Abstract
Recent evidence has emerged indicating that the maternal immune response can have a substantial deleterious impact on prenatal development (Croen et al., 2008). The maternal immune response is largely sequestered from the fetus. Maternal antibodies, specifically immunoglobulin G (IgG), are passed to the fetus to provide passive immunity throughout much of pregnancy. However, both protective and pathogenic autoantibodies have equal access to the fetus (Goines and Van de Water, 2010). If the mother has an underlying autoimmune disease or has reactivity to fetal antigens, autoantibodies produced before or during pregnancy can target tissues in the developing fetus. One such tissue is the fetal brain. The blood brain barrier (BBB) is developing during the fetal period allowing maternal antibodies to have direct access to the brain during gestation (Diamond et al., 2009; Braunschweig et al., 2011). It has been proposed that brain injury by circulating brain–specific maternal autoantibodies might underlie multiple congenital, developmental disorders (Lee et al., 2009). In this review, we will discuss the current state of research in the area of maternal autoantibodies and the development of autism.
Introduction
The fetus occupies a site protected by a non-immunogenic tissue barrier, the placenta, which promotes a local immunosuppressive response in the mother (Murphy, 2011). The placenta allows for the selective passage of nutritional and immune factors, while limiting the passage of potentially destructive molecules. Immunoglobulin G (IgG) crosses the placenta in part mediated by the neonatal Fc receptor, an IgG transport protein (Braunschweig et al., 2011; Murphy, 2011). Most antibodies are acquired during the third trimester and IgG levels in full-term infants often exceed those in the maternal circulation (Garty et al., 1994; Simister, 2003). Maternal IgG is also ingested by the newborn in its mother’s milk and colostrum, which enables maternal IgG to persist in the newborn through early infancy (Murphy, 2011). The transfer of maternal antibodies equips the immunologically naïve fetus with a subset of the maternal adaptive humoral immune system (Braunschweig et al., 2008). Maternal antibodies are passed without regard to their specificity, however, and maternal antibodies reactive to fetal antigens may be passed in addition to protective antibodies (Goines et al., 2011). Specifically, maternal antibodies reactive to fetal brain tissue could pose a significant risk to the developing fetus, as the window of exposure overlaps major processes in neurodevelopment such as cell migration, axonal elongation and dendritic tree maturation (Braunschweig et al., 2011).
Brain-reactive antibodies have been observed in mature patients with several neurological and psychiatric disorders and in healthy individuals (Diamond et al., 2009; Singer et al., 2009). It has been suggested that acquired changes or congenital impairments in cognition and behavior might be the consequence of these common, circulating brain-specific antibodies (Diamond et al., 2009). The mere presence of antibodies with potential brain reactivity in the serum does not necessarily correlate with CNS disease. Neuronal damage typically only occurs if there is a breakdown in the blood brain barrier (BBB). However, under conditions of BBB compromise and during fetal development, antibodies have greater access to the brain and thus have the potential to alter its function (Kowal et al., 2004; Diamond et al., 2009). If the BBB is abrogated due to infection, stress, catecholaminergic excess, or nicotine exposure or is not fully developed, as is the case with the developing fetus, these anti-brain antibodies can become pathologically significant (Kowal et al., 2004). Often, the symptoms of disease in the newborn infant disappear as the maternal antibody is catabolized over the first few months of life. But, in some cases, the antibodies cause chronic organ injury (Murphy, 2011). Furthermore, the potential effects of maternal antibodies on fetal brain development might be difficult to diagnose because of the variable time delay before the effects are manifested and the possibility that they might never become clinically evident in some individuals (Diamond et al., 2009). We have pursued the hypothesis that maternal antibodies directed at the fetal brain may disrupt aspects of normal brain development leading to one form of autism spectrum disorder. We provide an overview of the evidence adduced thus farin support of this hypothesis.
