Fig. 1D). The characteristic Muc5ac ladder pattern (16) confirmed proper maturation, secretion, and polymer formation of the transgene. In addition, Muc5ac-GFP–rich material was collected in the void fraction via HPLC and analyzed by refractometry (light scattering/refractive index) to determine molecular weight/mass (Mr = 5.1 × 107 g/mol) and radius of gyration/size (Rg = 228 nm), which were comparable to those of endogenous gel-forming mucins (e.g., Muc5b) (Fig. S2). Consistently, CsCl density gradient analysis confirmed that Muc5ac-GFP migrated through the gradient, which demonstrated that the exogenous mucin was multimerized and glycosylated (Fig. S3).
Fig. 2.
Mucin Western blots on BAL samples for protein function and quantification. BAL samples from WT, Tg, and ovalbumin-challenged (OVA) mice were separated via agarose electrophoresis gel and probed with antibodies, as indicated. Equal amounts of BAL were loaded on each lane. Fluorescent signal was detected for each gel but can be shown in black and white to optimize definition. (A) Unreduced BAL samples were analyzed for GFP migration pattern with anti-GFP antibody (goat) and Muc5b content with anti-Muc5b antibody (rabbit). (B) Validation of a unique Muc5ac antibody (UNC294) on reduced BAL samples. (C) Muc5ac protein quantification from reduced BALF with integrated signal intensities shown on the bar graph. Values are means ± SE (n = 3); *P < 0.05.
Fig. 2B demonstrates the specificity of a unique polyclonal anti-murine Muc5ac antibody (UNC294) in reduced BALF. The merged image shows almost complete overlap between GFP and Muc5ac antibody signals, with the exception of a small band detected by the Muc5ac antibody, which may correspond to a cleaved mucin fragment. The Muc5ac antibody (UNC294) was then used for protein quantification on BALF after reduction. Note that Muc5ac signal in WT BALF was barely detectable (Fig. 2C). However, a distinct Muc5ac signal was detected in BAL samples collected from WT animals sensitized by ovalbumin (OVA) challenges that was ∼10.6-fold stronger than in naive mice. Signal intensity in Muc5ac-Tg mice was 17.7-fold stronger than in naive mice and 1.7-fold above that in OVA-challenged animals (P < 0.05). These data indicate a robust up-regulation and secretion of Muc5ac protein in the lungs of transgenic mice.
Lung Histology and Pulmonary Inflammation.
We next evaluated the impact of Muc5ac overexpression in the murine lung on histology and survival. Muc5ac-Tg mice exhibited the expected Mendelian distribution of genotypes at birth, indicating that Muc5ac-GFP overexpression in the lungs had no adverse effect on fetal development or survival. Postnatal survival reached 96% at 40 d after birth for Muc5ac-Tg mice and was comparable to WT animal survival (Fig. S4). Growth rates were similar between the two genotypes, suggesting that gel-forming mucin overexpression alone did not increase mortality rates or delay growth in this animal model (Fig. S4).
Standard lung histopathology revealed only rare alcian blue periodic acid–Schiff (AB-PAS)–positive cells detected in airway surfaces of transgenic animals, resembling the histology of WT animals, and there was no evidence of intraluminal mucus plaques or plugs in Muc5ac-Tg animals (Fig. S4). In sharp contrast, an OVA-exposed WT mouse exhibits numerous enlarged AB-PAS–positive cells throughout the lungs, coupled with intraluminal mucus plaques.
Airway mucus hyperproduction is typically associated with inflammatory cell infiltrates in human lung diseases. Therefore, we tested whether the Muc5ac-GFP–overexpressing mouse model exhibited evidence of increased airways inflammation. Total inflammatory cell counts from BAL revealed no differences in the total number of cells in Muc5ac-Tg mice compared with control littermates (Fig. S5A). The majority of cells in BALF in both genotypes were macrophages with occasional neutrophils, eosinophils, or lymphocytes (Fig. S5B). The macrophage morphology was not different in either cohort, suggesting no overt macrophage activation when Muc5ac was overexpressed (Fig. S5C). These results, coupled with the absence of inflammatory cells in histologic sections (Fig. S4B), indicate that mucin hypersecretion alone does not initiate inflammation.
Muc5ac-GFP Organization and Localization in the Lungs.
