Abstract
BACKGROUND
Despite the increasing prevalence of type 2 diabetes in youth, there are few data to guide treatment. We compared the efficacy of three treatment regimens to achieve durable glycemic control in children and adolescents with recent-onset type 2 diabetes.
METHODS
Eligible patients 10 to 17 years of age were treated with metformin (at a dose of 1000 mg twice daily) to attain a glycated hemoglobin level of less than 8% and were randomly assigned to continued treatment with metformin alone or to metformin combined with rosiglitazone (4 mg twice a day) or a lifestyle-intervention program focusing on weight loss through eating and activity behaviors. The primary outcome was loss of glycemic control, defined as a glycated hemoglobin level of at least 8% for 6 months or sustained metabolic decompensation requiring insulin.
RESULTS
Of the 699 randomly assigned participants (mean duration of diagnosed type 2 diabetes, 7.8 months), 319 (45.6%) reached the primary outcome over an average follow-up of 3.86 years. Rates of failure were 51.7% (120 of 232 participants), 38.6% (90 of 233), and 46.6% (109 of 234) for metformin alone, metformin plus rosiglitazone, and metformin plus lifestyle intervention, respectively. Metformin plus rosiglitazone was superior to metformin alone (P = 0.006); metformin plus lifestyle intervention was intermediate but not significantly different from metformin alone or metformin plus rosiglitazone. Prespecified analyses according to sex and race or ethnic group showed differences in sustained effectiveness, with metformin alone least effective in non-Hispanic black participants and metformin plus rosiglitazone most effective in girls. Serious adverse events were reported in 19.2% of participants.
CONCLUSIONS
Monotherapy with metformin was associated with durable glycemic control in approximately half of children and adolescents with type 2 diabetes. The addition of rosiglitazone, but not an intensive lifestyle intervention, was superior to metformin alone. (Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others; TODAY ClinicalTrials.gov number, NCT00081328.)
Increases in childhood obesity have been accompanied by an increased incidence of type 2 diabetes in youth.1,2 Because the risk of microvascular and macrovascular complications in adults increases with both the duration of diabetes and lack of glycemic control,3,4 it is imperative to achieve and sustain metabolic control in youth. Addressing the physiological and psychological changes that normally occur during adolescence requires a high level of family involvement and makes the achievement of stringent treatment goals especially difficult in the case of adolescents with diabetes.5,6 These challenges are heightened in disadvantaged populations, which are over-represented among adolescents with type 2 diabetes.
METHODS
STUDY DESIGN
The Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study was a multicenter, randomized clinical trial funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) (members of the study group are listed in Section A in the Supplementary Appendix, available with the full text of this article at NEJM.org). The TODAY study compared metformin monotherapy with two alternative approaches, one combining metformin with a second pharmacologic agent (rosiglitazone) and one combining metformin with an intensive lifestyle-intervention program, to test the hypothesis that combination therapy initiated early in the course of youth-onset type 2 diabetes would maintain acceptable glycemic control better than metformin alone.
The rationale, design, and methods of the study have been reported previously7 and are summarized in Section B in the Supplementary Appendix. The randomization scheme was computer-generated at a 1:1:1 ratio according to study site, with a block size of 9. Participants were randomly assigned to metformin (at a dose of 1000 mg twice daily) given alone, metformin plus rosiglitazone (4 mg twice daily), or metformin plus a lifestyle-intervention program. The program, which focused on weight loss through family-based changes in eating and activity behaviors, was delivered in a series of in-person visits during the first 2 years, with continued contact at quarterly medical visits. Details of the lifestyle-intervention program have been reported previously8 and are summarized in Section B in the Supplementary Appendix. Site investigators, study personnel, and participants were not aware of the assignments to the metformin-alone and metformin-plus-rosiglitazone groups, and study medication was encapsulated to ensure masking; all participants took the same number of capsules each day.
