Abstract
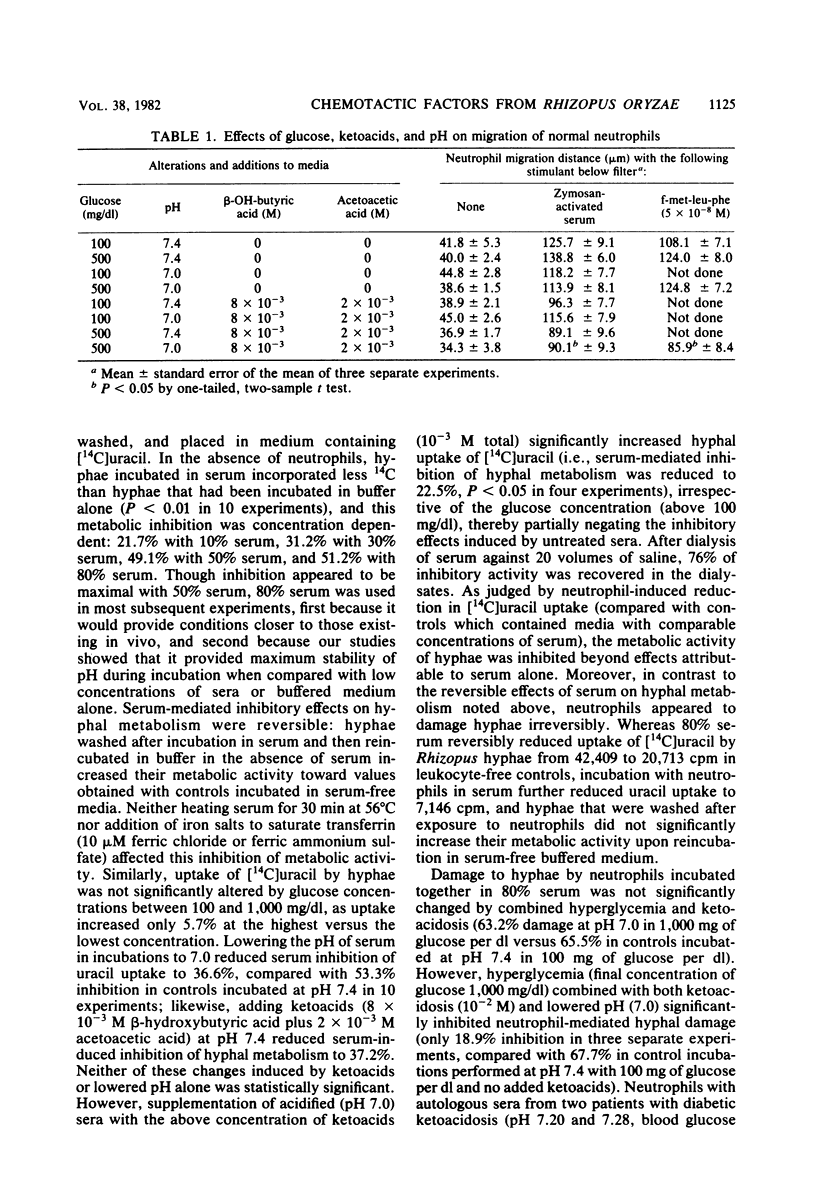
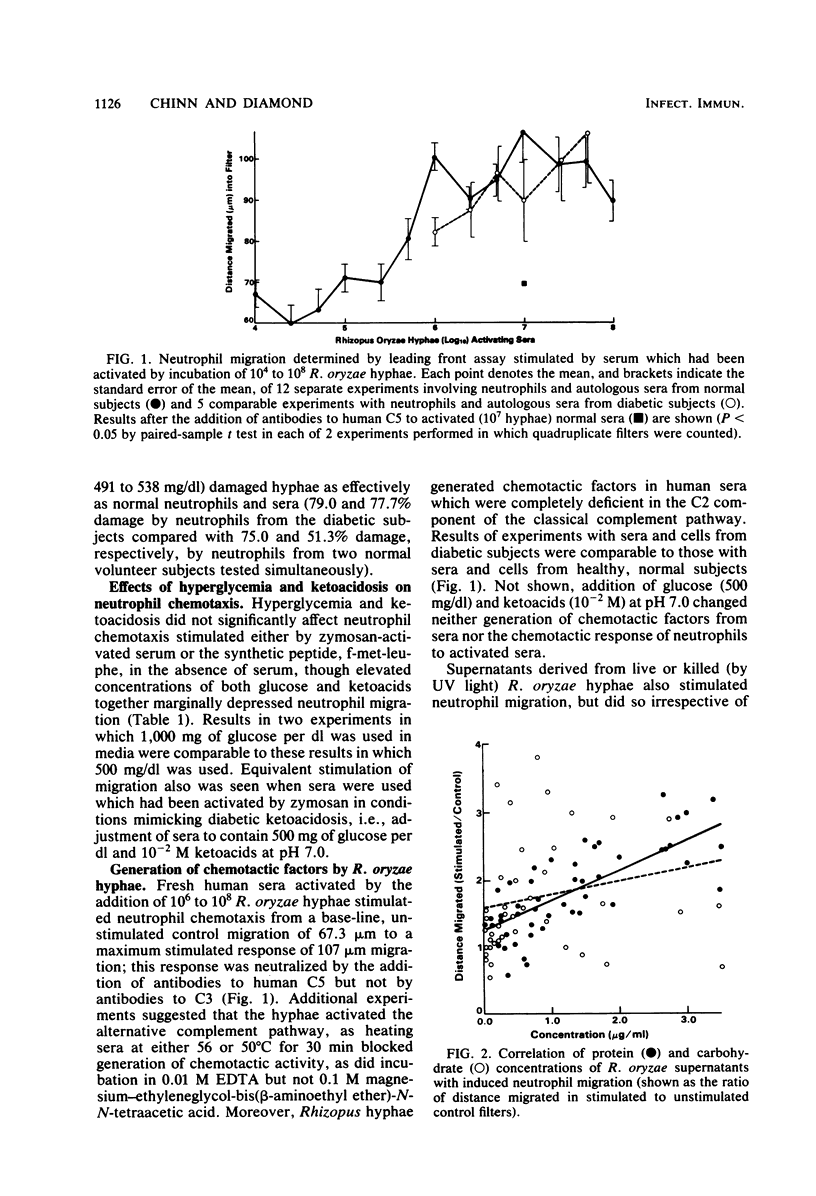
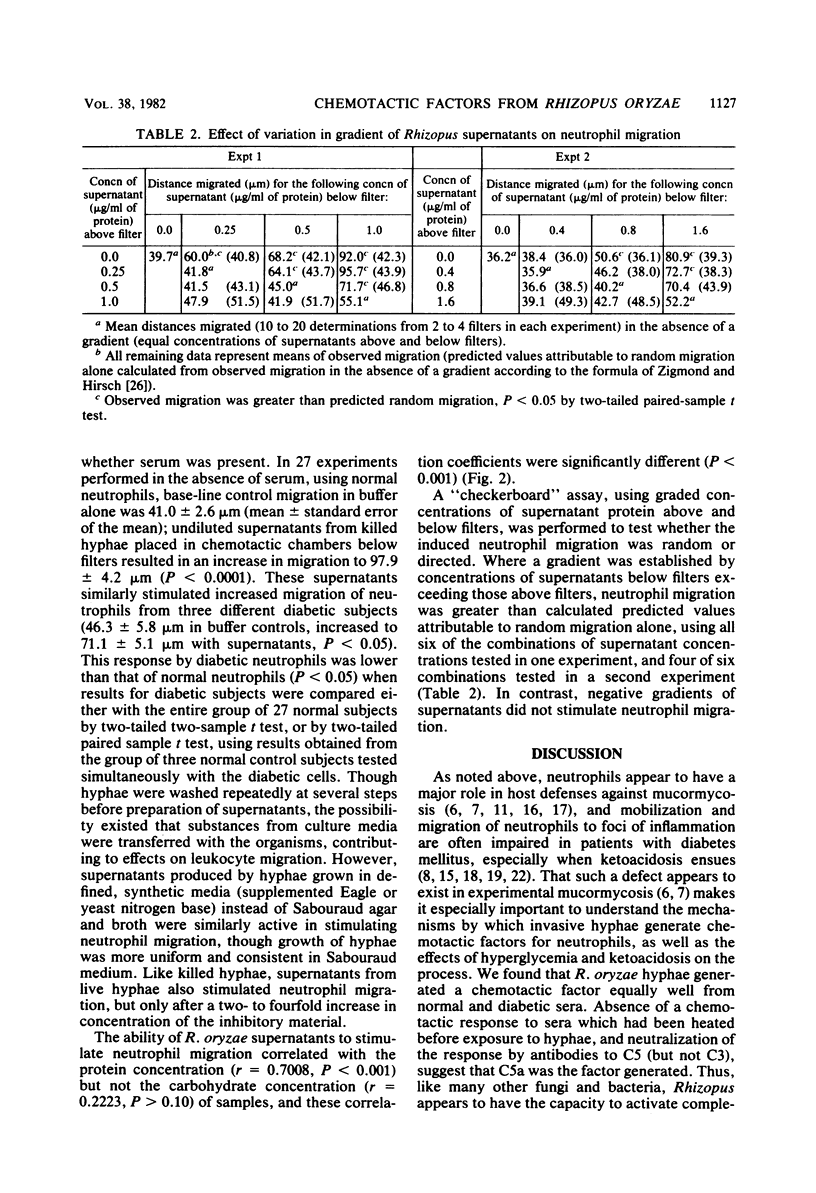
As our previous studies had shown that human neutrophils could kill Rhizopus oryzae hyphae in vitro, interactions of these hyphae with neutrophils and serum were further explored. Heated or fresh normal human sera suppressed hyphal metabolic activity as determined by [14C]uracil uptake, but severe ketoacidosis (8 X 10(-3) M beta-hydroxybutyric acid plus 2 X 10(-3) M acetoacetic acid at pH 7.0) negated this effect. Hyperglycemia (500 mg/dl) and severe ketoacidosis did not affect damage to hyphae by human neutrophils. Hyphae generated factors from sera which induced comparable chemotactic responses by neutrophils obtained from both normal and diabetic subjects, using a leading front assay performed in modified Boyden chambers. Zymosan-stimulated neutrophil chemotaxis was marginally depressed only by the combined elevation of both glucose (500 mg/dl) and ketoacids (10(-2)M) irrespective of pH (7.0 to 7.4), but not by any of these factors alone. Protein-containing supernatants from live or killed hyphae were chemotactic in the absence of serum based upon "checkerboard" assays varying the concentrations of hyphal supernatants above and below filters in the Boyden chambers. The supernatant-induced chemotactic response by neutrophils from diabetic subjects was minimally less than that of normal neutrophils (P less than 0.05). These findings indicate that R. oryzae hyphae can generate chemotactic factors which might prove to influence the inflammatory response to infections in vivo, and that severe hyperglycemia and ketoacidosis might affect interaction between the host and invading hyphae in mucormycosis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson E., Wilson D., Arky R. A. Rhinocerebral phycomycosis in association with diabetic ketoacidosis. Report of two cases and a review of clinical and experimental experience with amphotericin B therapy. Ann Intern Med. 1967 Apr;66(4):735–742. doi: 10.7326/0003-4819-66-4-735. [DOI] [PubMed] [Google Scholar]
