Abstract
In a continuing study of bevirimat (2), the anti-HIV-maturation clinical trials agent, 28 new betulinic acid (BA, 1) derivatives were designed and synthesized. Among these compounds, 17, with a C-28 MEM ester moiety, and 22, with a C-28 ethyl hexanoate, increased the anti-HIV replication activity compared with 2 by two-fold, while compounds 40–41 and 48–49, with C-28 piperazine or piperidine amide substitutions, increased the activity by three- to fifteen-fold. The best new compound 41 exhibited an anti-HIV IC50 value of 0.0059 μM, compared with 0.087 μM for 2. All of the active compounds showed only anti-maturation effects, as confirmed by TZM-bl assay, in blocking the HIV replication. The results suggest that proper C-28 substitutions can further enhance the anti-maturation activity of 2, without any anti-entry effects. Thus, 41 may serve as a promising new lead for development of anti-AIDS clinical trial candidates.
Introduction
Although introduction of highly active antiretroviral therapy (HAART) has significantly improved the treatment of HIV/AIDS,1–4 HIV incidence is still increasing in many countries and regions. Over 2.6 million new infections occurred in 2009 alone, contributing to the current global incidence of 33.3 million.5 New infections continue to outpace the number of people placed on treatments, and the virus is suppressed rather than eradicated.6–8 In addition, the efficacy of the treatments is hampered by the emergence of drug-resistant viral strains and severe drug-drug interactions.9–11 Therefore, novel potent antiretroviral agents with different targets are still urgently needed.
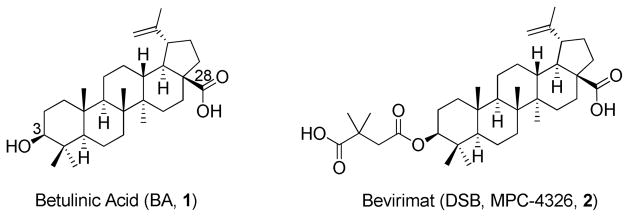
Triterpenes, such as betulinic acid (BA, 1),12 represent a promising class of anti-HIV agents with novel mechanisms. In our prior research on modified triterpenes, bevirimat [3-O-(3′,3′-dimethylsuccinyl)betulinic acid, 2] was found to exhibit remarkable anti-HIV-1 activity against primary and drug-resistant HIV-1 isolates (Figure 1).13, 14 Mechanism of action study revealed that 2 blocks the last step of viral gag precursor polyprotein processing from p25 (CA-SP1) to functional p24 (CA), resulting in the production of noninfectious immature HIV-1 particles.15 Therefore, 2 represents a unique first in a class of anti-HIV compounds termed maturation inhibitors (MIs), and it succeeded in Phase I and IIa clinical trials against sensitive viruses during 2007–2009.14, 16–20
Figure 1.
Introduction of a C-28 side chain into a C-3 modified BA-analog (e.g. 2) can generate a bi-functional HIV inhibitor with both anti-maturation and anti-entry activities.21 Consequently, in the current study, additional C-28 side chains were investigated. Interestingly, it was discovered that certain C-28 side chains could significantly enhance the anti-HIV maturation activity of 2 without introducing the second mechanism of anti-entry effects. Their syntheses, anti-HIV evaluation, and structure-activity relationship (SAR) correlations are reported in this paper.
Design
Prior C-28 modification of 1 focused only on the promotion of anti-HIV entry activity. It was found that C-28 amide functionality, a second aminoalkanoic acid group near the end of the C-28 side chain, and a 7–10 carbon alkane chain between these two amide moieties are important to the enhanced anti-entry potency.22 In order to design anti-maturation promoting C-28 side chains without an anti-entry effect, we first synthesized a group of BA derivatives (3, 4, 7–15, and 29) with a C-28 ester moiety rather than the prior amide functionality. Methoxymethyl (MOM), methoxyethoxymethyl (MEM), and tetraethylene glycol chains were incorporated to improve the hydrophilicity of the compounds (7, 8, and 29) and investigate the influence of a polar heteroatom (e.g. oxygen) present within the C-28 side chain, which was originally a pure carbon alkane chain. Bulky substitutions (9 and 12) and an unsaturated C-28 chain (11) were also synthesized. Different lengths of the C-28 alkane chains with or without a second ester group near the terminus were also investigated (14 vs 10, 13, and 15). These 28-modified BA derivatives were evaluated for anti-HIV replication activity to determine if these compounds function as HIV-1 entry inhibitors, before the C-3 anti-maturation pharmacophore, a dimethylsuccinyl side chain, was incorporated to generate the corresponding 28-substituted derivatives (16–24 and 30) of 2.
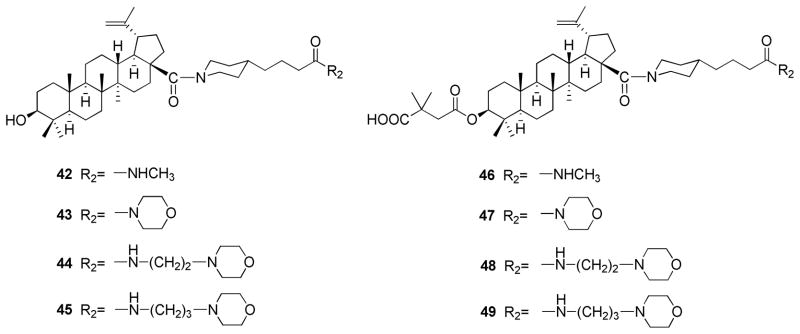
In addition, a cyclic secondary amine from piperazinebutyric acid or piperazinepentanoic acid, rather than a primary amine, was used to form the amide bond with the C-28 carboxylic acid of 1 to yield compounds 38 and 39. In this design, an additional polar heteroatom nitrogen was present within the side chain, and the substitution near the C-28 amide moiety was bulkier, in order to reduce the potential anti-entry activity and promote the anti-maturation effect. The corresponding C-3 succinylated compounds 40 and 41 were then prepared and evaluated in comparison with 2. Previously synthesized compounds 42–49, with C-28 piperidine side chains (Figure 2),23 were also tested due to their structural similarity to 38–41. The SAR of the anti-maturation promoting activity of C-28 side chains was then established.
Figure 2.
Chemistry
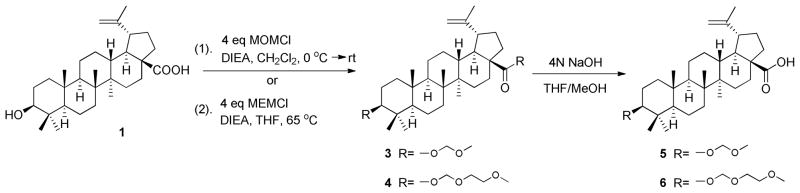
The syntheses of BA derivatives 3–6 were carried out according to Scheme 1. Four equivalents of MOMCl or MEMCl were reacted with 1 and DIEA in CH2Cl2 at room temperature overnight or in THF at 65 °C for 6 h to generate compounds 3 and 4, respectively, in 83% or 89% yield. Hydrolysis of 3 and 4 using 4N NaOH provided 5 and 6 in over 90% yield.
Scheme 1.
Scheme 2 depicts the synthetic pathway to compounds 7–24. Compounds 7 and 8 were obtained by reducing the amount of MOMCl or MEMCl to 1.1 equivalents in the reaction mixture. N-hydroxysuccinimide and 1 were reacted in the presence of EDCI and DMAP overnight to furnish 9 in 53% yield. Compounds 10–15 (67–98% yield) were obtained by treating 1 with Cs2CO3, followed by adding different halogenated alkanes or alkynes. Esterification of 7–11 and 13–15 with 2,2-dimethylsuccinic anhydride was conducted using microwave conditions rather than conventional oil-bath heating to yield compounds 16–23. A catalytic amount of PTSA and THF as solvent were used instead of pyridine resulting in yields of 40–55%. Compound 24 was synthesized in 75% yield by click reaction of 20 with AZT in the presence of CuI and N,N-diisopropylethylamine in DMF using a microwave synthesizer at 120 °C for 30min.
Scheme 2.