Eligibility criteria included an age of 10 to 17 years, type 2 diabetes according to American Diabetes Association criteria9 for less than 2 years, a body-mass index (BMI) at or above the 85th percentile for age and sex, a negative test for diabetes-related autoantibodies (glutamic acid decarboxylase 65 and tyrosine phosphatase10), a fasting C-peptide level of more than 0.6 ng per milliliter, and the availability of an adult caregiver who was willing to actively support study participation. Eligible children and adolescents entered a run-in period of 2 to 6 months, with the goals of weaning them from nonstudy diabetes medications, initiating treatment with metformin at a dose of up to 1000 mg twice daily but no less than 500 mg twice daily, attaining glycemic control (a glycated hemoglobin level of less than 8%, measured monthly for at least 2 months) with metformin alone, providing standard diabetes education and ensuring the participants’ mastery of the material,11 and confirming their adherence to the study medication regimen and attendance at scheduled visits. Enrollment started in July 2004 and ended in February 2009. Follow-up continued through February 2011, a predetermined stopping point that provided a minimum of 2 years of follow-up for all participants (mean, 3.8; maximum, 6.5). Laboratory assessments were performed in a central laboratory.7
The primary objective was to compare treatment groups with regard to the time to treatment failure, defined as a persistently elevated glycated hemoglobin level (≥8%) over a period of 6 months or persistent metabolic decompensation (defined as either the inability to wean the participant from insulin within 3 months after its initiation for decompensation or the occurrence of a second episode of decompensation within 3 months after discontinuation of insulin). Glycated hemoglobin testing was performed every 2 months in the first year and quarterly thereafter. Diabetes care was provided according to uniform study procedures7 (see Section B in the Supplementary Appendix). Adherence was determined on the basis of counts of pills in blister packs that were dispensed and returned full, partially full, or empty at each visit, with a target of at least 80% adherence.
The study protocol (available at NEJM.org) was approved by the institutional review board of each participating institution. The parents of children and adolescents who participated in the study provided written informed consent, and the children and adolescents provided their assent. Safety and risk management were monitored by the independent data and safety monitoring board. Serious adverse events were reported as they occurred. In addition to the standard monitoring of serious adverse events, three study-specific serious adverse events were tracked: severe hypoglycemia, diabetic ketoacidosis, and lactic acidosis.
The steering committee, composed of the principal investigator at each clinical site and at the data coordinating center and the project scientist from the sponsor (NIDDK), designed and implemented the study. The data coordinating center accumulated data in a central database during the study and performed data analyses according to a prespecified plan developed by the biostatisticians at the data coordinating center and approved by the steering committee and the data and safety monitoring board. The study investigators, who had access to all data analyses and wrote the manuscript, attest to the veracity and completeness of the data and the fidelity of the study to the protocol. The decision to submit the manuscript for publication was made by the steering committee, with no restrictions imposed by the sponsor. The data were analyzed by two members of the writing group. The drugs used in the study were donated by pharmaceutical manufacturers (listed at the end of the article), which had no role in the study design, data accrual, data analysis, or manuscript preparation.
STATISTICAL ANALYSIS
Treatment failure was analyzed with the use of time-to-event survival methods (PROC LIFEREG, SAS software, version 9.2, SAS Institute). All randomly assigned participants were included in the time-to-event analysis. For participants who had treatment failure, the time of failure was entered as an interval during which failure was known to have occurred rather than as the exact date of failure, which was not known. For participants who did not have treatment failure, the total amount of time in the study when the participant could be evaluated was entered; this was the time to the last visit in most cases, unless the participant withdrew, did not return for follow-up, underwent bariatric surgery, or chose to take insulin. A log-logistic distribution was specified for time to failure. The trial was powered for the three pairwise comparisons among treatment groups for the primary outcome, each at a significance level of 0.0167. Analyses of outcome according to sex and racial or ethnic subgroups were prespecified, including the three categories with adequate representation: non-Hispanic blacks (32.5% of the entire cohort), Hispanics (39.7%), and non-Hispanic whites (20.3%). Longitudinal data were tested with the use of general linear mixed models, which were used to test whether the change from baseline was equivalent across treatment groups, with adjustment for the baseline value. Log transformation was performed when appropriate to normalize skewed distributions. For secondary outcomes and analyses, a P value of 0.05 was considered to indicate statistical significance, with no adjustment for multiple testing.
RESULTS
STUDY COHORT
The study enrollment, randomization, and retention are shown in Figure 1. Of the 1211 children and adolescents who were screened, 927 (76.5%) entered the run-in phase and 699 (75.4%) were randomly assigned to a treatment group, for an overall enrollment rate of 57.7%. The mean duration of diagnosed type 2 diabetes was 7.8 months. The baseline demographic and clinical characteristics of this cohort have been reported in detail previously12 and are summarized in Section C in the Supplementary Appendix. Randomly assigned persons were not significantly different from those who were screened but not enrolled, in regard to sex, race or ethnic group, age, and BMI, but the glycated hemoglobin level was significantly lower among those who were not enrolled (7.5% vs. 8.0%, P<0.001). Participants were followed for an average of 3.86 years.
Figure 1. Enrollment, Randomization, and Retention of the Study Participants.