- BAKER R. D. Mucormycosis; a new disease? J Am Med Assoc. 1957 Mar 9;163(10):805–808. doi: 10.1001/jama.1957.02970450007003. [DOI] [PubMed] [Google Scholar]
- BAUER H., FLANAGAN J. F., SHELDON W. H. Experimental cerebral mucormycosis in rabbits with alloxan diabetes. Yale J Biol Med. 1955 Sep;28(1):29–36. [PMC free article] [PubMed] [Google Scholar]
- BAUER H., FLANAGAN J. F., SHELDON W. H. The effects of metabolic alterations on experimental Rhizopus oryzae (mucormycosis) infection. Yale J Biol Med. 1956 Sep;29(1):23–32. [PMC free article] [PubMed] [Google Scholar]
- BYBEE J. D., ROGERS D. E. THE PHAGOCYTIC ACTIVITY OF POLYMORPHONUCLEAR LEUKOCYTES OBTAINED FROM PATIENTS WITH DIABETES MELLITUS. J Lab Clin Med. 1964 Jul;64:1–13. [PubMed] [Google Scholar]
- Bagdade J. D., Root R. K., Bulger R. J. Impaired leukocyte function in patients with poorly controlled diabetes. Diabetes. 1974 Jan;23(1):9–15. doi: 10.2337/diab.23.1.9. [DOI] [PubMed] [Google Scholar]
- Bagdade J. D., Stewart M., Walters E. Impaired granulocyte adherence. A reversible defect in host defense in patients with poorly controlled diabetes. Diabetes. 1978 Jun;27(6):677–681. doi: 10.2337/diab.27.6.677. [DOI] [PubMed] [Google Scholar]
- Battock D. J., Grausz H., Bobrowsky M., Littman M. L. Alternate-day amphotericin B therapy in the treatment of rhinocerebral phycomycosis (mucormycosis). Ann Intern Med. 1968 Jan;68(1):122–137. doi: 10.7326/0003-4819-68-1-122. [DOI] [PubMed] [Google Scholar]