Antibody Generation
There are several potential mechanisms by which the maternal immune system could generate antibodies to fetal brain tissue. Many hypothesize maternal reactivity to fetal proteins may result from maternal environmental exposures (Zimmerman et al., 2007). It is thought that infectious agents that express epitopes resembling self-antigens may trigger autoantibody generation. Recent experiments have shown that autoantibodies produced as part of a protective response to infection also bind to brain antigens through molecular mimicry (Diamond et al., 2009; Murphy, 2011). For example, patients with rheumatic fever often produce lysoganglioside-specific antibodies that target an antigen that is expressed in the basal ganglia leading to obsessive-compulsive symptomatology (Diamond et al., 2009). Similarly, patients infected with C. jejuni produce ganglioside-specific antibodies that cross-react with, and impair, Schwann cell function (Diamond et al., 2009). In addition, many autoimmune disorders are caused by internal dysregulation of the immune system without the apparent participation of infectious agents. The production of anti-brain antibodies may be the result of a maternal immune reaction to fetal antigens during pregnancy (Silva et al., 2004; Zimmerman et al., 2007; Heuer et al., 2011). Additionally, autoantibodies may be generated as a result of an antibody-mediated autoimmune disease in the mother (Murphy, 2011). Furthermore, autoantibodies to nervous system antigens are detected in populations exposed to toxic, environmental, or occupational chemicals. Titers of antibodies against neurofilaments and myelin basic protein correlated with blood lead or urinary mercury levels and also correlated with sensorimotor deficits (Vojdani et al., 2002). Lastly, autoantibody generation may be the result of a genetic polymorphism. It has been found that the MET protein, which functions as a key regulator of immune function, is decreased in individuals possessing the MET ‘C’ allele. These individuals also have decreased levels of IL-10, an immunosuppressive cytokine, which may lead to a breakdown of maternal immune tolerance leading to the production of autoantibodies (Heuer et al., 2011). In addition to the numerous ways autoantibodies can be generated, there are also several mechanisms by which they can lead to neurodevelopmental disorders.
Mechanisms of Pathogenesis
There are three main mechanisms by which autoantibodies can lead to pathogenesis. First, autoantibodies may act as a receptor agonist or antagonist (Diamond et al., 2009). Autoantibodies can induce hyperactivity or excitotoxic death through excessive signaling by serving as ligands and binding to receptors (Wills et al., 2009). Moreover, autoantibodies to neural antigens can act as antagonists by blocking an essential pathway that may lead to abnormal nervous system function and/or development (Diamond et al., 2009). Second, autoantibodies may cause antigenic modulation by altering neuronal function, receptor density, or the release of neurotransmitters (Wills et al., 2009). Finally, autoantibodies might interact with diverse components of the immune system. Autoantibodies may mediate tissue destruction through complement-mediated or cell-mediated cytotoxicity by activating complement components or engaging Fc receptors (Diamond et al., 2009; Wills et al., 2009). It is also possible that some antibodies that recognize brain antigens are merely an epiphenomenon. However, maternal antibodies have been implicated in a number of congenital diseases and developmental disorders and likely provide an important biomarker for disease ontogeny (Diamond et al., 2009).
Autoantibodies in Disease
Many neonatal diseases are the result of antibody-mediated autoimmune diseases in the mother. Children born to mothers with systemic Lupus erythematosus (SLE), are often born with a congenital heart block due to the binding of maternal autoantibodies specific for ribosomal Ro ribonucleoprotein to fetal cardiomyocytes thereby preventing proper heart formation (Zimmerman et al., 2007; Murphy, 2011). Additionally, newborns born to mothers with Graves’ disease suffer from transient hyperthyroidism due to the transfer of maternal antibodies against the thyroid-stimulating hormone (TSH) receptor, which act as agonists of the receptor (Murphy, 2011). Similarly, infants born to mothers with Hashimoto’s thyroiditis suffer from hypothyroidism resulting from maternal autoantibodies against thyroid peroxidase (Braunschweig et al., 2008; Murphy, 2011). Lastly, other diseases may be the result of maternal immune system targeting paternal antigens. For example, in erythroblastosis fetalis, Rh-negative mothers make anti-Rh antibodies that target paternal Rh determinants on fetal red blood cells leading to hemolytic anemia in the fetus (Zimmerman et al., 2007; Murphy, 2011). These precedents of maternal antibodies leading to fetal disease raises the possibility that maternal antibodies specific for proteins highly expressed in fetal brain may play a role in neurodevelopmental disorders and more specifically, autism spectrum disorder.
