Abstract
Microglia are resident macrophages in the central nervous system (CNS) that play a major role in neuroinflammation and pathogenesis of several neurodegenerative diseases. Upon activation, microglia releases a multitude of pro-inflammatory factors that initiate and sustain an inflammatory response by activating various signalling pathways, including the NF-κB pathway in a feed forward cycle. In microglial cells, activation of NF-κB signalling is normally transient, while sustained NF-κB activation is associated with persistent neuroinflammation. RING finger protein 11 (RNF11), in association with A20 ubiquitin-editing complex, is one of the key negative regulators of NF-κB signalling pathway in neurons. In this study, we have demonstrated and confirmed this role of RNF11 in microglia, the immune cells of the CNS. Coimmunoprecipitation experiments showed that RNF11 and A20 interact in a microglial cell line, suggesting the presence of A20 ubiquitin-editing protein complex in microglial cells. Next, using targeted short hairpin RNA (shRNA) knockdown and over-expression of RNF11, we established that RNF11 expression levels are inversely related to NF-κB activation, as evident from altered expression of NF-κB transcribed genes. Moreover our studies, illustrated that RNF11 confers protection against LPS-induced cell cytotoxicity. Thus our investigations clearly demonstrated that microglial RNF11 is a negative regulator of NF-κB signalling pathway and could be a strong potential target for modulating inflammatory responses in neurodegenerative diseases.
Keywords: Inflammation, NF-κB, RNF11, Microglia, Cytotoxicity
1. Introduction
Microglia are resident macrophages of the central nervous system (CNS) that respond to injury and eliminate infection [1]. They regulate the innate immune response, communicate with other brain cells, and act as scavengers by removing dying cells [2, 3]. Any insult to the brain, exogenous or endogenous, that disturbs its homeostasis leads to microglial activation. Pro-inflammatory and cytotoxic factors, released by activated microglia, have been implicated in initiation and sustenance of neuroinflammatory responses that exacerbate the underlying neuropathology of several neurological diseases such as Huntington’s disease, Alzheimer’s disease (AD), and Parkinson’s disease (PD) [4, 5]. The microglial NF-κB signalling pathway is one of the key players in neuroinflammation [6–8]. In addition, p65 a subunit of NF-κB, was observed to be localized to the nuclei of activated microglia in PD brain tissue [8] implicating NF-κB activation in activated microglia.
In the CNS, NF-κB has crucial roles in both neuronal development and degeneration [9]. Dysregulation of NF-κB signalling can result in defective neuronal development and tumorigenesis, or chronic neuroinflammation and neuronal death [7, 8, 10–13] [14]. Thus the duration of NF-κB activation is tightly regulated for normal homeostasis through mechanisms such as phosphorylation, transcriptional modulation and protein-protein interaction [15]. For instance, NF-κB target genes, such as the ubiquitin-editing protein A20 (also known as tumor necrosis factor, alpha-induced protein 3 or TNFAIP3), IκBs (inhibitors of NF-κB), and cylindromatosis, act as inhibitors of NF-κB signalling [16]. Recent studies have demonstrated the critical role A20 plays in regulating NF-κB signalling cascade, in complex with Tax1 (human T-cell leukemia virus type 1) binding protein 1(TAX1BP1, also called TXBP151 or T6BP), Itch (also known as AIP4), and RNF11 [17–20].
RNF11 is a putative E3 ubiquitin ligase that has been associated with various types of cancers, as well as PD [18, 21]. Additionally, RNF11enhances TGF-β and EGFR endosomal signalling by interacting with Smad4 and Smurf2 [22]. Moreover, RNF11 is an essential component of the A20 ubiquitin editing complex and negatively regulates NF-κB signalling, as demonstrated in a monocytic cell line [20] and neurons [23]. In the current study, we investigated the association of RNF11 with the A20 ubiquitin-editing complex in microglia, the immune cells and mediators of neuroinflammation in the CNS, and determined its role in the NF-κB signalling pathway, inflammatory response and cell survival.
2. Materials and methods
2.1 Cultured cell lines
BV2 murine microglia cells were a kind gift from Dr. Elisabetta Blasi at University of Modena and Reggio Emilia in Italy [24]. Cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with L-glutamine (Lonza), inactivated fetal bovine serum (Lonza), and 1% penicillin/streptomycin (Bio Whittaker). Primary microglia cultures were prepared and harvested as described in [23]. All cultures were maintained at 37°C in 5% CO2.