- Brayton R. G., Stokes P. E., Schwartz M. S., Louria D. B. Effect of alcohol and various diseases on leukocyte mobilization, phagocytosis and intracellular bacterial killing. N Engl J Med. 1970 Jan 15;282(3):123–128. doi: 10.1056/NEJM197001152820303. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Gallin J. I., Kaplan A. P. The selective eosinophil chemotactic activity of histamine. J Exp Med. 1975 Dec 1;142(6):1462–1476. doi: 10.1084/jem.142.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Epstein B., Jao W. Damage to hyphal forms of fungi by human leukocytes in vitro. A possible host defense mechanism in aspergillosis and mucormycosis. Am J Pathol. 1978 May;91(2):313–328. [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Oppenheim F., Nakagawa Y., Krzesicki R., Haudenschild C. C. Properties of a product of Candida albicans hyphae and pseudohyphae that inhibits contact between the fungi and human neutrophils in vitro. J Immunol. 1980 Dec;125(6):2797–2804. [PubMed] [Google Scholar]
- Drachman R. H., Root R. K., Wood W. B., Jr Studies on the effect of experimental nonketotic diabetes mellitus on antibacterial defense. I. Demonstration of a defect in phagocytosis. J Exp Med. 1966 Aug 1;124(2):227–240. doi: 10.1084/jem.124.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE G. R., WELCH A. M. Studies of opportunistic fungi. I. Inhibition of Rhizopus oryzae by human serum. Am J Med Sci. 1961 May;241:604–612. [PubMed] [Google Scholar]
- Hill H. R., Sauls H. S., Dettloff J. L., Quie P. G. Impaired leukotactic responsiveness in patients with juvenile diabetes mellitus. Clin Immunol Immunopathol. 1974 Apr;2(3):395–403. doi: 10.1016/0090-1229(74)90057-9. [DOI] [PubMed] [Google Scholar]
- Meyer R. D., Rosen P., Armstrong D. Phycomycosis complicating leukemia and lymphoma. Ann Intern Med. 1972 Dec;77(6):871–879. doi: 10.7326/0003-4819-77-6-871. [DOI] [PubMed] [Google Scholar]