Autoantibodies in Development
Autoantibodies in Neurodevelopment
Elevated levels of circulating autoantibodies to nervous system components have been reported in a number of neurological disorders (Wills et al., 2009). Maternal autoantibodies against acetylcholine receptors produced as a result of myasthenia gravis can cause fetal development disorder and are sometimes associated with CNS abnormalities (Vincent et al., 1995; Dalton et al., 2003; Murphy, 2011). Furthermore, it is clearly established that mothers with systemic lupus erythematosus (SLE) transfer maternal autoantibodies to the fetus during pregnancy, which are reactive against the N-Methyl-D-aspartic acid receptor (NMDAR)(Kowal et al., 2004; Lee et al., 2009). These autoantibodies elicit NMDAR-mediated death of fetal neurons, causing congenital brain injuries and resulting in long-term cognitive deficits (Lee et al., 2009). Also, Pediatric Autoimmune Neuropsychiatric Disorders Associated with Streptococcal infections (PANDAS) are believed to result from anti-streptococcal antibodies that cross-react with basal ganglia tissue leading to neuropsychiatric symptoms such as obsessions and/or compulsions, tics and soft neurological signs (Guiseppe et al., 2009). Furthermore, autoantibodies to folate receptors have been observed in serum of women with a pregnancy complicated by neural tube defects (Cabrera et al., 2008). Lastly, a number of studies have found abnormal antibody responses to fetal brain antigens in mothers and children with Autism Spectrum Disorder (ASD).
Autoantibodies in Autism Spectrum Disorder
Autism spectrum disorder is a heterogeneous group of neurodevelopmental disorders that manifest in early childhood and are characterized by impairments in social interaction, verbal and nonverbal communication, and stereotyped behaviors and circumscribed interests (Ashwood et al., 2011). Children with ASD may have one or more of a series of co-morbid syndromes including epilepsy, sleep disorders, gastrointestinal disorders and developmental delays (Goines et al., 2011). An early study implicating maternal antibodies in the etiology of ASD found an association between autism and the presence of maternal antibodies reactive to paternal lymphocytes (Warren et al., 1990). It was subsequently demonstrated that serum antibodies from the mother of two children with autism identified rodent Purkinje cells and other neurons by immunohistochemistry (Dalton et al., 2003). This early work was followed by studies which examined serum reactivity to human fetal brain tissue using serum obtained from 61 mothers of children with autism and 102 control mothers. This case-controlled study revealed paired reactivity to a 37kDa band and a 73kDa band was observed only in mothers of children with autism and was observed in 7 of the 61 mothers (11.5%)(Braunschweig et al., 2008). Furthermore, another study found that serum from 11 women with autistic children reacted with proteins derived from prenatal rat brains in distinct patterns that were not observed with serum from 10 control mothers (Zimmerman et al., 2007). Moreover, the autism-associated antibodies were more reactive to proteins derived from fetal brain tissue than postnatal and adult brain tissue. Immunoblotting showed that the targets of these antibodies were not myelin basic protein and glial acidic fibrillary protein (GFAP), which had been shown to be targets for antibodies found in blood samples from autistic children (Zimmerman et al., 2007). Succeeding experiments further evaluated antibody reactivity towards human and rat fetal brain tissue using serum collected from 100 mothers of children with autistic disorder (MCAD) and 100 control mothers of unaffected, non-autistic children (MUC). Antibodies derived from MCAD had an increased reactivity to bands near 36kDa and 39kDa compared to MUC when human fetal brain tissue was used as the protein source. When using rodent embryonic tissue, MCAD had greater band specificity at 36kDa and 73kDa when compared to MUC (Singer et al., 2008). The aforementioned studies from two different laboratories, while not identical in their detailed findings, both presented data on the presence of highly specific antibodies in mothers of children with autism. The minor differences in band definition may be a result of the different tissue and western blot protocol used for analysis. One study used a single brain sample obtained from a human fetus at 17-weeks gestation, while the other used a pooled sample of 63 spontaneously aborted male and female fetuses at 20–40 weeks of gestation, which may have provided more potential antigens (Braunschweig et al., 2008; Singer et al., 2008). Additionally, one group used a 10% acrylamide gel and the other group used a 4–15% polyacrylamide gel, which may have allowed greater protein separation (Singer et al., 2006; Braunschweig et al., 2008). More recent studies using a larger sample size (560 subjects) replicated the original observation that there was a significant association between maternal IgG reactivity to proteins at 37kDa and 73kDa and a diagnosis of autism in the child; six percent of the mothers of children with autism showed paired reactivity to bands near 37kDa and 73kDa. A correlation between lower expressive language scores, and maternal plasma IgG reactivity to fetal brain proteins at 37kDa and 73kDa was also found in this study. This was the first report of an association between a behavioral correlate and the presence of maternal anti-fetal brain antibodies (Braunschweig et al., 2011). Reactivity to a band near 39kDa was also observed in this study; paired reactivity to proteins at 39kDa and 73kDa, which was also found in six percent of mothers of children with autism, correlated with broader diagnosis of ASD and increased irritability on the Aberrant Behavioral Checklist (Braunschweig et al., 2011).