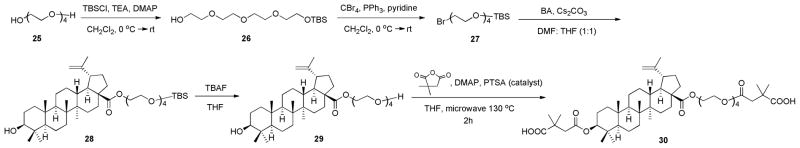
A tetraethylene glycol chain was also introduced at the C-28 position of 2, and compounds 29 and 30 were prepared as described in Scheme 3. Overall, 25 was first protected with TBSCl to yield 26, which was then converted to bromo-substituted 27 in 85% yield by reaction with CBr4 using PPh3 and pyridine. Reaction of 27 with 1 provided 28 in a yield of 72%, and deprotection of 28 using TBAF provided 29. Compound 30 was prepared by a microwave reaction as described above.
Scheme 3.
The syntheses of piperazine incorporated BA derivatives 38–41 were achieved according to Scheme 4. The C-28 piperazine side chains of 34 and 35 were prepared in toluene at 80 °C. C-3 acetyl protected BA (3-OAc-BA) was prepared previously23 and was activated by oxalyl chloride before reacting with 34 and 35 to furnish 36 and 37 in 89% and 100% yield, respectively. After hydrolysis, the resulting compounds 38 and 39 were reacted with 2,2-dimethylsuccinic anhydride in a microwave synthesizer to provide compounds 40 and 41 in approximately 40% yield.
Scheme 4.
Results and Discussion
The anti-HIV-1 replication activity of BA derivatives 3–24, 29, 30, and 38–49 was assessed in HIV-1NL4-3 infected MT-4 cell lines in parallel with AZT and 2, and the results are summarized in Table 1. The results indicated that compounds with MOM or MEM substitution at C-3 (5 and 6), C-28 (7 and 8), or both positions (3 and 4) of 1 did not show anti-HIV activity. Similarly, compounds 9–15 and 29 with a C-3 hydroxy group and C-28 ester substitutions of different length or size, or with heteroatoms within the side chains, did not show antiviral activity, suggesting that 1-derivatives bearing ester modifications at the C-28 position do not exhibit anti-HIV-1 activity and are not entry inhibitors. The results confirmed that C-3 dimethylsuccinyl substitution is essential to the anti-maturation activity of 2.
Table 1.
Anti-HIV-1 replication activities in HIV-1NL4-3 infected MT-4 cell lines. a
| Compound | IC50 (μM) | CC50 (μM) | TI |
|---|---|---|---|
| AZT | 0.030 | > 37 | 1,248 |
| Bevirimat (2) | 0.087 | 14.2 | 171 |
| 3 | NS b | NT c | – |
| 4 | NS | NT | – |
| 5 | NS | NT | – |
| 6 | NS | NT | – |
| 7 | NS | NT | – |
| 8 | NS | NT | – |
| 9 | NS | NT | – |
| 10 | NS | NT | – |
| 11 | NS | NT | – |
| 12 | NS | NT | – |
| 13 | NS | NT | – |
| 14 | NS | NT | – |
| 15 | NS | NT | – |
| 16 | 0.17 | 12.4 | 70.9 |
| 17 | 0.046 | 12.1 | 261.3 |
| 18 | 0.13 | 11.0 | 86.2 |
| 19 | 0.088 | 10.4 | 118.2 |
| 20 | 0.067 | 15.3 | 226.2 |
| 21 | 1.8 | 14.8 | 8.3 |
| 22 | 0.056 | 8.6 | 153.7 |
| 23 | NS | NT | – |
| 24 | 0.10 | 11.0 | 101.0 |
| 29 | NS | NT | – |
| 30 | 0.079 | 8.9 | 111.3 |
| 38 | NS | NT | – |
| 39 | NS | NT | – |
| 40 | 0.011 | 13.0 | 1,181 |
| 41 | 0.0059 | 13.2 | 2,237 |
| 42 | NS | NT | – |
| 43 | NS | NT | – |
| 44 | NS | NT | – |
| 45 | NS | NT | – |
| 46 | 0.24 | > 13.3 | > 55.5 |
| 47 | 0.07 | 10.9 | 155.8 |
| 48 | 0.035 | 10.0 | 286.1 |
| 49 | 0.031 | 10.0 | 321.2 |
All data presented are averages of at least three separate experiments. IC50: concentration that inhibits HIV-1NL4-3 replication by 50%. CC50: concentration that inhibits mock-infected MT-4 cell growth by 50%. TI = CC50/IC50.
NS: no suppression at the testing concentration (10 μM).
NT: not tested.
Diverse C-28 ester substituted 2-derivatives (16–24 and 30) were then investigated to establish the SAR of the C-28 side chain towards 2’s anti-maturation potency. Compounds 19, 21, and 23, with the C-28 alkane chain length increasing from propyl to hexyl to octyl, respectively, showed a decreasing activity trend. While 19 (IC50: 0.088 μM) and 2 (IC50: 0.087 μM) exhibited equivalent anti-HIV potency, compound 21 was approximately twenty-fold less potent (IC50: 1.8 μM), and 23 showed no inhibition of HIV-1 replication. Compound 20, with a C-28 prop-2-ynyl moiety, exhibited good antiviral activity (IC50: 0.067 μM), and 22, with a second ester substitution at the terminus of a C-28 hexyl chain, showed increased antiviral potency (IC50: 0.056 μM). For those compounds with one or two polar oxygen atoms present within their C-28 side chain, 16 (MOM ester) had an anti-HIV IC50 value of 0.17 μM, while 17 (MEM ester) had an IC50 of 0.046 μM, and thus, was 3.7-fold more potent than 16 and two-fold more potent than 2. Compound 30, with a C-28 tetraethylene glycol side chain terminating in a dimethylsuccinyl ester, in addition to the dimethyl succinyl ester at C-3, was equipotent (IC50: 0.079 μM) with 2. Compounds with bulky C-28 substituents, such as an N-succinimide ester (18), or conjugated to AZT through a triazole linkage (24) displayed slightly reduced anti-HIV-1 activity (IC50: 0.13 and 0.10 μM, respectively) relative to 2.
To further investigate the effect of C-28 side chains, cyclic secondary amines from piperazinebutyric acid, piperazinepentanoic acid, and piperidinebutyric acid, rather than a primary amine, were used in amidation reactions with the C-28 carboxylic acid of 3-acetylated BA, resulting in 38, 39, and 42–45. None of these 1-analogs exhibited anti-HIV replication activity in MT-4 lymphocytes, suggesting that bulkier C-28 amide moieties compromised the anti-entry effect of C-28 modified BAs.
Subsequently, the C-3 hydroxy group of 38, 39, and 42–45 was esterified with a dimethylsuccinyl moiety. Among the resulting compounds, 40, 41, 48, and 49 showed significantly increased antiviral activity (IC50 values ranging from 0.0059–0.035 μM). These results indicate that incorporating a cyclic secondary amide group with an extended side chain at the C-28 position can remarkably enhance the activity of 2. Specifically, a C-28 amide from piperidinebutyric acid with a second amide group formed from ethylmorpholine (48) or propylmorpholine (49) increased the antiviral activity of 2 by about three-fold, with IC50 values of 0.035 and 0.031 μM, respectively. The presence of additional polar nitrogen heteroatom within the C-28 amide side chain (from piperazinebutyric or piperazinepentanoic acid) significantly increase the potency by eight- to fifteen-fold, as shown by compound 40 (IC50: 0.011 μM) and 41 (IC50: 0.0059 μM), respectively.
A TZM-bl assay was used to confirm the mechanism of action of active compounds. This assay measures neutralization in TZM-bl cells as a function of a reduction in Tat-induced luciferase (Luc) reporter gene expression after a single round of virus infection. The TZM-bl cell line is a HeLa cell clone that was engineered to express CD4 and CCR5 and contains integrated reporter gene for firefly luciferase and E.coli β-galactosidase under control of an HIV-1 LTR. Expression of the reporter genes is induced in trans by viral Tat protein soon after infection, and the TZM-bl assay can detect HIV-entry inhibitors sensitively. However, because maturation inhibitors block HIV-1 replication only at the last step of the viral life cycle and, thus, still permit the production of immature non-infectious viral particles, the single round TZM-bl assay cannot detect HIV-maturation inhibitors, because the expression of reporter gene is not impaired in the first cycle. Therefore, all of the novel active 3,28-modified BAs were further screened in this assay system. As expected, none of the compounds were active in the single round TZM-bl assay, proving that they do not function as HIV entry inhibitors and, thus, their antiviral replication activity should result from an anti-maturation mechanism of action.