- Miller M. E., Baker L. Leukocyte functions in juvenile diabetes mellitus: humoral and cellular aspects. J Pediatr. 1972 Nov;81(5):979–982. doi: 10.1016/s0022-3476(72)80555-9. [DOI] [PubMed] [Google Scholar]
- Molenaar D. M., Palumbo P. J., Wilson W. R., Ritts R. E., Jr Leukocyte chemotaxis in diabetic patients and their nondiabetic first-degree relatives. Diabetes. 1976;25(2 Suppl):880–883. [PubMed] [Google Scholar]
- Mowat A., Baum J. Chemotaxis of polymorphonuclear leukocytes from patients with diabetes mellitus. N Engl J Med. 1971 Mar 25;284(12):621–627. doi: 10.1056/NEJM197103252841201. [DOI] [PubMed] [Google Scholar]
- Mucormycosis. Ann Intern Med. 1980 Jul;93(1):93–108. doi: 10.7326/0003-4819-93-1-93. [DOI] [PubMed] [Google Scholar]
- Nolan C. M., Beaty H. N., Bagdade J. D. Further characterization of the impaired bactericidal function of granulocytes in patients with poorly controlled diabetes. Diabetes. 1978 Sep;27(9):889–894. doi: 10.2337/diab.27.9.889. [DOI] [PubMed] [Google Scholar]
- PERILLIE P. E., NOLAN J. P., FINCH S. C. Studies of the resistance to infection in diabetes mellitus: local exudative cellular response. J Lab Clin Med. 1962 Jun;59:1008–1015. [PubMed] [Google Scholar]
- POLLI C., DIEKMANN H., KIS Z., ETTLINGER L. UBER DAS VORKOMMEN VON KETONREDUKTASEN BEI MIKROORGANISMEN. Pathol Microbiol (Basel) 1965;28:93–98. [PubMed] [Google Scholar]
- Zigmond S. H., Hirsch J. G. Leukocyte locomotion and chemotaxis. New methods for evaluation, and demonstration of a cell-derived chemotactic factor. J Exp Med. 1973 Feb 1;137(2):387–410. doi: 10.1084/jem.137.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oss C. J., Border J. R. Influence of intermittent hyperglycemic glucose levels on the phagocytosis of microorganisms by human granulocytes in vitro. Immunol Commun. 1978;7(6):669–676. doi: 10.3109/08820137809068727. [DOI] [PubMed] [Google Scholar]