2.2 Reagents
Cells were stimulated with 10ng/ml TNF-α (R& D Systems) and a range of doses for LPS (Sigma) as indicated in figure legends, for 24 hrs following a 1hr serum starvation period.
2.3 RNA interference
The constructs used for knockdown of RNF11 and overexpression of RNF11 have been previously described [23]. Lentiviruses were produced by the Emory University Viral Vector Core facility.
2.4 Immunoblotting and co-immunoprecipitation
As previously described, cells were grown for 24 hr, rinsed with PBS, and harvested as described in [18]. Immunoprecipitations (IPs) were performed as described in [23], using antibody against rabbit RNF11 (polyclonal antibody) described in [18]. Controls IPs were performed with beads alone to demonstrate specificity. Analysis of IPs completed using immunoblotting. Samples were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes. Primary antibodies against A20 (Abcam), β-actin (Abcam) and RNF11 were used for immunoblotting. Membranes were scanned using the Odyssey Image Station (LiCor).
2.5 Quantitative Real-time PCR
Quantitative Real-time PCR (qRT-PCR) was performed, as described previously [23], on a 7500 Fast RT-PCR instrument (Applied Biosystems) using TaqMan PCR master mix (Applied Biosystems) and gene-specific TaqMan probes (Applied Biosystems) against RNF11 (Mm00450014_m1), TNF-α (Mm00443258_m1), A20 (Mm00437121_m1), MCP-1 (Mm00441242_m1), IL1-B (Mm99999061_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (Applied Biosystems). RNA samples were run in triplicate. Gene expression was normalized to the house-keeping gene GAPDH and relative expression was calculated for each gene using 2−ΔΔCt method.
2.6 Cytotoxicity assay
Quantification of cell death and cell lysis was performed using the Roche Cytotoxicity Detection Kit. After 24hrs of LPS stimulation, the assay was performed and analyzed following manufacturer’s instructions. Culture medium was used as control.
2.7 Statistical analysis
Statistical analysis was performed using Graph Pad Prism version 4.03 software (Graph Pad Software, Inc). One-way analysis of variance (ANOVA) with Tukey’s posttest was performed for qRT-PCR analysis of RNF11 levels. Standard two-way ANOVA was used to analyze the mRNA expression of inflammatory markers, and cytotoxicity assay. Bonferroni post-tests were performed for all two-way ANOVAs. Statistical significance was set at P< 0.05. All experimental results are presented as mean ± SEM for at least three independent experiments.
3. Results
3.1 RNF11 mRNA expression levels are similar in primary microglia and BV2 microglial cell line
Weused BV2 cells, a murine microglia cell line [24] for our investigations since (1) they have been used extensively to study neuroinflammatory responses [4, 7], and (2) we could genetically manipulate RNF11 expression levels more efficiently. Furthermore, we established that BV2 cells had similar RNF11 expression levels as primary microglia, by measuring relative mRNA levels of RNF11 using qRT-PCR (Supplemental Fig. 1).
3.2 RNF11 associates with A20 and is a negative regulator of NF -κB pathway in microglial cell line
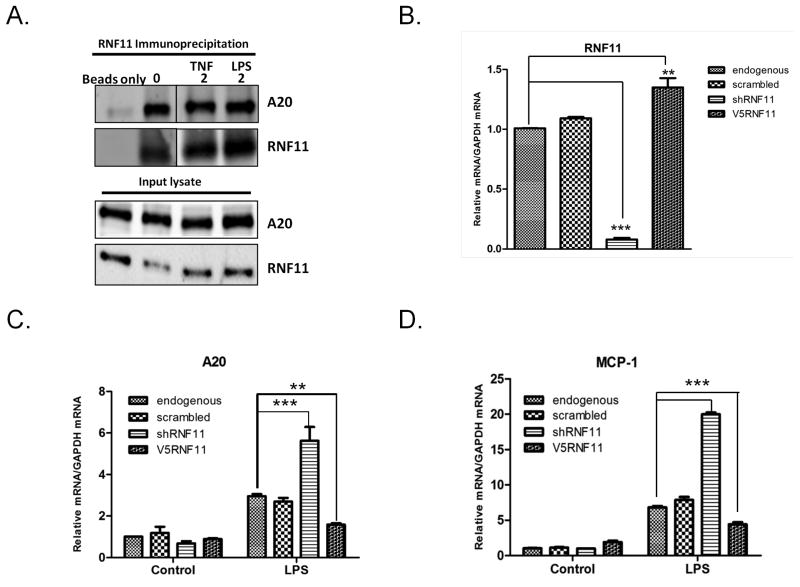
Previous studies in monocytic and neuronal cells suggested that interaction of RNF11 with the A20 ubiquitin-editing protein complex is crucial for mitigation of the canonical NF-κB signaling pathway [20, 23]. To determine if there is an interaction between RNF11 and A20 in microglia, we performed co-immunoprecipitation (co-IP) experiments in BV2 cells. Immunoprecipitates with RNF11 antibody from endogenous cells at steady state and following LPS and TNF-α stimulation were enriched with A20 immunoreactivity while control co-IPs, lacking RNF11 antibody, were absent for A20 immunoreactivity (Fig. 1A). LPS and TNF-α stimulation however did not enhance the association of A20 and RNF11 in BV2 cells with IP western (Fig. 1A). These findings indicate that RNF11 associates with A20 in microglia suggesting that RNF11 could modulate activation of NF-κB signaling pathway in microglia through the A20 ubiquitin-editing protein complex.