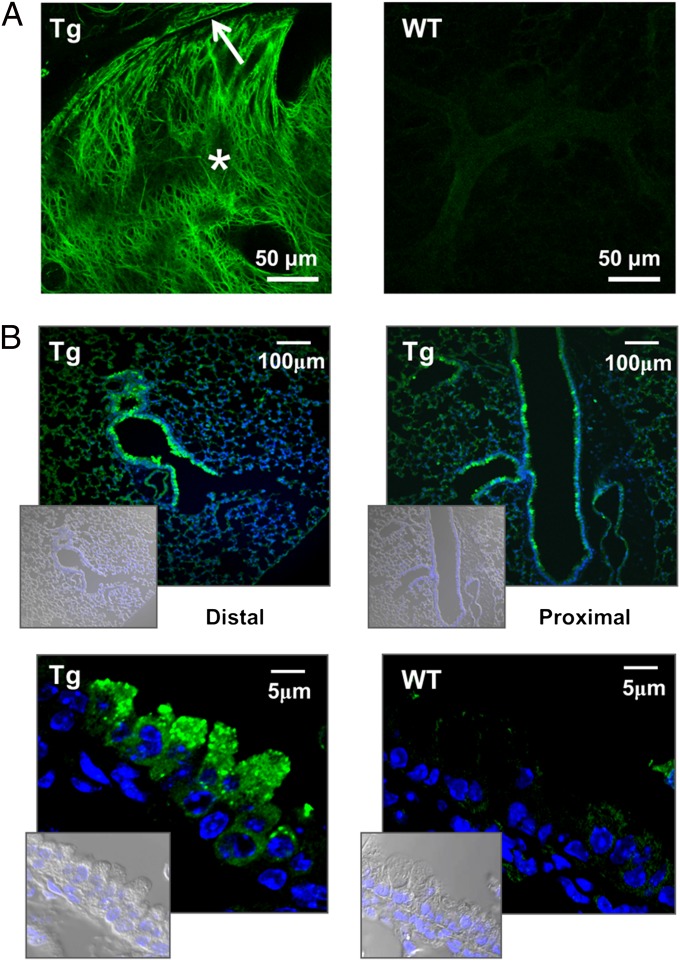
To test whether transgenic Muc5ac-GFP was able to organize in a characteristic gel-forming mucin network, i.e., mesh-like structure, the extracellular Muc5ac-GFP was allowed to accumulate postmortem in a trachea tied off at its proximal end over a 2-h period (Fig. 3A). The luminal contents of tracheal sections were observed in situ under a confocal microscope to visualize the native GFP organization. Transgenic animals displayed strong signals depicting complex networks of interpenetrating GFP-positive material, which resembles the entangled mesh of endogenous mucus (14, 17).
Fig. 3.
Muc5ac-GFP organization and protein expression. (A) Native Muc5ac-GFP signal from freshly harvested lungs to demonstrate polymer formation by the Muc5ac transgene, i.e., GFP meshwork in Tg animals. Images show cross-sections of mouse trachea, tied proximally for 2 h posteuthanasia to allow mucus to accumulate into the lumen (*). Arrow points at the mucus blanket lining the airways of Tg animals. WT, wild-type control. (B) Retrieved GFP signal from fixed lungs. Proximal and distal airway sections from transgenic mice were stained with a polyclonal anti-GFP antibody and DAPI, for nuclear stain. (Insets) Corresponding DIC/DAPI images are shown. WT image are shown as a control.
Localization of cells expressing GFP fluorescence in freshly excised or frozen sections was limited because of poor structural preservation and/or a weakened GFP signal (as shown in Fig. S6). Therefore, GFP immunohistochemistry was performed on fixed tissue. It has been shown that immunohistochemistry is a sensitive technique that can detect mucins in cells despite the lack of AB-PAS staining (18, 19). Note that the reported distribution of Muc5b-producing cells in WT mice, i.e., increasing numbers in the proximal airways (18), correlated with our real-time PCR data (Fig. 1D). Immunohistochemistry detected GFP-positive cells throughout the airways of Muc5ac-Tg mice (Fig. 3B). Consistent with previously published Clara cell distributions (15), large and small airways both exhibited significant numbers of GFP-positive cells. In rare cases, GFP-positive cells were also detected in alveolar regions (Fig. 3B and Fig. S6A). High-resolution images revealed the presence of GFP-positive granules in dome-shape–like cells, suggesting proper packaging of Muc5ac-GFP for maturation and regulated secretion by Clara cells. In the large airways of Muc5ac-Tg, but not of WT animals, the presence of an extracellular GFP- or Muc5ac-positive layer was detected (Figs. S7 and S8) and was further analyzed via electron microscopy.
Mucus Layer Depth and Effect on Mucociliary Clearance.