The following SAR conclusions summarize the effects of the C-28 substitutions to promote the anti-maturation activity of 2. C-28 ester side chains with a three to six carbons alkyl chain result in enhanced anti-HIV activity. The presence of a polar oxygen atom within the side chain and the introduction of a second ester functionality at the terminus of the C-28 ester chain can moderately enhance the anti-maturation activity of 2, as shown by 17 and 22. Meanwhile, while 1-analogs with C-28 primary amide substitutions function as anti-entry agents, 1-analogs with C-28 secondary cyclic amide groups do not. Instead, the latter compounds exhibit significant anti-maturation activity. Thus far, compound 41, which has an additional polar nitrogen heteroatom present in the C-28 amide (piperazinepentanoic acid) showed the best antiviral replication activity acting through an anti-maturation mechanism of action.
To evaluate its potential as a drug candidate, 41 was further assessed for its in vitro metabolic stability in pooled human liver microsomes (HLMs). The results revealed that 86.15% of 41 remained intact after 60 min of incubation in HLMs. Compound 41’s long in vitro half life (t1/2 of 327 min) and low clearance (CLint of 0.0021 mL/min/mg) proved that incorporation of C-28 cyclic secondary amide chains would not decrease the stability of 2, which has a long elimination half life (t1/2 of 56.3–69.5 h) in healthy volunteers.18
Overall, new BA derivatives were designed and synthesized in order to investigate the modulating effects of C-28 substitution towards 2’s anti-maturation activity. It was discovered that, certain ester or cyclic secondary amide substitutions at C-28 will enhance the antiviral activity of 2, acting only through an anti-maturation effect. The best new compound 41 showed excellent anti-HIV activity with an IC50 value of 0.0059 μM, fifteen-fold better than that of 2. Compound 41 also exhibited good in vitro metabolic stability in HLMs, and therefore, may serve as an attractive new lead for the development of a next generation of maturation inhibitors as anti-AIDS clinical trial candidates.
Experimental Section
Chemistry
The melting points were measured with a Fisher Johns melting apparatus without correction. 1H NMR spectra were measured on an Inova 400 MHz spectrometer using Me4Si (TMS) as internal standard. The solvent used was CDCl3, unless otherwise indicated. High resolution mass spectra (HRMS) were measured on a Shimadzu LCMS-IT-TOF with ESI interface. HPLC for purity determinations were conducted using a Shimadzu LCMS with a Grace Alltima 2.1mm × 150mm HP C18 5μ column and a Shimadzu SPD-M20A detector at 205 or 220 nm wavelength. The detailed solvent percentage conditions for each tested compound are listed in the supporting information. All target compounds were at least 95% pure. Optical rotations were measured with a Jasco Dip-2000 digital polarimeter at 25 °C at the sodium D line. Thin-layer chromatography (TLC) was performed on Merck precoated silica gel 60 F-254 plates. A Grace Reveleris® flash system equipped with RevealX(TM) detection allowing for multisignal (UV/ELSD) collection was used for medium pressure column chromatography. Reveleris® Navigator method optimizer and Grace Reveleris® flash silica cartridges were employed for high quality separations. Commercial chemicals were obtained from Sigma-Aldrich, Inc.
Syntheses of 3 and 7
Compound 1 (1 eq), DIEA (4 eq), and MOMCl (4 eq for 3 and 1.1 eq for 7) were dissolved in CH2Cl2 (5 mL) at 0 °C. The mixture was stirred overnight under N2, allowing a gradual warming to room temperature. The solution was then diluted with CH2Cl2 (20 mL) and washed with brine. The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds 3 and 7.
Methoxymethyl 3β-methoxymethoxy-lup-20(29)-en-28-oate (3)
149 mg (83% yield) starting from 179 mg of 1; white amorphous powder. Mp 99–102 °C. HRMS (ESI−) m/z: 543.3991 (C34H56O5 - H). 1H NMR (400 MHz, CDCl3): δ 5.28-5.22 (2H, app d, J = 5.6 Hz, -COOCH2O-), 4.80, 4.67 (1H each, app d, J = 6.4 Hz, -OCH2O-), 4.73, 4.61 (1H each, s, H-29), 3.48, 3.38 (3H each, s, -OCH3), 3.08-2.99 (2H, m, H-3, H-19), 1.69 (3H, s, H-30), 0.97, 0.95, 0.93, 0.83, 0.78 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.9, 16.2, 16.4, 16.5, 18.5, 19.6, 21.1, 24.4, 25.8, 28.2, 29.8, 30.8, 32.2, 34.6, 37.1, 37.3, 38.5, 38.9, 38.9, 41.0, 42.6, 47.1, 49.6, 50.8, 55.7, 56.0, 57.0, 57.9, 85.4, 90.1, 96.2, 109.9, 150.7, 175.8.
Methoxymethyl 3β-hydroxy-lup-20(29)-en-28-oate (7)
190 mg (86% yield) starting from 200 mg of 1; white amorphous powder. Mp 114–115 °C. HRMS (ESI−) m/z: 499.3745 (C32H52O4 - H). 1H NMR (400 MHz, CDCl3): δ 5.28-5.22 (2H, app q, J = 6.0, 5.6 Hz, -COOCH2O-), 4.73, 4.61 (1H each, s, H-29), 3.48 (3H, s, -OCH3), 3.18 (1H, dd, J = 11.2, 5.2 Hz, H-3), 3.05-2.98 (1H, m, H-19), 2.21-2.30 (2H, m, H-16), 1.69 (3H, s, H-30), 0.97, 0.96, 0.93, 0.82, 0.75 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.9, 15.6, 16.2, 16.3, 18.5, 19.6, 21.1, 25.7, 27.6, 28.2, 29.8, 29.9, 30.7, 32.2, 34.6, 37.1, 37.4, 38.4, 38.9, 39.0, 40.9, 42.6, 47.1, 49.6, 50.8, 55.6, 56.9, 57.9, 79.1, 90.1, 109.9, 150.6, 175.8.
Syntheses of 4 and 8
Compound 1 (1 eq), DIEA (4 eq), and MEMCl (4 eq for 4 and 1.1 eq for 8) were dissolved in THF (5 mL) at room temperature. For 4, the mixture was stirred at 65 °C under N2 for 6 h, and for 8, the mixture was stirred at 60 °C for 3 h. The solution was then diluted with water, extracted with CH2Cl2, and washed with brine. The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds 4 and 8.
Methoxyethoxymethyl 3β-methoxyethoxymethoxy-lup-20(29)-en-28-oate (4)
311 mg (89% yield) starting from 252 mg of 1; white amorphous powder. Mp 102–105 °C. HRMS (ESI+) m/z: 655.4579 (C38H64O7 + Na). 1H NMR (400 MHz, CDCl3): δ 5.37-5.30 (2H, app dd, J = 6.0, 5.8 Hz, -COOCH2O-), 4.81, 4.65 (1H each, app d, J = 6.8 Hz, -OCH2O-), 4.71, 4.58 (1H each, s, H-29), 3.80, 3.71, 3.57, 3.50 (2H each, m, 2 × -OCH2CH2O-), 3.38 (6H, s, 2 × -OCH3), 3.09-3.05 (1H, dd, J = 11.4, 4.4, 4.0 Hz, H-3), 3.01-2.94 (1H, m, H-19), 1.68 (3H, s, H-30), 0.97, 0.93, 0.91, 0.83, 0.77 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.91, 16.25, 16.38, 16.49, 18.51, 19.59, 21.14, 24.30, 25.78, 28.27, 29.88, 30.76, 32.20, 34.60, 37.10, 37.27, 38.45, 38.86, 38.89, 41.98, 42.64, 47.11, 49.62, 50.79, 55.94, 56.93, 59.26, 59.31, 67.19, 69.70, 71.76, 72.07, 85.33, 89.08, 95.13, 109.88, 150.70, 175.79.
Methoxyethoxymethyl 3β-hydroxy-lup-20(29)-en-28-oate (8)
219 mg (92% yield) starting from 200 mg of 1; white amorphous powder. Mp 119–121 °C. HRMS (ESI+) m/z: 567.4043 (C34H56O5 + Na). 1H NMR (400 MHz, CDCl3): δ 5.37-5.30 (2H, app dd, J = 6.4, 6.0 Hz, -COOCH2O-), 4.73, 4.60 (1H each, s, H-29), 3.80, 3.56 (2H each, t, J = 5.2 Hz, -OCH2CH2O-), 3.39 (3H, s, -OCH3), 3.18 (1H, m, H-3), 3.04-2.98 (1H, m, H-19), 1.68 (3H, s, H-30), 1.00, 0.97, 0.92, 0.82, 0.75 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.9, 15.6, 16.2, 16.4, 18.5, 19.6, 21.1, 25.8, 27.6, 28.2, 29.9, 30.8, 32.2, 34.6, 37.1, 37.4, 38.4, 39.0, 39.1, 41.0, 42.7, 47.1, 49.6, 50.8, 55.6, 56.9, 59.3, 69.7, 71.8, 79.2, 89.1, 109.9, 150.7, 175.8.