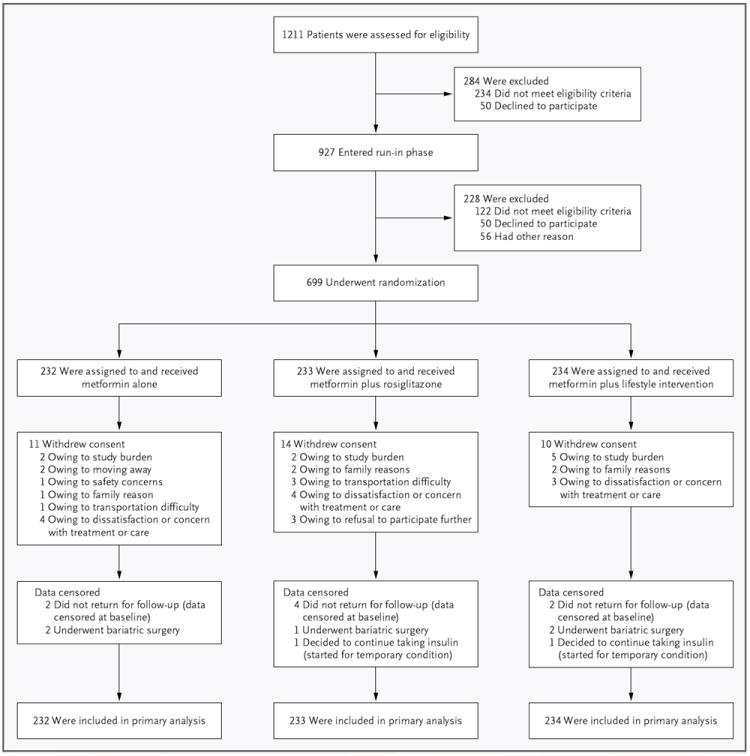
All randomly assigned participants were included in the primary outcome analysis (i.e., all participants contributed time in the study, although data for some participants were censored before the end of the study for the reasons shown in the figure).
PRIMARY OUTCOME
Of the 699 participants, 319 (45.6%) reached the primary outcome, with a median time to treatment failure of 11.5 months (range, <1 to 66). Overall rates of failure were 51.7% (95% confidence interval [CI], 45.3 to 58.2; 120 of 232 participants) with metformin alone, 38.6% (95% CI, 32.4 to 44.9; 90 of 233 participants) with metformin plus rosiglitazone, and 46.6% (95% CI, 40.2 to 53.0; 109 of 234 participants) with metformin plus lifestyle intervention (Fig. 2). Metformin plus rosiglitazone was associated with a 25.3% decrease in the occurrence of the primary outcome as compared with metformin alone (P = 0.006); the outcome with metformin plus lifestyle intervention was intermediate but did not differ significantly from the outcome with metformin alone or with metformin plus rosiglitazone. The reasons for treatment failure and the median time to failure did not differ significantly across treatments (see Section D in the Supplementary Appendix). The mean glycated hemoglobin levels according to duration of time in the study and to treatment group are shown in Section E in the Supplementary Appendix. A secondary covariate analysis with adjustment for sex, race or ethnic group, baseline BMI, and baseline glycated hemoglobin level did not modify the relationship between treatment group and primary outcome.
Figure 2. Overall Primary Outcome Results.
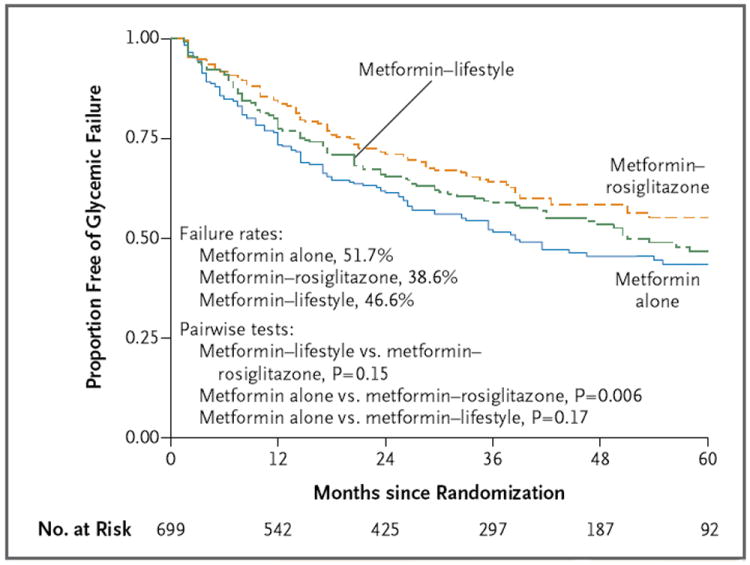
Survival curves for freedom from glycemic failure are shown. Data are shown for up to 60 months of follow-up (accounting for 98.4% of cases of glycemic failure), although the rates and analysis are based on the complete data set.