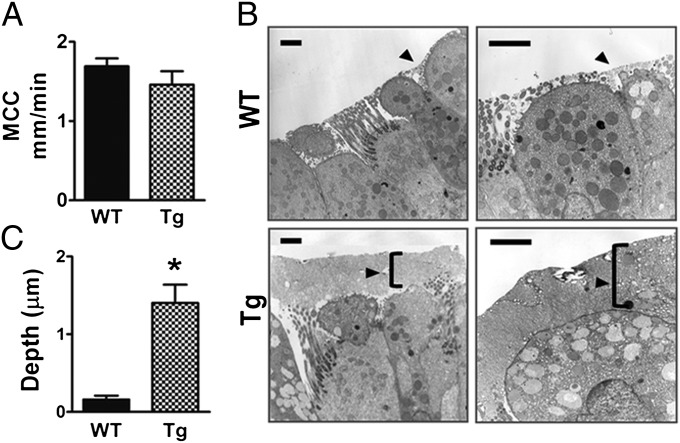
Biophysical modeling predicts that clearance rates will not be affected by mucus layer heights as long as the mucus concentration remains between 2% and 5% solid content (20). However, clearance rates are impaired above 6% solids, and clearance ceases at concentrations above 8% solids, i.e., in severe cases of dehydration. To test whether mucus clearance in vivo was affected by the overproduction of Muc5ac, we measured rates of clearance of fluorescent beads in tracheas of WT vs. Muc5ac-Tg mice (Fig. 4A). Clearance rates were measured during deep anesthesia and immediately after death because ciliary beat, and hence clearance, continues several hours postmortem (21). No significant differences in clearance rates were detected between the WT (1.69 ± 0.10 mm/min, n = 13) and Muc5ac-Tg (1.49 ± 0.17 mm/min, n = 11) animals, suggesting the presence of a sufficiently hydrated mucus that is removed efficiently from the surfaces. Thus, overexpression of Muc5ac is predicted, on the basis of these data, to form an expanded, i.e., “taller,” mucus layer rather than a hyperconcentrated layer.
Fig. 4.
Clearance rates and depth of the mucus layer. (A) Mucociliary clearance rates were measured by monitoring the transport of fluorescent beads across the trachea for WT (n = 13) and Tg (n = 11); P = 0.07. (B) WT and Tg lungs were fixed with perfluorocarbon-OsO4 to preserve the mucus layer (arrows) and observed with transmission electron microscope. (Scale bar, 2 μm.) (C) The graph shows the statistical analysis of the mucus layer depth revealed by EM images from 3 WT and 3 Tg animals; *P < 0.0001.
To measure the depth of the mucus layer, we used osmium perfluorocarbon to fix the lungs of WT and Muc5ac-Tg mice. Lung sections were initially stained with Richardson’s reagent, which revealed that a number of Muc5ac-Tg airways showed evidence of a “thicker” mucus layer (Fig. S9). Airways of the midsections of the lobes were randomly selected to be analyzed via electron microscopy. Fig. 4B shows the presence of a mucus layer of ∼2 μm in height lining the airways of Muc5ac-Tg animals whereas WT airways showed sporadic mucus patches whose depth did not exceed 0.5 μm. Statistical analysis revealed that an average 1.4-μm mucus layer lined the airways of Muc5ac-Tg mice vs. 0.2 μm in WT (Fig. 4C) (P < 0.0001); i.e., it was taller. Note that despite the lack of AB-PAS staining in Muc5ac-Tg mice, electron microscopy detected an increased number of electron-lucent granules characteristic of mucin-containing secretory granules (22) in dome-shape–like cells.
Resistance to Influenza PR8 Infection.
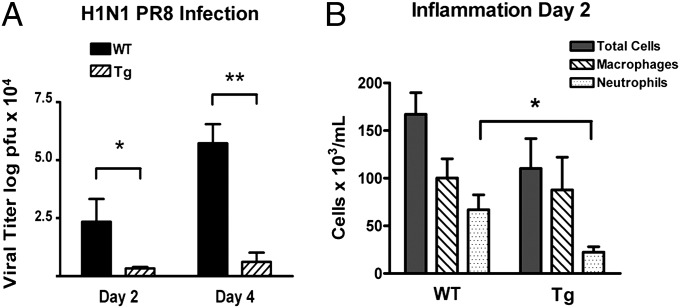
The expanded mucus layer in Muc5ac-Tg mice suggested that the protective function of the mucus layer against inhaled pathogens and particulates may be enhanced. Because secreted Muc5ac is highly sialylated and influenza virus infection requires binding of virus to sialic acid residues in airway epithelia, we tested the hypothesis that increased Muc5ac secretion may provide an enhanced mesh/adhesive barrier that would prevent influenza viruses from gaining access to the airway epithelium. In three different experiments, WT and Muc5ac-Tg littermates from three separate litters were inoculated via the nose with 700 plaque-forming units (pfu) of a mouse-adapted influenza virus PR8 (A/PR/8/34 H1N1) and infection was determined by measuring lung viral titers, using standard plaque assays (Fig. 5A). Consistently, Muc5ac-Tg mice exhibited significantly reduced viral loads in the lungs than control mice at days 2 (7.0-fold) and 4 (9.3-fold) postinoculation (p.i.). To determine whether infection-induced inflammation was also reduced in Muc5ac-Tg vs. WT animals, inflammatory cells were obtained from BALF and quantitated at day 2 p.i. (Fig. 5B). The total number of macrophages was increased approximately 4-fold after PR8 infection similarly for both genotypes. However, the number of BAL neutrophils was consistently decreased in Muc5ac-Tg animals, with an average 3-fold fewer neutrophils collected in Muc5ac-Tg BALF. These data indicate that Muc5ac overexpression serves as a protective barrier against influenza virus infection in vivo.