Syntheses of 34 and 35
Piperazine 31 (2 eq) was dissolved in toluene and heated to 80 °C. Compound 32 or 33 (1 eq) was added, and the reaction proceeded for 4 h. After cooling and filtering, the organic layer was concentrated under reduced pressure. The residue was chromatographed using a silica gel column to yield 500 mg of 34 (50%) and 498 mg of 35 (42%).
Syntheses of 36 and 37
Oxalyl chloride solution (2 M in CH2Cl2, 10 mL) was added to 3-OAc-BA (1 eq)22 in CH2Cl2 (10 mL) and stirred for 2 h. After concentration under vacuum, the residual mixture was treated with 34 or 35 (1.6 eq) and triethylamine (Et3N, 1.2 eq) in CH2Cl2. The mixture was stirred at room temperature overnight. The solution was then diluted with CH2Cl2 (20 mL) and washed with brine. The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds 36 and 37.
Methyl N-[3β-acetoxy-lup-20(29)-en-28-oyl]-4-piperazinebutanoate (36)
177.5 mg (89% yield) starting from 150 mg of 3-OAc-BA; white amorphous powder. Mp 203–205 °C. HRMS (ESI+) m/z: 667.5055 (C41H66N2O5 + H). 1H NMR (400 MHz, CDCl3): δ 4.72, 4.60 (1H each, s, H-29), 4.47 (1H, dd, J = 12.4, 5.6 Hz, H-3), 3.68 (3H, s, -COOCH3), 3.61-3.52 (4H, m, 28-CON(CH2CH2)2N-), 3.01 (1H, m, H-19), 2.91-2.76 (4H, m, 28-CON(CH2CH2)2N-), 2.46 (2H, m, -NCH2-), 2.05 (3H, s, OCOCH3), 1.68 (3H, s, H-30), 1.02, 0.99, 0.96, 0.85, 0.81 (3H each, s, 5 × CH3).
Methyl N-[3β-acetoxy-lup-20(29)-en-28-oyl]-5-piperazinepentanoate (37)
847 mg (100% yield) starting from 622.8 mg of 3-OAc-BA; white amorphous powder. Mp 217–218 °C. HRMS (ESI+) m/z: 681.5219 (C42H68N2O5 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.46 (1H, m, H-3), 3.67 (3H, s, -COOCH3), 3.58-3.47 (4H, m, 28-CON(CH2CH2)2N-), 3.00 (1H, m, H-19), 2.87-2.76 (4H, m, 28-CON(CH2CH2)2N-), 2.46 (2H, m, -NCH2-), 2.06 (3H, s, OCOCH3), 1.69 (3H, s, H-30), 0.98, 0.97, 0.90, 0.86, 0.83 (3H each, s, 5 × CH3).
Syntheses of 5–6 and 38–39
To a solution of the appropriate ester intermediates 3–4 and 36–37 (1 eq) in MeOH (8 mL) and THF (4 mL) was added 4 N NaOH (4 mL). The mixture was stirred overnight, and then neutralized with 20% HCl. The solution was dried under vacuum and reconstituted with CH2Cl2. The organic layer was washed with brine and dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds 5–6 and 38–39.
3β-Methoxymethoxy-betulinic acid (5)
85 mg (93% yield) starting from 100 mg of 3; white amorphous powder. Mp 117–119 °C. HRMS (ESI−) m/z: 499.3730 (C32H52O4 - H). 1H NMR (400 MHz, CDCl3): δ 4.75, 4.62 (1H each, app d, J = 6.4 Hz, -OCH2O-), 4.71, 4.59 (1H each, s, H-29), 3.36 (3H, s, -OCH3), 3.05-2.96 (2H, m, H-3, H-19), 1.71 (3H, s, H-30), 0.97, 0.95, 0.90, 0.83, 0.77 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.7, 16.1, 16.1, 16.3, 18.3, 19.4, 20.9, 24.2, 25.5, 28.0, 29.7, 30.6, 32.2, 34.3, 37.1, 38.4, 38.7, 40.7, 42.4, 47.0, 49.3, 50.5, 55.5, 55.7, 56.4, 77.3, 85.2, 96.0, 109.7, 150.4, 181.8.
3β-Methoxyethoxymethoxy-betulinic acid (6)
118 mg (92% yield) starting from 150 mg of 4; white amorphous powder. Mp 121–124 °C. HRMS (ESI+) m/z: 567.4033 (C34H56O5 + Na). 1H NMR (400 MHz, CDCl3): δ 4.80, 4.66 (1H each, app d, J = 6.8 Hz, -OCH2O-), 4.70, 4.58 (1H each, s, H-29), 3.69, 3.52 (2H each, t, J = 4.4 Hz, -OCH2CH2O-), 3.36 (3H, s, -OCH3), 3.07 (1H, dd, J = 12.0, 4.4 Hz, H-3), 3.00 (1H, m, H-19), 1.67 (3H, s, H-30), 0.94, 0.91, 0.89, 0.80, 0.73 (3H each, s, 5 × CH3).
N-[3β-Hydroxy-lup-20(29)-en-28-oyl]-4-piperazinebutyric acid (38)
91 mg (79% yield) starting from 125 mg of 36; white amorphous powder. Mp 153–154 °C. HRMS (ESI−) m/z: 609.4646 (C38H62N2O4 - H). 1H NMR (400 MHz, CDCl3): δ 4.71, 4.60 (1H each, s, H-29), 3.65-3.49 (4H, m, 28-CON(CH2CH2)2N-), 3.17 (1H, m, H-3), 3.01 (1H, m, H-19), 2.82-2.68 (4H, m, 28-CON(CH2CH2)2N-), 2.45 (2H, m, -NCH2-), 1.69 (3H, s, H-30), 1.01, 0.98, 0.89, 0.84, 0.78 (3H each, s, 5 × CH3).
N-[3β-Hydroxy-lup-20(29)-en-28-oyl]-5-piperazinepentanoic acid (39)
624 mg (85% yield) starting from 800 mg of 37; white amorphous powder. Mp 159–161 °C. HRMS (ESI−) m/z: 623.4781 (C39H64N2O4 - H). 1H NMR (400 MHz, CDCl3): δ 4.71, 4.58 (1H each, s, H-29), 3.64-3.51 (4H, m, 28-CON(CH2CH2)2N-), 3.19 (1H, m, H-3), 2.99 (1H, m, H-19), 2.89-2.72 (4H, m, 28-CON(CH2CH2)2N-), 2.46 (2H, m, -NCH2-), 1.68 (3H, s, H-30), 0.98, 0.95, 0.92, 0.82, 0.81 (3H each, s, 5 × CH3).
N-Succinimide 3β-hydroxy-lup-20(29)-en-28-oate (9)
A mixture of 1 (260 mg, 1 eq), N-hydroxysuccinimide (1.5 eq), EDCI (1.5 eq), and DMAP (0.1 eq) was dissolved in CH2Cl2 (5 mL) and stirred under N2 at room temperature overnight. The solution was then diluted with CH2Cl2 (20 mL) and washed with brine. The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield 167 mg of 9 (53% yield), white amorphous powder. Mp 189–191 °C. HRMS (ESI+) m/z: 576.3656 (C34H51NO5 + Na). 1H NMR (400 MHz, CDCl3): δ 4.72, 4.61 (2H, s, H-29), 3.18 (1H, dd, J = 11.2, 4.8 Hz, H-3), 2.95 (1H, m, H-19), 2.84, 2.82 (4H, d, J = 5.2 Hz, -COCH2CH2CO-), 2.47-2.42 (1H, m, H-13), 1.69 (3H, s, H-30), 0.98 (6H, s, 2 × CH3), 0.96, 0.82, 0.75 (3H each, s, 3 × CH3).