The above-mentioned studies used maternal samples collected anywhere from 2 to 18 years after the birth of the study child and it was not clear if the antibodies collected several years after delivery were present during pregnancy. Another study utilized archived mid-pregnancy blood specimens drawn during routine prenatal screening to determine maternal antibody reactivity. This study found that at least some mothers of children with autism (7%) had antibodies to 39kDa and 73kDa proteins during gestation (Croen et al., 2008). The studies carried out with human subjects find, therefore, that only women who have children with autism have antibodies directed at fetal brain that react with proteins both at 37/39kDa and 73kDa. What cannot be demonstrated in the human subjects is whether these antibodies cause autism. To marshal support in favor of this hypothesis, it is necessary to move to experimental animal studies.
Experimental Animal Studies
An initial attempt to determine whether antibodies derived from mothers of autistic children could have pathological effects on offspring was carried out in the mouse model. Plasma from one mother of multiple children with autism was injected into pregnant mice and the offspring demonstrated impaired exploratory behavior and motor coordination (Dalton et al., 2003). Another study purified IgG from several mothers of children with autism and injected the cocktail into pregnant mice. Abnormal behavior was again observed, which included increased anxiety and motor activity and abnormal startle reflexes and sociability (Singer et al., 2009). A more recent study determined the effects of gestational exposure to a single, intravenous dose of purified brain-reactive IgG antibodies from individual mothers of children with autism (MAU). The outcome revealed alterations in early growth trajectories, significantly impaired motor and sensory development, and increased anxiety in the offspring of treated dams. This study demonstrated, for the first time, that a single, low dose gestational exposure of IgG derived from individual MAU with specific reactivity to the 37 and 73 kDa fetal brain proteins had significant effects on the physical and social development of the gestationally-exposed offspring (Braunschweig et al., 2012). Further development of the murine model, including modifications to dosage, use of the specific antigen proteins, age at evaluation of social behavior and mouse strain, will allow for greater precision in determining the critical developmental windows that are perturbed in maternal antibody associated autism.
When one considers which animal model to employ to examine hypotheses of causality, it is important to consider which behaviors and which brain systems may be most involved in the disease process. Autism is a disorder of higher cognitive function that likely involves regions such as the frontal lobe that is not well developed in the rodent. Rhesus macaques (Macaca mulatta) demonstrate many similarities with human physiology, anatomy, and behavior. For example, despite the fact that a rhesus monkey brain is ten times smaller than a human brain, there are no regions of the human brain that have not been found in the rhesus monkey brain; the same cannot be said for the mouse brain.
The maternal antibody hypothesis of autism has also been investigated using a nonhuman primate model. The first study (Martin et al 2008) demonstrated the effects of exposing pregnant rhesus monkeys to IgG class antibodies from human mothers of multiple children with ASD obtained from the Autism Genetic Resource Exchange (AGRE). Rhesus monkeys prenatally exposed to this pool of maternal antibodies produced more whole body motor stereotypies and hyperactivity compared to both untreated control monkeys and monkeys prenatally exposed to IgG class antibodies from mothers of typically developing children (Martin et al., 2008). Perhaps even more intriguing, a second group of animals has now been treated with IgG derived from mothers who demonstrate the specific 37kDa and 73kDa associated with children who specifically have a diagnosis of ASD. Not only do these animals demonstrate inappropriate social behavior, but also magnetic resonance imaging analysis has demonstrated that they demonstrate a pattern of abnormal brain development that is characteristic of autistic children who have been exposed to the 37/73kDa antibodies in utero (Nordahl et al. IMFAR presentation; Bauman et al, submitted). This monkey model of antibody-induced behavioral and brain development impairment may prove to be a valuable adjunct to epidemiological and clinical data that abnormal antibody exposure during gestation may produce one form of autism. Equally important, this model opens up exciting new areas of exploration concerning the underlying pathology that leads to abnormal brain growth in autism. And, if this model continues to be replicated, it will provide a substrate for evaluating potential interventions and preventative measures.