Fig. 5.
Lung virus titers and inflammatory cell count following influenza infection. WT and Muc5ac-Tg mice were infected with 700 pfu of PR8 via intranasal instillation. Weight loss was monitored daily and did not exceed 25% for either group. (A) Mice were euthanized either at day 2 (n = 5 for each line) or at day 4 (n = 4 for each line) postinfection. Lung homogenates were analyzed by standard plaque assay for virus titers. Values are means ± SEM; *P < 0.05, **P < 0.001. (B) Mouse lungs (n = 5 for each line) were lavaged at day 2 postinfection and analyzed for inflammatory cell populations. Values are means ± SEM; *P = 0.014.
Infectivity of Influenza PR8 in Vitro Altered by Muc5ac.
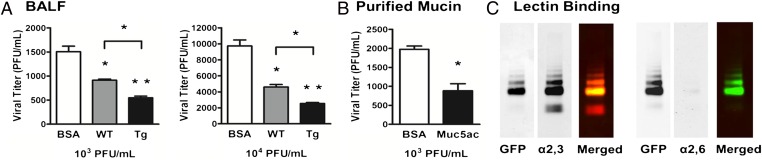
To distinguish whether Muc5ac protein provides (i) a physical (mesh-like) filtration barrier to influenza virus infection and/or (ii) an affinity barrier by virtue of the terminal sialic acids on Muc5ac acting as a decoy receptor, PR8 (100 or 1,000 pfu) was incubated in BALF (with endogenous mucins diluted at least 25-fold) from WT and Muc5ac-Tg mice. Infectivity was measured with Madin–Darby canine kidney (MDCK) cells (Fig. 6A), and PBS containing BSA at equal protein concentrations was used as a control. Muc5ac-Tg BALF significantly reduced the number of plaques by 1.7- and 1.8-fold compared with WT BALFs, respectively (P < 0.05).
Fig. 6.
Plaque reduction and lectin-binding assays. (A) MDCK cells were infected by mixing 100 or 1,000 PR8 pfu (or 103 or 104 pfu/mL solution), as indicated, with BSA, WT, or Mu5ac-Tg BALF (1 mg/mL) and plaque number was quantitated. (B) Muc5ac was purified from Muc5ac-Tg BALF, using denaturing agents and a density gradient. MDCK cells were infected by mixing 100 PR8 pfu (or 103 pfu/mL solution) with BSA or purified Muc5ac (2 mg/mL). Values are means ± SEM; *P < 0.05, **P < 0.001 (n = 3). (C) Western blots from a Muc5ac-Tg BALF were coimmunostained with a GFP antibody (in green) and α2,3- or α2,6-sialic acid-binding lectins (in red) to identify the sialic acid linkage for Muc5ac. Independent channels are shown in black and white.
A BALF contains a mixture of mucins, including Muc5b, and other natural defense molecules, limiting the ability to isolate the role of Muc5ac in viral neutralization. Accordingly, purified Muc5ac was prepared at a concentration similar to that in vivo (2 mg/mL) and was tested for protection against viral infection. Muc5ac exhibited a 2.2-fold inhibitory effect on viral infectivity at 100 pfu tested, supporting the notion that Muc5ac alone is able to protect against viral infection (Fig. 6B).
Influenza virus receptor specificity for airway epithelia is determined by cell surface terminal sialic acid linkages of the glycan chain that occur via carbon 3 or carbon 6, generating two distinct conformers: α2,3- and α2,6-linked sialic acids. It has been reported that PR8 preferentially binds to α2,3-linked sialic acids (23, 24), which are the most abundant conformer found on the surface of the avian digestive tract (25), but are not the predominant conformer in human lungs (26). Because Muc5ac reduced viral infectivity by an apparent binding mechanism, we investigated whether binding may reflect preferential expression of the α2,3-sialic acid conformer on murine Muc5ac protein. Lectin binding assays and Western blotting on Muc5ac-Tg BALF demonstrated that α2,3-, but not α2,6-linked, sialic acids colocalized with the Muc5ac-GFP signal (Fig. 6C). Note that neither lectin appeared to bind to the upper region of the gel where the Muc5b signal typically occurs in mouse BALF (Fig. 3). The removal of the sialic acids in Muc5ac-Tg BALF by treatment with neuraminidase restored PR8 infectivity in vitro, consistent with an important role of sialic acids in Muc5ac protection for PR8 infection (Fig. S10). This finding supports the notion that PR8 infection is reduced in Muc5ac-Tg animals at least in part from biochemical competition for PR8 binding by Muc5ac vs. surface α2,3-sialic acid receptors.