Figure 1. RNF11 associates with the A20 and is a negative regulator of NF-κB pathway in microglial cell line.
A) BV2 cell lysates (unstimulated and stimulated with 10ng/ml TNF and 100ng/ml of LPS for 2hrs) were subjected to immunoprecipitation (IP) with RNF11 antibody. Proteins were resolved by SDS-PAGE and immunoblotted with antibodies against A20 and RNF11. Control IP was performed by omitting RNF11 antibody. (B) RNA from lentiviral-transduced BV2 cells was analyzed by qRT-PCR and used to measure the efficiency of RNF11 knockdown and overexpression, relative to GAPDH. (C, D) RNA from untransduced (endogenous) and transduced BV2 (scrambled, shRNF11, V5RNF11) cells was analyzed by qRT-PCR to measure activation of NF-κB pathway markers A20 and MCP-1. Cells were exposed to 100ng/ml LPS for 24 hours. Values are expressed as mean 2 −ΔΔCT ± SEM of triplicate experiments. ** P <0.01, *** P<0.001.
Given the strong association of NF-kB activation with activated microglia and neuroinflammation [4, 13] we examined the role of microglial RNF11 on NF-κB signalling pathway. We transduced BV2 cells with lentivirus expressing shRNA targeted against RNF11 (shRNF11 cells), a scrambled shRNA sequence (scrambled cells), or wild type RNF11 tagged with V5 epitope (V5RNF11 cells) to manipulate RNF11 expression levels. The efficiency of RNF11 knockdown was measured by qRT-PCR. Endogenous RNF11 mRNA levels were reduced 92% in shRNF11 cells compared to endogenous (untransduced) cells (P<0.001) and scrambled cells (P<0.001). RNF11 over-expression increased mRNA levels by 34% compared to endogenous cells (P<0.01) (Fig. 1B). Next, we examined the transcriptional induction of several genes activated by NF-κB signalling. Peak A20 protein level (31% increase) and mRNA level was observed at 24hrs post LPS treatment, therefore, this time-point was chosen for further analysis (Supplemental Fig. 2A & 2B). To determine the effects of RNF11 expression on NF-κB activation, cells were exposed to 100ng/ml LPS for 24hrs. LPS, a component of the surface of gram-negative bacteria, acts as a potent inflammatory stimulus and activates the canonical NF-κB pathway [25, 26]. Specifically, we examined the mRNA expression levels of A20 [19, 27] and monocyte chemoattractant protein-1(MCP-1) by qRT-PCR. The gene for A20 ubiquitin-editing protein is transcribed following NF-κB activation [20], while MCP-1 is a chemokine produced following NF-κB activation [28]. LPS stimulation enhanced A20 mRNA levels in the shRNF11 cells to a greater extent than endogenous and scrambled cells (endogenous: 2.95, scrambled: 2.69, shRNF11: 5.63 fold change; p < 0.001) (Fig. 1C) while RNF11 over-expression reduced A20 mRNA levels (V5RNF11:1.58 fold change; p < 0.01). Similarly, MCP-1 mRNA expression increased with LPS stimulation to a higher degree in shRNF11 cells as compared to scrambled and endogenous cell lines (endogenous: 6.83, scrambled: 7.86, shRNF11:19.99 fold increase; (P < 0.001) while RNF11 over-expression decreased MCP-1 mRNA levels (V5RNF11: 4.41 fold change; (P < 0.001) (Fig. 1D). At steady state, expression levels of A20 and MCP-1 were not significantly different in the transduced cell lines (Fig. 1C, 1D). These results suggest that modulation of RNF11 in microglia influences mRNA levels of gene products downstream of NF-κB implying that RNF11 negatively regulates NF-κB signalling in microglial cells.