Conclusion
There is growing recognition that the intrauterine environment is important for the fetus because it can affect lifelong health outcomes (Wills et al., 2009). Maternal antibodies to fetal brain have been identified as one exposure during fetal life that may put a child at risk for autism spectrum disorder. Future studies will focus on identifying which fetal brain antigens the maternal antibodies identify. This may provide insight into the normal role for these proteins in fetal brain development and how interaction with the maternal antibodies may alter the course of brain and behavioral development.
Figure 1.
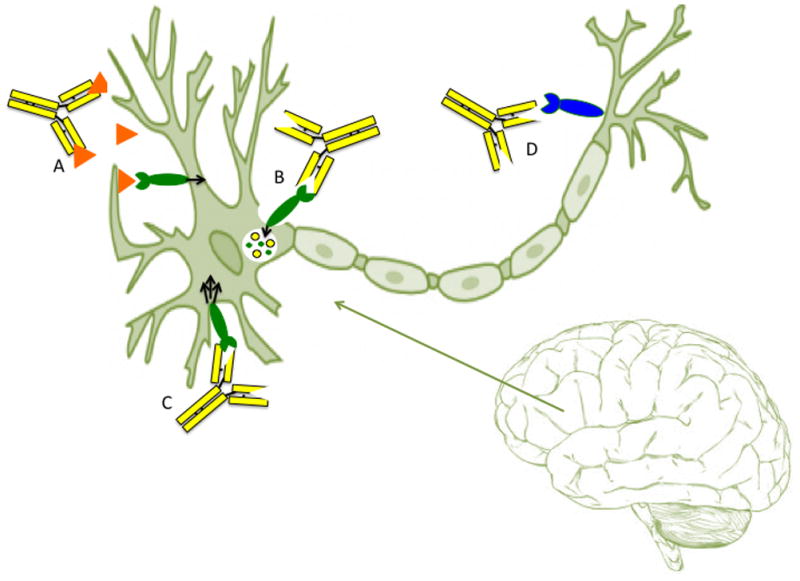
Table 1.
Maternal Antibodies Reactive Toward Fetal Tissues in Mothers of Children with ASD
| PBMC Antigen | Found an association between autism and the presence of maternal antibodies reactive to paternal lymphocytes | (Warren, 1990 #24) |
| Fetal Rat Brain Antigen | Demonstrated serum antibodies from the mother children with autism bound rodent Purkinje cells and other neurons by immunohistochemistry | (Dalton, 2003 #5) |
| Serum from women with autistic children reacted with proteins derived from rat brains and antibody reactivity occurred more often with proteins derived from fetal brain tissue than postnatal and adult brain tissue | (Zimmerman, 2007 #26) | |
| When fetal rat protein was used as an antigen, serum from mothers of children with autistic disorder had greater band specificity at 36kDa and 73 kDa when compared to serum from control mothers. | (Singer, 2006#20) | |
| Fetal Human Brain Antigen | IgG reactivity toward human fetal brain proteins at 37kDa and 73kDa was demonstrated using serum collected from mothers of children with ASD, but not serum from control mothers. | (Braunschweig, 2008 #2) |
| When fetal rat protein was used as an antigen, serum from mothers of children with autistic disorder had an increased reactivity to bands near 36kDa and 39kDa when compared to control mothers. | (Singer, 2006 #20) | |
| Fetal Monkey Brain Antigen | The significant association between maternal IgG reactivity to proteins 37kDa and 73kDa and a diagnosis of autism in the child was maintained with a larger sample size. It was also found this paired reactivity correlated with lower expressive language scores. Additionally, increased irritability on the Aberrant Behavioral Checklist correlated with paired reactivity to proteins at 39 kDa and 73 kDa. | (Braunschweig, 2011 #3) |
Table 2.
Animal Models: Gestational Exposure to Maternal Antibodies and Offspring Behavior
| Mouse Models | The serum from a mother of children with ASD was injected into mice during gestation. The resulting offspring had altered exploration and motor coordination with changes in cerebellar magnetic resonance spectroscopy. | (Dalton, 2003 #5) |
| The transplacental passage of IgG antibodies, collected from mothers of children with autism, is capable of inducing long-term behavioral changes in the resulting offspring. | (Singer, 2009 #19) | |
| Monkey Models | Pregnant rhesus monkeys were exposed maternal antibodies, collected from mothers of children with autism, during gestation and their offspring demonstrated significantly more stereotypies and higher levels of motor activity than control monkeys | (Martin, 2008 #15) |
Acknowledgments
The original research summarized here was supported, in part, by funding from the National Institute of Mental Health (R01MH80218) and NIEHS 1 P01 ES11269-01, the U.S. Environmental Protection Agency (U.S.EPA) through the Science to Achieve Results (STAR) program (Grant R829388). Additional support was provided by the base grant (RR00169) of the California National Primate Research Center.