Discussion
Mucins are secreted onto surfaces that are exposed to external pathogens, e.g., the respiratory, digestive, and reproductive tracts. Mucins have long been regarded as exerting a role as a barrier to prevent mucosal infection but data supporting this notion are few (27, 28). One of the main reasons for the absence of such data is the lack of biochemical tools to study these large and complex molecules in intact biological systems. The complexity of these high-molecular-weight glycoproteins resides in their size and the numerous domains distributed throughout the molecule, including the von Willebrand domains, the cysteine-rich regions, and the VNTRs. Indeed, the genomic region encoding for Muc5ac is 29 kb, including 49 exons, and is predicted to produce a 9-kb RNA transcript. The highly homologous tandem repeat units are located in the central region flanked by two identical non-VNTR sequences, which causes this region to be difficult to clone and/or sequence. Hence, molecular manipulations of mucins are challenging, and biochemical reagents and assays available to study these molecules are limited.
Partial cloning of the MUC5AC cDNA, mainly of the 5′ and 3′ ends, was achieved in the past, primarily to study domain homology (29, 30) and explore domain interactions (31). The cloning and tagging of the full-length Muc5ac cDNA as reported here provide a unique biological tool to study and/or monitor mucin in its physiologic environment and facilitate purification.
We achieved an approximate 20-fold increase in Muc5ac expression in murine large and small airways (Fig. 1D). It has been believed that mucins may exhibit reciprocal compensatory regulation and influence the expression of other mucins (32, 33). However, in Muc5ac-Tg mice, Muc5b expression was unaffected, suggesting that the expression of Muc5ac and Muc5b can be independently regulated in the absence of disease.
Muc5ac overexpression in transgenic mice was confirmed at the protein level (Fig. 2C). Several Muc5ac peptides distributed throughout the molecule were used to immunize rabbits but mucin antibodies are notoriously difficult to develop. Posttranslational modifications heighten the antigenic complexity of Muc5ac, e.g., the extensive O-glycosylation of the VNTR region, disulfide bonding, and dimer formation via cysteine residues. Upon exocytosis, mucin monomers form massive aggregates with molecular masses of roughly 1–10 million Da. The complex secondary structure and large carbohydrate backbone prevent mucins from migrating on traditional polyacrylamide gels and can impede antibody access to the mucin protein core. However, antibody UNC294 was able to detect Muc5ac in its reduced form in agarose gels (Fig. 2B). This antibody revealed a 17.7-fold increased Muc5ac secretion in BALs of transgenic animals compared with WT and approximately 2-fold over the OVA-challenged model (Fig. 2C).
Several mouse models have been engineered to study the role of mucin secretion but most of these models do not exhibit increased expression of a single mucin (34–36). The only pure gain-of-function model published to date is the Muc1 transgenic mouse that overexpressed the human MUC1 cDNA and was generated to investigate immunotherapy targets for breast cancer (37). Although the ovalbumin-challenged mouse is viewed as a mucin-overproducing model with goblet cell hyper/metaplasia, it exhibits up-regulation of multiple mucins and cannot be studied separately from the airway hyperresponsiveness and eosinophilia (38). The βENaC-overexpressing mouse is another model that develops a mucin hypersecretion phenotype with airway mucus plugging and goblet cell hyperplasia (14). However, this model again exhibits complex regulation of mucins and also exhibits neutrophilic inflammation, making it difficult to establish whether mucus overproduction triggered the neutrophilic response or vice versa. The Muc5ac-Tg mouse is a unique model to unequivocally test the relationship between mucin hypersecretion, airways obstruction, and inflammation. Muc5ac hypersecretion of ∼20-fold over WT levels produced (i) no evidence of airways obstruction in the small airways, despite an approximate 10-fold increase in total mucin expression, or in the large airways with an approximate 2-fold increase in total mucin expression, and (ii) no evidence of inflammation (Fig. S5).
Although Muc5ac-Tg Clara cells produced more Muc5ac and showed evidence of mucin granules via EM (Fig. 4 and Fig. S9), these cells did not stain with AB-PAS or adopt a metaplastic or enlarged goblet cell appearance (Fig. 3). These observations suggest that AB-PAS staining may detect static aspects of mucin-secreting cells, staining preferentially mucins that are stored in granules, but does not reflect mucin turnover. In normal conditions, some mucins, e.g., Muc5b in murine airway surfaces, may be continuously produced, secreted, and transported without a large storage pool (18). Because Muc5ac-Tg mice secreted more Muc5ac-Tg mucin without an apparent increase in goblet cell number or goblet cell metaplasia, it appears likely that Muc5ac-Tg mucins are secreted from Clara cells without a metaplastic change that produced AB-PAS–positive storage granules.