3.3 RNF11 modulates inflammatory response and confers protection against cell death in microglial cell line
To determine the effects of RNF11 expression on inflammatory responses, cells were exposed to 100ng/ml LPS for 24hrs. Tumor necrosis factor- α (TNF-α) is one of the cytokines produced by activated microglia in response to infection or injury [2]. Following LPS stimulation, TNF-α mRNA levels were increased in shRNF11 cells as compared to endogenous or scrambled cells (endogenous: 4.16, scrambled: 4.43, shRNF11: 7.56 fold change; p < 0.001) (Fig. 2A). IL1-β is one of the first responders of activated BV2 cells upon stimulation with LPS [29]. Similar to TNF-α, IL1-β mRNA levels were increased following LPS stimulation to a greater extent in shRNF11 cells as compared to the endogenous and scrambled cells (endogenous: 7.20, scrambled: 6.47, shRNF11: 20.33 fold change; p < 0.001) (Fig. 2B). RNF11 over-expression down-regulated both TNF-α (V5RNF11: 1.73 fold change; p < 0.01) (Fig. 2A) and IL1-β mRNA levels (V5RNF11: 1.23 fold change; p < 0.05) (Fig. 2B). At steady state, mRNA expression levels of TNF-α and IL1-β were not significantly different in transduced cell lines. These results implicate that RNF11 expression can modulate inflammatory responses following LPS treatment in microglia cells.
Figure 2. RNF11 modulates inflammatory response and confers protection against cell death in microglial cell line.
(A, B) RNA from untransduced (endogenous) and transduced BV2 (scrambled, shRNF11, V5RNF11) cells was analyzed by qRT-PCR to measure activation of inflammatorymarkers IL1-β and TNF -α. Cells were exposed to 100ng/ml LPS for 24 hours. Values are expressed as mean 2 −ΔΔCT± SEM of triplicate experiments. *P<0.05, **P <0.01, *** P<0.001. (C) Cytotoxicity was measured in untransduced (endogenous) and transduced BV2 (shRNF11, V5RNF11) following 24 hour treatment with different doses of LPS. LDH activity is expressed as % of cell death. *P<0.05, ** P<0.01, ***P <0.001.
To test whether modulation of RNF11 expression can influence the cytotoxic effects of LPS, transduced BV2 cells (endogenous, shRNF11, and V5RNF11) were exposed to various doses of LPS (750ng/ml-5μg/ml) LPS and cytotoxicity was measured after 24 hrs. Following LPS treatment we observed exaggerated cell death in shRNF11 cells (Fig 2C) as compared to endogenous cells: 750ng/ml (endogenous: 35%, shRNF11: 71% p<0.05), 1ug/ml (endogenous: 43%, shRNF11: 78% p<0.001), 2ug/ml (endogenous: 50%, shRNF11: 80%) and 5ug/ml (endogenous: 74%, shRNF11: 81%). V5RNF11 cells had significantly decreased cell death levels with all the different doses of LPS treatment compared to both endogenous and shRNF11 cells (V5RNF11: 0.8% to 2.5%; p<0.001) (Fig. 2C). Cells treated with vehicle did not show significant differences. Near complete protection observed from LPS-induced toxicity in microglial cells over-expressing RNF11, clearly demonstrated the protective role of RNF11. We propose that RNF11 likely contributes to the mitigation of cytotoxic inflammatory responses exerted by microglia themselves.
4. Discussion
The NF-κB pathway has a key role in immune responses and inflammatory processes by regulating transcription of various genes [30]. Diseases involving inflammation, including neurodegenerative diseases, have been associated with persistent activation of the NF-kB signalling pathway [30, 31]. Additionally, animal models of AD, PD, and ischemia have demonstrated that systemic inhibition of NF-κB prevents cell death and attenuation of neuroinflammation [8, 32, 33]. Since neuroinflammation in the brain is mediated mainly by microglia [1, 13], we investigated the role of RNF11, a modulator of NF-κB pathway, in a microglial cell line in this study. Our results show that microglial RNF11 interacts with A20 and also functions as a negative regulator of NF-κB signalling. Moreover, we demonstrated that RNF11 confers significant protection to microglial cells from LPS-induced toxicity. Together our findings suggest a critical role for RNF11 in the regulation of the NF-κB signalling in microglia.