WEIGHT LOSS AND BMI
BMI over time (up to 60 months) differed significantly according to the study treatment (P<0.001 for the overall comparison), and the results of all three pairwise comparisons between treatment groups were also significant. The metformin-plus-rosiglitazone group had the greatest increase in BMI and the metformin-plus-lifestyle group the least (Section F in the Supplementary Appendix). However, neither BMI at baseline nor BMI over time was a determinant of treatment failure. Percent overweight (defined as BMI minus BMI at the 50th percentile for age and sex, divided by BMI at the 50th percentile) was calculated to examine change in the critical first 6 months of treatment administration, when weight-loss interventions typically have their greatest effect. The average change in percent overweight at 6 months was −1.42 percentage points for metformin alone, 0.81 percentage points for metformin plus rosiglitazone, and −3.64 percentage points for metformin plus lifestyle intervention (P<0.001 for the overall comparison; all three pairwise comparisons were also significant). At 24 months, metformin plus rosiglitazone (0.89 percentage points) was still significantly different from both metformin alone (−4.42 percentage points) and metformin plus lifestyle intervention (−5.02 percentage points) (P<0.001 for both comparisons with metformin plus rosiglitazone), but metformin alone was not significantly different from metformin plus lifestyle intervention. A reduction of at least 7 percentage points in percent overweight was considered meaningful. The proportion of participants with such a reduction at 6 months was significantly higher in the metfor-min-plus-lifestyle group (31.2%) than in the met-formin-plus-rosiglitazone group (16.7%, P<0.001) but did not differ significantly from the proportion in the metformin-alone group (24.3%).
SUBGROUP ANALYSES
The results of prespecified exploratory subgroup analyses according to sex and race or ethnic group are shown in Figure 3. Overall failure rates were 44.3% among girls and 48.2% among boys. The interaction of treatment with sex was significant (P = 0.02). Metformin plus rosiglitazone was more effective in girls than in boys (P = 0.03). In addition, among girls, metformin plus rosiglitazone was more effective than metformin alone (P=0.002) and metformin plus lifestyle intervention (P=0.006), whereas in boys, metformin plus rosiglitazone was not more effective than either metformin alone or metformin plus lifestyle intervention. All other pairwise comparisons were nonsignificant.
Figure 3. Primary Outcome Results According to Sex and Race or Ethnic Group.
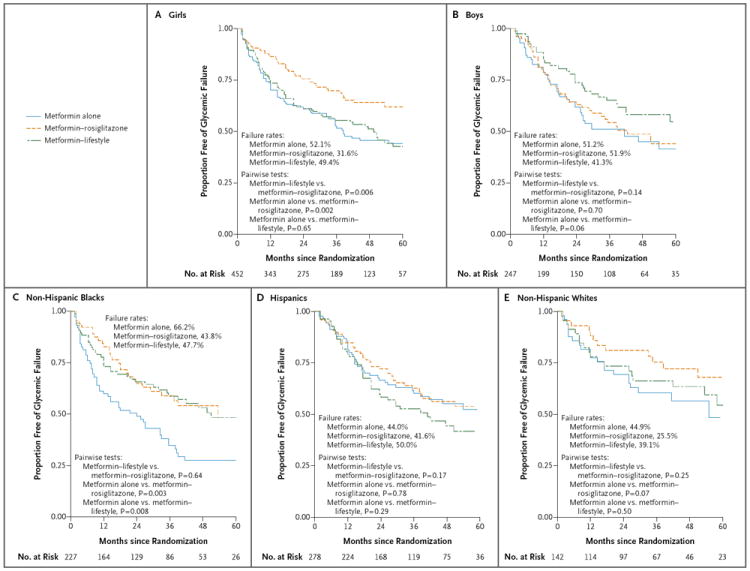
Survival curves for freedom from glycemic failure are shown. Data are shown for up to 60 months of follow-up (accounting for 98.4% of cases of glycemic failures), although the rates and analysis are based on the complete data set.
The interaction of treatment with race or ethnic group was not significant, but race or ethnic group alone had a significant effect on the outcome (P = 0.006). Overall failure rates among non-His-panic blacks, Hispanics, and non-Hispanic whites were 52.8%, 45.0%, and 36.6%, respectively. The failure rate among American Indians was 39.0%, although these participants were not included in the analysis by race or ethnic group owing to small numbers. Metformin alone was less effective in non-Hispanic blacks, with 66.2% reaching the primary outcome, than in either non-Hispanic whites (44.9%, P = 0.01) or Hispanics (44.0%, P<0.001); there were no significant differences with the other treatment regimens. Subgroup analyses that combined sex and race or ethnic group did not indicate any differential effects of the study treatments, although the numbers of participants in the subgroups were small.