The Muc5ac-Tg model showed that increased secreted mucin could be cleared from airway surfaces without accumulation or plugging. Our morphological data suggest that the approximate doubling of mucus (equivalent volume of Muc5ac and Muc5b) on proximal airway surfaces is handled by increasing the height of mucus layer, with presumably no change in concentration, rather than maintaining the same height but increasing the concentration (Fig. 4 B and C). These data are consistent with MCC data that revealed that MCC rates are comparable in WT and in Muc5ac-Tg animals (Fig. 4A). Note that recent data suggest that a doubling of mucin concentration would be associated with slowed MCC (20).
The Muc5ac-Tg model demonstrates that Muc5ac acts as a protective barrier against a specific influenza strain infection. Previous studies have suggested that mammalian viruses have evolved mechanisms to facilitate their penetration through the mucus barrier (39). Muc5ac-Tg mice were more resistant to PR8/H1N1 influenza in vivo, and incubation of PR8 with the purified mucin reduced the rate of infection in vitro, which suggests a direct “antiviral” effect of Muc5ac (Figs. 5A and 6A). Interestingly, the Muc5ac-GFP glycoprotein expresses terminal α2,3-sialic acids, the identified receptor for PR8 (Fig. 6A). Thus, we speculate that the increased Muc5ac with its sialic acid residues protected Muc5ac-Tg mice against specific influenza infections by acting as a “decoy” to bind virus in a mobile phase. This action prevented access of the virus to the airways surfaces, a necessary process to mediate cell infection.
Mucins may vary across species by expressing different sialic acid linkages that protect against viral infection. This notion is supported by the fact that α2,6-linked sialic acids, the preferred receptors for human influenza viruses, have been associated with mucins expressed in the human lung (40). Here, we have shown that α2,3-linked sialic acids, the preferred receptors for murine viruses, are associated with a secreted mucin (i.e., Muc5ac) in the murine lung. In addition, we have demonstrated that Muc5ac independently of its physical barrier role, i.e., in solution, and in absence of other defense molecules, i.e., purified Muc5ac, was able to protect against PR8 influenza infection (Fig. 6A), which reveals an “anti-infectivity” role per se for Muc5ac. Moreover, whether it is a direct result of the anti-infectivity role or an “anti-inflammatory” role of mucin, the neutrophilic response was reduced in Muc5ac-Tg mice after infection, which may provide an additional protective benefit to the lungs by limiting tissue damage (Fig. 5B).
Although mucin hypersecretion is perceived to contribute to morbidity and mortality in airway obstructive diseases, “mucoregulatory” therapy is infrequently used in patients with asthma, bronchitis, COPD, or cystic fibrosis, mostly because of its inefficiency. Importantly, the Muc5ac-Tg model supports the idea that increased mucus production might not be the primary cause for airway obstruction and, hence, a disease target. Therefore, the use of antisecretory therapies for preventive purposes may compromise the protective role of mucins, a concept supported by data from tethered mucin-deficient mice. Muc1-deficient mice are less resistant to Helicobacter pylori colonization than control littermates and develop a severe subsequent gastritis (41). Muc2-deficient mice spontaneously develop colitis (35) and have impaired resistance against enteric infection (42).
In conclusion, Muc5ac-Tg mice secrete ∼20 times more Muc5ac into their lungs, which leads to an expanded mucus layer lining the airway surfaces. The excess mucus is efficiently cleared from the lungs, demonstrating that mucin overproduction can occur without airways obstruction or airways inflammation. Resistance to PR8 influenza infection in mice overexpressing Muc5ac was increased as a result of, at least in part, biochemical competition for the viruses that bind to sialic acids decorating Muc5ac rather than receptors on cell surfaces. Therefore, future therapeutics aimed at reducing mucin production with the purpose of ameliorating airway obstruction may require careful titration to not compromise host defense.
Materials and Methods
The full methods and additional analysis and data are provided in SI Materials and Methods.
mRNAs were extracted from C57BL/6J mouse lungs and Muc5ac cDNA was internally tagged with GFP. Gene expression was measured by quantitative PCR. Bronchoalveolar lavages were performed to collect fluid and quantify mucin and lung inflammatory cells as previously described (18). The rate of mucociliary clearance was determined by measuring the movement of fluorescent beads along the trachea (21). Mice were inoculated via an intranasal route with the influenza virus A/PR/8/34 (H1N1) and virus titers were determined by standard plaque assays on MDCK cells.