Footnotes
The authors have no conflicts of interest to declare.
References
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J. Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. Journal of Neuroimmunology. 2011;232:196–199. doi: 10.1016/j.jneuroim.2010.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman MD, Iosif AM, Ashwood P, Braunschweig D, Van de Water J, Amaral, David G. Prenatal exposure of rhesus monkeys to autism-specific maternal antibodies alters brain growth and social behavior. doi: 10.1038/tp.2013.47. (submitted) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Croen LA, Pessah IN, Van de Water J. Autism: maternally derived antibodies specific for fetal brain proteins. Neurotoxicology. 2008;29:226–231. doi: 10.1016/j.neuro.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Duncanson P, Boyce R, Hansen R, Ashwood P, Pessah IN, Hertz-Picciotto I, Van de Water J. Behavioral Correlates of Maternal Antibody Status Among Children with Autism. Journal of Autism and Developmental Disorders. 2011 doi: 10.1007/s10803-011-1378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunschweig D, Golub MS, Koenig CM, Qi L, Pessah IN, Van de Water J, Berman RF. Maternal autism-associated IgG antibodies delay development and produce anxiety in a mouse gestational transfer model. Journal of Neuroimmunology. 2012 doi: 10.1016/j.jneuroim.2012.08.002. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera RM, Shaw GM, Ballard JL, Carmichael SL, Yang W, Lammer EJ, Finnell RH. Autoantibodies to folate receptor during pregnacy and nerual tube defect risk. Journal of Reproductive Immunology. 2008;79:85–92. doi: 10.1016/j.jri.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen LA, Braunschweig D, Haapanen L, Yoshida CK, Fireman B, Grether JK, Kharrazi M, Hansen RL, Ashwood P, Van de Water J. Maternal mid-pregnancy autoantibodies to fetal brain protein: the early markers for autism study. Biol Psychiatry. 2008;64:583–588. doi: 10.1016/j.biopsych.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton P, Deacon R, Blamire A, Pike M, McKinlay I, Stein J, Styles P, Vincent A. Maternal neuronal antibodies associated with autism and a language disorder. Ann Neurol. 2003;53:533–537. doi: 10.1002/ana.10557. [DOI] [PubMed] [Google Scholar]
- Diamond B, Huerta PT, Mina-Osorio P, Kowal C, Volpe BT. Losing your nerves? Maybe it’s the antibodies. Nat Rev Immunol. 2009 doi: 10.1038/nri2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garty BZ, Ludomirsky A, Danon YL, Peter JB, Douglas SD. Placental transfer of immunoglobulin G subclasses. Clin Diagn Lab Immunol. 1994;1:667–669. doi: 10.1128/cdli.1.6.667-669.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Haapanen L, Boyce R, Duncanson P, Braunschweig D, Delwiche L, Hansen R, Hertz-Picciotto I, Ashwood P, Van de Water J. Autoantibodies to cerebellum in children with autism associate with behavior. Brain Behav Immun. 2011;25:514–523. doi: 10.1016/j.bbi.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Van de Water J. The immune system’s role in the biology of autism. Curr Opin Neurol. 2010;23:111–117. doi: 10.1097/WCO.0b013e3283373514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goines P, Zimmerman AW, Ashwood P, Van de Water J. The Immune System, Autoimmunity, Allergy and Autism. In: Amaral D, Geschwind DH, Geraldine D, editors. Autism Spectrum Disorders. New York, New York: Oxford University Press, Inc; 2011. p. 1456. [Google Scholar]
- Guiseppe M, Albert U, Bogetto F, Borghese C, Berro AC, Mutani R, Ferdinando R, Vigliani MC. Anti-brain antibodies in adult patients with obsessive-compulsive disorder. Journal of Affective Disorders. 2009;116:192–200. doi: 10.1016/j.jad.2008.11.019. [DOI] [PubMed] [Google Scholar]