Syntheses of 26 and 27
A mixture of tetraethylene glycol (25, 35g, 5 eq), TEA (1.5 eq), and DMAP (0.1 eq) was dissolved in CH2Cl2 (200 mL) at 0 °C. TBSCl (5 g, 1 eq) in CH2Cl2 (50 mL) was added dropwise and reaction continued overnight. The solution was diluted with CH2Cl2 and washed with mildly acidic water and brine. The organic layer was dried over anhydrous MgSO4, filtered and concentrated under reduced pressure. The crude product was purified by column chromatography to yield 50 g of TBS-protected tetraethylene glycol 26 (90%).24 In the next step, a mixture of 26 (852 mg, 1 eq) and CBr4 (2 eq) was dissolved in CH2Cl2 (200 mL) at 0 °C. Pyridine (2.5 mL, 10 eq) was added, followed by PPh3 (2 eq). The mixture was stirred overnight with a gradual warming to room temperature. The suspension was filtered through Celite and the organic layer was concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield 872 mg of bromo-substituted TBS-protected tetraethylene glycol 27 (85% yield).25
Syntheses of 10–15 and 28
Compound 1 (1 eq) and CS2CO3 (3 eq) were dissolved in a 1:1 mixed solution of DMF and THF (4 ml) and stirred for 30 min at room temperature. Bromo- or iodo-substituted compound (R-X) or 27 (3 eq) was then added, and the reaction was carried out overnight at room temperature. Water was added to stop the reaction, and the solution was extracted with EtOAc. The organic layer was washed further with brine, dried over anhydrous MgSO4, and concentrated under reduced pressure. The residue was chromatographed using a silica gel column to yield the pure target compounds 10–15 and 28.
Propyl 3β-hydroxy-lup-20(29)-en-28-oate (10)
56 mg (86% yield) starting from 60 mg of 1; white amorphous powder. Mp 106107 °C. HRMS (ESI+) m/z: 499.4155 (C33H54O3 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.09 (2H, m, 28-COOCH2-), 3.18 (1H, dd, J = 11.2, 4.8 Hz, H-3), 3.01 (1H, m, H-19), 1.68 (3H, s, H-30), 1.01 (6H, s, 2 × CH3), 0.94 (3H, s, CH3), 0.91 (3H, m, CH3), 0.82, 0.76 (3H each, s, 2 × CH3).
Prop-2′-ynyl 3β-hydroxy-lup-20(29)-en-28-oate (11)
47 mg (72% yield) starting from 60 mg of 1; white amorphous powder. Mp 131–133 °C. HRMS (ESI+) m/z: 495.3826 (C33H50O3 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.61 (2H, s, H-29), 4.69, 4.65 (2H, d, J = 2.2 Hz, 28-COOCH2-), 3.18 (1H, m, H-3), 3.01 (1H, m, H-19), 2.43 (1H, t, J = 2.2 Hz, -C3CH), 1.69 (3H, s, H-30), 0.97 (6H, s, 2 × CH3), 0.93, 0.82, 0.75 (3H each, s, 3 × CH3).
3′-Phenylpropyl 3β-hydroxy-lup-20(29)-en-28-oate (12)
72 mg (96% yield) starting from 60 mg of 1; white amorphous powder. Mp 169–172 °C. HRMS (ESI+) m/z: 575.4465 (C39H58O3 + H). 1H NMR (400 MHz, CDCl3): δ 7.31-7.26, 7.22-7.18 (5H, m, -C6H5), 4.73, 4.61 (2H, s, H-29), 4.09 (2H, m, 28-COOCH2-), 3.18 (1H, m, H-3), 3.02 (1H, m, H-19), 2.72 (1H, t, J = 6.4 Hz, -CH2C6H5), 1.69 (3H, s, H-30), 0.97, 0.96, 0.91, 0.81, 0.75 (3H each, s, 5 × CH3).
Hexyl 3β-hydroxy-lup-20(29)-en-28-oate (13)
48 mg (67% yield) starting from 60 mg of 1; white amorphous powder. Mp 124–125 °C. HRMS (ESI+) m/z: 541.4629 (C36H60O3 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.59 (2H, s, H-29), 4.07 (2H, m, 28-COOCH2-), 3.18 (1H, dd, J = 11.8, 4.4 Hz, H-3), 3.02 (1H, m, H-19), 1.68 (3H, s, H-30), 0.97 (6H, s, 2 × CH3), 0.92 (3H, s, CH3), 0.90 (3H, m, CH3), 0.82, 0.75 (3H each, s, 2 × CH3).
Ethyl O-[3β-hydroxy-lup-20(29)-en-28-oyl]-6-hexanoate (14)
77 mg (98% yield) starting from 60 mg of 1; white amorphous powder. Mp 191–194 °C. HRMS (ESI+) m/z: 599.4649 (C38H62O5 + H). 1H NMR (400 MHz, CDCl3): δ 4.72, 4.59 (2H, s, H-29), 4.16-4.02 (4H, m, 28-COOCH2-, -COOCH2CH3), 3.41 (1H, t, J = 6.8 Hz, -OH), 3.17 (1H, dd, J = 11.8, 4.4 Hz, H-3), 3.00 (1H, m, H-19), 2.31 (2H, m, -CH2COO-), 1.68 (3H, s, H-30), 1.26 (3H, t, J = 7.2 Hz, -COOCH2CH3), 0.97 (6H, s, 2 × CH3), 0.91, 0.82, 0.75 (3H each, s, 3 × CH3).
Octyl 3β-hydroxy-lup-20(29)-en-28-oate (15)
70 mg (94% yield) starting from 60 mg of 1; white amorphous powder. Mp 146–147 °C. HRMS (ESI+) m/z: 569.4936 (C38H64O3 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.07 (2H, m, 28-COOCH2-), 3.17 (1H, dd, J = 12.0, 4.0 Hz, H-3), 3.02 (1H, m, H-19), 1.68 (3H, s, H-30), 0.96 (6H, s, 2 × CH3), 0.92 (3H, s, CH3), 0.89 (3H, t, J = 6.4 Hz, CH3), 0.82, 0.75 (3H each, s, 2 × CH3).
2-(2-(2-(2-O-tert-Butyldimethylsilylethoxy)ethoxy)ethoxy)ethyl 3β-hydroxy-lup-20(29)-en-28-oate (28)
142 mg (72% yield) starting from 120 mg of 1; white amorphous powder. Mp 203–206 °C. HRMS (ESI+) m/z: 747.5603 (C44H78O7Si + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.62 (2H, s, H-29), 4.25 (2H, m, -COOCH2CH2O-), 3.96-3.54 (2H each, m, -COOCH2CH2O-, 3 × -OCH2CH2O-), 3.17 (1H, dd, J = 11.2, 4.8 Hz, H-3), 3.01 (1H, m, H-19), 1.68 (3H, s, H-30), 0.98 (12H, s, CH3, -Si(CH3)2C(CH3)3), 0.91, 0.89, 0.82, 0.75 (3H each, s, 4 × CH3), 0.21 (6H, s, -Si(CH3)2C(CH3)3).
2-(2-(2-(2-Hydroxyethoxy)ethoxy)ethoxy)ethyl 3β-hydroxy-lup-20(29)-en-28-oate (29)
To a solution of 28 (170 mg, 1eq) in THF (5 mL) was added TBAF (1.5 eq). The mixture was stirred overnight at room temperature. The organic layer was concentrated to dryness under reduced pressure and the residue was chromatographed using a silica gel column to yield 166 mg of 29 (98% yield), white amorphous powder. Mp 178–180 °C. HRMS (ESI+) m/z: 633.4743 (C38H64O7 + H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.61 (2H, s, H-29), 4.25 (2H, m, -COOCH2CH2O-), 3.65-3.44 (2H each, m, -COOCH2CH2O-, 3 × -OCH2CH2O-), 3.17 (1H, dd, J = 11.6, 4.8 Hz, H-3), 3.01 (1H, m, H-19), 1.68 (3H, s, H-30), 0.98, 0.97, 0.89, 0.82, 0.76 (3H each, s, 5 × CH3).
Syntheses of 16–23, 30, and 40–41
The appropriate BA derivative (1 eq), 2,2-dimethylsuccinic anhydride (5 eq), DMAP (1.6 eq), and PTSA (catalytic amount) was dissolved in THF (1.5 mL) and stirred at 130 °C for 2 h using a microwave synthesizer (Biotage). Water was added, and the mixture was then extracted with CH2Cl2 (15 mL). The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield pure target compounds 16–23, 30, 40, and 41.
Methoxymethyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (16)
25 mg (40% yield) starting from 52 mg of 7; white amorphous powder. Mp 107–109 °C. HRMS (ESI−) m/z: 627.4247 (C38H60O7 - H). 1H NMR (400 MHz, CDCl3): δ 11.11 (1H, br s, -COOH), 5.28-5.22 (2H, app d, J = 5.8 Hz, -COOCH2O-), 4.72, 4.60 (1H each, s, H-29), 4.47 (1H, dd, J = 11.2, 5.6 Hz, H-3), 3.48 (3H, s, -OCH3), 3.00 (1H, m, H-19), 2.64-2.42 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.30, 1.28 (3H each, s, 2 × CH3-3′), 0.98, 0.97, 0.92, 0.81, 0.75 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.9, 16.2, 16.4, 16.7, 18.4, 19.6, 21.1, 23.8, 25.3, 25.7, 25.8, 28.1, 29.9, 30.8, 32.2, 34.5, 37.1, 37.3, 38.0, 38.4, 38.6, 40.7, 41.0, 42.6, 44.9, 47.1, 49.6, 50.7, 55.7, 57.0, 57.9, 81.8, 90.1, 109.9, 150.6, 171.2, 175.8, 182.6.
Methoxyethoxymethyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (17)
30.5 mg (41% yield) starting from 60 mg of 8; white amorphous powder. Mp 115–118 °C. HRMS (ESI−) m/z: 671.4511 (C40H64O8 - H). 1H NMR (400 MHz, CDCl3): δ 11.16 (1H, br s, -COOH), 5.37-5.30 (2H, app d, J = 6.0 Hz, -COOCH2O-), 4.72, 4.59 (1H each, s, H-29), 4.47 (1H, t, J = 7.6 Hz, H-3), 3.80, 3.56 (2H each, t, J = 5.2 Hz, -OCH2CH2O-), 3.42 (3H, s, -OCH3), 3.01 (1H, m, H-19), 2.67-2.46 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.29, 1.28 (3H each, s, 2 × CH3-3′), 1.00, 0.97, 0.92, 0.82, 0.76 (3H each, s, 5 × CH3). 13C NMR (100 MHz, CDCl3): δ 14.9, 16.2, 16.4, 16.7, 18.4, 19.6, 21.1, 23.8, 25.3, 25.7, 25.8, 28.1, 29.9, 30.7, 32.2, 34.5, 37.1, 37.3, 37.9, 38.4, 38.6, 40.7, 41.0, 42.6, 45.0, 47.1, 49.6, 50.7, 55.7, 56.9, 59.3, 69.7, 71.7, 81.8, 89.1, 109.9, 150.6, 171.2, 175.8, 183.0. [α]D 25 −13.8 ° (c = 0.28, MeOH).
N-Succinimide 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (18)
17 mg (39% yield) starting from 35 mg of 9; light yellow amorphous powder. Mp 164–166 °C. HRMS (ESI−) m/z: 680.4161 (C40H59NO8 - H). 1H NMR (400 MHz, CDCl3): δ 4.72, 4.60 (2H, s, H-29), 4.47 (1H, dd, J = 11.2, 5.2 Hz, H-3), 2.99 (1H, m, H-19), 2.84, 2.82 (4H, d, J = 5.2 Hz, -COCH2CH2CO-), 2.64-2.41 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.28 (6H, s, 2 × CH3-3′), 0.98 (6H, s, 2 × CH3), 0.91, 0.82, 0.75 (3H each, s, 3 × CH3).
Propyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (19)
23 mg (46% yield) starting from 40 mg of 10; white amorphous powder. Mp 109–111 °C. HRMS (ESI−) m/z: 625.4465 (C39H62O6 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.48 (1H, dd, J = 11.0, 4.8 Hz, H-3), 4.09 (2H, m, 28-COOCH2-), 3.00 (1H, m, H-19), 2.58-2.39 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.28, 1.27 (3H each, s, 2 × CH3-3′), 1.00, 0.98, 0.93 (3H each, s, 3 × CH3), 0.89 (3H, t, J = 6.4 Hz, CH3), 0.82, 0.75 (3H each, s, 2 × CH3).
Prop-2′-ynyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (20)
28 mg (55% yield) starting from 40 mg of 11; light yellow amorphous powder. Mp 127–129 °C. HRMS (ESI−) m/z: 621.4175 (C39H58O6 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.69, 4.66 (2H, d, J = 2.4 Hz, 28-COOCH2-), 4.47 (1H, dd, J = 11.8, 5.6 Hz, H-3), 3.00 (1H, m, H-19), 2.62-2.40 (3H, m, H-2′, -C3CH), 1.69 (3H, s, H-30), 1.30, 1.28 (3H each, s, 2 × CH3-3′), 0.97 (6H, s, 2 × CH3), 0.93, 0.81, 0.75 (3H each, s, 3 × CH3).
Hexyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (21)
26 mg (52% yield) starting from 40 mg of 13; white amorphous powder. Mp 118–120 °C. HRMS (ESI−) m/z: 667.4931 (C42H68O6 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.47 (1H, dd, J = 11.2, 4.8 Hz, H-3), 4.07 (2H, m, 28-COOCH2-), 3.01 (1H, m, H-19), 2.54-2.41 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.31, 1.28 (3H each, s, 2 × CH3-3′), 0.97 (6H, s, 2 × CH3), 0.92 (3H, s, CH3), 0.89 (3H, m, CH3), 0.81, 0.75 (3H each, s, 2 × CH3).
Ethyl O-[3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oyl]-6-hexanoate (22)
28 mg (46% yield) starting from 50 mg of 14; light yellow amorphous powder. Mp 179–181 °C. HRMS (ESI−) m/z: 725.4989 (C44H70O8 - H). 1H NMR (400 MHz, CDCl3): δ 4.72, 4.59 (2H, s, H-29), 4.48 (1H, t, J = 7.8 Hz, H-3), 4.18-4.02 (4H, m, 28-COOCH2-, -COOCH2CH3), 3.01 (1H, m, H-19), 2.62-2.43 (2H, m, H-2′), 2.31 (2H, m, -CH2COO-), 1.68 (3H, s, H-30), 1.31, 1.28 (3H each, s, 2 × CH3-3′), 1.26 (3H, t, J = 7.2 Hz, -COOCH2CH3), 0.98 (6H, s, 2 × CH3), 0.91, 0.82, 0.75 (3H each, s, 3 × CH3). [α]D25 −16.6 ° (c = 0.22, MeOH).
Octyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (23)
31 mg (51% yield) starting from 50 mg of 15; white amorphous powder. Mp 136–137 °C. HRMS (ESI−) m/z: 695.5248 (C44H72O6 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.47 (1H, t, J = 7.6 Hz, H-3), 4.07 (2H, m, 28-COOCH2-), 3.01 (1H, m, H-19), 2.61-2.40 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.28 (6H, s, 2 × CH3-3′), 0.97 (6H, s, 2 × CH3), 0.92 (3H, s, CH3), 0.89 (3H, t, J = 6.4 Hz, CH3), 0.82, 0.75 (3H each, s, 2 × CH3).
2-[2-[2-[2-O-(3′,3′-Dimethylsuccinyl)ethoxy]ethoxy]ethoxy]ethyl 3β-O-(3′,3′-dimethyl succinyl)-lup-20(29)-en-28-oate (30)
50 mg (41% yield) starting from 100 mg of 29; light yellow amorphous powder. Mp 165–167 °C. HRMS (ESI−) m/z: 759.5013 (C44H72O10 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (2H, s, H-29), 4.48 (1H, dd, J = 11.4, 4.8 Hz, H-3), 4.26 (2H, m, -COOCH2CH2O-), 3.68-3.41 (2H each, m, -COOCH2CH2O-, 3 × -OCH2CH2O-), 3.01 (1H, m, H-19), 2.64-2.42 (2H, m, H-2′), 1.68 (3H, s, H-30), 1.30, 1.28 (3H each, s, 2 × CH3-3′), 0.98, 0.97, 0.91, 0.82, 0.76 (3H each, s, 5 × CH3).
N-[3β-O-(3′,3′-Dimethylsuccinyl)-lup-20(29)-en-28-oyl]-4-piperazinebutyric acid (40)
45 mg (53% yield) starting from 70 mg of 38; light yellow amorphous powder. Mp 127–129 °C. HRMS (ESI−) m/z: 737.5109 (C44H70N2O7 - H). 1H NMR (400 MHz, CDCl3): δ 4.71, 4.60 (1H each, s, H-29), 4.47 (1H, dd, J = 11.6, 4.8 Hz, H-3), 3.65-3.49 (4H, m, 28-CON(CH2CH2)2N-), 3.01 (1H, m, H-19), 2.82-2.68 (4H, m, 28-CON(CH2CH2)2N-), 2.64-2.40 (3H, m, H-2′, -NCH2-), 1.68 (3H, s, H-30), 1.31, 1.28 (3H each, s, 2 × CH3-3′), 1.01, 0.98, 0.91, 0.84, 0.76 (3H each, s, 5 × CH3). [α]D25 −17.5 ° (c = 0.28, MeOH).