Supplementary Material
Acknowledgments
The authors thank Drs. C. W. Davis, L. H. Abdullah, and J. K. Sheehan for their assistance with mucin gels and the generation of mucin antibodies; Dr. M. Kesimer and Ms. G. DeMaria for assistance with refractometry, density gradients, and mucin purification; Drs. M. Chua and N. Kramarcy and Ms. W. Salmon for their assistance with confocal microscopy; and Ms. K. Burns for histology assistance. This work was supported by Cystic Fibrosis Foundation Grants EHRE06I0, EHRE08I0, and R026-CR11; National Institutes of Health Grants 2P30DK065988 and 1-P01-HL8808-01A1; and National Heart, Lung, and Blood Institute Grant 5 P50HL 107168-01.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1206552109/-/DCSupplemental.
References
- 1.Audie JP, et al. Expression of human mucin genes in respiratory, digestive, and reproductive tracts ascertained by in situ hybridization. J Histochem Cytochem. 1993;41:1479–1485. doi: 10.1177/41.10.8245407. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, et al. MUC5B and MUC7 are differentially expressed in mucous and serous cells of submucosal glands in human bronchial airways. Am J Respir Cell Mol Biol. 1998;19:30–37. doi: 10.1165/ajrcmb.19.1.3054. [DOI] [PubMed] [Google Scholar]
- 3.Wickström C, Davies JR, Eriksen GV, Veerman EC, Carlstedt I. MUC5B is a major gel-forming, oligomeric mucin from human salivary gland, respiratory tract and endocervix: Identification of glycoforms and C-terminal cleavage. Biochem J. 1998;334:685–693. doi: 10.1042/bj3340685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hovenberg HW, Davies JR, Carlstedt I. Different mucins are produced by the surface epithelium and the submucosa in human trachea: Identification of MUC5AC as a major mucin from the goblet cells. Biochem J. 1996;318:319–324. doi: 10.1042/bj3180319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Groneberg DA, et al. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- 6.Young HW, et al. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′ elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gray T, et al. Regulation of MUC5AC mucin secretion and airway surface liquid metabolism by IL-1beta in human bronchial epithelia. Am J Physiol Lung Cell Mol Physiol. 2004;286:L320–L330. doi: 10.1152/ajplung.00440.2002. [DOI] [PubMed] [Google Scholar]
- 8.Shao MX, Nakanaga T, Nadel JA. Cigarette smoke induces MUC5AC mucin overproduction via tumor necrosis factor-alpha-converting enzyme in human airway epithelial (NCI-H292) cells. Am J Physiol Lung Cell Mol Physiol. 2004;287:L420–L427. doi: 10.1152/ajplung.00019.2004. [DOI] [PubMed] [Google Scholar]
- 9.Rogers DF. Chronic Obstructive Pulmonary Disease: Pathogenesis to Treatment, Novartis Foundation Symposium 234. UK: Wiley, Chichester; 2001. Mucus hypersecretion in chronic obstructive pulmonary disease; pp. 65–83. [DOI] [PubMed] [Google Scholar]
- 10.Rose MC, Nickola TJ, Voynow JA. Airway mucus obstruction: Mucin glycoproteins, MUC gene regulation and goblet cell hyperplasia. Am J Respir Cell Mol Biol. 2001;25:533–537. doi: 10.1165/ajrcmb.25.5.f218. [DOI] [PubMed] [Google Scholar]
- 11.Morcillo EJ, Cortijo J. Mucus and MUC in asthma. Curr Opin Pulm Med. 2006;12:1–6. doi: 10.1097/01.mcp.0000198064.27586.37. [DOI] [PubMed] [Google Scholar]
- 12.Rogers DF. Physiology of airway mucus secretion and pathophysiology of hypersecretion. Respir Care. 2007;52(9) 1134–1146; discussion 1146–1149. [PubMed] [Google Scholar]
- 13.Singer M, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–196. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- 14.Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 15.Evans CM, et al. Mucin is produced by clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheehan JK, et al. Physical characterization of the MUC5AC mucin: A highly oligomeric glycoprotein whether isolated from cell culture or in vivo from respiratory mucous secretions. Biochem J. 2000;347:37–44. [PMC free article] [PubMed] [Google Scholar]