- Heuer L, Braunschweig D, Ashwood P, Van de Water J, Campbell DB. Association of a MET Genetic Variant with Autism-Associated Maternal Autoantibodies to Fetal Brain Proteins and Cytokine Expression Translational Psychiatry. 2011 doi: 10.1038/tp.2011.48. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowal C, DeGiorgio LA, Nakaoka T, Hetherington H, Huerta PT, Diamond B, Volpe BT. Cognition and immunity; antibody impairs memory. Immunity. 2004;21:179–188. doi: 10.1016/j.immuni.2004.07.011. [DOI] [PubMed] [Google Scholar]
- Lee JY, Huerta PT, Zhang J, Kowal C, Bertini E, Volpe BT, Diamond B. Neurotoxic autoantibodies mediate congenital cortical impairment of offspring in maternal lupus. Nat Med. 2009;15:91–96. doi: 10.1038/nm.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LA, Ashwood P, Braunschweig D, Cabanlit M, Van de Water J, Amaral DG. Stereotypies and hyperactivity in rhesus monkeys exposed to IgG from mothers of children with autism. Brain Behav Immun. 2008;22:806–816. doi: 10.1016/j.bbi.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. Janeway’s Immunobiology. New York: Garland Science; 2011. p. 888. [Google Scholar]
- Silva SC, Correia C, Fesel C, Barreto M, Coutinho AM, Marques C, Miguel TS, Ataide A, Bento C, Borges L, Oliveira G, Vicente AM. Autoantibody repertoires to brain tissue in autism nuclear families. J Neuroimmunol. 2004;152:176–182. doi: 10.1016/j.jneuroim.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Simister NE. Placental transport of immunoglobulin G. Vaccine. 2003;21:3365–3369. doi: 10.1016/s0264-410x(03)00334-7. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris C, Gause C, Pollard M, Zimmerman AW, Pletnikov M. Prenatal exposure to antibodies from mothers of children with autism produces neurobehavioral alterations: A pregnant dam mouse model. J Neuroimmunol. 2009;211:39–48. doi: 10.1016/j.jneuroim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Gause CD, Gillin PK, Crawford S, Zimmerman AW. Antibodies against fetal brain in sera of mothers with autistic children. J Neuroimmunol. 2008;194:165–172. doi: 10.1016/j.jneuroim.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Singer HS, Morris CM, Williams PN, Yoon DY, Hong JJ, Zimmerman AW. Antibrain antibodies in children with autism and their unaffected siblings. Journal of Neuroimmunology. 2006;178:149–155. doi: 10.1016/j.jneuroim.2006.05.025. [DOI] [PubMed] [Google Scholar]
- Vincent A, Roberts M, Willison H, Lang B, Newsom-Davis J. Autoantibodies, neurotoxins and the nervous system. J Physiol Paris. 1995;89:129–136. doi: 10.1016/0928-4257(96)80110-0. [DOI] [PubMed] [Google Scholar]
- Vojdani A, Campbell AW, Anyanwu E, Kashanian A, Bock K, Vojdani E. Antibodies to neuron-specific antigens in children with autism: possible cross-reaction with encephalitogenic proteins from milk, Chlamydia pneumonaie, and Streptococcus group A. Journal of Neuroimmunology. 2002;129:168–177. doi: 10.1016/s0165-5728(02)00180-7. [DOI] [PubMed] [Google Scholar]
- Warren RP, Cole P, Odell JD, Pingree CB, Warren WL, White E, Yonk J, Singh VK. Detection of maternal antibodies in infantile autism. J Am Acad Child Adolesc Psychiatry. 1990;29:873–877. doi: 10.1097/00004583-199011000-00005. [DOI] [PubMed] [Google Scholar]
- Wills S, Cabanlit M, Bennett J, Ashwood P, Amaral DG, Van de Water J. Detection of autoantibodies to neural cells of the cerebellum in the plasma of subjects with autism spectrum disorders. Brain Behav Immun. 2009;23:64–74. doi: 10.1016/j.bbi.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman AW, Connors SL, Matteson KJ, Lee LC, Singer HS, Castaneda JA, Pearce DA. Maternal antibrain antibodies in autism. Brain Behav Immun. 2007;21:351–357. doi: 10.1016/j.bbi.2006.08.005. [DOI] [PubMed] [Google Scholar]