Previous reports have used BV2 cells to demonstrate the role of microglia in neuroinflammation [4, 6, 7, 34]. In our study we used the BV2 cells to alter RNF11 levels since primary microglial cultures resist transfection or transduction protocols. By altering expression levels and measuring mRNA expression of NF-κB target genes [35–37], inflammatory markers [7, 8, 29, 38] and LPS-induced cell death, we have demonstrated the role of microglial RNF11 as a modulator of 1) NF-κB signalling pathway, 2) inflammatory response and 3) cell survival.
The ubiquitin-editing protein A20 functions in a complex with TAX1BP, Itch and RNF11, in a negative feed-back loop to limit NF-κB activation [20]. Additionally, RNF11 has been reported to be associated with A20, and TAX1BP1 in fibroblasts, macrophages, and HEK cells [20]. The A20 ubiquitin-editing protein complex and the NF-κB family of proteins are highly expressed in peripheral immune system [30, 39, 40]. We have recently reported that components of the A20 complex are present in microglia and neurons in the human brain [41]. In this investigation, we demonstrate that RNF11 not only interacts with A20, but also negatively regulates NF-κB activation in microglia-like cells, as previously reported in peripheral and neuronal system [20, 23]. Furthermore, our data establishes the functionality of the regulatory A20 ubiquitin-editing complex in glial cells by demonstrating that microglial RNF11 can modulate NF-κB activation and inflammatory responses.
Increased expressions of cytokines and chemokines by microglia have been correlated with microglial activation and associated nueroinflammatory responses [6, 8, 13]. In our study we demonstrated that RNF11 modulates inflammatory responses by regulating expression of inflammatory cytokines. Inflammatory responses and persistent activation of NF-κB pathway have been associated with cytotoxicity in various cell types [42, 43]. Though LPS-induced cell death has rarely been reported in microglial cells [4, 44], here we discovered that RNF11 can not only protect against LPS-induced toxicity but that RNF11 knock down can exaggerate LPS toxicity in BV2 cells. Our study thus implies that, RNF11, as a negative regulator of NF-κB pathway, is critical not only in modulating inflammatory responses but also crucial for the survival of microglia cells.
Given the growing evidence that chronic dysregulation of NF-κB signalling pathway results in developmental and degenerative disorders in the CNS [13, 45, 46], the role of microglial RNF11 in modulating NF-κB activation will be vitally important.
Conclusion
From the data presented above, we conclude that RNF11 is a negative regulator of NF-κB activation and inflammatory responses in microglia. Microglia play a dynamic functional role in initiation and subsequent exacerbation of neuroinflammation and is one of most common pathological markers associated with neurodegenerative disease. This work will potentiate understanding of the mechanisms involved in controlling persistent NF-κB activation and subsequent chronic neuroinflammation.
Supplementary Material
RNA from murine primary microglia and BV2 cells was analyzed by qRT-PCR for RNF11 expression, relative to GAPDH. Values are expressed as mean comparative cycle threshold ± SEM.
(A) BV2 cells were stimulated for 0, 2, 4, 6, 16 and 24hrs with 100ng/ml LPS and proteins were resolved with SDS-PAGE, and blotted for A20. Image J was used to quantify the ratio of the densitometry of the A20 bands to that of β-actin. (B) BV2 mRNA stimulated with 100ng/ml LPS for 0, 2, 4, 6 and 24hrs was analyzed by qRT-PCR for A20 levels, relative to GAPDH. Values are expressed as mean 2 −ΔΔCT ± SEM of triplicate experiments.
Highlights.
RNF11 associates with A20 in the glial system
RNF11 is a negative regulator of NF-κB signalling pathway in microglia
Overexpression of RNF11 is protective against LPS-induced cytotoxicity
Acknowledgments
We thank Dr Jeremy H. Herskowitz for constructive discussion, Dr Yepes laboratory for primary microglia cultures. This research was supported by NIEHS ES015777 (RSB), NIEHSES012870 (ELP), NINDSNS007480 (ELP), and Emory Viral Vector Core, P30NS055077.
Abbreviations
- AD
Alzheimer’s disease
- CNS
central nervous system
- co-IP
coimmunoprecipitation
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- IκBs
inhibitors of NF-κB
- IP
Immunoprecipitation
- LPS
lipopolysaccharide
- MCP-1
monocyte chemoattractant protein-1
- PD
Parkinson’s disease
- PCR
polymerase chain reaction
- qRT-PCR
quantitative real-time PCR
- RNF11
RING finger protein 11
- SDS-PAGE
sodium dodecyl sulphate polyacrylamide gel electrophoresis
- Tax1
human T-cell leukemia virus type 1
- TAX1BP1
Tax1 binding protein 1
- TLR
Toll-like receptors
- TNF
tumor necrosis factor
- TNFAIP3
tumor necrosis factor, alpha-induced protein 3
- TNFR
tumour necrosis factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nirjari V. Dalal, Email: nvdalal@emory.edu.