ADHERENCE
Adherence to the medication regimen before the primary outcome was reached or the study was completed ranged from 84% at month 8 to 57% at month 60 but did not differ significantly across treatments (Section G in the Supplementary Appendix). Sex, race or ethnic group, age, baseline BMI, and baseline glycated hemoglobin level did not differ significantly between participants who adhered to the study regimen and those who did not. When the analysis of the primary outcome included only participants who adhered to the medication regimen, the findings were unchanged.
The rate of attendance at lifestyle program visits during the first 24 months was 75.2%; 53.6% of participants met the preplanned target of attending 75% or more of visits over these 2 years. There was no significant difference in the occurrence of glycemic failure or BMI change between participants who met the target for visits and those who did not.
SECONDARY OUTCOMES
Median values for metabolic outcomes (lipid levels, blood pressure, albumin:creatinine ratio, insulin secretion and sensitivity, and body composition) at baseline and 2 years, the maximum time that all participants were evaluated for secondary outcomes, are reported in Section H in the Supplementary Appendix. The change in fat mass from baseline differed significantly across the treatment groups (P<0.05) because of a significant difference between the metformin-plus-rosiglitazone and metformin-plus-lifestyle groups. There were no significant between-group differences in the change from baseline for any other outcome.
Risk factors for cardiovascular disease — hypertension, high low-density lipoprotein cholesterol levels, hypertriglyceridemia, and microalbuminuria — at baseline and at the end of the study are shown in Table 1. The proportions of participants with these risk factors increased over time and by the end of study were 33.8%, 10.3%, 28.2%, and 16.6%, respectively. There were no significant differences across treatment groups in newly identified risk factors over equivalent person-years of follow-up. No clinically apparent cardiac, renal, neurovascular, or ophthalmologic complications occurred during the trial.
Table 1.
Major Coexisting Conditions at Baseline and during the Study, According to Treatment Group.*
| Condition | Overall (N = 699) | Metformin Alone (N = 232) | Metformin plus Rosiglitazone (N = 233) | Metformin plus Lifestyle Intervention (N = 234) |
|---|---|---|---|---|
| number of participants (percent) | ||||
| Hypertension | ||||
| Cases at baseline | 81 (11.6) | 28 (12.1) | 27 (11.6) | 26 (11.1) |
| New cases during study | 155 (22.2) | 57 (24.6) | 53 (22.7) | 45 (19.2) |
| Dyslipidemia | ||||
| Elevated LDL cholesterol | ||||
| Cases at baseline | 23 (3.3) | 9 (3.9) | 8 (3.4) | 6 (2.6) |
| New cases during study | 49 (7.0) | 18 (7.8) | 16 (6.9) | 15 (6.4) |
| Triglyceridemia | ||||
| Cases at baseline | 127 (18.2) | 51 (22.0) | 38 (16.3) | 38 (16.2) |
| New cases during study | 70 (10.0) | 20 (8.6) | 28 (12.0) | 22 (9.4) |
| Microalbuminuria | ||||
| Cases at baseline | 44 (6.3) | 21 (9.1) | 8 (3.4) | 15 (6.4) |
| New cases during study | 72 (10.3) | 25 (10.8) | 27 (11.6) | 20 (8.5) |
Diagnosis of a coexisting condition was made on the basis of an out-of-range value repeated within 6 months or the use of appropriate medication in lieu of a laboratory value. Cutoff values for hypertension were a blood-pressure level at or above the 95th percentile, systolic pressure of 130 mm Hg or more, or diastolic pressure of 80 mm Hg or more; for dyslipidemia, a low-density lipoprotein (LDL) cholesterol level of 130 mg per deciliter (3.4 mmol per liter) or more or a triglyceride level of 150 mg per deciliter (1.7 mmol per liter) or more; and for microalbuminuria, a urinary albumin:creatinine ratio of 30 or more, with albumin measured in milligrams per deciliter and creatinine in grams per deciliter.
ADVERSE EVENTS
Serious adverse events were reported by 19.2% of participants, including 18.1% in the metformin-alone group, 14.6% in the metformin-plus-rosiglitazone group, and 24.8% in the metformin-plus-lifestyle group (P = 0.02). Overall, 227 serious adverse events were reported in 134 participants, of which 87% were not considered to be related to the study treatment (Table 2). Hospitalizations accounted for more than 90% of serious adverse events. Severe hypoglycemia occurred in 1 patient in the metformin-alone group, 1 in the metformin-plus-rosiglitazone group, and 2 in the metformin-plus-lifestyle group, and there was 1 case of confirmed, nonfatal transient lactic acidosis in a participant in the metformin-alone group who was hospitalized for asthma exacerbation. No effects of rosiglitazone on bone mineral content or rate of fracture were noted.
Table 2.