- 17.Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–161. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Y, et al. Munc13-2-/- baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–1992. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy MG, et al. Mucin production during prenatal and postnatal murine lung development. Am J Respir Cell Mol Biol. 2011;44:755–760. doi: 10.1165/rcmb.2010-0020OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Button B, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–941. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ostrowski LE, et al. Conditional deletion of dnaic1 in a murine model of primary ciliary dyskinesia causes chronic rhinosinusitis. Am J Respir Cell Mol Biol. 2010;43:55–63. doi: 10.1165/rcmb.2009-0118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- 23.Nagai T, Suzuki Y, Yamada H. Comparison of substrate specificities of sialidase activity between purified enzymes from influenza virus A (H1N1 and H3N2 subtypes) and B strains and their original viruses. Biol Pharm Bull. 1995;18:1251–1254. doi: 10.1248/bpb.18.1251. [DOI] [PubMed] [Google Scholar]
- 24.Meng B, Marriott AC, Dimmock NJ. The receptor preference of influenza viruses. Influenza Other Respir Viruses. 2010;4:147–153. doi: 10.1111/j.1750-2659.2010.00130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pillai SP, Saif YM, Lee CW. Detection of influenza A viruses in eggs laid by infected turkeys. Avian Dis. 2010;54:830–833. doi: 10.1637/9102-101209-Reg.1. [DOI] [PubMed] [Google Scholar]
- 26.Shinya K, et al. Avian flu: Influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- 27.Chen CC, Baylor M, Bass DM. Murine intestinal mucins inhibit rotavirus infection. Gastroenterology. 1993;105:84–92. doi: 10.1016/0016-5085(93)90013-3. [DOI] [PubMed] [Google Scholar]
- 28.McGuckin MA, Lindén SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–278. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- 29.van de Bovenkamp JH, et al. Molecular cloning of human gastric mucin MUC5AC reveals conserved cysteine-rich D-domains and a putative leucine zipper motif. Biochem Biophys Res Commun. 1998;245:853–859. doi: 10.1006/bbrc.1998.8535. [DOI] [PubMed] [Google Scholar]
- 30.Inatomi T, Tisdale AS, Zhan Q, Spurr-Michaud S, Gipson IK. Cloning of rat Muc5AC mucin gene: Comparison of its structure and tissue distribution to that of human and mouse homologues. Biochem Biophys Res Commun. 1997;236:789–797. doi: 10.1006/bbrc.1997.7051. [DOI] [PubMed] [Google Scholar]
- 31.Perez-Vilar J, Mabolo R, McVaugh CT, Bertozzi CR, Boucher RC. Mucin granule intraluminal organization in living mucous/goblet cells. Roles of protein post-translational modifications and secretion. J Biol Chem. 2006;281:4844–4855. doi: 10.1074/jbc.M510520200. [DOI] [PubMed] [Google Scholar]
- 32.Wang IJ, Yu CJ, Hu FR. Alteration of ocular surface mucins in MUC5AC-DTA transgenic mice. Mol Vis. 2009;15:108–119. [PMC free article] [PubMed] [Google Scholar]
- 33.Malmberg EK, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G203–G210. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- 34.Spicer AP, Rowse GJ, Lidner TK, Gendler SJ. Delayed mammary tumor progression in Muc-1 null mice. J Biol Chem. 1995;270:30093–30101. doi: 10.1074/jbc.270.50.30093. [DOI] [PubMed] [Google Scholar]
- 35.Van der Sluis M, et al. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Cheon DJ, et al. CA125/MUC16 is dispensable for mouse development and reproduction. PLoS ONE. 2009;4:e4675. doi: 10.1371/journal.pone.0004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rowse GJ, Tempero RM, VanLith ML, Hollingsworth MA, Gendler SJ. Tolerance and immunity to MUC1 in a human MUC1 transgenic murine model. Cancer Res. 1998;58:315–321. [PubMed] [Google Scholar]
- 38.Tomkinson A, et al. Temporal association between airway hyperresponsiveness and airway eosinophilia in ovalbumin-sensitized mice. Am J Respir Crit Care Med. 2001;163:721–730. doi: 10.1164/ajrccm.163.3.2005010. [DOI] [PubMed] [Google Scholar]
- 39.Bisaillon M, Sénéchal S, Bernier L, Lemay G. A glycosyl hydrolase activity of mammalian reovirus sigma1 protein can contribute to viral infection through a mucus layer. J Mol Biol. 1999;286:759–773. doi: 10.1006/jmbi.1998.2495. [DOI] [PubMed] [Google Scholar]
- 40.Kesimer M, et al. Characterization of exosome-like vesicles released from human tracheobronchial ciliated epithelium: A possible role in innate defense. FASEB J. 2009;23:1858–1868. doi: 10.1096/fj.08-119131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McGuckin MA, Eri R, Simms LA, Florin TH, Radford-Smith G. Intestinal barrier dysfunction in inflammatory bowel diseases. Inflamm Bowel Dis. 2009;15:100–113. doi: 10.1002/ibd.20539. [DOI] [PubMed] [Google Scholar]
- 42.Hasnain SZ, et al. Mucin gene deficiency in mice impairs host resistance to an enteric parasitic infection. Gastroenterology. 2010;138:1763–1771. doi: 10.1053/j.gastro.2010.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.