N-[3β-O-(3′,3′-Dimethylsuccinyl)-lup-20(29)-en-28-oyl]-5-piperazinepentanoic acid (41)
199 mg (55% yield) starting from 300 mg of 39; white amorphous powder. Mp 135–137 °C. HRMS (ESI−) m/z: 751.5251 (C45H72N2O7 - H). 1H NMR (400 MHz, CDCl3): δ 4.73, 4.60 (1H each, s, H-29), 4.47 (1H, dd, J = 11.2, 4.8 Hz, H-3), 3.64-3.51 (4H, m, 28-CON(CH2CH2)2N-), 3.00 (1H, m, H-19), 2.89-2.72 (4H, m, 28-CON(CH2CH2)2N-), 2.61-2.40 (3H, m, H-2′, -NCH2-), 1.69 (3H, s, H-30), 1.30, 1.28 (3H each, s, 2 × CH3-3′), 0.98, 0.97, 0.92, 0.82, 0.75 (3H each, s, 5 × CH3). [α]D25 −18.4 ° (c = 0.30, MeOH).
[1-(3′-Deoxythymidine)-1H-1,2,3-triazol-4-yl]methyl 3β-O-(3′,3′-dimethylsuccinyl)-lup-20(29)-en-28-oate (24)
Compound 20 (25 mg, 1.3 eq), AZT (1 eq), CuI (0.2 eq), and i-Pr2EtN (2 eq) were dissolved in DMF (1 mL) and stirred at 120 °C for 30 min using a microwave synthesizer (Biotage). The solution was diluted with EtOAc, and then washed with water, sat NaHCO3 and brine. The organic layer was dried over anhydrous MgSO4 and concentrated to dryness under reduced pressure. The residue was chromatographed using a silica gel column to yield 20 mg of 24 (75%), white amorphous powder. Mp 186–189 °C. HRMS (ESI+) m/z: 890.5227 (C49H71N5O10 + H). 1H NMR (400 MHz, CDCl3): δ 9.31 (1H, br, s, -NH), 7.79 (1H, s, Triazol-H), 7.48, 7.44 (1H, d, J = 16 Hz, Thy-H), 6.21 (1H, t, J = 5.6 Hz, Ribose-CH-2′), 5.42 (1H, m, Ribose-CH-4′), 5.28-5.20 (2H, m, 28-COOCH2-), 4.72, 4.60 (2H, s, H-29), 4.46 (1H, m, H-3), 4.04, 3.80-3.73 (4H, m, Ribose-CH-5′-CH2-OH), 3.06-2.96 (3H, m, H-19, Ribose-CH2-3′), 2.70-2.56 (2H, m, H-2′), 1.94 (3H, s, Thy-CH3), 1.67 (3H, s, H-30), 1.30, 1.28 (3H each, s, 2 × CH3-3′), 1.01 (6H, s, 2 × CH3), 0.93, 0.81, 0.75 (3H each, s, 3 × CH3).
HIV-1NL4-3 Replication Inhibition Assay in MT-4 Lymphocytes
A previously described HIV-1 infectivity assay was used.26, 27 A 96-well microtiter plate was used to set up the HIV-1NL4-3 replication screening assay. NL4-3 variants at a multiplicity of infection (MOI) of 0.01 were used to infect MT4 cells. Culture supernatants were collected on day 4 PI for the p24 antigen capture using an ELISA kit from ZeptoMetrix Corporation (Buffalo, New York). The 50% inhibition concentration (IC50) was defined as the concentration that inhibits HIV-1NL4-3 replication by 50%.
Cytotoxicity Assay
A CytoTox-Glo cytotoxicity assay (Promega) was used to determine the cytotoxicity of the synthesized BA derivatives. Mock-infected MT-4 cells were cultured in the presence of various concentrations of the compounds for 2 days. Percent of viable cells was determined by following the protocol provided by the manufacturer. The 50% cytotoxic concentration (CC50) was defined as the concentration that caused a 50% reduction of cell viability.
TZM-bl Assay
Anti-HIV-1 activity was measured as reduction in Luc reporter gene expression after a single round of virus infection of TZM-bl cells. HIV-1 at 200 TCID50 and test samples were mixed in a total volume of 100 mL growth medium in 96-well black solid plates (Corning- Costar). After 48 h incubation, culture medium was removed from each well and 100 mL of Bright Glo luciferase reagent was added to each culture well. The luciferase activity in the assay wells was measured using a Victor 2 luminometer. The 50% inhibitory dose (IC50) was defined as the sample concentration that caused a 50% reduction in Relative Luminescence Units (RLU) compared to virus control wells after subtraction of background RLU.
Microsomal Stability Assay
Stock solutions of 41, as well as reference compounds propranolol and terfenadine (1 mg/mL), were prepared by dissolving the pure compound in DMSO and were stored at 4 °C. Before the assay, the stock solution was diluted with ACN to a concentration of 0.1 mM. For measurement of metabolic stability, all compounds were brought to a final concentration of 1 μM with 0.1 M potassium phosphate buffer at pH 7.4, which contained 0.1 mg/mL human liver microsomes and 5 mM MgCl2. The incubation volumes were 300 μL, and reaction temperature was 37 °C. Reactions were started by adding 60 μL of NADPH (final concentration of 1.0 mM) and quenched by adding 600 μL of ice-cold ACN to stop the reaction at 5, 15, 30, and 60 min time points. Samples at 0 min time point were prepared by adding 600 μL ice-cold ACN first, followed by 60 μL NADPH. Incubations of all samples were conducted in duplicate. After quenching, all samples were centrifuged at 12,000 rpm for 5 min at 0 °C. The supernatant was collected, and 30 μL of the supernatant was injected directly onto a Shimadzu LC-MS-2010 system by auto-sampler. The HPLC-MS analysis was carried out with an electrospray ionization source (ESI). An Alltima C18 column (5 μm, 150 mm × 2.1 mm) was used for HPLC with a gradient elution at a flow rate of 0.3 mL/min. The elution conditions for 41 were ACN (B) in water (A) at 30% for 0–3 min, 95% for 3–9 min, and 30% for 9–12 min. The MS conditions were optimized to detector voltage +1.7 kV, positive acquisition mode with selected ion monitoring (SIM) of the appropriate molecular weights of the testing compounds. The CDL temperature was 250 °C and heat block temperature was 200 °C. The neutralizing gas flow was 1.5 L/min. The peak heights of test compounds at different time points were converted to percentage of remaining, and the peak height values at initial time (0 min) served as 100%. The slope of the linear regression from log percentage remaining versus incubation time relationships (−k) was used to calculate in vitro half-life (t1/2) value by the formula of in vitro t1/2 = 0.693/k, regarded as first-order kinetics. Conversion to in vitro clearance (CLint in units of mL/min/mg protein) was calculated by the formula28: CLint = (0.693/in vitro t1/2) × (mL incubation/mg microsomes). Fast and moderate metabolizing reference compounds terfenadine and propranolol, respectively, have t1/2 of 21.14 and 40.82 min, respectively, in these assay conditions.
Supplementary Material
Acknowledgments
This investigation was supported by Grant AI-077417 from the National Institute of Allergy and Infectious Diseases (NIAID) awarded to K.H.L. I.D.B. thanks the NIAID for a Minority Research Supplement grant. Thanks are also due to the support of Taiwan Department of Health Clinical Trial and Research Center of Excellence (DOH100-TD-B-111-004). Efficient purification of all the synthetic BA derivatives was performed with a Grace Reveleris® flash chromatography system equipped with RevealX(TM) detection allowing for multisignal (UV/ELSD) collection to low mg quantities. Reveleris® Navigator method optimizer and Grace Reveleris® flash silica cartridges were employed for high quality separations.
ABBREVIATIONS USED
- ACN
acetonitrile
- AIDS
acquired immunodeficiency syndrome
- HIV-1
human immunodeficiency virus type 1
- BA
betulinic acid
- HAART
highly active antiretroviral therapy
- MI
maturation inhibitor
- P24 (CA)
capsid
- P25 (CA-SP1)
capsid precursor
- Bevirimat
3-O-(3′,3′-dimethylsuccinyl)-betulinic acid
- AZT
zidovudine
- MOMCl
methoxymethyl chloride
- MEMCl
methoxyethoxymethyl chloride
- DIEA
diisopropylethylamine
- THF
tetrahydrofuran
- EDCI
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride
- DMAP
4-(dimethylamino)pyridine
- TBS
tert-butyldimethylsilyl
- TEA
triethylaluminum
- PTSA
p-toluenesulfonic acid
- TBAF
tetra-n-butylammonium fluoride
- HLMs
human liver microsomes
- PI
post-infection
Footnotes
The authors declare no competing financial interest.