Adverse Events and Clinical and Biochemical Assessments According to Treatment Group.*
| Event or Assessment | Metformin Alone (N = 232) | Metformin plus Rosiglitazone (N = 233) | Metformin plus Lifestyle Intervention (N = 234) | P Value |
|---|---|---|---|---|
| no. of participants with ≥1 episode | ||||
| Events | ||||
| Serious adverse events | 42 | 34 | 58 | 0.02 |
| Diabetic ketoacidosis | 5 | 3 | 3 | 0.70 |
| Hyperglycemia | 3 | 5 | 2 | 0.49 |
| Hypoglycemia | 1 | 1 | 2 | 1.00 |
| Lactic acidosis | 1 | 0 | 0 | 0.33 |
| Adverse events | ||||
| Rash on physical examination | 108 | 101 | 95 | 0.43 |
| Gastrointestinal symptoms† | 129 | 100 | 136 | 0.002 |
| Edema on physical examination | 17 | 17 | 17 | 1.00 |
| Hyperglycemia symptoms‡ | 115 | 98 | 103 | 0.24 |
| Infection requiring medical attention | 149 | 120 | 151 | 0.005 |
| Sprain or fracture requiring medical attention | 66 | 53 | 64 | 0.33 |
| Muscle ache or pain | 68 | 53 | 77 | 0.05 |
| Mild hypoglycemia | 10 | 19 | 8 | 0.05 |
| Clinical and biochemical assessments | ||||
| Anemia§ | 71 | 58 | 52 | 0.11 |
| Renal impairment¶ | 0 | 1 | 1 | 1.00 |
| Elevation of liver enzymes | ||||
| 1.5–2.5× upper limit of normal | 70 | 47 | 57 | 0.04 |
| >2.5× upper limit of normal | 39 | 20 | 35 | 0.02 |
Event categories included both adverse events and serious adverse events. Serious adverse events were reported by investigators as they occurred. A serious adverse event was defined as satisfying one or more of the following criteria: inpatient hospitalization, prolongation of a hospital stay, permanent or severe disability, death, congenital anomaly in the offspring of a participant, overdose (either accidental or intentional) of the study medication, and life-threatening event. Three study-specific serious adverse events were identified: severe hypoglycemia, diabetic ketoacidosis, and lactic acidosis. There were no deaths. Participants were asked about a list of targeted adverse events at each scheduled study visit. P values are based on chi-square or Fisher’s exact tests.
Gastrointestinal symptoms included frequent stomach pains, bloating, nausea, diarrhea, and loss of appetite.
Symptoms of hyperglycemia included nocturia more than once a night on a regular basis, enuresis, increased thirst, and more frequent urination than usual.
Anemia was defined as a hematocrit of less than 30% or a hemoglobin level of less than 10 mg per deciliter.
Renal impairment was defined as an estimated creatinine clearance of less than 70 ml per minute or a serum creatinine level of more than 1.5 mg per deciliter (133 μmol per liter).
DISCUSSION
The primary results of our study show first, that metformin monotherapy provided durable glycemic control in only half the participants; second, that the combination of metformin and rosiglitazone improved the durability of glycemic control; and third, that metformin combined with lifestyle intervention was no better than metformin alone in maintaining glycemic control. The differential effect among treatments did not appear to be due to differences in adherence and could not be explained by baseline characteristics, differential effects on BMI, or treatment-group differences in insulin secretion, insulin sensitivity, or body composition.
The rate of treatment failure with metformin monotherapy was higher in this cohort than in similar cohorts of adults treated with metformin,13-15 although the definition of failure differed among the studies. The reason for the decreased durability of glycemic control with metformin is unclear but is unlikely to be due to poor medication adherence, since adherence was more than 80% during the first year of the study, when half the patients had treatment failure, and there was no significant difference in the rate of loss of glycemic control between participants who adhered to the medication regimen and those who did not. Further analysis is required to determine whether the apparent decrease in the durability of glycemic control with metformin in adolescents as compared with adults reflects biologic differences, pathophysiological differences, or both.
The combination of rosiglitazone and metformin reduced the rate of treatment failure as compared with metformin monotherapy, despite a small increase in BMI and fat mass in the rosiglitazone-treated participants. Whether the effect shown in this study is specific for rosiglitazone, a more general effect of thiazolidinediones, or a feature of combination therapy is unclear. This question is of particular importance, given the currently restricted status of rosiglitazone in the United States and Europe. The absence of adverse events related to rosiglitazone, including the absence of an identified effect of rosiglitazone on bone density in this cohort of children and adolescents, an age group characterized by skeletal growth, should be interpreted cautiously, given the limited sample size.
The lifestyle program developed for the study was a multicomponent intervention based on the best available evidence, individually delivered by trained personnel, and with good participant adherence to visits. Although metformin plus lifestyle intervention significantly decreased percent overweight, this did not translate into sustained glycemic control, as compared with metformin monotherapy. Further analysis of the effect of various components of the lifestyle intervention is needed to understand the current findings and identify ways to effectively integrate behavioral self-management in the ongoing care of youth with type 2 diabetes.