Elaine L. Pranski, Email: epransk@emory.edu.
Malu G. Tansey, Email: malu.tansey@emory.edu.
James J. Lah, Email: jlah@emory.edu.
Allan I. Levey, Email: alevey@emory.edu.
Ranjita S. Betarbet, Email: rbetarb@emory.edu.
References
- 1.Perry VH, Nicoll JA, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6(4):193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 2.Liu B, Hong JS. Role of microglia in inflammation-mediated neurodegenerative diseases: mechanisms and strategies for therapeutic intervention. J Pharmacol Exp Ther. 2003;304(1):1–7. doi: 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- 3.Puntambekar SS, Doose JM, Carson MJ. Microglia: A CNS-Specific Tissue Macrophage. In: Lane T, Carson M, Bergmann C, Wyss-Coral T, editors. Central Nervous System Diseases and Inflammation. Vol. 1. New York: Springer; 2008. pp. 1–12. [Google Scholar]
- 4.Kim BW, Koppula S, Kim JW, Lim HW, Hwang JW, Kim IS, Park PJ, Choi DK. Modulation of LPS-stimulated neuroinflammation in BV-2 microglia by Gastrodia elata: 4-hydroxybenzyl alcohol is the bioactive candidate. J Ethnopharmacol. 2012;139(2):549–557. doi: 10.1016/j.jep.2011.11.048. [DOI] [PubMed] [Google Scholar]
- 5.Nelson PT, Soma LA, Lavi E. Microglia in diseases of the central nervous system. Ann Med. 2002;34(7–8):491–500. doi: 10.1080/078538902321117698. [DOI] [PubMed] [Google Scholar]
- 6.Lee JK, Chung J, McAlpine FE, Tansey MG. Regulator of G-protein signaling-10 negatively regulates NF-kappaB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats. J Neurosci. 2011;31(33):11879–11888. doi: 10.1523/JNEUROSCI.1002-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tran T, McCoy M, Sporn M, Tansey M. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. Journal of Neuroinflammation. 2008;5(1):14. doi: 10.1186/1742-2094-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghosh A, Roy A, Liu X, Kordower JH, Mufson EJ, Hartley DM, Ghosh S, Mosley RL, Gendelman HE, Pahan K. Selective inhibition of NF-κB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proceedings of the National Academy of Sciences. 2007;104(47):18754–18759. doi: 10.1073/pnas.0704908104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Neill LA, Kaltschmidt C. NF-kappa B: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20(6):252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- 10.Bonini SA, Ferrari-Toninelli G, Uberti D, Montinaro M, Buizza L, Lanni C, Grilli M, Memo M. Nuclear factor kappaB-dependent neurite remodeling is mediated by Notch pathway. J Neurosci. 2011;31(32):11697–11705. doi: 10.1523/JNEUROSCI.1113-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaltschmidt B, Kaltschmidt C. NF-kappaB in the nervous system. Cold Spring Harb Perspect Biol. 2009;1(3):a001271. doi: 10.1101/cshperspect.a001271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mattson MP, Camandola S. NF-κB in neuronal plasticity and neurodegenerative disorders. The Journal of Clinical Investigation. 2001;107(3):247–254. doi: 10.1172/JCI11916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140(6):918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mattson MP, Meffert MK. Roles for NF-kappaB in nerve cell survival, plasticity, and disease. Cell Death Differ. 2006;13(5):852–860. doi: 10.1038/sj.cdd.4401837. [DOI] [PubMed] [Google Scholar]
- 15.Magne N, Toillon RA, Bottero V, Didelot C, Houtte PV, Gerard JP, Peyron JF. NF-kappaB modulation and ionizing radiation: mechanisms and future directions for cancer treatment. Cancer Lett. 2006;231(2):158–168. doi: 10.1016/j.canlet.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 16.Harhaj EW, Dixit VM. Deubiquitinases in the regulation of NF-kappaB signaling. Cell Res. 2011;21(1):22–39. doi: 10.1038/cr.2010.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee EG, Boone DL, Chai S, Libby SL, Chien M, Lodolce JP, Ma A. Failure to regulate TNF-induced NF-kappaB and cell death responses in A20-deficient mice. Science. 2000;289(5488):2350–2354. doi: 10.1126/science.289.5488.2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson LR, Betarbet R, Gearing M, Gulcher J, Hicks AA, Stefansson K, Lah JJ, Levey AI. PARK10 candidate RNF11 is expressed by vulnerable neurons and localizes to Lewy bodies in Parkinson disease brain. J Neuropathol Exp Neurol. 2007;66(10):955–964. doi: 10.1097/nen.0b013e3181567f17. [DOI] [PubMed] [Google Scholar]