Supporting Information Available: Additional information on compound purity, high-resolution mass spectroscopic data, and HPLC analysis results of the target compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT. Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des. 2005;11:1805–1843. doi: 10.2174/1381612053764869. [DOI] [PubMed] [Google Scholar]
- 2.Ho DD, Neumann AU, Perelson AS, Chen W, Leonard JM, Markowitz M. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature. 1995;373:123–126. doi: 10.1038/373123a0. [DOI] [PubMed] [Google Scholar]
- 3.Hammer SM, Katzenstein DA, Hughes MD, Gundacker H, Schooley RT, Haubrich RH, Henry WK, Lederman MM, Phair JP, Niu M, Hirsch MS, Merigan TC. A trial comparing nucleoside monotherapy with combination therapy in HIV-infected adults with CD4 cell counts from 200 to 500 per cubic millimeter. AIDS Clinical Trials Group Study 175 Study Team. N Engl J Med. 1996;335:1081–1090. doi: 10.1056/NEJM199610103351501. [DOI] [PubMed] [Google Scholar]
- 4.Gulick RM, Meibohm A, Havlir D, Eron JJ, Mosley A, Chodakewitz JA, Isaacs R, Gonzalez C, McMahon D, Richman DD, Robertson M, Mellors JW. Six-year follow-up of HIV-1-infected adults in a clinical trial of antiretroviral therapy with indinavir, zidovudine, and lamivudine. AIDS. 2003;17:2345–2349. doi: 10.1097/00002030-200311070-00009. [DOI] [PubMed] [Google Scholar]
- 5.HIV/AIDS strategy. 2011 http://www.who.int/topics/hiv_aids/en/
- 6.Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, Kuo YH, Brookmeyer R, Zeiger MA, Barditch-Crovo P, Siliciano RF. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 7.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 8.Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 9.Piscitelli SC, Flexner C, Minor JR, Polis MA, Masur H. Drug interactions in patients infected with human immunodeficiency virus. Clin Infect Dis. 1996;23:685–693. doi: 10.1093/clinids/23.4.685. [DOI] [PubMed] [Google Scholar]
- 10.Louie M, Markowitz M. Goals and milestones during treatment of HIV-1 infection with antiretroviral therapy: a pathogenesis-based perspective. Antiviral Res. 2002;55:15–25. doi: 10.1016/s0166-3542(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 11.Boden D, Hurley A, Zhang L, Cao Y, Guo Y, Jones E, Tsay J, Ip J, Farthing C, Limoli K, Parkin N, Markowitz M. HIV-1 drug resistance in newly infected individuals. JAMA. 1999;282:1135–1141. doi: 10.1001/jama.282.12.1135. [DOI] [PubMed] [Google Scholar]
- 12.Fujioka T, Kashiwada Y, Kilkuskie RE, Cosentino LM, Ballas LM, Jiang JB, Janzen WP, Chen IS, Lee KH. Anti-AIDS agents, 11. Betulinic acid and platanic acid as anti-HIV principles from Syzigium claviflorum, and the anti-HIV activity of structurally related triterpenoids. J Nat Prod. 1994;57:243–247. doi: 10.1021/np50104a008. [DOI] [PubMed] [Google Scholar]
- 13.Kashiwada Y, Hashimoto F, Cosentino LM, Chen CH, Garrett PE, Lee KH. Betulinic acid and dihydrobetulinic acid derivatives as potent anti-HIV agents. J Med Chem. 1996;39:1016–1017. doi: 10.1021/jm950922q. [DOI] [PubMed] [Google Scholar]
- 14.Kanamoto T, Kashiwada Y, Kanbara K, Gotoh K, Yoshimori M, Goto T, Sano K, Nakashima H. Anti-human immunodeficiency virus activity of YK-FH312 (a betulinic acid derivative), a novel compound blocking viral maturation. Antimicrob Agents Chemother. 2001;45:1225–1230. doi: 10.1128/AAC.45.4.1225-1230.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li F, Goila-Gaur R, Salzwedel K, Kilgore NR, Reddick M, Matallana C, Castillo A, Zoumplis D, Martin DE, Orenstein JM, Allaway GP, Freed EO, Wild CT. PA-457: a potent HIV inhibitor that disrupts core condensation by targeting a late step in Gag processing. Proc Natl Acad Sci US A. 2003;100:13555–13560. doi: 10.1073/pnas.2234683100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qian K, Nitz TJ, Yu D, Allaway GP, Morris-Natschke SL, Lee KH. Anti-AIDS agents 76. From natural product to clinical trials: bevirimat, a plant-derived anti-AIDS drug. In: Buss T, Butler M, editors. Natural Product Chemistry for Drug Discovery, for the Royal Society of Chemistry Biomolecular Sciences Series. Merlion Pharmaceuticals Pte Ltd Press; Singapore: 2009. pp. 374–391. [Google Scholar]
- 17.Smith PF, Ogundele A, Forrest A, Wilton J, Salzwedel K, Doto J, Allaway GP, Martin DE. Phase I and II study of the safety, virologic effect, and pharmacokinetics/pharmacodynamics of single-dose 3-O-(3′,3′-dimethylsuccinyl)betulinic acid (bevirimat) against human immunodeficiency virus infection. Antimicrob Agents Chemother. 2007;51:3574–3581. doi: 10.1128/AAC.00152-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin DE, Blum R, Doto J, Galbraith H, Ballow C. Multiple-dose pharmacokinetics and safety of bevirimat, a novel inhibitor of HIV maturation, in healthy volunteers. Clin Pharmacokinet. 2007;46:589–598. doi: 10.2165/00003088-200746070-00004. [DOI] [PubMed] [Google Scholar]
- 19.Martin DE, Blum R, Wilton J, Doto J, Galbraith H, Burgess GL, Smith PC, Ballow C. Safety and pharmacokinetics of bevirimat (PA-457), a novel inhibitor of human immunodeficiency virus maturation, in healthy volunteers. Antimicrob Agents Chemother. 2007;51:3063–3066. doi: 10.1128/AAC.01391-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://investors.myriadpharma.com/releasedetail.cfm?ReleaseID=427302.
- 21.Huang L, Ho P, Lee KH, Chen CH. Synthesis and anti-HIV activity of bi-functional betulinic acid derivatives. Bioorg Med Chem. 2006;14:2279–2289. doi: 10.1016/j.bmc.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Qian K, Morris-Natschke SL, Lee KH. HIV entry inhibitors and their potential in HIV therapy. Med Res Rev. 2009;29:369–393. doi: 10.1002/med.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qian K, Yu D, Chen CH, Huang L, Morris-Natschke SL, Nitz TJ, Salzwedel K, Reddick M, Allaway GP, Lee KH. Anti-AIDS agents. 78. Design, synthesis, metabolic stability assessment, and antiviral evaluation of novel betulinic acid derivatives as potent anti-human immunodeficiency virus (HIV) agents. J Med Chem. 2009;52:3248–3258. doi: 10.1021/jm900136j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang ZX, Yu YB. The design and synthesis of highly branched and spherically symmetric fluorinated oils and amphiles. Tetrahedron. 2007;63:3982–3988. doi: 10.1016/j.tet.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang W, Oya S, Kung MP, Hou C, Maier DL, Kung HF. F-18 Polyethyleneglycol stilbenes as PET imaging agents targeting Aβ aggregates in the brain. Nucl Med Biol. 2005;32:799–809. doi: 10.1016/j.nucmedbio.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 26.Zhu CB, Zhu L, Holz-Smith S, Matthews TJ, Chen CH. The role of the third beta strand in gp120 conformation and neutralization sensitivity of the HIV-1 primary isolate DH012. Proc Natl Acad Sci US A. 2001;98:15227–15232. doi: 10.1073/pnas.261359098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qian K, Kuo RY, Chen CH, Huang L, Morris-Natschke SL, Lee KH. Anti-AIDS agents 81. Design, synthesis, and structure-activity relationship study of betulinic acid and moronic acid derivatives as potent HIV maturation inhibitors. J Med Chem. 2010;53:3133–3141. doi: 10.1021/jm901782m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obach RS, Baxter JG, Liston TE, Silber BM, Jones BC, MacIntyre F, Rance DJ, Wastall P. The prediction of human pharmacokinetic parameters from preclinical and in vitro metabolism data. J Pharmacol Exp Ther. 1997;283:46–58. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.