Subgroup analyses suggest that metformin plus rosiglitazone was more effective in girls than in boys and that metformin alone was less effective in non-Hispanic black participants than in other racial or ethnic groups. The reasons for these differences in response to treatments according to sex and race or ethnic group are not clear, although differences in insulin sensitivity and insulin secretion,16-18 adiponectin,19 fitness,20 and routine activity levels have been reported. Further analysis of these variables, as well as other covariates, such as socioeconomic status and psychosocial functioning, is needed to more fully understand these findings.
Metformin alone was effective in maintaining durable glycemic control in only half the study participants, and the addition of rosiglitazone, but not intensive lifestyle intervention, was superior to metformin alone. These results suggest that a majority of youth with type 2 diabetes may require combination treatment or insulin therapy within a few years after diagnosis.
Supplementary Material
Acknowledgments
Funded by the National Institute of Diabetes and Digestive and Kidney Diseases and others;
Supported by grants from the NIDDK (U01-DK61212, U01-DK61230, U01-DK61239, U01-DK61242, and U01-DK61254) and the National Center for Research Resources (NCRR) (M01-RR00036, to the Washington University School of Medicine; M01-RR00043-45, to Children’s Hospital Los Angeles; M01-RR00069, to the University of Colorado Denver; M01-RR00084, to Children’s Hospital of Pittsburgh; M01-RR01066, to Massachusetts General Hospital; M01-RR00125, to Yale University; and M01-RR14467, to the University of Oklahoma Health Sciences Center); by NCRR Clinical and Translational Science Awards (UL1-RR024134, to Children’s Hospital of Philadelphia; UL1-RR024139, to Yale University; UL1-RR024153, to Children’s Hospital of Pittsburgh; UL1-RR024989, to Case Western Reserve University; UL1-RR024992, to Washington University in St. Louis; UL1-RR025758, to Massachusetts General Hospital; and UL1-RR025780, to the University of Colorado Denver); and by donations from Becton Dickinson, Bristol-Myers Squibb, Eli Lilly, GlaxoSmithKline, LifeScan, Pfizer, and Sanofi-Aventis.
Dr. Arslanian reports receiving consulting fees from Sanofi-Aventis, Novo Nordisk, and Bristol-Myers Squibb and serving on the data and safety monitoring board of Boehringer Ingelheim. Dr. Copeland reports receiving consulting fees from Novo Nor-disk (advisory board membership) and from Daiichi Sankyo (Clinical Trial Steering Committee). Dr. Kaufman reports receiving consulting fees from DPS Health and being an employee of Medtronic. Dr. Wilfley reports receiving consulting fees from UnitedHealth Group, Jenny Craig, and Nestlé and receiving grant support from Shire on behalf of her institution. Dr. Zeitler reports receiving consulting fees from Daiichi Sankyo, Merck, and Bristol-Myers Squibb on behalf of his institution.
We thank the American Indian partners associated with the clinical center at the University of Oklahoma Health Sciences Center, including members of the Absentee Shawnee Tribe, Cherokee Nation, Chickasaw Nation, Choctaw Nation of Okla-homa, and Oklahoma City Area Indian Health Service, for their participation and guidance.
The members of the writing group — Phil Zeitler, M.D., Ph.D., University of Colorado Denver, Aurora; Kathryn Hirst, Ph.D., and Laura Pyle, M.S., George Washington University, Washington, DC; Barbara Linder, M.D., Ph.D., National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD; Kenneth Copeland, M.D., University of Oklahoma Health Sciences Center, Oklahoma City; Silva Arslanian, M.D., Children’s Hospital of Pittsburgh, Pittsburgh; Leona Cuttler, M.D., Case Western Reserve University, Cleveland; David M. Nathan, M.D., Massachusetts General Hospital, Boston; Sherida Tollefsen, M.D., Saint Louis University, and Denise Wilfley, Ph.D., Washington University — both in St. Louis; and Francine Kaufman, M.D., Children’s Hospital Los Angeles, Los Angeles — assume responsibility for the overall content and integrity of the article.
Footnotes
The opinions expressed in this article are those of the authors and do not necessarily reflect the views of the tribal and Indian Health Service institutional review boards or their members.
No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Materials used in the TODAY standard diabetes education program and the lifestyle-intervention program are available for download at https://today.bsc.gwu.edu.