- 19.Shembade N, Ma A, Harhaj EW. Inhibition of NF-kappaB signaling by A20 through disruption of ubiquitin enzyme complexes. Science. 2010;327(5969):1135–1139. doi: 10.1126/science.1182364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shembade N, Parvatiyar K, Harhaj NS, Harhaj EW. The ubiquitin-editing enzyme A20 requires RNF11 to downregulate NF-[kappa]B signalling. EMBO J. 2009;28(5):513–522. doi: 10.1038/emboj.2008.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramaniam V, Li H, Wong M, Kitching R, Attisano L, Wrana J, Zubovits J, Burger AM, Seth A. The RING-H2 protein RNF11 is overexpressed in breast cancer and is a target of Smurf2 E3 ligase. Br J Cancer. 2003;89(8):1538–1544. doi: 10.1038/sj.bjc.6601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li H, Seth A. An RNF11: Smurf2 complex mediates ubiquitination of the AMSH protein. Oncogene. 2004;23(10):1801–1808. doi: 10.1038/sj.onc.1207319. [DOI] [PubMed] [Google Scholar]
- 23.Pranski EL, Dalal NV, Herskowitz JH, Orr AL, Roesch LA, Fritz JJ, Heilman C, Lah JJ, Levey AI, Betarbet RS. Neuronal RING finger protein 11 (RNF11) regulates canonical NF-kappaB signaling. J Neuroinflammation. 2012;9(1):67. doi: 10.1186/1742-2094-9-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- 25.Andreakos E, Sacre SM, Smith C, Lundberg A, Kiriakidis S, Stonehouse T, Monaco C, Feldmann M, Foxwell BM. Distinct pathways of LPS-induced NF-kappa B activation and cytokine production in human myeloid and nonmyeloid cells defined by selective utilization of MyD88 and Mal/TIRAP. Blood. 2004;103(6):2229–2237. doi: 10.1182/blood-2003-04-1356. [DOI] [PubMed] [Google Scholar]
- 26.Sharif O, Bolshakov VN, Raines S, Newham P, Perkins ND. Transcriptional profiling of the LPS induced NF-kappaB response in macrophages. BMC Immunol. 2007;8:1. doi: 10.1186/1471-2172-8-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krikos A, Laherty CD, Dixit VM. Transcriptional activation of the tumor necrosis factor alpha-inducible zinc finger protein, A20, is mediated by kappa B elements. J Biol Chem. 1992;267(25):17971–17976. [PubMed] [Google Scholar]
- 28.Ueda A, Ishigatsubo Y, Okubo T, Yoshimura T. Transcriptional regulation of the human monocyte chemoattractant protein-1 gene. Cooperation of two NF-kappaB sites and NF-kappaB/Rel subunit specificity. J Biol Chem. 1997;272(49):31092–31099. doi: 10.1074/jbc.272.49.31092. [DOI] [PubMed] [Google Scholar]
- 29.Kim YJ, Hwang SY, Oh ES, Oh S, Han IO. IL-1beta, an immediate early protein secreted by activated microglia, induces iNOS/NO in C6 astrocytoma cells through p38 MAPK and NF-kappaB pathways. J Neurosci Res. 2006;84(5):1037–1046. doi: 10.1002/jnr.21011. [DOI] [PubMed] [Google Scholar]
- 30.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- 31.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 32.Cao S, Theodore S, Standaert DG. Fcgamma receptors are required for NF-kappaB signaling, microglial activation and dopaminergic neurodegeneration in an AAV-synuclein mouse model of Parkinson’s disease. Mol Neurodegener. 2010;5:42. doi: 10.1186/1750-1326-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Kooij MA, Nijboer CH, Ohl F, Groenendaal F, Heijnen CJ, van Bel F, Kavelaars A. NF-kappaB inhibition after neonatal cerebral hypoxia-ischemia improves long-term motor and cognitive outcome in rats. Neurobiol Dis. 2010;38(2):266–272. doi: 10.1016/j.nbd.