References
- 1.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr. 2005;146:693–700. doi: 10.1016/j.jpeds.2004.12.042. [DOI] [PubMed] [Google Scholar]
- 2.Fagot-Campagna A, Pettitt DJ, Engelgau MM, et al. Type 2 diabetes among North American children and adolescents: an epidemiologic review and a public health perspective. J Pediatr. 2000;136:664–72. doi: 10.1067/mpd.2000.105141. [DOI] [PubMed] [Google Scholar]
- 3.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HAW. 10-Year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577–89. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 4.Turner R, Cull C, Holman R. United Kingdom Prospective Diabetes Study 17: a 9-year update of a randomized, controlled trial on the effect of improved metabolic control on complications in non-insulin-dependent diabetes mellitus. Ann Intern Med. 1996;124:136–45. doi: 10.7326/0003-4819-124-1_part_2-199601011-00011. [DOI] [PubMed] [Google Scholar]
- 5.Pinhas-Hamiel O, Standiford D, Hamiel D, Dolan LM, Cohen RM, Zeitler PS. The type 2 family: a setting for development and treatment of adolescent type 2 diabetes mellitus. Arch Pediatr Adolesc Med. 1999;153:1063–7. doi: 10.1001/archpedi.153.10.1063. [DOI] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O, Zeitler P. Barriers to the treatment of adolescent type 2 diabetes — a survey of provider perceptions. Pediatr Diabetes. 2003;4:24–8. doi: 10.1034/j.1399-5448.2003.00027.x. [DOI] [PubMed] [Google Scholar]
- 7.TODAY Study Group. Zeitler P, Epstein L, et al. Treatment options for type 2 diabetes in adolescents and youth: a study of the comparative efficacy of metformin alone or in combination with rosiglitazone or lifestyle intervention in adolescents with type 2 diabetes. Pediatr Diabetes. 2007;8:74–87. doi: 10.1111/j.1399-5448.2007.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.TODAY Study Group. Design of a family-based lifestyle intervention for youth with type 2 diabetes: the TODAY study. Int J Obes (Lond) 2010;34:217–26. doi: 10.1038/ijo.2009.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2006;29(Suppl 1):S43–S48. [PubMed] [Google Scholar]
- 10.Klingensmith GJ, Pyle L, Arslanian S, et al. The presence of GAD and IA-2 antibodies in youth with a type 2 diabetes phenotype: results from the TODAY study. Diabetes Care. 2010;33:1970–5. doi: 10.2337/dc10-0373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grey M, Schreiner B, Pyle L. Development of a diabetes education program for youth with type 2 diabetes. Diabetes Educ. 2009;35:108–16. doi: 10.1177/0145721708325156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copeland KC, Zeitler P, Geffner M, et al. Characteristics of adolescents and youth with recent-onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab. 2011;96:159–67. doi: 10.1210/jc.2010-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner RC, Cull CA, Frighi V, Holman RR. Glycemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49) JAMA. 1999;281:2005–12. doi: 10.1001/jama.281.21.2005. [DOI] [PubMed] [Google Scholar]
- 14.Brown JB, Conner C, Nichols GA. Secondary failure of metformin monotherapy in clinical practice. Diabetes Care. 2010;33:501–6. doi: 10.2337/dc09-1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kahn SE, Haffner SM, Heise MA, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355:2427–43. doi: 10.1056/NEJMoa066224. Erratum, N Engl J Med 2007;356:1387-8. [DOI] [PubMed] [Google Scholar]
- 16.Bacha F, Saad R, Gungor N, Janosky J, Arslanian SA. Obesity, regional fat distribution, and syndrome X in obese black versus white adolescents: race differential in diabetogenic and atherogenic risk factors. J Clin Endocrinol Metab. 2003;88:2534–40. doi: 10.1210/jc.2002-021267. [DOI] [PubMed] [Google Scholar]
- 17.Hasson RE, Adam TC, Davis JN, et al. Ethnic differences in insulin action in obese African-American and Latino adolescents. J Clin Endocrinol Metab. 2010;95:4048–51. doi: 10.1210/jc.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bacha F, Gungor N, Lee S, Arslanian SA. Type 2 diabetes in youth: are there racial differences in β-cell responsiveness relative to insulin sensitivity? Pediatr Diabetes. 2011 Sep 20; doi: 10.1111/j.1399-5448.2011.00820.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SJ, Bacha F, Gungor N, Arslanian SA. Racial differences in adiponectin in youth: relationship to visceral fat and insulin sensitivity. Diabetes Care. 2006;29:51–6. doi: 10.2337/diacare.29.1.51. [DOI] [PubMed] [Google Scholar]
- 20.Andreacci J, Robertson RJ, Dubé JJ, Aaron DJ, Dixon CB, Arslanian SA. Comparison of maximal oxygen consumption between obese black and white adolescents. Pediatr Res. 2005;58:478–82. doi: 10.1203/01.pdr.0000176909.66057.a3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