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 34.Chinta SJ, Ganesan A, Reis-Rodrigues P, Lithgow GJ, Andersen JK. Anti-Inflammatory Role of the Isoflavone Diadzein in Lipopolysaccharide-Stimulated Microglia: Implications for Parkinson’s Disease. Neurotox Res. 2012 doi: 10.1007/s12640-012-9328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Hallenbeck JM, Ruetzler C, Bol D, Thomas K, Berman NEJ, Vogel SN. Overexpression of Monocyte Chemoattractant Protein 1 in the Brain Exacerbates Ischemic Brain Injury and Is Associated With Recruitment of Inflammatory Cells. J Cereb Blood Flow Metab. 2003;23(6):748–755. doi: 10.1097/01.WCB.0000071885.63724.20. [DOI] [PubMed] [Google Scholar]
- 36.Conductier G, Blondeau N, Guyon A, Nahon JL, Rovere C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J Neuroimmunol. 2010 doi: 10.1016/j.jneuroim.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Shembade N, Harhaj E. A20 inhibition of NFkappaB and inflammation: targeting E2:E3 ubiquitin enzyme complexes. Cell Cycle. 2010;9(13):2481–2482. doi: 10.4161/cc.9.13.12269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee JK, Tran T, Tansey MG. Neuroinflammation in Parkinson’s disease. J Neuroimmune Pharmacol. 2009;4(4):419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shembade N, Harhaj NS, Parvatiyar K, Copeland NG, Jenkins NA, Matesic LE, Harhaj EW. The E3 ligase Itch negatively regulates inflammatory signaling pathways by controlling the function of the ubiquitin-editing enzyme A20. Nat Immunol. 2008;9(3):254–262. doi: 10.1038/ni1563. [DOI] [PubMed] [Google Scholar]
- 40.Shembade N, Harhaj NS, Liebl DJ, Harhaj EW. Essential role for TAX1BP1 in the termination of TNF-alpha-, IL-1- and LPS-mediated NF-kappaB and JNK signaling. EMBO J. 2007;26(17):3910–3922. doi: 10.1038/sj.emboj.7601823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pranski EL, Van Sanford CD, Dalal NV, Orr AL, Karmali D, Cooper DS, Costa N, Heilman CJ, Gearing M, Lah JJ, Levey AI, Betarbet RS. Comparative distribution of protein components of the A20 ubiquitin-editing complex in normal human brain. Neurosci Lett. 2012 doi: 10.1016/j.neulet.2012.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Muroya M, Chang K, Uchida K, Bougaki M, Yamada Y. Analysis of cytotoxicity induced by proinflammatory cytokines in the human alveolar epithelial cell line A549. Biosci Trends. 2012;6(2):70–80. [PubMed] [Google Scholar]
- 43.Cardoso FL, Kittel A, Veszelka S, Palmela I, Toth A, Brites D, Deli MA, Brito MA. Exposure to Lipopolysaccharide and/or Unconjugated Bilirubin Impair the Integrity and Function of Brain Microvascular Endothelial Cells. PLoS One. 2012;7(5):e35919. doi: 10.1371/journal.pone.0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheong MH, Lee SR, Yoo HS, Jeong JW, Kim GY, Kim WJ, Jung IC, Choi YH. Anti-inflammatory effects of Polygala tenuifolia root through inhibition of NF-kappaB activation in lipopolysaccharide-induced BV2 microglial cells. J Ethnopharmacol. 2011;137(3):1402–1408. doi: 10.1016/j.jep.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nat Rev Neurosci. 2002;3(3):216–227. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- 46.Wyss-Coray T, Mucke L. Inflammation in neurodegenerative disease--a double-edged sword. Neuron. 2002;35(3):419–432. doi: 10.1016/s0896-6273(02)00794-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RNA from murine primary microglia and BV2 cells was analyzed by qRT-PCR for RNF11 expression, relative to GAPDH. Values are expressed as mean comparative cycle threshold ± SEM.
(A) BV2 cells were stimulated for 0, 2, 4, 6, 16 and 24hrs with 100ng/ml LPS and proteins were resolved with SDS-PAGE, and blotted for A20. Image J was used to quantify the ratio of the densitometry of the A20 bands to that of β-actin. (B) BV2 mRNA stimulated with 100ng/ml LPS for 0, 2, 4, 6 and 24hrs was analyzed by qRT-PCR for A20 levels, relative to GAPDH. Values are expressed as mean 2 −ΔΔCT ± SEM of triplicate experiments.